ABSTRACT
Fecal calprotectin (FC) has drawn attention as a biomarker in the evaluation of Crohn’s disease (CD). However, few reports have provided a detailed examination of the relationship between small-bowel CD lesions and FC levels. The present study aimed to examine the entire small bowel using double-balloon endoscopy (DBE) and to determine the relationship between the endoscopic activity in small-bowel CD and FC levels. Twenty small-bowel CD patients, who underwent DBE, were prospectively enrolled. Endoscopic evaluation was based on the simple endoscopic score for CD, with the small bowel divided into four regions. This score was defined as the double-balloon endoscopic score for CD (DES-CD). Furthermore, to focus on mucosal membrane damage, we used the partial DES-CD (pDES-CD), in which presence of stenosis was excluded from DES-CD. DES-CD revealed a correlation with FC (γ = 0.691, P = 0.001) and C-reactive protein (CRP) (γ = 0.631, P = 0.003) levels. Furthermore, pDES-CD showed a correlation with the FC level (γ = 0.747, P < 0.001), erythrocyte sedimentation rate (γ = 0.492, P = 0.028), and the CRP level (γ = 0.605, P = 0.005). CD Activity Index and endoscopic score showed no correlation. Our results revealed a correlation between the endoscopic activity in small-bowel CD and FC levels. Furthermore, pDES-CD showed a strong correlation with FC levels. This may be because FC levels were elevated due to mucosal membrane damages, rather than stenoses.
Key Words: Crohn’s disease, fecal calprotectin, double-balloon endoscopy
INTRODUCTION
Crohn’s disease (CD) is characterized by a chronic course of repeated flare-ups and remission, during which lesions can develop throughout the gastrointestinal tract. Depending on the part of gastrointestinal tract involved, the types of CD are classified into small-bowel, ileocolonic, and colonic. For example, by definition, small-bowel CD has lesions in the small bowel, but not in the large bowel.
Over time, most CD patients develop complications, such as stenoses, anal fistulas, and abscesses, which increase the possibility of requiring surgery.1-3) In CD patients, mucosal healing (MH) is important, because it is associated with long-term prognosis. In previous studies, MH has been shown to inhibit CD flare-ups, thereby lowering the risk of hospital admission and surgery.4-7)
More than 60% of CD patients have small-bowel lesions, indicating the importance of examinations of the small bowel.8-10) However, compared with the stomach and large bowel, the small bowel is difficult to examine. There are various modalities available to examine the small bowel, including endoscopic examination, abdominal ultrasound, computed tomography, and magnetic resonance imaging.11) Of these, endoscopic examination enables the direct observation and evaluation of the state of the mucous membrane. Therefore, it is considered to be the gold standard for evaluating MH. At present, endoscopic examinations to evaluate the small-bowel CD lesions include double-balloon endoscopy (DBE) and capsule endoscopy (CE).12,13) These examinations are useful; however, performing them repeatedly in the short term is associated with invasiveness and cost issues.
Recently, fecal calprotectin (FC) has drawn attention as a potential new biomarker. Calprotectin is a calcium-binding protein, consisting of two types of peptides (S100A8/A9). It is primarily found in neutrophils, but has also been found in monocytes and epithelial cells; it accounts for approximately 60% of the soluble cytoplasmic fraction of neutrophils. When inflammation occurs in the gastrointestinal tract, leucocytes mobilize to the inflammation site. From there, they are translocated to the gut lumen, and calprotectin is released into the feces.14-17) Therefore, FC is considered a biomarker reflecting inflammation in the gastrointestinal tract. Compared with the conventional symptom evaluation methods, such as CD Activity Index (CDAI) and serum C-reactive protein (CRP) levels, FC levels exhibit a strong correlation with endoscopic activity.18,19) However, only a limited number of reports have examined, in detail, small-bowel CD lesions. The present study aimed to examine the correlation between FC levels and endoscopic activity in small-bowel CD patients.
MATERIALS AND METHODS
Patients
This prospective study included 20 small-bowel CD patients who underwent DBE at Nagoya University Hospital between May 2016 and July 2017. Within a week of DBE with the transoral and transanal approaches, all patients underwent observation of the entire small bowel. When this observation by DBE was not accomplished, an additional endoscopic water-soluble contrast study was performed. Prior to examination, the CDAI, erythrocyte sedimentation rate (ESR), and CRP levels were measured. Fecal samples were collected within a week before undergoing DBE.
Patients were excluded if fecal samples could not be collected and/or if neoplastic lesions were observed in the large intestine. In the subject sample, there were no patients who took aspirin or non-steroidal anti-inflammatory drugs daily.
Measurement of FC Levels
Fecal samples collected from patients were stored at −30˚C until measurement. FC levels were measured using enzyme-linked immunosorbent assay (Immundiagnostik AG, Bensheim, Germany), according to manufacturer’s guidelines.
Endoscopic Evaluation Method
Conventional endoscopic evaluations included measuring the simple endoscopic score for CD (SES-CD) by colonoscopy20) and the Lewis score (LS) by CE.21) In addition, SES-CD is measured in the large bowel by dividing it into four areas: 1) rectum, 2) left hemicolon, 3) transverse colon, and 4) right hemicolon. The small bowel includes only one region (i.e., the ileum terminal); therefore, most of the small bowel is not evaluated. Furthermore, the entire small bowel can be evaluated with LS, whereas in CD patients with severe stenosis, CE cannot be performed; therefore, evaluation is not possible.
In the present study, to perform endoscopic evaluations limited to the small bowel, the latter was divided into four areas: 1) upper jejunum, 2) lower jejunum, 3) upper ileum, and 4) lower ileum. As with SES-CD, four items were evaluated: 1) size of the ulcer, 2) ulcerated surface, 3) affected surface, and 4) presence of stenosis. This new endoscopic evaluation is defined as the double-balloon endoscopic score for CD (DES-CD) (Table 1). Furthermore, to focus on the damage to the mucosal membrane, the presence of stenosis was excluded from the four items; a separate evaluation was conducted using the partial DES-CD (pDES-CD), which evaluated the remaining three items (Table 2). DES-CD ranged from 0 to 46, and pDES-CD ranged from 0 to 36.
Table 1.
Definitions of the DES-CD
| Endoscopic value | ||||
|---|---|---|---|---|
| Variable | 0 | 1 | 2 | 3 |
| Size of ulcers | None | Aphthous ulcers
(Ø 0.1 to 0.5 cm) |
Large ulcers
(Ø 0.5 to 2 cm) |
Very large ulcers
(Ø >2 cm) |
| Ulcerated surface | None | <10% | 10–30% | >30% |
| Affected surface | None | <50% | 50–75% | >75% |
| Presence of stenoses | None | Single,
can be passed |
Multiple,
can be passed |
Cannot be passed |
Ø, Diameter
DES-CD, double-balloon endoscopic score for Crohn’s disease
Table 2.
Definitions of the pDES-CD
| Endoscopic value | ||||
|---|---|---|---|---|
| Variable | 0 | 1 | 2 | 3 |
| Size of the ulcer | None | Aphthous ulcers
(Ø 0.1 to 0.5 cm) |
Large ulcers
(Ø 0.5 to 2 cm) |
Very large ulcers
(Ø >2 cm) |
| Ulcerated surface | None | <10% | 10–30% | >30% |
| Affected surface | None | <50% | 50–75% | >75% |
Ø, Diameter
pDES-CD, partial double-balloon endoscopic score for Crohn’s disease
When the scope of DBE could not be inserted deeply due to adhesion and stenosis, the unobservable small bowel was evaluated by inserting a contrast medium via DBE. As a result, patients without clear abnormal findings were included. Patients were excluded if the range of contrast enhancement exceeded 50 cm. EN-580T (Fujinon Inc., Saitama City, Japan) was used as the scope.
Statistical Analysis
The statistical software package SPSS for Windows (SPSS, Chicago, IL, USA) was used to analyze statistical data. The correlations between the FC level and the CDAI, CRP level, ESR, and endoscopic activity (DES-CD and pDES-CD) were estimated with Spearman’s rank order correlation coefficient (γ ). In all analyses, a P-value of <0.05 was considered to indicate significance.
RESULTS
Patient Backgrounds
The clinical backgrounds of the patients are presented in Table 3. Overall, there were 20 patients (16 male patients), with a median age of 33 years at diagnosis. The median disease duration was 8.5 years. The median CDAI was 69 (range: 0–360.2), median ESR was 6 mm/h (range: 1–36 mm/h), and median CRP level was 0.04 mg/l (range: 0–2.74 mg/l). No lesions were observed in the large bowel, including the anus, in any patient. There were 10 patients with a history of intestinal resection. There were five patients taking infliximab, seven patients taking adalimumab, and six patients taking azathioprine.
Table 3.
Patient characters
| Number of patients | 20 | |
| Gender: Male/Female | 16/4 | |
| Age at diagnosis (years): median, range | 33 (19–65) | |
| Disease duration (years): median, range | 8.5 (0–23) | |
| Prior surgery (%) | 10 (50) | |
| CDAI: median, range | 69 (0–360.2) | |
| Smoking (%) | 1 (5) | |
| Medication | ||
| 5-ASA (%) | 18 (90) | |
| Elemental diet (%) | 15 (75) | |
| Infliximab (%) | 5 (25) | |
| Adalimumab (%) | 7 (35) | |
| Azathioprine (%) | 6 (30) | |
| ESR (mm/h): median, range | 6 (1–36) | |
| CRP (mg/l): median, range | 0.04 (0–2.74) | |
| FC (μg/g): median, range | 35.4 (9–1506.9) | |
| DES-CD: median, range | 7 (0–16) | |
| pDES-CD: median, range | 5 (0–13) | |
| Distribution of CD lesions | ||
| Jejunum (%) | 2 (10) | |
| Ileum (%) | 12 (60) | |
| both (%) | 6 (30) | |
| Stenosis (%) | 17 (85) | |
| Cannot be passed (%) | 11 (55) |
DBE, double-balloon endoscopy; CDAI, Crohn’s Disease Activity Index; ASA, aminosalicylic acid; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; DES-CD, double-balloon endoscopic score for Crohn’s disease; pDES-CD, partial double-balloon endoscopic score for Crohn’s disease
Endoscopic Findings by DBE
The lesions were distributed in the jejunum (two patients), in the ileum (12 patients), and in both areas (six patients). The entire small bowel could be examined by DBE, without contrast enhancement of the small bowel in six patients. Stenosis was observed in 17 patients, among whom the scope could not be passed for DBE in 11. There were no patients in whom the entire small bowel could be examined only by the one-way approach.
Correlation between the FC level and the CRP Level, ESR, and CDAI
The median FC level was 35.4 μg/g (range: 9–1506.9 μg/g). A correlation was observed between the FC and CRP levels (γ = 0.767, P < 0.001) (Fig. 1). No correlation was observed between the FC level and the ESR and CDAI.
Fig. 1.
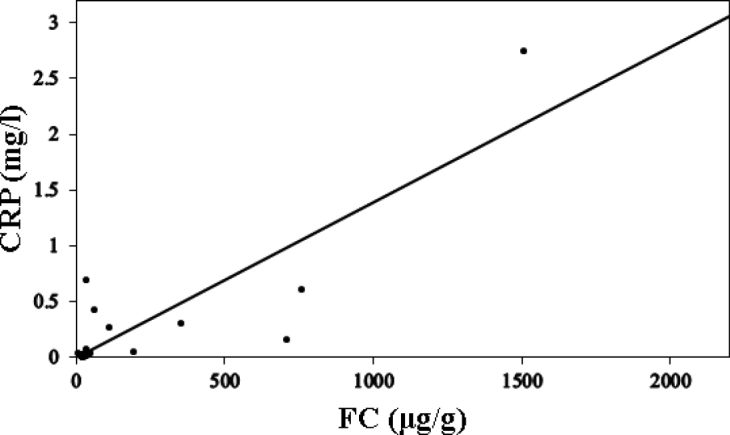
Correlation between the FC and CRP levels
Correlation between the FC and CRP levels. Spearman rank correlation coefficient γ was 0.767 (P < 0.001).
Correlation between the Endoscopic Score Obtained by DBE and the FC Level, CRP Level, ESR, and CDAI
The median DES-CD was 7 (range: 0–16). A correlation was observed with the FC (γ = 0.691, P = 0.001) and CRP (γ = 0.631, P = 0.003) levels (Fig. 2A and 2B). There was no correlation observed with the ESR and CDAI.
Fig. 2.
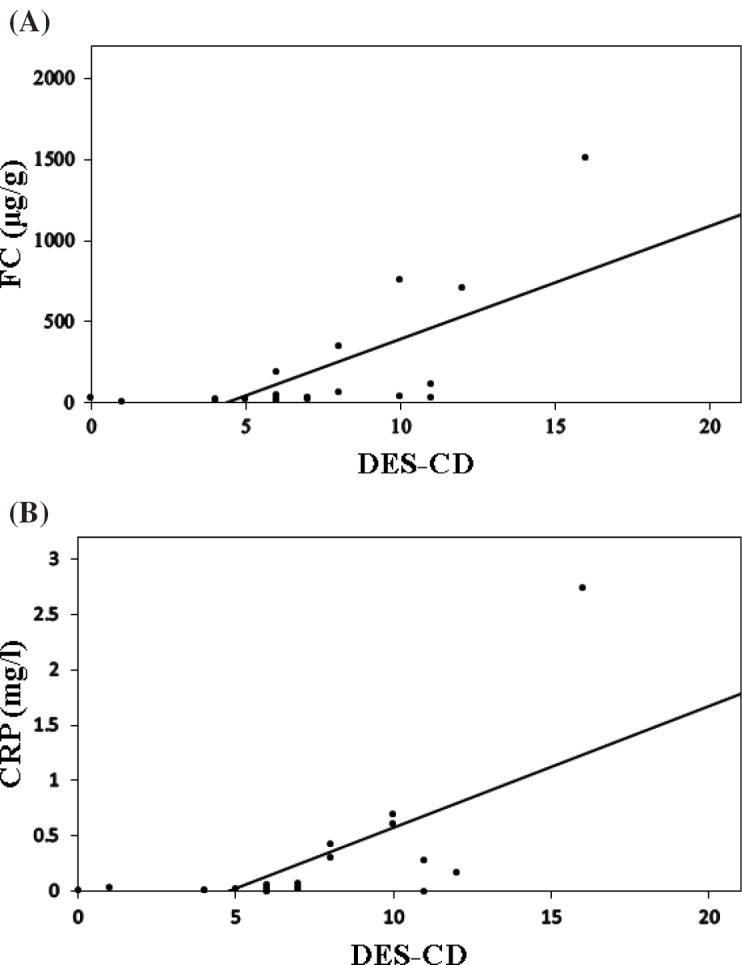
Correlation between the DES-CD, FC level, and CRP level
(A) Correlation between the DES-CD and FC level. Spearman rank correlation coefficient γ was 0.691 (P = 0.001).
(B) Correlation between the DES-CD and CRP level. Spearman rank correlation coefficient γ was 0.631 (P = 0.003).
Furthermore, the median pDES-CD was 5 (range: 0–13). A correlation was observed with the FC level (γ = 0.747, P < 0.001), ESR (γ = 0.492, P = 0.028), and CRP level (γ = 0.605, P = 0.005) (Fig. 3A, 3B, and 3C). There was no correlation observed with CDAI.
Fig. 3.
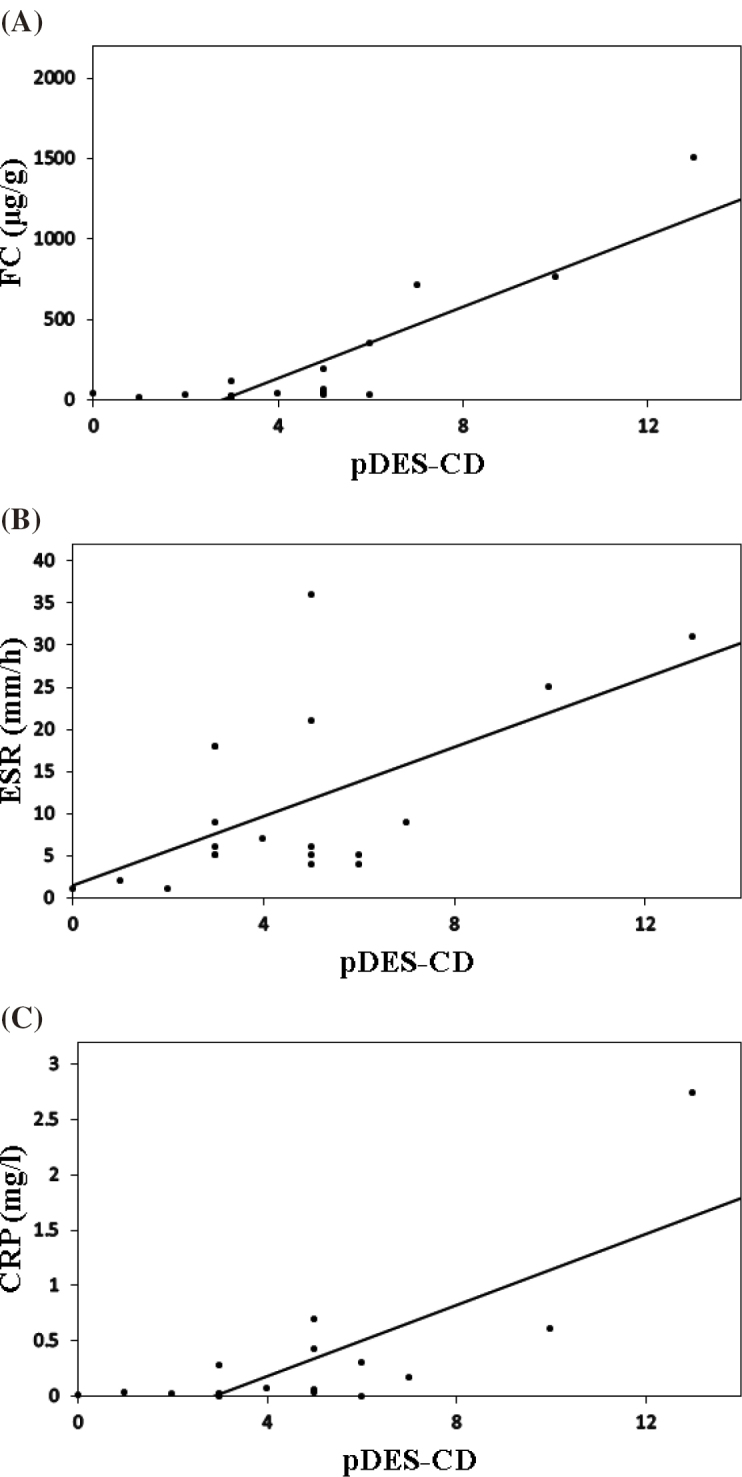
Correlation between the pDES-CD, FC level, ESR, and CRP level
(A) Correlation between the pDES-CD and FC level. Spearman rank correlation coefficient γ was 0.747 (P < 0.001).
(B) Correlation between the pDES-CD and ESR. Spearman rank correlation coefficient γ was 0.492 (P = 0.028).
(C) Correlation between the pDES-CD and CRP level. Spearman rank correlation coefficient γ was 0.605 (P = 0.005).
The endoscopic activity analysis revealed that pDES-CD and FC levels had the strongest correlation. Moreover, CDAI showed no correlation in any of the analyses.
DISCUSSION
In the present study, we evaluated the correlation between endoscopic activity and FC levels in small-bowel CD patients. The endoscopic activity correlates with FC levels in small- and large-bowel CD patients.18,19) Conversely, some reports have indicated that there is no such correlation in small-bowel CD patients, and other studies have suggested that FC levels are low in small-bowel CD patients.22-24) Therefore, it is possible that in small-bowel CD patients, no correlation is observed between disease activity and FC levels. Few reports have evaluated the correlation between disease activity and FC levels in small-bowel CD patients; therefore, it is important to perform such an evaluation. In the past, small-bowel lesions were primarily evaluated by colonoscopy; therefore, evaluation of small-bowel lesions was limited. Recently, it has become possible to evaluate the small bowel more extensively using CE, magnetic resonance enterography (MRE), and DBE. With each of these examinations, a correlation between disease activity and FC levels has been observed25-27); however, CE cannot be performed on patients with severe stenosis, thereby limiting the number of patients. Moreover, MRE is superior in terms of the evaluation of lesions other than those in the intestinal mucosa; however, it is difficult to detect superficial lesions and small mucosal lesions using this method.28) Furthermore, although DBE is superior in terms of mucosal lesion detection, to date, only one study27) has exclusively used the transanal approach, which makes the evaluation of the upper small bowel inadequate.
In the present study, we examined disease activity by DBE to evaluate, in detail, lesions in the mucous membrane. To observe the entire small bowel, we conducted our examination using the transoral and transanal approaches. Based on these results, in small-bowel CD patients, a correlation was observed between endoscopic activity and the FC levels. Furthermore, a strong correlation was observed between the pDES-CD and FC levels, which suggested that stenosis has little involvement in the elevation of FC levels and that mucous membrane damage (e.g., ulcers) causes FC levels to increase. Compared with conventional indices (e.g., ESR, CRP level, and CDAI), FC levels exhibit a strong correlation with endoscopic disease activity. Therefore, it appears that FC is more useful than conventional indices for the evaluation of mucous membrane damage.
Rutgeerts et al. reported that in CD patients, endoscopic flare-ups occur first, and then flare-ups appear in other data (e.g., blood tests). This is followed by flare-up of clinical symptoms.29) If treatment can be intensified at the endoscopic flare-up stage, the prognosis of CD patients may improve. However, from the perspective of convenience in clinical practice, clinical activity indices (e.g., CDAI) and serum biomarkers (e.g., CRP level) are often used to evaluate disease activities and determine therapeutic outcomes. Moreover, imaging examinations are not regularly conducted at present. The method to evaluate FC levels is simple and easy to perform. If FC levels reflect damage to the mucous membrane, endoscopic flare-ups can be monitored through FC levels, and they can also predict the timing required to intensify treatment.
A limitation of the present study was the very small sample size of patients who underwent observation of the entire small bowel by DBE. In addition, when observation was difficult by DBE, the observation was performed using contrast imaging of the small bowel. Moreover, evaluation by endoscopy was not directly possible for all patients.
In conclusion, FC levels showed a correlation with endoscopic activity in small-bowel CD patients, which was stronger than that for the ESR, CRP level, and CDAI.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1).Wolters FL, Russel MG, Sijbrandij J, Ambergen T, Odes S, Riis L, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut, 2006; 55: 1124–1130. [DOI] [PMC free article] [PubMed]
- 2).Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis, 2011; 17: 1415–1422. [DOI] [PMC free article] [PubMed]
- 3).Watanabe K, Sasaki I, Fukushima K, Futami K, Ikeuchi H, Sugita A, et al. Long-term incidence and characteristics of intestinal failure in Crohn’s disease: a multicenter study. J Gastroenterol, 2014; 49: 231–238. [DOI] [PubMed]
- 4).Frøslie KF, Jahnsen J, Moum BA, Vatn MH, and the IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology, 2007; 133: 412–422. [DOI] [PubMed]
- 5).Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Scientific Committee of the European Crohn’s and Colitis Organization. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis, 2011; 5: 477–483. [DOI] [PubMed]
- 6).Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology, 2010; 138: 463–468. [DOI] [PubMed]
- 7).Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis, 2009; 15: 1295–1301. [DOI] [PubMed]
- 8).Steinhardt HJ, Loeschke K, Kasper H, Holtermüller KH, Schäfer H. European Cooperative Crohn’s Disease Study (ECCDS): clinical features and natural history. Digestion, 1985; 31: 97–108. [DOI] [PubMed]
- 9).Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. European Crohn’s and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis, 2010; 4: 63–101. [DOI] [PubMed]
- 10).Voderholzer WA, Beinhoelzl J, Rogalla P, van der Woude CJ, Sturm A, De Vos M, et al. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut, 2005; 54: 369–373. [DOI] [PMC free article] [PubMed]
- 11).D'Incà R, Caccaro R. Measuring disease activity in Crohn’s disease: what is currently available to the clinician. Clin Exp Gastoenterol, 2014; 7: 151–161. [DOI] [PMC free article] [PubMed]
- 12).Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol, 2004; 2: 1010–1016. [DOI] [PubMed]
- 13).Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy, 2015; 47: 352–376. [DOI] [PubMed]
- 14).Poullis A, Foster R, Northfield TC, Mendall MA. Review article: faecal markers in the assessment of activity in inflammatory bowel disease. Aliment Pharmacol Ther, 2002; 16: 675–681. [DOI] [PubMed]
- 15).Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut, 2006; 55: 426–431. [DOI] [PMC free article] [PubMed]
- 16).Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut, 2009; 58: 859–868. [DOI] [PubMed]
- 17).Judd TA, Day AS, Lemberg DA, Turner D, Leach ST. Update of fecal markers of inflammation in inflammatory bowel disease. J Gastroenterol Hepatol, 2011; 26: 1493–1499. [DOI] [PubMed]
- 18).Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol, 2010; 105: 162–169. [DOI] [PubMed]
- 19).D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis, 2012; 18: 2218–2224. [DOI] [PubMed]
- 20).Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc, 2004; 60: 505–512. [DOI] [PubMed]
- 21).Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther, 2008; 27: 146–154. [DOI] [PubMed]
- 22).Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther, 2008; 28: 1221–1229. [DOI] [PubMed]
- 23).Gecse KB, Brandse JF, van Wilpe S, Löwenberg M, Ponsioen C, van den Brink G, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol, 2015; 50: 841–847. [DOI] [PubMed]
- 24).Stawczyk-Eder K, Eder P, Lykowska-Szuber L, Krela-Kazmierczak I, Klimczak K, Szymczak A, et al. Is faecal calprotectin equally useful in all Crohn’s disease locations? A prospective, comparative study. Arch Med Sci, 2015; 11: 353–361. [DOI] [PMC free article] [PubMed]
- 25).Kopylov U, Yablecovitch D, Lahat A, Neuman S, Levhar N, Greener T, et al. Detection of Small Bowel Mucosal Healing and Deep Remission in Patients With Known Small Bowel Crohn’s Disease Using Biomarkers, Capsule Endoscopy, and Imaging. Am J Gastroenterol, 2015; 110: 1316–1323. [DOI] [PubMed]
- 26).Cerrillo E, Beltrán B, Pous S, Echarri A, Gallego JC, Iborra M, et al. Fecal Calprotectin in Ileal Crohn’s Disease: Relationship with Magnetic Resonance Enterography and a Pathology Score. Inflamm Bowel Dis, 2015; 21: 1572–1579. [DOI] [PubMed]
- 27).Inokuchi T, Kato J, Hiraoka S, Takashima S, Nakarai A, Takei D, et al. Fecal Immunochemical Test Versus Fecal Calprotectin for Prediction of Mucosal Healing in Crohn’s Disease. Inflamm Bowel Dis, 2016; 22: 1078–1085. [DOI] [PubMed]
- 28).Takenaka K, Ohtsuka K, Kitazume Y, Nagahori M, Fujii T, Saito E, et al. Correlation of the Endoscopic and Magnetic Resonance Scoring Systems in the Deep Small Intestine in Crohn’s Disease. Inflamm Bowel Dis, 2015; 21: 1832–1838. [DOI] [PubMed]
- 29).Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology, 1990; 99: 956–963. [DOI] [PubMed]


