ABSTRACT
Left ventricular hypertrophy (LVH) and proteinuria are known as independent predictors of cardiovascular death in hypertension. However, LVH and its association with proteinuria have not been investigated in adult hypertensive patients in Afghanistan. The objective of this research was to determine the prevalence of LVH and the correlation between LVH and proteinuria among the Afghan adult hypertensive population visiting an outpatient clinic in Afghanistan. We retrospectively evaluated 789 hypertensive patients (mean age is 56 years and 46% were men) who visited the clinic between December 2014 and August 2016. Patient characteristics and laboratory and clinical findings were recorded. The rate of LVH among hypertensive patients was 54.4%. Patients with proteinuria had a significantly higher LVH percentage compared to those without proteinuria (73.2% versus 55.8%; P<0.001). There was a significant correlation between LVH and proteinuria among hypertensive patients (r=0.182, P<0.001). Based on a multivariate regression analysis, age (odds ratio [OR], 1.04; 95% confidence interval [CI], 1.02–1.05), proteinuria (OR, 1.69; 95% CI, 1.19–2.41), and female sex (OR, 0.09; 95% CI, 0.06–0.13) were significant factors. In conclusion, the prevalence of LVH was more than 50% in the Afghan adult hypertensive population. This study indicates that there is a significant relationship between LVH detected by ECG and the presence of proteinuria among such subjects.
Key Words: proteinuria, left ventricular hypertrophy, hypertensive patients, Andkhoy, Afghanistan
INTRODUCTION
Cardiovascular disease (CVD) has been found to be the main cause of all global deaths, resulting in around 30% of deaths per year, while 50% of these deaths are due to CVD directly associated with hypertension.1) The high prevalence of hypertension and poor blood pressure control are important factors in the rise of CVD burden in the developing countries.2)
Left ventricular hypertrophy (LVH) is the earliest cardiac complication of hypertension and is known to be a major risk factor for cardiac failure, sudden death, stroke, and myocardial infarction.3-4) LV mass is strongly associated with cardiovascular morbidity and mortality.5-8) Studies indicate that there is an increase in the levels of LVH with age, obesity, and blood pressure.9-12)
A high rate of proteinuria was seen in the Afghan adult hypertensive population,13) and the presence of proteinuria has recently been shown to be a cardiovascular risk marker in hypertensive patients.14) Increased urinary albumin excretion has been considered a marker of renal dysfunction.15) Similarly, hypertensive patients with microalbuminuria have higher levels of LVH.16)
There is a lack of information on the prevalence of LVH and its relationship with proteinuria in the Afghan adult hypertensive population. Thus, this study was designed to determine the prevalence of LVH and the relationship between LVH and proteinuria among adult hypertensive patients visiting an outpatient clinic in Afghanistan.
MATERIAL AND METHODS
Between December 2014 and August 2016, a total of 789 consecutive hypertensive patients visiting an outpatient clinic in Andkhoy, Afghanistan, were enrolled in the study. The patients were aged ≥18 years and were currently being treated for, were with undiagnosed, or were untreated for hypertension. Hypertension was defined as a systolic blood pressure (SBP)≥140 mmHg or diastolic blood pressure (DBP)≥90 mmHg, with anti-hypertensive medication.17) Our exclusion criteria included intense physical activity over the last 24 h and pregnancy. Precise enrolled criteria and definition of variables have been already described elsewhere.13)
The results from these measurements were entered into each patient’s file. The Sokolow-Lyon index, which is recommended as a diagnostic screening method for LVH, was calculated from the patient’s ECG.18)
Statistical Analysis
Qualitative variables are shown with their frequency distribution, while quantitative variables are shown as means±standard deviations (SD). Student’s t-test was used for comparison of the quantitative variables, and chi-squared test was used to compare qualitative variables. A multiple regression model was used to determine the significance of certain predictors of LVH and other variables. P-values<0.05 were considered significant. Data were analyzed using SPSS version 20.0 (SPSS, Chicago, IL). Ethical approval for this study was obtained from the Ethics Committee of Balkh Regional Hospital.
RESULTS
The comparison of baseline sociodemographic and clinical characteristics of all participants is presented in Table 1. The mean age of patients with LVH (60.2 [±11.6] years) was significantly different from that of patients without LVH (56.4 [±12.7] years) (P=0.006). The male-to-female ratio among patients with LVH was 2.1, while that in patients without LVH was 0.24. Subjects with MI had significantly higher LVH levels than those without MI (32.4% versus 6.9%, P<0.001). Physical activity had an inverse effect on LVH (71.1% versus 25.0%, P<0.001). The prevalence rate of LVH among patients with proteinuria was greater than the overall prevalence rate of LVH (73.2% versus 54.4%, P<0.001) (Table 2) in the study. A significant correlation was observed between proteinuria and LVH (r=0.182, P<0.001).
Table 1.
Comparison of demographic and clinical characteristics of hypertensive patients with and without LVH
| No LVH
(n=360) |
LVH
(n=429) |
P-value | |
|---|---|---|---|
| Age, mean, years, mean±SD | 56.4±12.7 | 60.2±11.6 | 0.006 |
| Female, % | 289 (80.3%) | 137 (31.9%) | <0.001 |
| Smokers, % | 100 (27.8%) | 138 (32.2%) | 0.332 |
| Systolic blood pressure, mmHg, mean±SD | 152.6±21.5 | 154.0±27.4 | <0.001 |
| Diastolic blood pressure, mmHg, mean±SD | 93.6±11.9 | 95.3±14.7 | 0.001 |
| Body mass index, kg/m2, mean±SD | 23.5±4.0 | 24.0±4.4 | <0.001 |
| Fasting blood sugar, mean ±SD | 103.5±13.8 | 105.7±17.1 | 0.516 |
| Blood pressure controlled, % | 74 (20.6%) | 93 (21.7%) | 0.701 |
| Myocardial infarction, % | 25 (6.9%) | 139 (32.4%) | <0.001 |
| Diabetes mellitus, % | 47 (13.1%) | 75 (17.5%) | 0.087 |
| Total cholesterol≥190 mg/dL, % | 173 (48.1%) | 254 (59.2%) | 0.002 |
| Lack of regular physical exercise, % | 90 (25.0%) | 305 (71.1%) | <0.001 |
| Stroke, % | 14 (3.9%) | 22 (5.1%) | 0.406 |
| Heart failure, % | 45 (12.5%) | 54 (12.6%) | 0.971 |
| Family history of essential hypertension, % | 190 (52.8%) | 254 (59.2%) | 0.064 |
| Family history of coronary artery disease, % | 76 (21.1%) | 131 (30.5%) | 0.003 |
| Family history of diabetes mellitus, % | 65 (18.1%) | 73 (17.0%) | 0.702 |
Table 2.
Prevalence of LVH among patients with proteinuria
| LVH | Total | P-value | |||
|---|---|---|---|---|---|
| Present | Absent | ||||
| Proteinuria | Present | 314 (73.2%) | 201 (55.8%) | 515 (65.3%) | <0.001 |
| Absent | 115 (26.8%) | 159 (44.2%) | 274 (34.7%) | <0.001 | |
| Total | 429 (54.4%) | 360 (45.6%) | 789 (100%) | ||
Table 3 illustrates the results of the multivariate logistic regression analysis determining the relationship between LVH, age, sex, proteinuria, SBP, and DBP. LVH was considered a dependent variable. Patients with a higher level of proteinuria have a high likelihood of having LVH (OR, 1.69; 95% CI, 1.19–2.41). The probability of LVH was estimated to be 1.04 times greater for one-year incremental increase in patient age (95% CI, 1.02–1.05). Women may have a lower risk of having LVH (OR, 0.09; 95% CI, 0.06–0.13). The association between the prevalence of LVH and proteinuria is shown in Fig. 1
Table 3.
Multivariate logistic regression analysis for prediction of LVH
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Age | 1.04 | 1.02–1.05 | <0.001 |
| Female gender | 0.09 | 0.06–0.13 | <0.001 |
| Proteinuria | 1.69 | 1.19–2.41 | 0.003 |
| Systolic blood pressure | 1.00 | 0.99–1.01 | 0.416 |
| Diastolic blood pressure | 1.00 | 0.98–1.02 | 0.891 |
Abbreviations: OR, odds ratio; CI, confidence interval
Fig. 1.
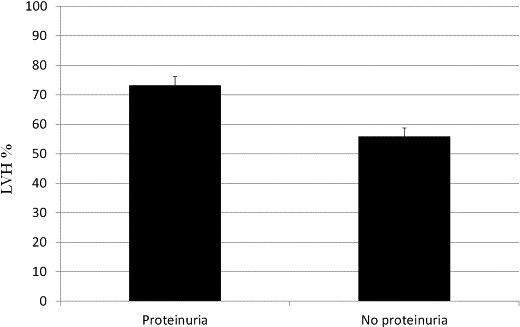
Association between the prevalence of LVH and proteinuria
DISCUSSION
As far as the authors are aware, this is the first investigation to evaluate the prevalence of LVH as determined through an ECG and its correlation with proteinuria in the Afghan adult hypertensive population. This correlation confirms that there is a considerable group of adult hypertensive patients at a higher risk for CVD-related death. Furthermore, both proteinuria and LVH levels increase with advanced stages of hypertension. LVH is considered a marker of target organ damage, as well as an independent cardiovascular risk factor that can result in a rise in fibrinogen values and is associated with the development of atherosclerosis.19-20) The prevalence rates of LVH among hypertensive patients in different countries ranged between 20% and 70% according to the study population and criteria used for LVH.6-8) The prevalence rate of LVH at 54.4% among the hypertensive population in the current study falls within these prevalence rates. However, it was higher than that of Japan and the United States, which had reported levels of 15% and 19%, respectively.21) These differences could arise from the method used to index LV mass and the criteria used for the identification of LVH in the different populations.
We found a positive correlation between proteinuria and LVH. This correlation was demonstrated in other studies.22) Wachtell et al. suggested that angiotensin II is a growth factor that results in LVH as well as renal vascular changes, resulting in an increase in vascular permeability and microalbuminuria.16)
The present study showed that male sex appears to be a stronger risk factor for LVH. This finding concurs with previous studies that reported that men could have a higher likelihood of having LVH than women.23) However, other studies have shown that female sex was an independent predictor for LVH.24-26) A previous study of Losartan Intervention For Endpoint Reduction in Hypertension Study (LIFE) patients with Sokolow-Lyon product LVH indicated a male predominance.26) Further investigations are needed from this context.
The results of the study show that there is a relationship between LVH and age among hypertensive adult patients. Our finding is consistent with a previous result.27) However, another study found an inverse relationship between increasing age and the presence of LVH.24) The determining factors of ECG-LVH among hypertensive subjects were shown to differ based on the methods and criteria used.18)
Some limitations in the study need to be addressed. First, the sample size consisted of only a single-center data source. Therefore, the results of the study would not be generalized. Second, the blood pressure was measured at the outpatient clinic, and there was a lack of data for ambulatory blood pressure assessment during the previous 24 h. Additionally, there was a paucity of laboratory data to assess the correlation between LVH and sCr or eGFR in our study. Finally, there is the lack of information on the duration of blood pressure control as well as a paucity of data about the burden of LVH and the association between LVH and proteinuria in Afghanistan. This is an important examination to evaluate hypertensive patients with proteinuria that will help identify patients at high risk for the treatment. Moreover, the results of our study are in agreement with other studies and should be beneficial in the accumulation of data regarding this issue.
In conclusion, we found a high prevalence rate of LVH and an association between LVH and proteinuria in hypertensive patients in an outpatient clinic in Andkhoy, Afghanistan. Therefore, predicting LVH through the screening of proteinuria using a relatively simple and cheap test at the primary care clinics helps to identify patients at high risk for essential hypertension.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest in this manuscript.
ACKNOWLEDGEMENTS
This project was supported, in part, by the non-profit organization Epidemiological and Clinical Research Information Network (ECRIN). MSH is supported by the Fellowship of Takeda Science Foundation. We would like to thank Dr. Mirwais Rabi and Dr. Ahmad Arsalan Karimi for their cooperation in this study.
REFERENCES
- 1).Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. J Cardiovasc Dis 2013; 1: 1–2.
- 2).Tibazarwa KB, Damasceno AA. Hypertension in developing countries. Can J Cardiol 2014; 30: 527–533. [DOI] [PubMed]
- 3).Monfared A, Salari A, Mirbolok F, Momeni M, Shafighnia S, Shakiba M, et al. Left ventricular hypertrophy and microalbuminuria in patients with essential hypertension. Iran J Kidney Dis 2013; 7(3): 192–197. [PubMed]
- 4).Rodilla E, Pascual JM, Costa JA, Martin J, Gonzalez C, Redon J. Regression of left ventricular hypertrophy and microalbuminuria changes during antihypertensive treatment. J Hypertens 2013; 31: 1683–1691. [DOI] [PubMed]
- 5).Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322: 1561–1566. [DOI] [PubMed]
- 6).Ruilope LM, Schmieder RE. Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 2008; 21: 500–508. [DOI] [PubMed]
- 7).Knöll R, Iaccarino G, Tarone G, Hilfiker-Kleiner D, Bauersachs J, Leite-Moreia AF, et al. Towards a re-definition of “cardiac hypertrophy” through a rational characterization of left ventricular phenotypes: a position paper of the working group “Myocardial Function” of the ESC. Eur J Heart Fail 2011; 13: 811–819. [DOI] [PubMed]
- 8).Adebiyi AA, Ogah OS, Aje A, Ojji DB, Adebayo AK, Oladapo OO, OjjiDB,Adebayo AK, Ojadapo OO, et al. Echocardiographic partition values and prevalence of left ventricular hypertrophy in hypertensive Nigerians. BMC Med Imaging [Internet] 2006; 6: 10. [DOI] [PMC free article] [PubMed]
- 9).Lozano J V, Redon J, Cea-Calvo L, Fernández-Pérez C, Navarro J, Bonet A, et al. [Left ventricular hypertrophy in the Spanish hypertensive population. The ERIC-HTA study]. Rev Esp Cardiol 2006; 59: 136–142. [PubMed]
- 10).Mancia G, Carugo S, Grassi G, Lanzarotti A, Schiavina R, Cesana G, et al. Prevalence of left ventricular hypertrophy in hypertensive patients without and with blood pressure control: data from the PAMELA population. Pressioni Arteriose Monitorate E Loro Associazioni. Hypertension 2002; 39(3): 744–749. [DOI] [PubMed]
- 11).de Simone G, Devereux RB, Roman MJ, Alderman MH, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension 1994; 23: 600–606. [DOI] [PubMed]
- 12).Hammond IW, Devereux RB, Alderman MH, Laragh JH. Relation of blood pressure and body build to left ventricular mass in normotensive and hypertensive employed adults. J Am Coll Cardiol 1988; 12: 996–1004. [DOI] [PubMed]
- 13).Hamrah MS, Hamrah MH, Ishii H, Suzuki S, Hamrah MHU, Hamrah MHA, Yisireyili M et al. Association between proteinuria and cardiovascular risk factors among hypertensive patients in Andkhoy, Afghanistan. Nagoya J Med Sci 2016; 78: 377–385. [DOI] [PMC free article] [PubMed]
- 14).Bigazzi R, Bianchi S, Campese VM, Baldari G. Prevalence of microalbuminuria in a large population of patients with mild to moderate essential hypertension. Nephron 1992; 61: 94–97. [DOI] [PubMed]
- 15).Cerasola G, Cottone S, Mulé G, Nardi E, Mangano MT, Andronico G, et al. Microalbuminuria, renal dysfunction and cardiovascular complication in essential hypertension. J Hypertens 1996; 14: 915–920. [DOI] [PubMed]
- 16).Wachtell K, Olsen MH, Dahlöf B, Devereux RB, Kjeldsen SE, Nieminen MS, et al. Microalbuminuria in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. J Hypertens 2002; 20: 405–412. [DOI] [PubMed]
- 17).Carretero OM, Oparil S. Essential Hypertension Part I: definition and etiology. Circulation 2000; 101:329–335. [DOI] [PubMed]
- 18).Antikainen RL, Grodzicki T, Palmera J, Beevers DG, Webster J, Bulpitt CJ. Left ventricular hypertrophy determined by Sokolow-Lyon criteria: a different predictor in women than in men? J Hum Hypertens 2006; 20: 451–459. [DOI] [PubMed]
- 19).Palmieri V, Celentano A, Roman MJ, de Simone G, Lewis MR, Best L, et al. Fibrinogen and preclinical echocardiographic target organ damage: the strong heart study. Hypertension 2001; 38: 1068–1074. [DOI] [PubMed]
- 20).Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35: 580–586. [DOI] [PubMed]
- 21).Chen CH, Ting CT, Lin SJ, Hsu TL, Chou P, Kuo HS, et al. Relation between diurnal variation of blood pressure and left ventricular mass in a Chinese population. Am J Cardiol 1995; 75: 1239–1243. [PubMed]
- 22).Wachtell K, Palmieri V, Olsen MH, Bella JN, Aalto T, Dahlöf B, et al. Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Losartan Intervention for Endpoint Reduction. Am Heart J 2002; 143: 319–326. [DOI] [PubMed]
- 23).Ching SM, Chia YC, Wan Azman WA. Prevalence and determinants of left ventricular hypertrophy in hypertensive patients at a primary care clinic. Malays Fam Physician 2012; 7: 2–9. [PMC free article] [PubMed]
- 24).Jafary FH, Jafar TH. Disproportionately high risk of left ventricular hypertrophy in Indo-Asian women: a call for more studies. Echocardiography 2008; 25: 812–819. [DOI] [PubMed]
- 25).Gerdts E, Okin PM, De Simone G, Cramariuc D, Wachtell K, Boman K, et al. Gender differences in left ventricular structure and function during antihypertensive treatment: the losartan intervention for endpoint reduction in hypertension study. Hypertension 2008; 51:1109–1114. [DOI] [PubMed]
- 26).Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Dahlöf B. Baseline characteristics in relation to electrocardiographic left ventricular hypertrophy in hypertensive patients: the losartan intervention for endpoint reduction (LIFE) in hypertension study. The Life Study Investigators. Hypertension. 2000; 36: 766–773. [DOI] [PubMed]
- 27).Owusu IK, Acheamfour-Akowuah E. Determinants of left ventricular hypertrophy in hypertensive patients seen in a teaching hospital in Ghana. J Hypertension. 2017; 6: 238. doi:10.4172/2167-1095.1000238.


