Abstract
Objective
A rapidly accumulating body of research suggests that exercise can improve symptoms and wellbeing in patients suffering from psychosis. Exercise may also promote neurogenesis in the hippocampus, a structure that plays an important role in the pathophysiology of psychosis. To date, there has not been an intervention focused on exercise prior to the onset of psychosis, a critical time for prevention of more serious illness.
Method
In this pilot study, 12 young adults at ultrahigh risk (UHR) for psychosis were enrolled in a 12-week open-label exercise intervention. Participants were randomized to exercise 2 or 3 times each week and exercised between 65–85% of maximal oxygen capacity (VO2max) for 30 minutes each session under the supervision of an exercise physiologist. Positive and negative symptoms, social and role function, performance on neurocognitive tests, cardiovascular fitness, and hippocampal structure and functional connectivity were evaluated before and after the trial.
Results
A total of 9 participants completed the exercise intervention. Participants showed improved positive and negative symptoms and social and role functioning; improvement in multiple areas of cognition; and increased functional connectivity between the left hippocampus and occipital cortex after 12 weeks of exercise.
Conclusion
The results of this study suggest that exercise interventions are feasible in an UHR sample and may promote improvement in clinical, social, and cognitive domains as well as changes to brain function in regions impacted by the development of psychosis. These findings set the stage for an ongoing phase-II randomized controlled trial.
Clinical Trials Registration
Keywords: Exercise, Hippocampus, Prodrome, Intervention, Open-Label
INTRODUCTION
Regular physical activity is an important component to maintaining physical and mental health.1 A growing body of innovative research suggests that exercise could be an impactful supplement to traditional treatments for psychosis.2, 3 Recent reviews illustrate a variety of aerobic, anaerobic and combined exercise interventions for patients with psychosis including running, walking, team sports, weight lifting, and yoga.3–5 Indeed, there have been exciting developments in exercise interventions in schizophrenia populations, and findings suggest that exercise may improve symptom, cognitive, cardiovascular, social and role functioning, and neurobiological domains; potentially facilitating greater quality of life and well-being for people with debilitating mental illnesses.3, 6–11 Given the demonstrated benefits of exercise interventions in those with a formal psychotic disorder, the current study aims to test the feasibility of an aerobic exercise intervention prior to the onset of the illness.
The period preceding the onset of psychosis is characterized by attenuated psychotic symptoms in both positive and negative domains and a decline in functioning.12 Studying individuals at ultrahigh risk (UHR) for psychosis can be an important point of intervention, as the disorder typically develops in early adulthood and remains a chronic problem throughout life; intervening early in the course of the illness may help prevent or decrease the significant costs and distress caused by the illness.13
Previous work provides an impetus for exercise interventions prior to the onset of psychosis. Cross sectional and prospective studies show that UHR individuals on average spend more time being sedentary, engage in less intense exercise, and have poorer markers of cardiorespiratory fitness compared to those who did not develop the disorder or healthy controls.14,15,16 In addition, UHR individuals report less motivation for getting exercise, and engage in exercise that requires little social interaction compared to healthy individuals.17,18 Adolescents at risk for psychosis are less physically active and often engage in poor health behaviors compared to typically developing adolescents, including an increased rate of tobacco and alcohol use.19 Taken together, individuals at risk for psychosis appear to be less physically active than typically developing young adults and this may have an impact on other markers of health and functioning.
One possible mechanism by which exercise improves cognition, as well as positive and negative symptoms, is by promoting neuroplasticity in the hippocampus.20–22 The hippocampus has been widely cited as a major brain region impaired in psychosis.23 This impairment has far ranging implications for the neurobiology underlying signs and symptoms of psychosis, as the brain region plays an important role in both modulating the biological stress response and higher order cognitive functions.24 Furthermore, there is evidence that the structure and connections to the hippocampus are impaired prior to the onset of psychosis.25, 26 Importantly, cross sectional research with UHR individuals suggests that smaller temporal lobe volume is related to decreased physical activity.16 Examining the structure and function of the hippocampus may provide important insight into neurobiological effects of exercise in UHR participants.
We recruited 12 UHR participants to complete 3 months of moderate-to-vigorous intensity aerobic exercise. The participants underwent structured clinical interviews, assessment of social and role function, cognitive testing, cardiovascular fitness testing, and structural and resting state functional neuroimaging of the hippocampus before and after the exercise intervention. The major objective of this pilot study was to establish the feasibility of an exercise intervention for UHR participants. Based on the noted results from patients with schizophrenia,2, 3, 27 we conducted exploratory analysis of positive and negative symptoms, social and role function, cognition, and physical fitness. In addition, we examined change in hippocampal volume and anatomical seed based functional connectivity.
METHODS
About the participants
Adolescent and young adult UHR participants between 16 and 24 years of age (M = 19.42, SD = 1.16) were recruited to the University of Colorado Boulder’s Adolescent Development and Preventive Treatment (ADAPT) research program. An inclusion criterion for the study was that the participant reported a predominantly sedentary lifestyle of no more than 60 minutes of at least moderate physical activity per week for the past six months. Exclusion criteria consisted of head injury, the presence of a neurological disorder, lifetime substance dependence, current antipsychotic medication use, and the presence of any contraindication to the magnetic resonance imaging (MRI) environment (e.g. pregnant or metal in the body). The presence or lifetime history of an Axis I psychotic disorder was also an exclusion criterion. The study was approved by the University of Colorado Boulder Institutional Review Board, written consent or assent was obtained, and the study was registered at ClinicalTrials.gov (identifier: NCT02155699).
Clinical Interviews
The Structured Interview for Prodromal Syndromes (SIPS)28, 29 was administered before and after the exercise intervention to diagnose a prodromal syndrome and track positive and negative symptom changes. A total sum score for the positive and negative symptom domain is used as an indicator of the respective dimensions of symptomatology. The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID-IV)30 was also administered to rule out a psychotic disorder diagnosis. Interviewers were kept blind to the prescribed exercise condition for each participant. See supplemental material for more information regarding UHR criteria and training of clinical interviewers.
Assessment of social/role functioning
Social and role functioning was assessed by trained graduate students using scales specifically designed for adolescents/young adults: the Global Functioning Scale: Social (GFS:S)31 and the Global Functioning Scale: Role (GFS:R).32,33 These scales provide ratings on two separate 10-point Likert scales, which are scored independent of symptom severity and higher scores correspond to better functioning.
Cognitive Testing
Participants completed the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery of cognitive tasks before and after the exercise intervention.34 The MATRICS battery includes tests for speed of processing, verbal and visual learning, working memory, reasoning and problem solving, attention, and social cognition. The MATRICS was administered by trained graduate students in a quiet room. Raw scores were transformed to T scores using established standard scores, which were used in analyses concerning pre/post differences.
Physical Fitness Assessment
Baseline and follow-up cardiovascular fitness was measured with maximum oxygen uptake (VO2max), which also served as the basis for individually tailored exercise prescriptions.35 The participants ran on a treadmill and the speed remained the same throughout the test but the incline of the treadmill belt increased 2% every 2 min (or 2.5% for speeds 6 mph or greater). The treadmill speed was determined using participant heart rate and ratings of perceived exertion (RPE). The initial speed was set to 70% of age-predicted max heart rate and an RPE rating of around 13 (“somewhat hard”). Staying within these parameters generally yielded an 8–12 min test; the recommended target for VO2max testing.36 VO2max was determined using a valid primary criterion of having achieved a plateau in VO2 as well as secondary criteria outlined by Pimentel and colleagues37 including respiratory exchange ratio (RER)max≥1.1, RPEmax≥18, and age predicted heart rate max ±10bpm.
Exercise Intervention
Participants were randomly assigned to one of two conditions: moderate or vigorous. The moderate condition required exercise 2 days a week at 65% of their VO2max for a total of 24 sessions. The vigorous condition required exercise 3 days a week at 85% of the participant’s VO2max for a total of 36 sessions. Participants wore a Polar FT1 heart rate monitor (https://www.polar.com/us-en) throughout each exercise session, and an exercise physiologist monitored the participant’s exercise in order to keep the participant’s heart rate at ± 5% of the prescribed target intensity. Initial exercise sessions lasted 15 minutes at 55% of VO2max intensity and were gradually increased to 30 minutes and target intensity within the first 3 weeks. The remaining exercise sessions lasted 30 minutes and were conducted at prescribed exercise intensity. Participants were given the choice to ride stationary bikes, run/walk on treadmills, or use elliptical machines at each session. At the end of each exercise session, participants were compensated.
Structural and Resting state functional connectivity MRI processing
Structural MRI scans were acquired on a Siemens 3-Tesla Magnetom TIM Trio MRI scanner (Siemens AG, Munich, Germany) with a 12-channel head coil. Left and right hippocampus and total intracranial volume were segmented using the FreeSurfer 5.3.0 suite of automated tools.38 Each structure was divided by the participant’s TICV to control for whole brain volume.
A resting state functional connectivity MRI (fcMRI) scan was acquired. Data were preprocessed in FSL (v.5; http://fsl.fmrib.ox.ac.uk/fsl), and fcMRI analysis was performed in the Conn toolbox v.15b.39 Connectivity between the left or right hippocampal seed ROI was calculated with all other voxels in the brain. For more detailed methods of the structural and fcMRI analysis see the supplemental material.
Data Analysis
Following a similar strategy to Nuechterlein and colleagues7 for examining the magnitude of changes following exercise in a small sample only the effect size estimates (Cohen’s d) were calculated for pre/post outcome variables.
RESULTS1
Participants
A total of 12 participants (6 male, 6 female) were recruited to the exercise study, which took place over a 24-month recruitment period. Of those participants, 9 (4 male, 5 female; 75% of sample) participants completed the exercise intervention and returned for follow-up assessment. There were 7 participants in the moderate condition that completed 24 sessions and 2 participants in the vigorous condition that completed 36 sessions. The 3 drop-outs were in the vigorous condition and completed 8, 18, and 31 sessions. Because there were few participants who completed the vigorous condition, subsequent analyses of the exercise intervention collapsed across conditions.
Social/Role functioning and symptom improvement post trial
There was a small to medium improvement in social functioning (d = .45) and role functioning (d = .33). Participants reported a medium to large decrease in positive symptoms post exercise, (d = −.61) as well as a small to medium decrease in negative symptoms, (d = −.47). See Table 1.
Table 1.
Behavioral outcome measures for the exercise intervention. Mean (SD) of outcome variables to the exercise study. Cohen’s d is used to measure the change in outcome variable pre to post exercise intervention.
| Variable | Pre - Exercise Mean (SD) |
Post - Exercise Mean (SD) |
d |
|---|---|---|---|
| Symptoms | |||
| Positive Symptoms | 12.33 (4.91) | 9.89 (4.04) | −.61 |
| Negative Symptoms | 12.75 (9.29) | 9 (6.16) | −.47 |
| Social and Role Functioning | |||
| Global Functioning: Social | 6.83 (1.19) | 7.33 (1.5) | .45 |
| Global Functioning Role | 6.83 (1.4) | 7.22 (1.09) | .33 |
| Cognition | |||
| Speed of Processing | 52.22 (9.3) | 58.22 (13.38) | 1.3 |
| Attention | 46.38 (7.84) | 51 (6.08) | .76 |
| Working Memory | 49 (8.22) | 51.78 (8.76) | .92 |
| Verbal Learning | 48.22 (9.4) | 51.11 (9.61) | .63 |
| Visual Learning | 44.56 (8.43) | 51.56 (5.96) | .76 |
| Reasoning and Problem Solving | 51.56 (4.85) | 55 (5.81) | .47 |
| Social Cognition | 47.22 (7.31) | 44.22 (12.83) | −.31 |
| Composite | 48.25 (8.41) | 52.89 (9.21) | 1.74 |
| Hippocampus Volume | |||
| Left Hippocampus | .0025 (.00024) | .0025 (.00019) | .18 |
| Right Hippocampus | .0026 (.00025) | .0026 (.00019) | .31 |
| Physical health | |||
| VO2-max | 41.16 (8.27) | 39.52 (8.58) | −.28 |
Cognitive improvement
Participants exhibited substantial post trial improvement in a number of cognitive domains including Working Memory (d = .92), Verbal Learning (d = .63), Visual Learning (d = .76), Speed of Processing (d = 1.3), Attention/Vigilance (d = .76), and Reasoning and Problem Solving (d = .47). There was no improvement in social cognition (d = −.31). Overall, the participants showed significant improvement on the MATRICS cognitive battery, with a large composite score improvement (d = 1.74).
Physical fitness
The participants did not show improvements to physical fitness as measured by VO2max (d = −.28).
Structural imaging and resting state functional connectivity
There were no changes to hippocampal volume post exercise for either the left (d = .18) or right (d = .31) hippocampus. Using the left hippocampus seed, participants showed increased functional connectivity to bilateral occipital cortices after the exercise intervention. The right hippocampus seed did not show changes in connectivity. See Table 2 and Figure 1.
Table 2.
Results of the left hippocampal seed based connectivity analysis. MNI coordinates for the peak cluster location, the peak cluster size (in voxels) are included.
| Direction of Connectivity | Region | Cluster Size | MNI Coordinates | t-value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| X | Y | Z | ||||
| Baseline < Post Exercise | Left Occipital Pole | 526 | −16 | −104 | 4 | 9.71*** |
| Right Occipital Pole | 256 | 32 | −98 | 0 | 10.79*** | |
| Lateral Occipital Cortex | 63 | 52 | −76 | −6 | 10.96*** | |
FDR corrected, cluster level p-values are noted for all t-values as
p≤.05,
p≤.01,
p≤.001.
Figure 1.
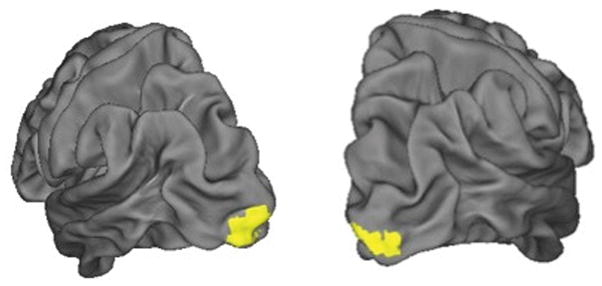
Occipital regions showing increased connectivity to the left hippocampal anatomical seed after the exercise intervention.
DISCUSSION
In the first open-label exercise intervention for youth at risk for psychosis, we showed that exercise led to a reduction in positive and negative symptoms, improved social and role function, and improved cognition. These results suggest that exercise interventions are feasible within UHR samples and may help to improve important domains that are affected during the development of psychosis. Unique to this study from others in psychosis is the examination of brain structure and functional connectivity, suggesting that exercise may lead to changes in the functional organization of cortico-hippocampal networks.
As noted, we recruited a total of 12 participants to the exercise intervention. Of the 9 participants who completed the study, each attended 100% of the exercise sessions. It is important to note that the retention of participants and adherence to the aerobic exercise guidelines was in line with and in some cases better than other exercise interventions in adolescent and adult samples.40–42 While the sample size in this study is small, it is similar to other aerobic exercise interventions in schizophrenia that have reported sample sizes between 8 and 16 participants.2 Anecdotally, many of the participants were interested in being in the study to become more physically active and to improve their overall health. This may have been an important motivational tool to seeking help initially, as exercise has positive social benefits and does not carry the same stigma as psychotherapy or pharmacological treatment.43, 44 Social factors regarding the high value of exercise in the community in which the study was conducted (Boulder, CO) as well as monetary and other incentives may have also played a role in motivating participants to complete the intervention. For those who did not complete the exercise intervention, low motivation and loss of contact during the exercise intervention remained a substantial barrier. Innovative efforts to address adherence to exercise regimens in patients with psychosis have suggested that initial sessions of an exercise intervention include psychoeducation around exercise and goal setting.45–47
An important outcome of this study is the improvement of positive and negative symptoms, as well as social and role functioning. One mechanism by which exercise may improve symptoms and increase social and role function is through behavioral activation.48, 49 Another explanation from exercise interventions in schizophrenia patients suggests that exercise may promote self-esteem and confidence, leading to further engagement in the world and better well-being.50 It is also possible that regression to the mean accounted for these results, and it will be important to conduct a controlled trial to ensure the changes are related to the intervention. The UHR period is heterogeneous in terms of symptom presentation and course; fluctuations in the intensity and distress of symptoms are common in this population.51 Importantly, we have begun recruitment for a follow-up randomized controlled trial (RCT) study incorporating a wait-listed group of UHR and healthy matched controls, which may provide further understanding of the mechanisms by which exercise can help ameliorate features of psychosis development.
Cognitive function improvement is consistent with a large body of work in human and non-human exercise studies.6, 8, 52–54 However, there remains some controversy about whether aerobic or anaerobic exercise produces improved cognition in patients with psychosis.48 A promising avenue of research combines high intensity exercise and cognitive remediation training.7 Future work examining aerobic and anaerobic exercise to improve cognition in UHR youth would be helpful for developing personalized medicine and exercise prescriptions for those at risk for psychosis.
The exercise intervention was calibrated to each individual’s physical fitness level using VO2max. We were surprised to see that VO2max did not improve after the exercise intervention. However, individual differences in genetic heritability for cardiovascular performance, response to training, body composition of lipids, and glucose metabolism could potentially affect these results.55, 56 Although the finding that VO2max didn’t change is consistent with a number of other aerobic exercise interventions in schizophrenia patients, these results also encourage future interventions to consider including higher intensity exercise. A wealth of evidence now indicates that higher intensity aerobic exercise – particularly high intensity interval (HIIT) training – improves cardiovascular fitness and related metabolic measures.57 The current sample included cardiovascularly healthy but sedentary participants who exercised at two different intensities (i.e., 65% and 85% of VO2max), and most exercised at the lower intensity, thus it is not terribly surprising that we did not see large improvements in cardiovascular fitness. However, even without notable improvements in cardiovascular fitness in the UHR group, the results suggest that there are substantial benefits to this exercise intervention in other functional domains critical to quality of life in this population. This study thus represents an important starting point for exercise prescriptions for UHR individuals.
Consistent with other UHR studies we did not find changes to hippocampus volume for either the left or right hippocampus.9, 58 Importantly, we used a multimodal neuroimaging approach and the finding that increased hippocampal functional connectivity of the hippocampus is an innovative development to understanding the role of the hippocampus in the neurodevelopment of psychosis. The direction and magnitude of the change in hippocampal connectivity is difficult to interpret given the lack of comparison subjects. However, it has been suggested in other studies that increased occipital-hippocampal connectivity is associated with the task positive network and spatial memory performance.59, 60
A diathesis stress model of psychosis suggests that neurodevelopmental abnormalities begin early in adulthood, and then brain maturational factors and stress from the environment interact with these vulnerabilities to promote the emergence of psychosis.61–63 As noted, exercise may target brain regions thought to be a part of the abnormal neurodevelopment in psychosis. One brain region that is central to the neural diathesis stress model of psychosis – the hippocampus – is susceptible to neurogenesis and improved synaptic plasticity through regular exercise.6, 64 The current results support this model of exercise intervening on both neurodevelopmental and behavioral levels of risk for psychosis. Importantly, we found that the exercise intervention attenuated psychotic symptoms, improved social and role function, and improved cognition. In addition, we saw increased functional connectivity between the hippocampus and the occipital cortex. Replication of these results and further examination of the links between these results in larger samples of UHR participants using a higher intensity exercise stimulus may produce a helpful treatment prescription for those at risk for psychosis.
There are a number of strengths and limitations to this study. The final sample size was small, did not include enough participants to compare exercise conditions, and the participants did not show changes in cardiovascular fitness. The planned RCT will improve on these limitations by including a wait list clinical and healthy control group, require two weekly sessions (as 3 sessions was less tolerable), and incorporate HIIT training, which should be a more powerful exercise stimulus for improving V02max.65 We assessed the participants directly after they finished the exercise intervention, and importantly, none of the participants transitioned to psychosis during the intervention. Current research suggests that as many as 35% of UHR individuals will transition to psychosis and others may experience problems with mood, anxiety, and other mental health issues. 51, 66 Thus, longitudinal studies incorporating multiple domains of physical and mental health assessment are needed to see if the benefits of exercise interventions continue to improve the health and well-being of people at risk for psychosis.
Supplementary Material
Clinical Points.
There are few interventions that can help with all of the early signs of psychosis; aerobic exercise may be a powerful treatment option with holistic benefits.
Consider exercise in the treatment planning for young adults showing early signs of psychosis.
Acknowledgments
This project was supported by NIMH R21/33 MH103231 (Mittal and Bryan). The funding agencies did not contribute to the development, data analysis, or interpretation of the results of this study.
Footnotes
Previous Presentation: Poster presentation at the Society for Research in Psychopathology meeting, Baltimore, Maryland, September 29 – October 2, 2016.
CONFLICTS OF INTEREST
Dr. Mittal is a consultant with Takeda Pharmaceuticals. No other authors have conflicts to disclose.
References
- 1.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008 Jan;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 2.Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015 May;45(7):1343–1361. doi: 10.1017/S0033291714003110. [DOI] [PubMed] [Google Scholar]
- 3.Stanton R, Happell B. A systematic review of the aerobic exercise program variables for people with schizophrenia. Curr Sports Med Rep. 2014 Jul-Aug;13(4):260–266. doi: 10.1249/JSR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 4.Martin H, Beard S, Clissold N, Andraos K, Currey L. Combined aerobic and resistance exercise interventions for individuals with schizophrenia: A systematic review. Mental Health and Physical Activity. 2017 [Google Scholar]
- 5.Mittal VA, Vargas T, Juston Osborne K, et al. Exercise Treatments for Psychosis: a Review. Current Treatment Options in Psychiatry. 2017:1–15. doi: 10.1007/s40501-017-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimhy D, Vakhrusheva J, Bartels MN, et al. The Impact of Aerobic Exercise on Brain-Derived Neurotrophic Factor and Neurocognition in Individuals With Schizophrenia: A Single-Blind, Randomized Clinical Trial. Schizophr Bull. 2015 Jul;41(4):859–868. doi: 10.1093/schbul/sbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuechterlein KH, Ventura J, McEwen SC, Gretchen-Doorly D, Vinogradov S, Subotnik KL. Enhancing Cognitive Training Through Aerobic Exercise After a First Schizophrenia Episode: Theoretical Conception and Pilot Study. Schizophr Bull. 2016 Jul;42(Suppl 1):S44–52. doi: 10.1093/schbul/sbw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oertel-Knochel V, Mehler P, Thiel C, et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2014 Oct;264(7):589–604. doi: 10.1007/s00406-014-0485-9. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum S, Lagopoulos J, Curtis J, et al. Aerobic exercise intervention in young people with schizophrenia spectrum disorders; improved fitness with no change in hippocampal volume. Psychiatry Res. 2015 May 30;232(2):200–201. doi: 10.1016/j.pscychresns.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Malchow B, Reich-Erkelenz D, Oertel-Knochel V, et al. The effects of physical exercise in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci. 2013 Sep;263(6):451–467. doi: 10.1007/s00406-013-0423-2. [DOI] [PubMed] [Google Scholar]
- 11.Scheewe TW, Backx FJ, Takken T, et al. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatr Scand. 2013 Jun;127(6):464–473. doi: 10.1111/acps.12029. [DOI] [PubMed] [Google Scholar]
- 12.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004 Apr 1;67(2–3):131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 13.Waghorn G, Chant D, White P, Whiteford H. Delineating disability, labour force participation and employment restrictions among persons with psychosis. Acta Psychiatr Scand. 2004 Apr;109(4):279–288. doi: 10.1046/j.1600-0447.2003.00269.x. [DOI] [PubMed] [Google Scholar]
- 14.Koivukangas J, Tammelin T, Kaakinen M, et al. Physical activity and fitness in adolescents at risk for psychosis within the Northern Finland 1986 Birth Cohort. Schizophr Res. 2010 Feb;116(2–3):152–158. doi: 10.1016/j.schres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Deighton S, Addington J. Exercise practices in individuals at clinical high risk of developing psychosis. Early Interv Psychiatry. 2015 Aug;9(4):284–291. doi: 10.1111/eip.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal VA, Gupta T, Orr JM, et al. Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J Abnorm Psychol. 2013 Nov;122(4):1101–1110. doi: 10.1037/a0034085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodgekins J, French P, Birchwood M, et al. Comparing time use in individuals at different stages of psychosis and a non-clinical comparison group. Schizophr Res. 2015 Feb;161(2–3):188–193. doi: 10.1016/j.schres.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Deighton S, Addington J. Exercise practices of young people at their first episode of psychosis. Schizophr Res. 2014 Jan;152(1):311–312. doi: 10.1016/j.schres.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Carney R, Cotter J, Bradshaw T, Firth J, Yung AR. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: A systematic review and meta-analysis. Schizophr Res. 2016 Feb;170(2–3):290–300. doi: 10.1016/j.schres.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 21.Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010 Feb;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- 22.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004 Jun;174(1):151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 24.Phillips LJ, McGorry PD, Garner B, et al. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. 2006 Sep;40(9):725–741. doi: 10.1080/j.1440-1614.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- 25.Dean DJ, Orr JM, Bernard JA, et al. Hippocampal Shape Abnormalities Predict Symptom Progression in Neuroleptic-Free Youth at Ultrahigh Risk for Psychosis. Schizophr Bull. 2015 Jun 25; doi: 10.1093/schbul/sbv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard JA, Orr JM, Mittal VA. Abnormal hippocampal-thalamic white matter tract development and positive symptom course in individuals at ultra-high risk for psychosis. NPJ Schizophr. 2015:1. doi: 10.1038/npjschz.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faulkner G, Soundy AA, Lloyd K. Schizophrenia and weight management: a systematic review of interventions to control weight. Acta Psychiatr Scand. 2003 Nov;108(5):324–332. doi: 10.1034/j.1600-0447.2003.00218.x. [DOI] [PubMed] [Google Scholar]
- 28.McGlashan T, Walsh B, Woods S. The psychosis-risk syndrome: handbook for diagnosis and follow-up. Oxford University Press; 2010. [Google Scholar]
- 29.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL: SCID-I/P Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 31.Auther A, Smith C, Cornblatt B. Global Functioning: Social Scale (GF: Social) Glen Oaks, NY: Zucker-Hillside Hospital; 2006. [Google Scholar]
- 32.Niendam TA, Bearden CE, Johnson JK, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res. 2006;84(1):100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Cornblatt BA, Auther AM, Niendam T, et al. Preliminary Findings for Two New Measures of Social and Role Functioning in the Prodromal Phase of Schizophrenia. Schizophr Bull. 2007 Apr 19;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004 Dec 15;72(1):1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer K, Lobelo F, Ward DS, Pate RR. Endurance trainability of children and youth. 2008. [Google Scholar]
- 36.Yoon B-K, Kravitz L, Robergs R. VO2max, protocol duration, and the VO2 plateau. Med Sci Sports Exerc. 2007;39(7):1186–1192. doi: 10.1249/mss.0b13e318054e304. [DOI] [PubMed] [Google Scholar]
- 37.Pimentel AE, Gentile CL, Tanaka H, Seals DR, Gates PE. Greater rate of decline in maximal aerobic capacity with age in endurance-trained than in sedentary men. J Appl Physiol (1985) 2003 Jun;94(6):2406–2413. doi: 10.1152/japplphysiol.00774.2002. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002 Jan 31;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 39.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 40.Stephens S, Feldman BM, Bradley N, et al. Feasibility and effectiveness of an aerobic exercise program in children with fibromyalgia: results of a randomized controlled pilot trial. Arthritis Rheum. 2008 Oct 15;59(10):1399–1406. doi: 10.1002/art.24115. [DOI] [PubMed] [Google Scholar]
- 41.Perri MG, Anton SD, Durning PE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002 Sep;21(5):452–458. [PubMed] [Google Scholar]
- 42.Richardson CR, Avripas SA, Neal DL, Marcus SM. Increasing lifestyle physical activity in patients with depression or other serious mental illness. J Psychiatr Pract. 2005 Nov;11(6):379–388. doi: 10.1097/00131746-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Mittal VA, Dean DJ, Mittal J, Saks ER. Ethical, Legal, and Clinical Considerations when Disclosing a High-Risk Syndrome for Psychosis. Bioethics. 2015 Feb 17; doi: 10.1111/bioe.12155. [DOI] [PubMed] [Google Scholar]
- 44.Tsang HW-h, Fung KM-t, Chung RC-k. Self-stigma and stages of change as predictors of treatment adherence of individuals with schizophrenia. Psychiatry Res. 2010 Nov 30;180(1):10–15. doi: 10.1016/j.psychres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Firth J, Rosenbaum S, Stubbs B, Gorczynski P, Yung AR, Vancampfort D. Motivating factors and barriers towards exercise in severe mental illness: a systematic review and meta-analysis. Psychol Med. 2016 Oct;46(14):2869–2881. doi: 10.1017/S0033291716001732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vancampfort D, De Hert M, Broderick J, et al. Is autonomous motivation the key to maintaining an active lifestyle in first-episode psychosis? Early Interv Psychiatry. 2016 Sep 4; doi: 10.1111/eip.12373. [DOI] [PubMed] [Google Scholar]
- 47.Beebe LH, Smith K, Burk R, et al. Effect of a motivational intervention on exercise behavior in persons with schizophrenia spectrum disorders. Community Ment Health J. 2011 Dec;47(6):628–636. doi: 10.1007/s10597-010-9363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dauwan M, Begemann MJ, Heringa SM, Sommer IE. Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr Bull. 2016 May;42(3):588–599. doi: 10.1093/schbul/sbv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vancampfort D, Probst M, Scheewe T, Knapen J, De Herdt A, De Hert M. The functional exercise capacity is correlated with global functioning in patients with schizophrenia. Acta Psychiatr Scand. 2012 May;125(5):382–387. doi: 10.1111/j.1600-0447.2011.01825.x. [DOI] [PubMed] [Google Scholar]
- 50.Vancampfort D, Probst M, Sweers K, Maurissen K, Knapen J, De Hert M. Relationships between obesity, functional exercise capacity, physical activity participation and physical self-perception in people with schizophrenia. Acta Psychiatr Scand. 2011 Jun;123(6):423–430. doi: 10.1111/j.1600-0447.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- 51.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012 Mar;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 52.Hotting K, Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013 Nov;37(9 Pt B):2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Knochel C, Oertel-Knochel V, O’Dwyer L, et al. Cognitive and behavioural effects of physical exercise in psychiatric patients. Prog Neurobiol. 2012 Jan;96(1):46–68. doi: 10.1016/j.pneurobio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Malchow B, Keller K, Hasan A, et al. Effects of Endurance Training Combined With Cognitive Remediation on Everyday Functioning, Symptoms, and Cognition in Multiepisode Schizophrenia Patients. Schizophr Bull. 2015 Jul;41(4):847–858. doi: 10.1093/schbul/sbv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hecksteden A, Kraushaar J, Scharhag-Rosenberger F, Theisen D, Senn S, Meyer T. Individual response to exercise training - a statistical perspective. J Appl Physiol (1985) 2015 Jun 15;118(12):1450–1459. doi: 10.1152/japplphysiol.00714.2014. [DOI] [PubMed] [Google Scholar]
- 56.Bouchard C. Genomic predictors of trainability. Exp Physiol. 2012 Mar;97(3):347–352. doi: 10.1113/expphysiol.2011.058735. [DOI] [PubMed] [Google Scholar]
- 57.Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015 May;45(5):679–692. doi: 10.1007/s40279-015-0321-z. [DOI] [PubMed] [Google Scholar]
- 58.Walter A, Suenderhauf C, Harrisberger F, et al. Hippocampal volume in subjects at clinical high-risk for psychosis: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2016 Oct 20;71:680–690. doi: 10.1016/j.neubiorev.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Cao X, Liu Z, Xu C, et al. Disrupted resting-state functional connectivity of the hippocampus in medication-naive patients with major depressive disorder. J Affect Disord. 2012 Dec 10;141(2–3):194–203. doi: 10.1016/j.jad.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Y, Shu N, Liu Y, et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008 Mar;100(1–3):120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 61.Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997 Oct;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- 62.Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29(4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 63.Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 64.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007 Sep;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Helgerud J, Hoydal K, Wang E, et al. Aerobic High-Intensity Intervals Improve VO~ 2~ m~ a~ x More Than Moderate Training. Medicine and science in sports and exercise. 2007;39(4):665. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 66.Hengartner MP, Heekeren K, Dvorsky D, Walitza S, Rossler W, Theodoridou A. Course of psychotic symptoms, depression and global functioning in persons at clinical high risk of psychosis: Results of a longitudinal observation study over three years focusing on both converters and non-converters. Schizophr Res. 2017 Jan 27; doi: 10.1016/j.schres.2017.01.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


