Abstract
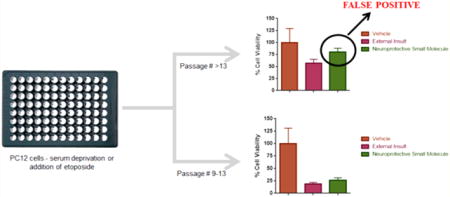
The PC12 cell line is a widely used in vitro model for screening the neuroprotective activity of small molecule libraries. External insult due to serum deprivation or addition of etoposide induces cell death by apoptosis. While this screening method is commonly used in early stage drug discovery no protocol accounting for cell passage number effect on neuroprotective activity has been disclosed. We herein report that passage variation results in false-positive/false-negative identification of neuroprotective compounds; undifferentiated PC12 cells with high passage number are less sensitive to injury induced by serum-deprivation or etoposide treatment. In contrast, NGF differentiated PC12 cells of later passage number are more sensitive to injury induced by etoposide than lower passage number but only after 72 h. Passage number also affects the adherence phenotype of the PC12 cells, complicating screening assays. We report an optimized protocol for screening the neuroprotective activity of small molecules in PC12 cells, which accounts for passage number variations.
Keywords: PC12 cells, neuroprotection assay, screening, cell viability, protocol, apoptosis
Graphical abstract
1. INTRODUCTION
Cell-based assays for high, medium and low throughput screening of bioactive small molecules are a mainstay of the drug discovery process. In the area of neurodegenerative drug discovery the PC12 cell line has become almost ubiquitous as an early stage in vitro model system, capable of mimicking the disease state of many disorders depending on the external insult and pathophysiology modeled. PC12 is a rat pheochromocytoma cell line, derived from the adrenal gland, the cell line is noradrenergic in nature and differentiates into mature neurons in response to nerve growth factor (NGF).1 The use of NGF is known to induce neuroprotection against serum deprivation, camptothecin and etoposide-induced cellular death.2,3 PC12 cells offer several advantages over primary cultured cortical neurons, including the ability to provide high throughput culturing output and retention of a mature neuron phenotype, which can be lost in primary neurons isolated from embryonic brain.
The PC12 cell line has been employed as a general in vitro model to determine the neuroprotective ability of small molecules through induction of cell death by external insult using serum deprivation,2 camptothecin,2 hydrogen peroxide,4 or etoposide.3 The cell line has seen widespread use as a model of neurodegenerative diseases. Parkinson’s disease can be modeled by inducing injury using 6-hydroxydopamine (6-OHDA) or 1-methyl-4-phenylpyridinium (MPP).5 Alzheimer’s disease can be modeled by external insult using β-amyloid peptide-(1–42).6 Amyotrophic lateral sclerosis (ALS) can be modeled by using cells transfected to express fluorescent protein fused mutant SOD1G93A, which induces cell death.7,8 Ischemic stroke can be modeled by external insult through oxygen and glucose deprivation.9
Of the myriad of agents and conditions available to induce external insult, serum deprivation and exposure of the cell line to the cytotoxic agent etoposide represent excellent choices to determine the general ability of compounds under investigation to protect neuronlike PC12 cells from apoptotic cell death, a major, but not sole, process of neurodegenerative disease pathophysiology.10 Serum deprivation induces apoptosis in PC12 cells by activating caspases, particularly caspase-2 and capsase-3.11–13 Etoposide induces apoptosis in PC12 cells without oligonucleosomal DNA fragmentation.14 These processes and conditions are particularly useful when studying a disease with poorly defined pathophysiology as neuronal apoptosis plays a significant role in neurodegeneration.10,15
The protective ability of small molecules in PC12 cellular assays is quantified by cell viability measurement using a number of fluorescent or nonfluorescent dyes. Popular cell viability agents include Resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide),16 the tetrazolium dyes 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),17 ((3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)) (MTS),18 and 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT),19 and the water-soluble tetrazolium salts, propidium iodide staining,20 or the diazo dye Trypan Blue.21 The recently disclosed PrestoBlue dye possess several advantages over the tetrazolium-based and resazurin dyes including very high sensitivity that make it ideal for use as a cell viability agent.22
Protocols detailing external conditions and agents to induce cell death and the use of dyes to quantify cell viability have been extensively published. However, comprehensive details are often omitted and very few protocols report on the PC12 passage number of the cells employed.
Herein we report significant passage variation in PC12 cells that leads to inconsistent susceptibility to externally induced apoptosis. Relatively small changes in passage number lead to a shift in phenotype of the PC12 cells that results in reduced effect of serum deprivation and etoposide-induced cell death in undifferentiated cells and enhanced susceptibility in differentiated PC12 cells. This may result in false-positive or false-negative identification of neuroprotective compounds. While others have reported passage variation in different aspects of PC12 cell behavior,23–26 this is the first report of undifferentiated PC12 cells experiencing decreased cell death (greater “protection”) upon exposure to external insult by etoposide or serum deprivation as a result of later passage number and the observation of greater susceptibility to etoposide-induced apoptosis in differentiated PC12 cells of later passage number. We report an optimized protocol for screening neuroprotective molecules against serum deprivation and etoposide-induced cell death in PC12 cells using PrestoBlue as a model cell viability agent.
2. RESULTS AND DISCUSSION
2.1. Optimization of PC12 Seeding Density and Incubation Time of Cell Viability Agent
PC12 cells were plated in a 96-well plate at 25 × 103, 50 × 103, 100 × 103, and 200 × 103 cells per well and allowed to adhere overnight. The plate was excluded from light, PrestoBlue cell viability agent was added, and fluorescence measured at excitation 560 ± 9 nm and emission 590 ± 9 nm every 10 min over the course of 1 h. The optimum cell density based on the magnitude of fluorescence response in relative fluorescent units (RFU) (Figure 1a) and considering the 48 h doubling time of PC12 cells (to avoid cells in the wells from reaching confluence under normal conditions) (Figure 1b) was determined to be 50 × 103 cells/well.
Figure 1.
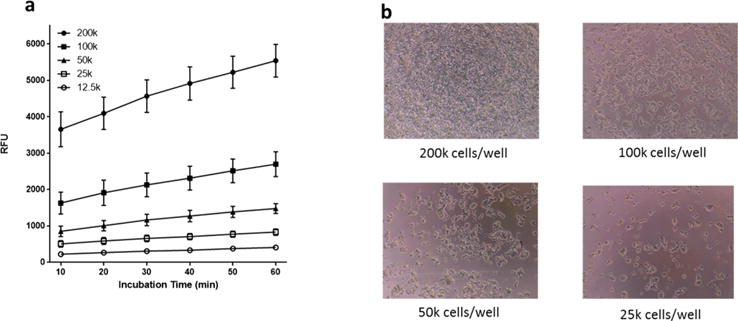
Optimization of PC12 seeding density and PrestoBlue incubation time. (a) Number of PC12 cells incubated and relative fluorescence. (b) Density of PC12 cells in a 96-well plate. Magnification 10×.
The optimum incubation time of PrestoBlue cell viability agent for 50 × 103 PC12 cells/well was determined to be 30 min. A linear range of fluorescence was observed until 30 min with a slight plateau beyond 30 min. The fluorescence readings for 50 × 103 cells/well were statistically significant at 30 (p < 0.001), 40 (p < 0.0001), 50 (p < 0.0001), and 60 (p < 0.0001) minutes when compared to the initial 10 min reading (data not shown).
2.2. Effect of PC12 Cell Passage Number on Cell Viability under Serum Deprivation Conditions
PC12 cells grow as a suspension in culture media, and are adherent in collagen coated flasks. Due to the tendency of PC12 cells to detach during the assay procedure, even on collagen coated flasks, the assay and subsequent experimental conditions were optimized irrespective of the use of adherent or suspension phenotype cells. PrestoBlue cell viability reagent provides a strategic advantage over other reagents when continuous assessment of cell viability is required. PrestoBlue is a cell permeable nonfluorescent reagent that is rapidly taken up by cells. The reducing environment within viable cells converts Prestoblue to a red-fluororescent cell permeable dye. In assays using PrestoBlue the change in fluorescent intensity can be detected either by directly reading the cell plates or by reading supernatant media aliquots, unlike in the MTT assay which requires the dissolution of formazan crystals formed within the cells and termination of the experiment.
We adopted a published method of inducing cell death in PC12 cells using serum deprivation.2 When PC12 cells (passage 17–19) were exposed to serum-free (0% serum containing media) conditions for 60 h, we observed 57% RFU compared to vehicle control (Figure 2a). Interestingly, when the same experiment was performed using earlier passages of PC12 cells (passage 6 and 7), we observed 19% RFU compared to vehicle control (Figure 2b), a decrease of 38% compared to values obtained in passage 17–19 PC12 cells, which was statistically significant (p < 0.0001) (Figure 2c); RFU values of vehicle control were comparable at 7000 and 6200 for passage 17–19 and passage 6–7 PC12 cells, respectively. If these two sets of results were included in a screening assay, it would lead to the erroneous conclusion that compounds screened in the later passage number PC12 cells possess greater protective activity than those screened in lower passage number cells and hence a high false-positive rate. While suitable controls would reduce this occurrence the experiments would need to be repeated in lower passage number cells, resulting in a significant loss of time and resources.
Figure 2.
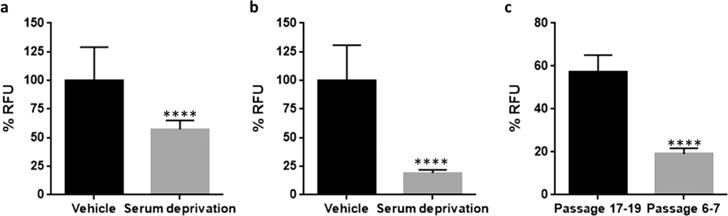
PC12 cell viability in serum free media when measured after 60 h. 50 × 103 cells/well. (a) PC12 cells (passage 17–19). For serum deprivation group, experiments were repeated six times with four replicates per experiment (two experiments per passage). Values are represented as mean ± SD. Unpaired two-tailed t test, 95% CI, ****p < 0.0001. (b) PC12 cells (passage 6 and 7). For serum deprivation group, experiments were repeated four times with four replicates per experiment (two experiments per passage). Values are represented as mean ± SD. Unpaired two-tailed t test, 95% CI, ****p < 0.0001. (c) Statistical comparison of cell viability by RFU for serum deprivation in passage 17–19 and passage 6–7 PC12 cells. Unpaired two-tailed t test, 95% CI, ****p < 0.0001.
2.3. Optimization of Serum Deprivation Experiments
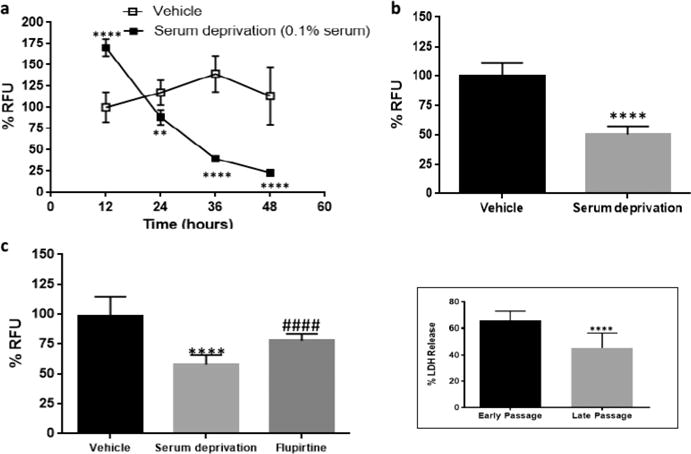
Based on the preceding studies, we further optimized the serum deprivation conditions in PC12 cells. PC12 cells were cultured in two different concentrations of serum (0.5% and 0.1%) containing culture medium. Passage 14 PC12 cells were plated in a 96-well plate with 0.5% serum containing media to deprive the cells of serum. Surprisingly, after 96 h under reduced serum conditions cell viability remained significantly high at 254% compared to control, suggesting exponential cell proliferation even under 0.5% serum conditions. Cell viability between control and serum deprived cells was significantly (p < 0.01) reduced only at 96 h (Figure 3a). When PC12 cells (passage 15) were plated in 0.1% serum containing media we observed ∼50% cell viability after 60 h (Figure 3b). When these conditions (0.1% serum, 60 h time point) were repeated in passage 12 PC12 cells we observed 18% RFU in the serum deprivation group (Figure 3c) after 60 h. The known neuroprotective compound flupirtine27 at 3 μM demonstrated a protective effect, increasing cell viability to approximately 26%, which was statistically significant (p < 0.01) compared to nontreated serum deprived cells (Figure 3c). Although the observed magnitude of increase in cell viability was statistically significant a greater magnitude is desirable for comparative purposes, to this end we chose to use the 24 h incubation time point under reduced 0.1% serum conditions (Figure 4).
Figure 3.

Serum deprivation optimization using PC12 cells with a passage number > 11. (a) PC12 cells (passage 14), 0.5% serum. Six replicates per experiment. Values are represented as mean ± SD. Multiple t test, Holm-Sidak method, alpha = 5.000%, **p < 0.01. (b) PC12 cells (passage 15), 0.1% serum. Six replicates per experiment. Values are represented as mean ± SD. Multiple t test, Holm-Sidak method, alpha = 5.000%, **p < 0.01, ****p < 0.0001. (c) PC12 cells (passage 12), 0.1% serum. RFU measured after 60 h. Flupirtine at 3 μM concentration rescued the cells from apoptosis. Experiment repeated twice with four replicates per experiment. Values are represented as mean ± SD. Unpaired two-tailed t test, 95% CI, ****p < 0.0001 in comparison to vehicle. ##p < 0.01 in comparison to serum deprivation.
Figure 4.
Optimization and validation of serum deprivation in PC12 cells using 0.1% serum and 50 × 103 cells/well. (a) PC12 cells (passage 12). Values are represented as mean ± SD. Multiple t test, Holm-Sidak method, alpha = 5.000%, **p < 0.01, ****p < 0.0001. (b) PC12 cells (passage 13). Fluorescence measured after 24 h. Values are represented as mean ± SD. Unpaired two-tailed t test, 95% CI, ****p < 0.0001. (c) PC12 cells (passage 9–13). Flupirtine at 3 μM concentration rescued the cells from apoptosis. Values are represented as mean ± SD. Unpaired two-tailed t test, 95% CI, ****p < 0.0001 in comparison to vehicle. ####p < 0.0001 in comparison to serum deprivation. (Inset) Early passage (passage 9–13) and late passage (passage 17–19) PC12 cells grown in 0.1% serum media. LDH release measured after 60 h. Twelve replicates per experiment. Values are represented as mean ± SD. Unpaired two-tailed t test, 95% CI, ****p < 0.0001.
To obtain a cell viability of approximately 50%, PC12 cells (passage 12) were plated in wells containing 0.1% serum and cell viability determined across several time points. Approximately 50% cell death was observed between 24 and 36 h when measured using PrestoBlue (Figure 4a and b). Using these optimized conditions (0.1% serum, 24 h time point), cell viability was measured in PC12 cells (passage 9–13) to determine the effect of the known neuroprotective compound flupirtine. Again approximately 50% cell death was observed at 24 h and flupirtine (3 μM) demonstrated protective effect which was statistically significant (p < 0.0001 compared to serum deprivation) (Figure 4c). Based on the observed passage variations in the prior experiments, PC12 cells with a strict passage range of 9 to 13 retain constant cell viability when exposed to serum deprivation conditions (Figure 4c). An increase in RFU was observed at 12 h in PC12 cells growing under 0.1% serum (Figures 3b and 4a) which may be due to increased metabolic activity in the cells to compensate for decreased serum levels. The results obtained from the PrestoBlue assay were further verified by employing a lactate dehydrogenase (LDH) release assay. Early (passage 9–13) and late (passage 17–19) passage PC12 cells were grown in 0.1% serum containing medium and LDH release was measured after 60 h. A similar trend was observed in the LDH assay and the results were statistically significant (p < 0.0001) between early and late passage number (Figure 4, inset).
2.4. Optimization of Etoposide-Induced Apoptosis in PC12 Cells
PC12 cells (passage 9–13) were used to optimize an etoposide-based protection assay. Etoposide is a known anticancer agent, which can induce apoptosis by inhibiting topoisomerase II.28 Etoposide at 15 μg/mL concentration was used for this experiment based on a previously reported method.29 PC12 cells (passage 9–13) were plated in a 96-well plate and were cultured overnight. The cells were then treated with 15 μg/mL etoposide. Cell viability was measured using PrestoBlue dye every 24 h for 72 h. Approximately 50% viable cells remained after 48 h of treatment (Figure 5a). The assay was validated using flupirtine (3 μM) in PC12 cells (passage 9–13), which after 48 h resulted in an increase of cell viability by 38% compared to etoposide treatment, demonstrating statistically significant protection (p < 0.0001) (Figure 5b).
Figure 5.
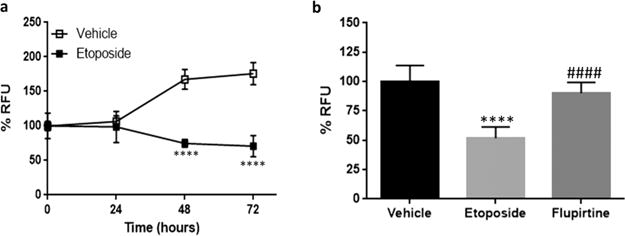
Optimization and validation of etoposide-induced cell death in PC12 cells (passage 9–13). 50 × 103 cells/well. (a) Approximately 50% cell death was observed after 48 h. Four replicates per experiment. Values are represented as mean ± SD. Multiple t test, Holm-Sidak method, alpha = 5.000%, ****p < 0.0001. (b) Fluorescence measured after 48 h. Flupirtine at 3 μM concentration rescued the cells from apoptosis. Experiment repeated thrice. Values are represented as mean ± SD. Unpaired two-tailed t test, 95% CI, ****p < 0.0001 in comparison to vehicle. ####p < 0.0001 in comparison to etoposide treatment.
NGF is known to be cytoprotective against several insults including serum deprivation, hydrogen peroxide, oxygen and glucose deprivation.2,30,31 NGF mediated differentiation of PC12 cells occurs over a period of five to eight days, limiting the use of differentiated PC12 cells for high throughput screening. We sought to verify if a significant difference of sensitivity to induced insult exists in differentiated PC12 cells due to passage number as observed in undifferentiated PC12 cells. PC12 cells were differentiated by treatment with NGF (100 ng/mL) and incubated for eight days,1 the cells were then treated with etoposide (15 μg/mL) to induce apoptosis. The observed reduction in RFU was statistically significant compared to 0 h (p < 0.0001) at 48 and 72 h after treatment with etoposide in both early and late passage (Figure 6a, b). No statistical difference was observed in RFU at 24 and 48 h between early and late passage differentiated PC12 cells. However, a statistically significant difference (p < 0.0001) was obtained between the RFU at 72 h between early and late passage cells with an absolute RFU of 48% in the early passage and 38% in the later passage cells (Figure 6c).
Figure 6.
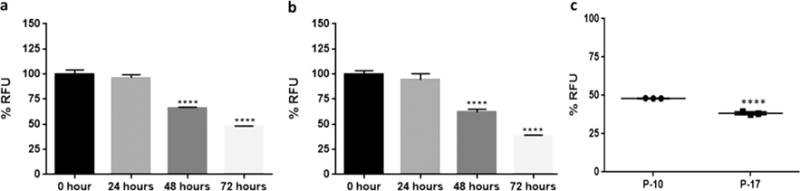
NGF differentiated PC12 cells show a statistically significant difference in sensitivity to apoptosis induced by etoposide between early and late passages at 72 h post insult. (a) Passage 10. Three replicates per experiment. Values are represented as mean ± SD. One-way ANOVA, Dunnett test, 95% CI. ****p < 0.0001. (b) Passage 17. Three replicates per experiment. Values are represented as mean ± SD. One-way ANOVA, Dunnett test, 95% CI. ****p < 0.0001. (c) Comparison of 72 h etoposide treatment in passage 10 and 17 PC12 cells differentiated using NGF. Unpaired two-tailed t test, 95% CI, ****p < 0.0001.
3. CONCLUSION
We report the optimization and validation of a medium to high throughput neuroprotection assay using PrestoBlue dye in PC12 cells. The PrestoBlue cell viability agent has many advantages over other commonly employed cell viability determinants.22,32 PrestoBlue represents a feasible cell viability agent for both adherent and suspension cell lines while the dye can be removed by washing with PBS allowing the cells to be reused for other experiments.33
A significant passage number variation was shown to exist in PC12 cells, influencing the susceptibility of this cell line to external insult. Later passage numbers (above 14) reduce the sensitivity of undifferentiated PC12 cells to insults from both etoposide and serum deprivation, providing an inherently greater “protective” effect. Similar results have been reported against injury induced by 6-OHDA where cells with passage number 5 were more sensitive to the injury compared to cells with passage number 16.23 The same group also reported that higher passage number of PC12 cells were more sensitive to injury induced by lead and arsenic compared to lower passage.23
Optimized protocols have been developed for both serum deprivation and etoposide-induced apoptosis of PC12 cells (passage 9–13) using PrestoBlue. The accuracy of these protocols were confirmed by observance of the neuroprotective effect of the known neuroprotective compound flupirtine. It is recommended to screen molecules of interest in at least two different types of assay using different methods to induce external insult. Molecules that show a protective effect under both assay conditions would ensure that screened compounds do not act by replacing etoposide at its binding site but are in fact neuroprotective.
It should be noted that NGF prevents PC12 cells from undergoing cell death under serum deprivation conditions,2 preventing the use of differentiated PC12 cells in the serum deprivation experiments. However, we show that NGF differentiated PC12 cells do exhibit variable sensitivity to etoposide-induced apoptosis. Later passage number cells are more sensitive to etoposide-induced apoptosis than earlier passage differentiated cells, the opposite trend as seen in undifferentiated cells, albeit only after 72 h. A large number of phenotypic changes occur upon differentiation of PC12 cells, possibly accounting for this observed sensitivity to externally induced apoptosis. Differentiated PC12 cells have been shown to be more sensitive to EtOH-induced apoptosis than their undifferentiated counterparts,34 a similar trend was noted upon exposure to HgCl2.35 In other accounts, differentiated PC12 cells were shown to be less sensitive to apoptosis induced by pteridine exposure than differentiated cells.36 We propose that passage number is a critical factor in determining sensitivity to externally induced apoptosis across both differentiated and undifferentiated PC12 cells and a passage range of 9–13 provides sufficient accuracy and invariability to determine neuroprotective efficacy of candidate compounds.
In conclusion, we report for the first time an optimized protocol for the screening of neuroprotective activity of small molecules in PC12 cells using PrestoBlue cell viability agent. This protocol accounts for the variation of the inherent “protective effect” demonstrated by later passage number undifferentiated PC12 cells and the greater sensitivity of later passage number differentiated PC12 cells to etoposide-induced apoptosis, an observation that has critical implications in drug discovery screening but one that has never previously been reported for the common etoposide and serum deprivation conditions employed to induce cell death. These studies provide consideration of an additional parameter (passage number) to enhance the scientific rigor of neuroprotective screens using PC12 cells in neurologic-based drug discovery processes.
4. METHODS
4.1. Cell Culture and Media
PC12 cells were obtained from ATCC (CRL-1721) and cultured in RPMI 1640 (Corning, 10–040-CV) supplemented with 10% heat inactivated horse serum (Corning, 35-030-CV), 5% fetal bovine serum (Corning, 35-010-CV), 1% penicillin streptomycin (Corning, 30-002-CI) and maintained as monolayer cultures in a humidified atmosphere containing 5% CO2 at 37 °C. Complete media was diluted appropriately to make serum deprived media containing 0.5% or 0.1% serum. T-75 flasks and 96-well plates were coated with 100 μg/mL Rat Tail Collagen I (Corning, 354249) prepared in 0.02 N acetic acid solution. The flasks and plates were coated for 4 h and washed with sterile double distilled water. The coated flasks and plates can be stored for up to a week at 4 °C. The cells obtained from ATCC were considered as passage 1 and the subsequently cultured cells as passage 2 and onward. The protocols for culturing and subculturing were used from published protocols.5 Etoposide (Chem-Impex International, 28435) was stored as a working stock solution of 150 μg/mL at −20 °C for up to 3 months. Etoposide is sensitive to freeze–thaw cycles and thus the number of free-thaw cycles was limited to one. The LDH assay was performed based on the manufacturer’s recommended protocol (ThermoFisher Scientific, Pierce LDH Cytotoxicity Assay Kit, 88954).
4.2. Serum Deprivation Assays in a 96-Well Plate
All experiments are conducted in undifferentiated PC12 cells (passage 9–13).
The media from the T75 flask was removed and the cells were washed with 5 mL of PBS (without calcium or magnesium) to remove traces of serum.
A volume of 3 mL of 0.25% trypsin-EDTA solution was added. The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 3 min, and then 7 mL of complete media was added to neutralize the effect of trypsin.
The entire cell suspension was collected and then centrifuged at 1000 rpm for 5 min. The media was removed from the centrifuge tube, fresh media was added, and the cells were resuspended.
The cells were counted using a hemocytometer. Appropriate dilutions were made using 0.1% media such that there were 50 000 cells/well (∼150 000 cells/cm2) and the cells were plated in a 96-well black plate with clear bottom using a multichannel pipet.
The cells were treated with the desired concentrations of test compound using vehicle with complete serum as control.
The plate was incubated for 24 h in a humidified atmosphere containing 5% CO2 at 37 °C.
After 24 h, 10 μL of 10× PrestoBlue dye was added in the dark. The plate was covered with aluminum foil (as PrestoBlue is light sensitive) and incubated for 30 min in a humidified atmosphere containing 5% CO2 at 37 °C.
RFU was measured at excitation 560 ± 9 nm and emission 590 ± 9 nm using a microplate reader (Biotek Synergy Mx, Reader model SMATBL).
For calculation of cell viability, all values were normalized to cells growing in normal serum containing media considering its RFU as 100%.
4.3. Etoposide-Induced Apoptosis Assays in a 96-Well Plate
All experiments are conducted in undifferentiated PC12 cells (passage 9–13). Steps (1)–(4) were followed as per the serum deprivation protocol above.
-
(5)
The cells were allowed to adapt overnight in a humidified atmosphere containing 5% CO2 at 37 °C.
-
(6)
The cells were pretreated for 4 h with the desired concentrations of test compound. Etoposide (15 μg/mL) and vehicle were used as controls.
-
(7)
After 4 h, 10 μL of a 150 μg/mL etoposide solution was added to make the final concentration of etoposide in the well 15 μg/mL.
-
(8)
The plate was incubated for 48 h in a humidified atmosphere containing 5% CO2 at 37 °C. After 48 h, 10 μL of 10× PrestoBlue dye was added in the dark. The plate was covered with aluminum foil (as PrestoBlue is light sensitive) and incubated for 30 min in a humidified atmosphere containing 5% CO2 at 37 °C.
-
(9)
RFU was measured at excitation 560 ± 9 nm and emission 590 ± 9 nm using a microplate reader (Biotek Synergy Mx, Reader model SMATBL).
-
(10)
For calculation of cell viability, all values were normalized to cells growing in normal serum containing media considering its RFU as 100%.
4.4. Etoposide-Induced Apoptosis Assays in Differentiated PC12 Cells
PC12 cells were plated at a density of 150 000 cells/cm2 in rat tail collagen I coated 12-well plates. The cells were allowed to adapt overnight and exposed to NGF (100 ng/mL) for 8 days to induce differentiation. On day nine, etoposide (15 μg/mL) was added.1 PrestoBlue dye was added in the dark at 24, 48, or 72 h time points corresponding to the desired incubation time of etoposide. The plate was covered with aluminum foil (as PrestoBlue is light sensitive) and incubated for 30 min in a humidified atmosphere containing 5% CO2 at 37 °C. RFU was measured at excitation 560 ± 9 nm and emission 590 ± 9 nm using a microplate reader (Biotek Synergy Mx, Reader model SMATBL).
4.5. Data Analysis
Data reported are mean values ± SD from the indicated number of experiments. For single comparisons, the significance of differences between means was assessed by Student’s t test; for multiple comparisons, data were analyzed by analysis of variance and post hoc t tests. A level of p = 0.05 or lower was considered statistically significant.
Acknowledgments
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001105. The authors acknowledge the Rare Genomics Institute and Collaborative Drug Discovery for the award of a BeHEARD challenge grant.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors declare no competing financial interest.
References
- 1.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene LA. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978;78:747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima M, Kashiwagi K, Ohta J, Furukawa S, Hayashi K, Kawashima T, Hayashi Y. Nerve growth factor and epidermal growth factor rescue PC12 cells from programmed cell death induced by etoposide: Distinct modes of protection against cell death by growth factors and a protein-synthesis inhibitor. Neurosci Lett. 1994;176:161–164. doi: 10.1016/0304-3940(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 4.Gassen M, Pergande G, Youdim MB. Antioxidant properties of the triaminopyridine, flupirtine. Biochem Pharmacol. 1998;56:1323–1329. doi: 10.1016/s0006-2952(98)00126-9. [DOI] [PubMed] [Google Scholar]
- 5.Grau CM, Greene LA. Use of PC12 cells and rat superior cervical ganglion sympathetic neurons as models for neuroprotective assays relevant to Parkinson’s disease. Methods Mol Biol. 2012;846:201–211. doi: 10.1007/978-1-61779-536-7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei H, Leeds PR, Qian Y, Wei W, Chen R-w, Chuang DM. β-Amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur J Pharmacol. 2000;392:117–123. doi: 10.1016/s0014-2999(00)00127-8. [DOI] [PubMed] [Google Scholar]
- 7.Trippier PC, Benmohamed R, Kirsch DR, Silverman RB. Substituted pyrazolones require N2 hydrogen bond donating ability to protect against cytotoxicity from protein aggregation of mutant superoxide dismutase 1. Bioorg Med Chem Lett. 2012;22:6647–6650. doi: 10.1016/j.bmcl.2012.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto G, Stojanovic A, Holmberg CI, Kim S, Morimoto RI. Structural properties and neuronal toxicity of amyotrophic lateral sclerosis-associated Cu/Zn superoxide dismutase 1 aggregates. J Cell Biol. 2005;171:75–85. doi: 10.1083/jcb.200504050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabakman R, Lazarovici P, Kohen R. Neuroprotective effects of carnosine and homocarnosine on pheochromocytoma PC12 cells exposed to ischemia. J Neurosci Res. 2002;68:463–469. doi: 10.1002/jnr.10228. [DOI] [PubMed] [Google Scholar]
- 10.Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Haviv R, Lindenboim L, Yuan J, Stein R. Need for caspase-2 in apoptosis of growth-factor-deprived PC12 cells. J Neurosci Res. 1998;52:491–497. doi: 10.1002/(SICI)1097-4547(19980601)52:5<491::AID-JNR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Stefanis L, Park DS, Yan CY, Farinelli SE, Troy CM, Shelanski ML, Greene LA. Induction of CPP32-like activity in PC12 cells by withdrawal of trophic support. Dissociation from apoptosis. J Biol Chem. 1996;271:30663–30671. doi: 10.1074/jbc.271.48.30663. [DOI] [PubMed] [Google Scholar]
- 13.Mah SP, Zhong LT, Liu Y, Roghani A, Edwards RH, Bredesen DE. The Protooncogene bcl-2 Inhibits Apoptosis in PC12 Cells. J Neurochem. 1993;60:1183–1186. doi: 10.1111/j.1471-4159.1993.tb03275.x. [DOI] [PubMed] [Google Scholar]
- 14.Saura J, MacGibbon G, Dragunow M. Etoposide-induced PC12 cell death: apoptotic morphology without oligonucleosomal DNA fragmentation or dependency upon de novo protein synthesis. Mol Brain Res. 1997;48:382–388. doi: 10.1016/s0169-328x(97)00105-8. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Han T, Nijhawan D, Theodoropoulos P, Naidoo J, Yadavalli S, Mirzaei H, Pieper AA, Ready JM, McKnight SL. P7C3 Neuroprotective Chemicals Function by Activating the Rate-Limiting Enzyme in NAD Salvage. Cell. 2014;158:1324–1334. doi: 10.1016/j.cell.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed SA, Gogal RM, Jr, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 19.Paull KD, Shoemaker RH, Boyd MR, Parsons JL, Risbood PA, Barbera WA, Sharma MN, Baker DC, Hand E, Scudiero DA, Monks A, Alley MC, Grote M. The synthesis of XTT: A new tetrazolium reagent that is bioreducible to a water-soluble formazan. J Heterocycl Chem. 1988;25:911–914. [Google Scholar]
- 20.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 21.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.ima03bs21. Appendix 3. [DOI] [PubMed] [Google Scholar]
- 22.Lall N, Henley-Smith CJ, De Canha MN, Oosthuizen CB, Berrington D. Viability Reagent, PrestoBlue, in Comparison with Other Available Reagents, Utilized in Cytotoxicity and Antimicrobial Assays. Int J Microbiol. 2013;2013:420601. doi: 10.1155/2013/420601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mejia M, Salgado-Bustamante M, Castillo CG, Jimenez-Capdeville ME. Passage determines toxicity and neuronal markers expression in PC12 cells with altered phenotype. Toxicol Res. 2013;2:388–396. [Google Scholar]
- 24.Xiao J, Zhou Q, Liu Y. Variant PC12 cell line that spontaneously differentiates and extends neuritic processes. J Neurosci Res. 2002;69:104–109. doi: 10.1002/jnr.10260. [DOI] [PubMed] [Google Scholar]
- 25.Shoji-Kasai Y, Morishima M, Kuwahara R, Kondo S, Itakura M, Takahashi M. Establishment of variant PC12 subclones deficient in stimulation–secretion coupling. Biochim Biophys Acta, Mol Cell Res. 2001;1499:180–190. doi: 10.1016/s0167-4889(00)00103-8. [DOI] [PubMed] [Google Scholar]
- 26.Quinn JP. Variation in the composition of the AP1 complex in PC12 cells following induction by NGF and TPA. Mol Cell Neurosci. 1991;2:253–258. doi: 10.1016/1044-7431(91)90052-p. [DOI] [PubMed] [Google Scholar]
- 27.Seyfried J, Evert BO, Rundfeldt C, Schulz JB, Kovar KA, Klockgether T, Wullner U. Flupirtine and retigabine prevent L-glutamate toxicity in rat pheochromocytoma PC 12 cells. Eur J Pharmacol. 2000;400:155–166. doi: 10.1016/s0014-2999(00)00397-6. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr Med Chem: Anti-Cancer Agents. 2005;5:363–372. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- 29.Hatayama T, Yamagishi N, Minobe E, Sakai K. Role of hsp105 in Protection against Stress-Induced Apoptosis in Neuronal PC12 Cells. Biochem Biophys Res Commun. 2001;288:528–534. doi: 10.1006/bbrc.2001.5802. [DOI] [PubMed] [Google Scholar]
- 30.Satoh T, Sakai N, Enokido Y, Uchiyama Y, Hatanaka H. Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis. J Biochem. 1996;120:540–546. doi: 10.1093/oxfordjournals.jbchem.a021447. [DOI] [PubMed] [Google Scholar]
- 31.Oeltgen PR, Bishop PD, Brown SA. Deltorphin-D and Nerve Growth Factor Induce Cell Protection in PC12 Cells Subjected to Ischemia. FASEB J. 2011;25:612.5. [Google Scholar]
- 32.Boncler M, Rozalski M, Krajewska U, Podsedek A, Watala C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J Pharmacol Toxicol Methods. 2014;69:9–16. doi: 10.1016/j.vascn.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 34.Oberdoerster J, Rabin RA. NGF-Differentiated and undifferentiated PC12 cells vary in induction of apoptosis by ethanol. Life Sci. 1999;64:PL267–PL272. doi: 10.1016/s0024-3205(99)00166-6. [DOI] [PubMed] [Google Scholar]
- 35.Parran DK, Mundy WR, Barone S., Jr Effects of methylmercury and mercuric chloride on differentiation and cell viability in PC12 cells. Toxicol Sci. 2001;59:278–290. doi: 10.1093/toxsci/59.2.278. [DOI] [PubMed] [Google Scholar]
- 36.Enzinger C, Wirleitner B, Spottl N, Bock G, Fuchs D, Baier-Bitterlich G. Reduced pteridine derivatives induce apoptosis in PC12 cells. Neurochem Int. 2002;41:71–78. doi: 10.1016/s0197-0186(01)00134-6. [DOI] [PubMed] [Google Scholar]


