Abstract
Oxidative stress is known to be associated with various age-related diseases. D-galactose (D-gal) has been considered a senescent model which induces oxidative stress response resulting in memory dysfunction. Pyrroloquinoline quinone (PQQ) is a redox cofactor which is found in various foods. In our previous study, we found that PQQ may be converted into a derivative by binding with amino acid, which is beneficial to several pathological processes. In this study, we found a beneficial glutamate mixture which may diminish neurotoxicity by oxidative stress in D-gal induced mouse. Our results showed that PQQ may influence the generation of proinflammatory mediators, including cytokines and prostaglandins during aging process. D-gal-induced mouse showed increased MDA and ROS levels, and decreased T-AOC activities in the hippocampus, these changes were reversed by PQQ supplementation. Furthermore, PQQ statistically enhanced Superoxide Dismutase SOD2 mRNA expression. PQQ could ameliorate the memory deficits and neurotoxicity induced by D-gal via binding with excess glutamate, which provide a link between glutamate-mediated neurotoxicity, inflammation and oxidative stress. In addition, PQQ reduced the up-regulated expression of p-Akt by D-gal and maintained the activity of GSK-3β, resulting in a down-regulation of p-Tau level in hippocampus. PQQ modulated memory ability partly via Akt/GSK-3β pathway.
Introduction
Aging is a complicated multifactorial process that results in a gradual slow decline in physiological function and the ability of an organism to survive. Oxidative damage has been implicated to be a major factor in the decline in physiologic function that occurs during the aging process1,2. Several studies have suggested that accumulation of reactive oxygen species (ROS) and elevated oxidative stress are associated with neuronal dysfunction in various age-related neurodegenerative disorders3–5. Mitochondria have been revealed as an important link between the age-related oxidative damage and the alterations in physiologic function associated with aging6–8. Furthermore, studies reported that oxidative damage to mitochondria would increase the generation of ROS, and lead to impaired cognition6,9–11. Oxidative damage stimulates the generation of free radicals and release high level of glutamate that may aggravate damage to brain cells12,13. Glutamate is the critical excitatory neurotransmitter in the central nervous system. However, excessive glutamate stimulation can produce ROS, which will contribute to neuronal damage14–17. In addition, the cumulate of Glutamate and continuous increase of ROS trigger neuroinflammatory pathways, increasing the chemokines, cytokines, and prostaglandins18–23. Moreover, reports demonstrated that glutamate decreased the expression of p-GSK-3β, which would increase GSK-3β activity during glutamate-induced neurotoxicity24. Pharmacological treatments, which inhibit GSK-3β, have been reported to reduce cognitive impairment in AD mice25.
D-galactose (D-gal) has been considered an artificial aging model which induces oxidative stress and inflammatory response resulting in memory and synaptic dysfunction26–28. Chronic systemic administration of D-gal in rodents has been extensively used as an animal model for brain aging in various anti-aging studies29–31. It has been reported that animals receiving chronic successive administration of D-gal (50–500 mg/kg) for 4–8 weeks experienced cognitive and memory dysfunction24,25,30. It has been reported that D-gal induced behavioral and neurochemical changes that could mimic many characteristics of the natural process of brain aging26,32,33. This model exhibits accelerated aging in tissues especially brain34–38. The brain is particularly vulnerable to oxidative damage because of its high oxygen demand, high unsaturated lipid content, and relative deficiency in anti-oxidative defense mechanisms. Thus, initial studies suggested that antioxidant therapy is crucial to prevent aging or age-related neurodegenerative disorders39,40.
Pyrroloquinoline quinone (PQQ) is a redox cofactor. It has been found in various foods, including vegetable and animal tissues41–43. PQQ not only serves to mediate redox reactions in the mitochondrial respiratory chain, but also plays a potential role of scavenging ROS and attenuating oxidative stress in mitochondria44. Accumulating evidence shows that PQQ can protect neurons against glutamate-induced damage by scavenging ROS17.
In the present study, we investigated that the protective effects of PQQ against D-gal induced oxidative stress, cognitive impairment, glutamate level and inflammation factors in mouse brain. We further explored the link between oxidative stress, neurotoxicity, neuroinflammation, and its potential contribution in aging related disease.
Results
Effects of PQQ on cognitive impairment induced by D-gal
Y-maze test
The spatial working memory using spontaneous alteration behavior percentage (%) was analyzed using Y-maze task. Our results indicated that D-gal had a lower percentage of spontaneous alteration than control group, indicating less working memory (P < 0.05). However, the spontaneous alteration was significantly increased in mice who received PQQ either alone or in combination with D-gal (P < 0.05), indicating that PQQ improved memory in D-gal treated mice (Fig. 1).
Figure 1.
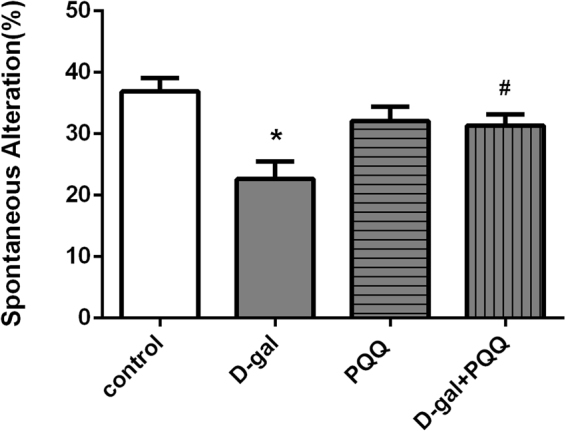
The percentage of spontaneous alteration in Y-maze of behavior test. The data are shown as a mean ± S.E.M. *P < 0.05 relative to control group. #P < 0.05 relative to D-gal treated mice group.
Passive avoidance test
The results showed that PQQ significantly ameliorated cognitive ability of D-gal-treated mice for Passive avoidance test (Fig. 2). The model group mice had more error times and shorter latency for retention than those of control group mice (*P < 0.05, **P < 0.01 relative to control group, respectively). PQQ contributed to decreased number of mistakes (#P < 0.05, ##P < 0.01 relative to D-gal group, respectively) during the training session both alone and combining with D-gal. In the retention session, PQQ treatment with D-gal group mice showed longer latency and less mistakes compared with the model group mice (##P < 0.01 relative to D-gal group).
Figure 2.
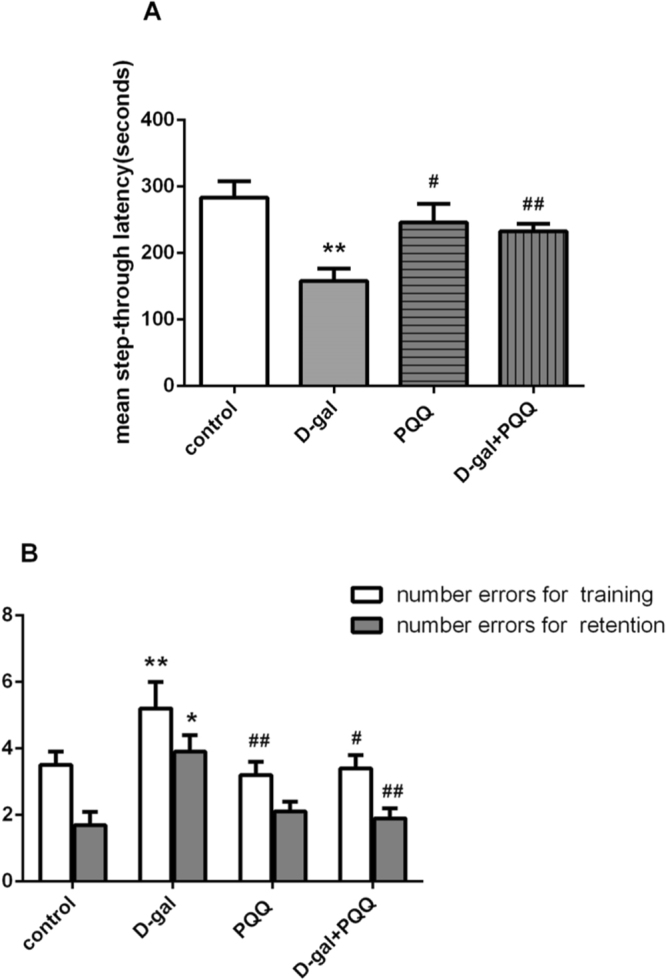
Effect of PQQ on learning and memory abilities of D-gal-treated mice in passive avoidance test. *P < 0.05, **P < 0.01 relative to control group. #P < 0.05, ##P < 0.01 relative to D-gal group.
PQQ ameliorated D-gal-induced oxidative stress and restored T-AOC activity in D-gal-induced aging mice
Treatment with PQQ reduced the hippocampal MDA level, and thus reduced oxidative stress (Fig. 3A). In addition, the ROS levels were significantly higher in the hippocampal (p < 0.01) of D-gal-induced mice compared to the control mice. Treatment with PQQ inhibited the increase of levels of ROS in hippocampal (p < 0.01) (Fig. 3B). T-AOC activities were significantly lower in the hippocampal (p < 0.05) of D-gal-induced mice compared with normal control mice. Treatment with PQQ significantly ameliorated the reduction in hippocampal T-AOC activity (p < 0.05) (Fig. 3C). In addition, PQQ alone had no effect on MDA, ROS level or T-AOC activity in normal mice.
Figure 3.

Effects of PQQ on MDA (A), ROS level (B) and T-AOC activities (C) in hippocampus of the D-gal-treated mice. *P < 0.05, **P < 0.01 relative to control group. #P < 0.05, ##P < 0.01 relative to D-gal group.
Effect of PQQ on SOD expression in D-gal-induced mice
Semiquantitative RT-PCR (Fig. 4A, Supplementary Info 2) was carried out to determine the levels of SOD1 and SOD2 expression. The mean levels of SOD1 mRNA and, to a greater extent, SOD2 mRNA were lower in D-gal mice than in the control group (P < 0.01) (Fig. 4). PQQ combine with D-gal treatment did not alter mRNA expression of SOD1 compared to the D-gal group, but statistically enhanced SOD2 expression (P < 0.01).
Figure 4.
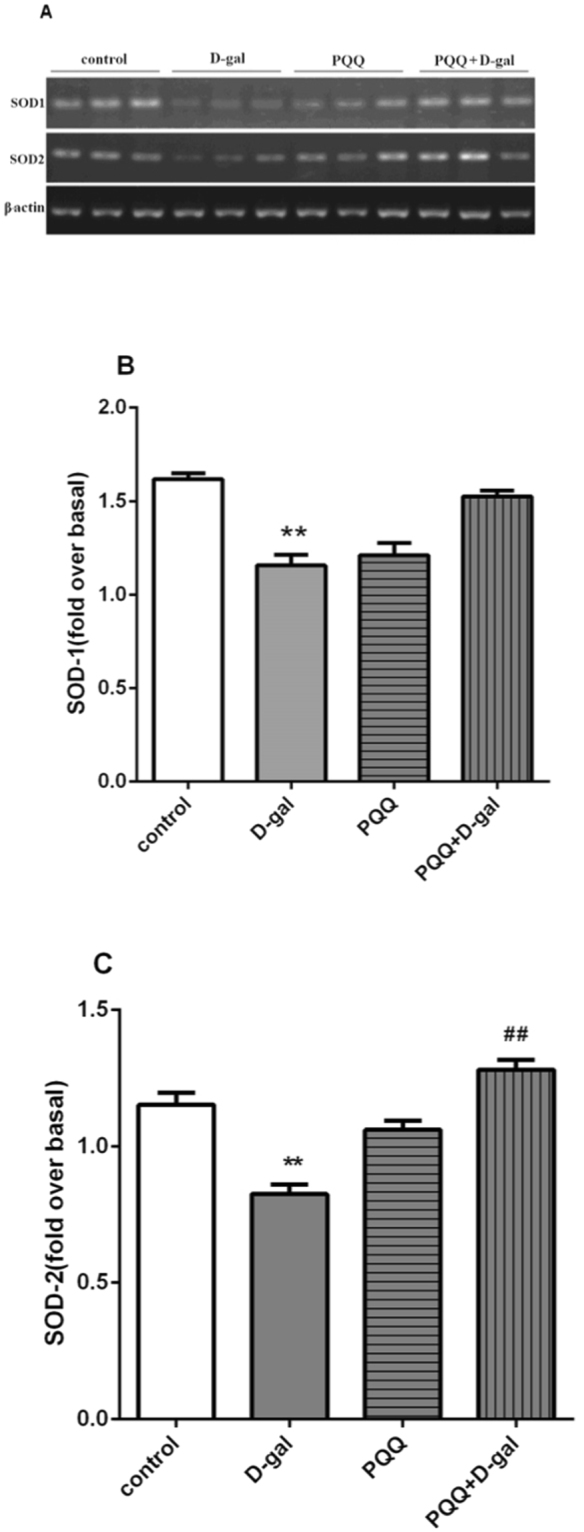
(A) RT-PCR analysis of superoxide dismutase (SOD) isoenzymes mRNA levels in the brain. (B,C) Densitometric analysis of gel images. Intensity of each band was quantified, normalized to the intensity of β-actin bands, and expressed as relative intensity; the results are shown as an average of seven subjects in each group. **P < 0.01 compared with control group. ##P < 0.01 compared with D-gal group.
Effect of PQQ on inflammatory factors include IL-2, IFN-γ levels and the production of Prostaglandin E2 (PGE2) in D-gal-induced mice
The effects of PQQ on IL-2 and IFN-γ levels in serum were detected by ELISA (Fig. 5A,B). D-gal treatment significantly increased IL-2 and IFN-γ levels 1.8- and 2.4-times (P < 0.01), respectively. Combining D-gal treatment with PQQ significantly decreased IL-2 from 2.63 ± 0.08 pg/ml in the D-gal group to 1.26 ± 0.12 pg/ml in the D-gal + PQQ group (P < 0.01). IFN-γ secretion was decreased from 42.35 ± 2.12 pg/ml in the D-gal group to 11.26 ± 1.18 pg/ml in the D-gal + PQQ group (P < 0.01). Figure 5C showed the effect of PQQ on the production of PGE2 in the hippocampus of D-gal-induced mice. As compared to control group, D-gal significantly increased the production of PGE2 to 6.41 times (P < 0.01). Treating the mice with PQQ significantly depressed the increase of production of PGE2 (p < 0.05), as compared to the D-gal-treated group. The production of PGE2 level in hippocampus were lower in PQQ treated mice than D-gal treated group (p < 0.01). These data indicate that PQQ reduced the enhanced IL-2 and IFN-γ caused by D-gal and regulated the production of PGE2.
Figure 5.
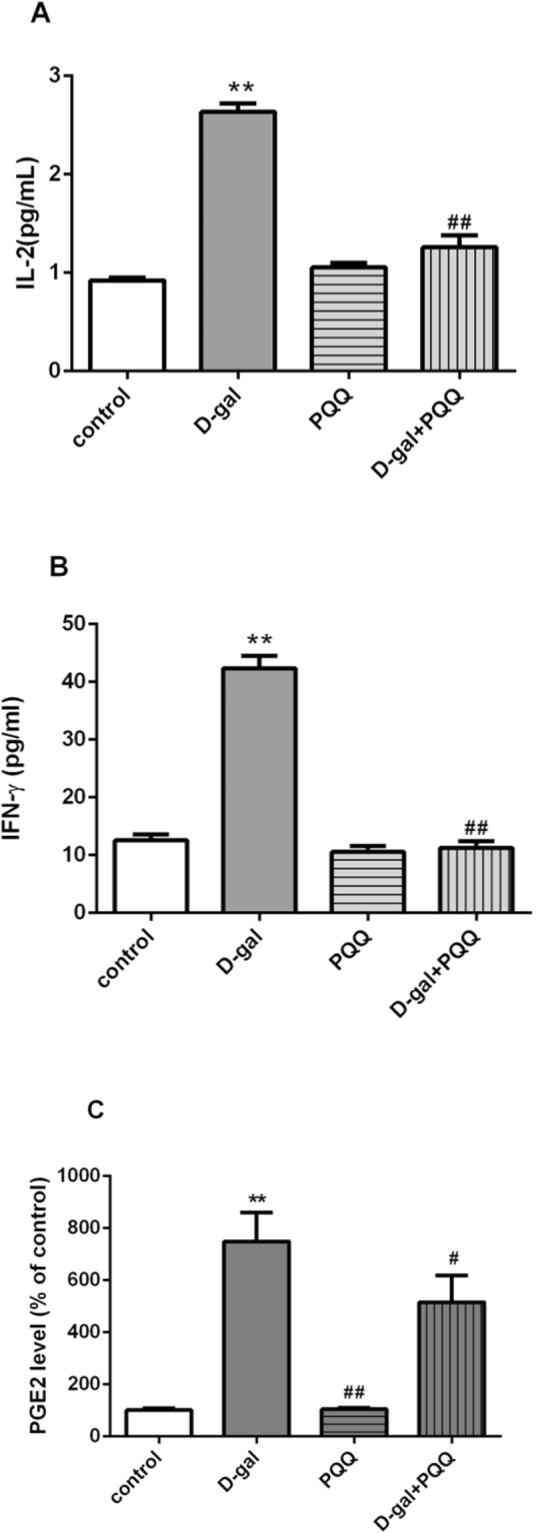
Effect of PQQ on IL-2 (A), IFN-γ (B) levels in serum and the production of PGE2 (C) in the hippocampus of the D-gal-treated mice. **P < 0.01, *P < 0.05 relative to control group; ##P < 0.01, #P < 0.05 relative to D-gal group.
Effects of PQQ on glutamate content in D-gal-induced mice
Increased glutamate concentration in hippocampus were found in D-gal induced mice compared to control groups (25.14% vs p < 0.05). Only PQQ treated, the glutamate levels were not significant compared to control mouse. Glutamate level in hippocampus was lower in PQQ treated mouse than D-gal treated mouse (27.86% vs p < 0.05). The increased glutamate level in D-gal treatment were ameliorated significantly by PQQ treatment in hippocampus (23.32% vs p < 0.05) (Fig. 6A). Additionally, there was no significant difference in cortex and cerebellumin between these groups (Data not shown).
Figure 6.
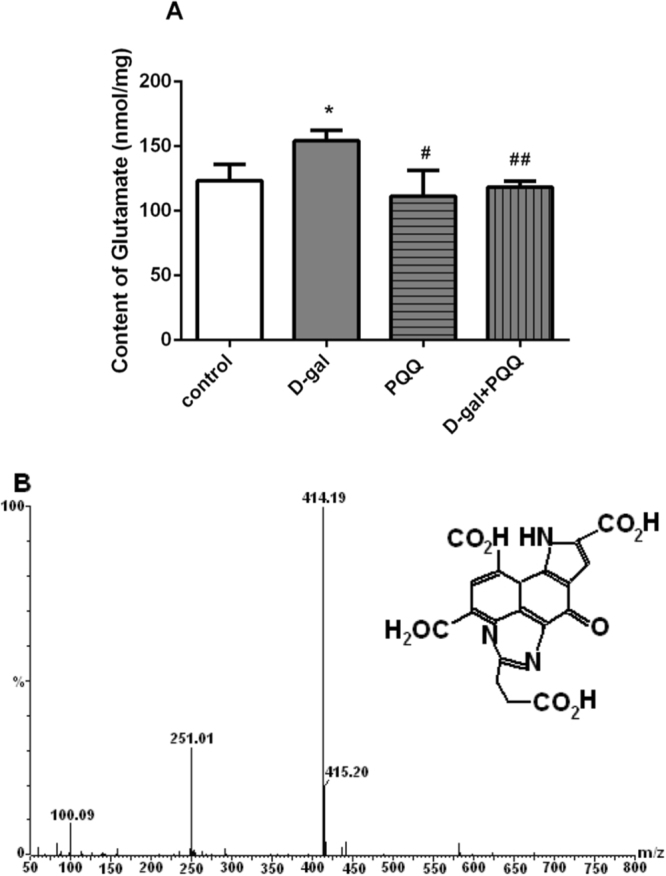
Effects of PQQ on glutamate content (A) in hippocampus of the D-gal-treated mice. *P < 0.05, **P < 0.01 relative to control group. #P < 0.05, ##P < 0.01 relative to D-gal group. Negative-mode electrospray ionization mass spectra of production PQQ-glu (B) by UPLC.
Furthermore, in molecular masses spectrum (Fig. 6B), the ions at m/z 414.19 ([M + H]+), 415.2([M + 2 H]+) was found in brain tissue of PQQ treatment group. We infer that the production has a molecular mass of 413 Da. These results are agreement with the previously reporters that PQQ can easily react with amino acids to form stable imidazolopyrroloquinoline29,30. Accordingly, the production with 413 Da molecular weight was tentatively identified as imidazolopyrroloquinoline PQQ-Glu (Fig. 6B). Given the evidence that imidazolopyrroloquinoline have redox and antioxidant activity as well as growth-promoting properties, a clear demonstration that complexes PQQ binding with amino acids exist in tissues has considerable importance. Further study on the beneficial effects of PQQ-Glu in neural function is necessary.
Effect of PQQ on expressions of Akt/GSK3β signaling markers
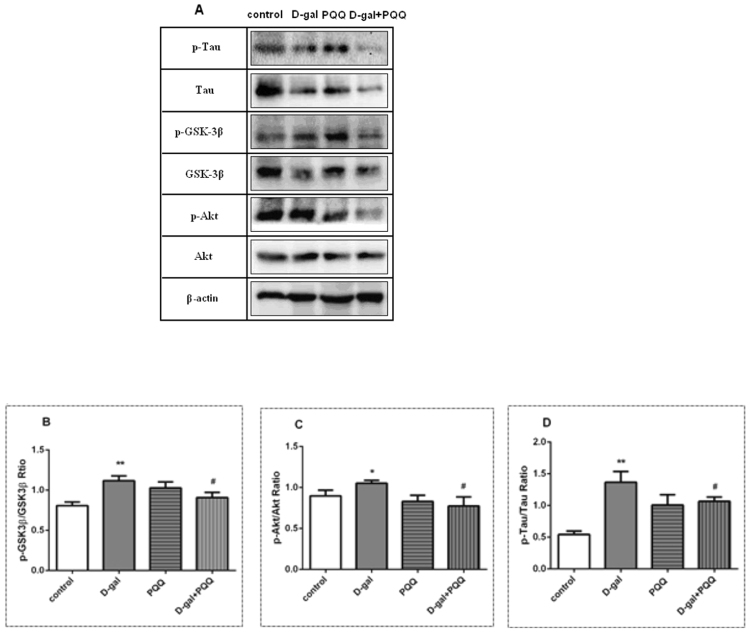
Western blot analysis (Fig. 7A, Supplementary Info 1) was used to assess the effect of PQQ on the Akt/GSK3β pathway in hippocampus subjected to D-gal. The results show that D-gal had no significant effect on the overall expression of Tau in hippocampus (p > 0.05). But mice treated with D-gal exhibited significant stimulation in the expression of p-Tau in hippocampus (p < 0.01), whereas the expressions of p-Tau in hippocampus were significantly attenuated by co-treatment with PQQ (p < 0.05, Fig. 7D). Although PQQ failed to modulate the expression of Akt in hippocampus, it was able to reduce the p-Akt expression in D-gal treated groups so as to decrease the ratio of p-Akt/Akt from 1.05 to 0.77 (p < 0.05, Fig. 7C). Likewise, PQQ was able to reduce the ratio of p-GSK-3β/GSK-3β from 1.11 to 0.91, which was beneficial to regulate the expression of GSK-3β during D-gal-induced aging (p < 0.05, Fig. 7A,B). However, there were no significant differences in cortex and cerebellumin in these groups (Data not shown).
Figure 7.
Western blot (A) analysis the effects of PQQ on GSK-3β (B), Akt (C) and Tau (D) phosphorylation in the hippocampus of the D-gal-treated mice. *P < 0.05, **P < 0.01 relative to control group. #P < 0.05, ##P < 0.01 relative to D-gal group.
Discussion
The present study aimed to investigate the protective effect of PQQ on cognitive impairment in the aging mouse induced by D-gal and the possible underlying mechanisms.
The chronic administration of D-gal induces changes that resemble natural aging in animals, such as cognitive dysfunction, oxidative stress and neurodegradation. D-gal induced senescence acceleration has been accepted as a rodent model for use in research on aging studies45,46. D-gal can cause the accumulation of ROS in vivo, ultimately result in oxidative stress37. In addition, oxidative stress contributes to a chronic inflammatory process during aging and causes age-associated diseases22,47. It is well known that glutamate is the critical excitatory neurotransmitter in the central nervous system. Oxidative stress increases the generation of ROS and release excess glutamate48,49. Excess glutamate stimulation is a common pathway in brain injuries and degenerative diseases, and ROS production is usually considered to be involved in glutamate stimulation50,51. Furthermore, oxidative injury, excitotoxicity and inflammatory seem to be three important factors involved in process of aging.
Some studies had already confirmed that PQQ prevents oxidative damage in the brain and reduces the cognitive deficit caused by oxidative stress in rats during aging52. In our previous study, we found that PQQ may be converted into a derivative by binding with amino acid, which is beneficial to several pathological processes53,54. However, which derivative promotes antioxidant in aging brain and the underlying mechanism is yet unclear. For the first time, we found a beneficial glutamate mixture which may diminish neurotoxicity by oxidative stress. As well, we will discuss the relevant aspects concerning possible neuroprotection of PQQ in the oxidative stress and neuroinflammatory changes observed in aging.
Antioxidation
The evidence showed that PQQ treatment can affect learning ability and memory function during oxidative stress in rats55. It is well known that PQQ is a well-characterized, free and redox cycling planar orthoquinone. Similar to other polyphenolic biofactors, there is strong evidence PQQ may play an important role in pathways to antioxidant potential56. Our results showed D-gal seriously impaired the learning and memory abilities. We found that this behavioral impairment was reversed when D-gal treated mice were simultaneously administered PQQ for the duration of the treatment (Fig. 1). The results are supported by previous studies, using MK-801 treatment in mice54 and hypoxia treatment in rats55, which proved that PQQ would prevent the cognitive deficit resulting from the oxidative stress.
We also observed that oxidative damage occurred in the D-gal induced aging mouse brain, and this damage was reversed by PQQ intervention. The consequences of oxidative stress can be measured by markers of damage including ROS, MDA and T-AOC. It has been suggested that accumulation of ROS induces severe damage to neuronal system. An increase in MDA is another important marker for lipid peroxidation37, which is a well-known indicator of oxidative damage of membranes under conditions of oxidative stress. In addition, total antioxidant capacity (T-AOC), as a non-enzymatic antioxidant, also indirectly reflects the level of oxidative stress57. According to our researches, PQQ decreased MDA (Fig. 3A) levels, and reduced ROS in D-gal aging model (Fig. 3B). Furthermore, in our T-AOC test, PQQ could renew the activity of T-AOC in the brain of D-gal-induced aging mice (Fig. 3C).
Apart from amelioration of MDA and ROS, PQQ also increased the activity of SOD, a major mitochondrial antioxidant enzyme that converts toxic superoxide to hydrogen peroxide. SOD is the first and the most important line of antioxidant enzyme defense against ROS protecting cells and tissues58. SOD2 null mice have been known to have short survival. B-Sod2−/− showed brain lipid peroxidation significantly increased, causing severe growth retardation59. Further more, mitochondrial SOD2 is thought to play an important role in cellular defense against oxidative damage by ROS60.
In this study, PQQ statistically enhanced SOD2 mRNA expression compared to the D-gal group (Fig. 4). Our findings provide direct supportive experimental evidence supporting that PQQ might have anti-aging effects due to its antioxidant role.
Anti-inflammatory
In addition, the development of neuroinflammation has also been related to cognitive changes in aging related diseases61–63. Neuroinflammation in aging is characterized by the accumulation of many cytokines, in which the most obvious change is IL-2 production64. IL-2 and IFN-γ are produced by activated T cells, and aging disorders have been related to these cytokines exceedingly. Furthermore, chronic hypobaric hypoxia prompts the release of cytokines/chemokines viz. IFN-γ and IL-2 activating the signal transduction pathway related to oxidative stress65. In this study, the production of important cytokines such as IL-2 and IFN-γ was also investigated. D-gal increased the levels of the pro-inflammatory cytokines IL-2 and IFN-γ. PQQ decreased cytokines IL-2 (52.1%), and IFN-γ (73.4%) from D-gal treatment mice (Fig. 5). The results are agreed with the report that PQQ deprivation bring about decline immune function66. This study indicated that PQQ markedly reduce the inflammation in D-gal-treated brain, and thus protect the nervous system against oxidative damage.
More over, prostaglandins are major components of the neuroinflammatory process. Prostaglandin E2 (PGE2), a product of oxidative stress, is one of the most reliable biomarkers of lipid peroxidation in the human body, and of aging related diseases67,68.
It has been reported that prostaglandin levels are higher in the brains of AD patients than in control brains69,70. Our results showed that hippocampus PGE2 was significantly increased in D-gal treated mouse. Treatment with PQQ suppressed hippocampus level of PGE2. As the levels of pro-inflammatory prostaglandins increase, the capability of clearance must also be improved to maintain low level prostaglandin and prevent additional inflammation. Our results suggest that PQQ may influence the generation of proinflammatory mediators, including cytokines and prostaglandins during in aging process.
Anti-excitotoxicity
Glutamate is the main excitatory neurotransmitter in the central nervous system (CNS) and the toxicity of glutamate has been shown to induce neuronal cell death through oxidative stress71. In mammalian brain, Glu is by far the most prevalent neurotransmitter, which is excitatory working on over 90% of the synapses in the brain72. It is considered to be the major mediator of excitatory signals and is probably involved in cognitive domain of the central nervous system73. Glutamatergic neurotransmission has already been adopted as a promising target for neuro disorder drug development. Furthermore, Liang et al. reported that reduction of the SOD2 level increased the levels of glutamate at the synapse and led to pathological conditions due to the downregulation of glutamate transporter 1 that clear glutamate from synapse74. In the brains of B-Sod2−/−, the potently downregulated expression of GLT-1 could induce a resultant increase in the glutamate concentration at synapse, leading to excitotoxicity. Therefore, it is of critical importance that the extracellular glutamate concentration must be kept enough low due to the toxicity of glutamate in high concentrations. It should be revealed that the distribution of glutamate in brain is a dynamic equilibrium with a rapid turnover in normal condition. Extracellular glutamate levels are maintained through a balance between release and uptake in the normal brain function. Abnormal glutamate uptake has been shown to be involved in the pathogenesis of neurological disorders where glutamate neurotoxicity plays a major role. Glutamate uptake is one of the main mechanisms responsible for keeping glutamate concentration at low level to resistant glutamate toxicity in normal intact brain tissue for the long-term75. However, there is no match to the capabilities of glutamate uptake in abnormal condition. The previous research was focused on the ability of glutamate transporter function for promoting protective effects against glutamate excitotoxicity to neurons. It has been proved that glutamate bound to various proteins is likely to reduce the concentration of free glutamate in glutamate transport cycles76. Inspired by the successful studies of binding with proteins, it seemed tempting to look for effective compounds binding to glutamate for the normal brain function. It is well known that PQQ has special chemical structure and characteristics and easy to combine with different substances42. Our previous studies found that PQQ was easily converted into derivatives with neurotransmitters amino acid which have beneficial capabilities to several physiological processes53,77. The concentrations of amino acid in the central nervous system tissue vary according to the physiological state. From Fig. 6A, it can be seen that the concentrations of glutamate in D-gal-induced mice were significantly higher than those in control group. D-gal is a reducing sugar having affinity for free amines of amino acid in proteins which lead to accumulation of advanced glycation end products, subsequently lead to elevated oxidative stress78. Because of the competitive interaction with free amines of amino acid, the potential efficacy of PQQ in inhibiting the production of glycation end products by binding with amino acid, and prevented the oxidative stress. Moreover, the “bingding” reduced excitability toxicity by decreasing the content of glutamate. From Fig. 6B, new production was found and identified in the brain of PQQ treated mouse compared with untreated ones by UPLC/MS. The results indicated that the complex may be beneficial for anti-aging effects of PQQ, which were related to the action of mechanism. PQQ inhibits excitotoxicity by which it may produce complex via binding with free glutamate in the brain, providing new insights into future research of central nervous system and drug discovery.
Akt/GSK-3β signaling
Glycogen Synthase Kinase-3β (GSK-3β) is a protein kinase involved in memory formation which impairs memory through excessive phosphorylation of substrates, such as tau protein79,80. GSK-3β has been revealed to provide an opportunistic target for learning and memory related disorders in aging process81. Several studies have demonstrated the link between GSK-3β and the neuropathology of AD82,83. Sintoni also reported that GSK-3β dysregulation in the hippocampus play a key role in memory impaired rat models84. In addition, the regulation of transcription process by GSK-3β is an important survival pathway against oxidative stress85. Pharmacological treatments, which inhibit GSK-3β, have been reported to repair cognitive impairment in AD mice25. GSK-3β acts as a downstream regulator, and its activity is mainly regulated by phosphorylation or dephosphorylation via signaling pathways86. In addition, decreased the expression of p-GSK-3β was also reported during glutamate-induced neurotoxicity24. It has been documented that protein kinase B (Akt) is a major kinases to inactivate GSK-3βthrough phosphorylation87. The high enzymatic activity is inhibited upon phosphorylation on residue ser9 of GSK-3β by Akt88. Therefore, drugs that are able to down-regulate the activity of GSK-3β hold high promise of therapeutic effects for aging-related diseases.
Obviously, PQQ has been proved to attenuate oxidative stress in mitochondria44,89, and antagonize glutamate-induced neuronal injury both in vitro and in vivo17,90,91. It has been observed that phosphorylated Akt was regulated by PQQ treatment in glutamate-injured hippocampal neurons17. The molecular mechanisms underlying these effects have not been fully elucidated. Although it was well proposed that PQQ might have antioxidant role, the direct supportive experimental evidences linking the mechanism of action with anti-aging have rarely been reported so far. The biological effects of PQQ have been intensively studied, but it is not clear whether the neuroprotective effects of PQQ against oxidative stress is mediated through reducing glutamate and suppressing neuroinflammatory mediators by Akt/GSK-3β signal pathway interruption.
In this experiment, we found D-gal up-regulated the expression of p-AKT in the hippocampus, the active form of AKT, leading to decrease the activity of GSK-3β. Fortunately, the expression of p-Akt was markedly reduced and maintained the activity of GSK-3β by PQQ, resulting in an down-regulation of p-Tau level in hippocampus. Moreover, our present study indicated that PQQ prevents cognitive impairment of D-gal induced mouse. Interestingly, PQQ reduced the phosphorylation of Tau and maintained the activity of GSK-3β, which was essential for memory and learning in aging process. In addition, the inhibition of MDA activity and ROS production might be involved in the anti-oxidative stress effect of PQQ. Its mechanism may be related to the down-regulation the expression of SOD2. The decline of SOD2 activity can lead to the disruption of oxidative stress balance, which damages mitochondrial function and causes aging related diseases. This effect is likely related to the reduction of oxidative stress via the Akt/GSK-3β pathway. Therefore we inferred that PQQ modulated memory ability partly via Akt/GSK-3β pathway. Our results showed that antioxidant capability of PQQ might be mediated by the inactivation of GSK-3β in an increase of p-GSK-3β. Furthermore, for the first time, we revealed that PQQ could ameliorate the memory deficits and neurotoxicity induced by D-gal via binding with excess glutamate, inhibiting oxidative stress, and eliminating inflammation through GSK-3β/Akt signaling pathway. This is in agreement with the evidence that PQQ protected cultured hippocampal neurons against glutamate excitotoxicity90,91
In conclusion, our findings demonstrate that PQQ effectively improves D-gal-induced cognitive dysfunction provide a link between Glu-mediated neurotoxicity, inflammation and oxidative stress. Taken together, our results suggest that PQQ is beneficial in preventing cognitive deficits during aging process. More work remains to be done before we elucidate these mechanisms.
Materials and Methods
Reagents and drugs
D-gal (Sigma-Aldrich, USA) and PQQ (Shanghai Med. Co., China) solutions were freshly prepared in sterile water. MDA (catalog no. S0131) were purchased from Beyotime Institute of Biotechnology (China). T-AOC (catalog no. S0116, Beyotime Institute of Biotechnology, China). IL-2 and IFN-γ production were measured using ELISA Ready-SET-Go! ® kits (eBioscience, USA). PGE2 were tested using PGE2 EIA kit (Cayman Chemical Co., Ann Arbor, MI, USA). Total RNA Extraction Reagent was purchased from Beyozol (China); High-Capacity cDNA Reverse Transcription Kits were purchased from Applied Biosystems (China). Semiquantitative RT-PCR was performed using primers designed by and obtained from Sangon Biotech, LTD (China). Glu were purchased from ACROS (Belgium, USA). Methanol was of HPLC grade and obtained from CNW Technologies GmbH (Hanau, Germany).
Animals and treatment
ICR mice were supplied from Cavens Lab Animal Co. (Changzhou, China). The mice were aged 3 months old, weighed 32 ± 2 g. Animals were housed at a temperature of 25 ± 1 °C and relative humidity of 55%–60% with a controlled light-dark cycle. The mice had free access to food and water. The mice were divided into four groups of 10 male mice at random. Two groups of mice received daily subcutaneous injection of D-gal at dose of 150 mg·kg−1 · d−1 for 6 weeks, and the third group of mice served as normal control was injected with saline (0.9% NaCl) only. Meanwhile, one group of D-gal-treated mice received PQQ at a dose of 100 μg · kg−1 · d−1. At the same time, the other group of D-gal-treated mice and the control group of mice were given distilled water, and the fourth group received PQQ (100 μg·kg−1 · d−1) only. The dosages and pretreatment duration for the compounds were used based on the previous studies56–58. All experiments were approved by the Animal Care and Ethics Committee of Jiangsu Institute of Nuclear Medicine and carried out according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Behavioral tests
Y-Maze Test
Y-maze constructed from black painted wood with three arms of 50 cm long, 20 cm high, and 10 cm wide at the bottom and at the top was used for behavioral analysis. The mice were placed at the center and were allowed to move freely for three 8-min sessions. The test was performed in a sound-isolated and dark room. The arm entries series was noted for observation. Spontaneous alteration was defined as the successive entry of the mice into the three arms in overlapping triplet sets. Alteration behavior (%) was calculated as [successive triplet sets(entries into three different arms consecutively)/total number of arms entries −2] × 10059.
Passive avoidance test
The passive avoidance apparatus contains two chambers (20 × 25 × 30 cm each) of equal size, one is an illuminated by a 4 W fluorescent lamp and other is a dark one. Both of these compartments are linked with a door which allows mice to cross freely from one compartment to another. These compartments contain a grid floor that consists of rods having a distance of 0.5 cm between the rods. Initially the animals were trained by placing them in the illuminated compartment, and an electric shock (40 V, 0.5 A, 1 s) was delivered to their paws via the rods of the grid floor, causing them to move into the dark chamber. Mice were then immediately removed and returned to their home cages. During the retention trial that occurred 24 h later, mice were placed in the illuminated chamber, and the latency to enter the dark chamber was recorded (i.e., step-through latency). In this session, the number of repeated step-down in 300 s was counted as errors.
Measurement of ROS, MDA levels, and total antioxidant capacity (T-AOC)
MDA, ROS, and T-AOC, were determined by using commercially available kits. ROS was measured as described previously with some modification, based on oxidation of 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) to 2,7-dichlorodihydrofluorescein60. The data are expressed as picomole DCF formed per minute per milligram protein. The thiobarbituric acid reaction method was used to determine MDA content. MDA reacts with thiobarbituric acid to form a stable chromophoric molecule that can be measured at the wavelength of 532 nm61. MDA content was expressed as nanomoles per milligram of tissue protein. The Ferric reducing power (FRAP) test method was used to quantify the T-AOC of the organs62. After mixing the FRAP reagent with the organ sample, the absorbance was determined every 1 min for a total of 10 min. Fe (II) standard solution was tested in parallel.
RNA extraction and RT-PCR analysis
T RNA was extracted from brain using the kit as directed by the manufacturer’s protocol. Using the High-Capacity cDNA Reverse Transcription Kits, 1.5 μg of RNA was transcribed into cDNA according to the manufacturer’s instructions, and cDNA samples were stored at −70 °C. Semiquantitative RT-PCR was performed using the primers obtained from Sangon Biotech, LTD (China). The sequences of the primers are designed as follows: SOD1(352 bp), Forward 5′-ACCATCCACTTCGAGCAG-3′, Reverse 5′-TTTCTTCATTTCCACCTTTG-3′; SOD2(396 bp), Forward, 5′-GCACCACAGCAAGCACC-3′, Reverse, 5′-CCCAGCAGCGGAATAAG-3′; β-actin(384 bp), Forward, 5′-GGGAAATCGTGCGTGACAT-3′, Reverse, 5′-CAGGAGGAGCAATGATCTT-3′.
ELISA analysis for IL-2, IFN-γ and PGE2
IL-2 and IFN-γ production were measured using ELISA Ready-SET-Go! ® kits with sensitivity of 2 and 4 pg/ml, respectively as described by the manufacturer. The absorbance of the reaction was measured at 450 nm with subtraction of background at 570 nm using a microplate reader. PGE2 concentrations were measured using a commercially available PGE2 EIA kit according to manufacturer’s instructions as the previous described31.
Western blot analysis
The Western blot assay was performed as previously described24,88. Briefly, the hippocampus was homogenized in lysis buffer (Beyotime Biotechnology, Haimen, China) and centrifuged at 12,000 rpm for 10 min at 4 °C. The concentration of hippocampal protein was measured using BCA assay kit (Sangon, Shanghai, China). The dilution of primary antibodies as follows: β-actin (1:4000, Abcam, UK); GSK-3β; p-GSK-3β; AKT; p-AKT; Tau, p-Tau (1:2000, Cell Signaling Technology, USA). Each membrane was rinsed three times for 15 min and incubated with the secondary antibodies (1:8000, Abcam, UK). β-actin was used as the loading reference for data analysis.
Measurement of glutamate
The levels of glutamate in brain tissues were analyzed using HPLC system as the our confirmed method. Furthermore, a related marker for the protection of glutamate neurotoxicity in hippocampus has been explored by UPLC/MS. The brain tissues were homogenized in ice-cold 0.5 M formic acid (5 mL/g) and the mixtures were centrifuged 15,000 g for 30 min at 4 °C. The supernatant was collected and run through solid-phase extraction columns (Styre Screen® H2P) with the aid of vacuum. Extraction cartridges were pretreated by rinsing with 1 ml of ethanol, followed by 1 ml deionized water contain 0.1% trifluoroacetic acid (TFA). After the samples were loaded onto columns, the columns were washed with 1 ml deionized water, followed by 2 ml of ethanol/o.1%TFA (60/40) in water. Eluants were evaporated to dryness by vacuum freeze-drying and waiting for analysis. The content of glutamate were determinated using UPLC-MS49. Molecular masses were carried out on a Waters Acquity ultraperformance liquid chromatography system (Waters, MA, USA) with a SQ Detector 2 mass spectrometer equipped with diode-array detector. Data were collected and processed with Waters MassLynx. A Waters BEH C18 column (2.1 × 100 mm, 1.7 um) was applied for reverse phase chromatographic separation. The optimized MS parameterswere set as follows: Desolvation temperature 400 °C, desolvation gas flow 600 L/h, source temperature 110 °C, cone gas flow 50 L/h, capillary voltage 3000 V, cone voltage 30 V. The initial mobilephase composition was held at 5/95 ACN/water contain 0.1% TFA (v/v) for 5 min followed by a linear gradient to 75:25 ACN:water contain 0.1% TFA (v/v) at 25 min.
Statistical analysis
All statistical tests were performed with the SPSS v16.0 statistical software. One way analysis of variance was applied using Tukey’s posthoc comparisons. The data were expressed as mean ± SD of triplicate experiments. P-values below 0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81371590, 30770602).
Author Contributions
X.-q.Z. and Y.P. conceived and designed the project. X.-q.Z., Z.-w.Y., Y.P., S.-s.M., D.X., X.-f.Q. and R.-j.Z. conducted the experiments. X.-q.Z. and Z.-w.Y. wrote the main manuscript text. All authors read and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Xing-qin Zhou and Zhi-wen Yao contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26962-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van RH, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol. 2001;36(7):957–968. doi: 10.1016/S0531-5565(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 2.Dröge W. Oxidative stress and aging. Adv Exp Med Biol. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- 3.Castegna A, et al. Proteomicidentification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy terminal hydrolase L-1. Free Radic. Biol. Med. 2002;33:562–571. doi: 10.1016/S0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE, Armbrecht HJ, Farr SA, Kumar VB. The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer’s disease. Biochim Biophys Acta. 2012;1822:650–656. doi: 10.1016/j.bbadis.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Nunomura A, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 6.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 7.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenaz G, et al. Mitochondrial bioenergetics in aging. Biochimica et Biophysica Acta. 2000;1459:397–404. doi: 10.1016/S0005-2728(00)00177-8. [DOI] [PubMed] [Google Scholar]
- 9.Bonda DJ, et al. Neuronal failure in Alzheimer’s disease: a view through the oxidative stress looking-glass. Neurosci Bull. 2014;30:243–252. doi: 10.1007/s12264-013-1424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, et al. Puerarin alleviates cognitive impairment and oxidative stress in APP/PS1 transgenic mice. Int J Neuropsychopharmacol. 2014;17:635–644. doi: 10.1017/S146114571300148X. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, et al. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 13.Vandresen-Filho S, et al. Atorvastatin prevents cell damage via modulation of oxidative stress, glutamate uptake and glutamine synthetase activity in hippocampal slices subjected to oxygen/glucose deprivation. Neurochemistry International. 2013;62:948–955. doi: 10.1016/j.neuint.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl Acad. Sci. USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca++ from intracellular stores and is prevented by estradiol. Eur. J. Neurosci. 2006;24:3008–3016. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luetjens CM, et al. Delayed mitochondrial dysfunction in excitotoxic neuron death: cytochrome c release and a secondary increase in superoxide production. J. Neurosci. 2000;20:5715–5723. doi: 10.1523/JNEUROSCI.20-15-05715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Shen M, Ding M, Shen D, Ding F. The neuroprotective action of pyrroloquinoline quinone against glutamate-induced apoptosis in hippocampal neurons is mediated through the activation of PI3K/Akt pathway. Toxicology and Applied Pharmacology. 2011;252:62–72. doi: 10.1016/j.taap.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Troost D, Van denOord JJ, Vianney de Jong JM. Immunohistochemical characterization of the inflammatory infiltrate in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 1990;16:401–410. doi: 10.1111/j.1365-2990.1990.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 19.Lampson LA, Kushner PD, Sobel RA. Major histocompatibility complex antigen expression in the affected tissues in amyotrophic lateral sclerosis. Ann. Neurol. 1990;28:365–372. doi: 10.1002/ana.410280311. [DOI] [PubMed] [Google Scholar]
- 20.Schiffer D, Cordera S, Cavalla P, Migheli A. Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. J. Neurol. Sci. 1996;139:27–33. doi: 10.1016/0022-510X(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 22.Franceschi C, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanism of Aging & Development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Choi K, Zhuang H, Crain B, Doré S. Expression and localization of prostaglandin transporter in Alzheimer disease brains and age-matched controls. Journal of Neuroimmunology. 2008;195:81–87. doi: 10.1016/j.jneuroim.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen X, et al. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: involvement of the Akt/GSK-3βpathway. Neuroscience. 2015;303:558–568. doi: 10.1016/j.neuroscience.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Zou Y, et al. Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: involvement of the GSK-3beta/Nrf2 signaling pathway. Free Radic Res. 2013;47:55–63. doi: 10.3109/10715762.2012.742518. [DOI] [PubMed] [Google Scholar]
- 26.Cui X, et al. Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J. Neurosci. Res. 2006;84:647–654. doi: 10.1002/jnr.20899. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XL, Jiang B, Li ZB, Hao S, An LJ. Catalpol ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Pharmacol. Biochem. Behav. 2007;88:64–72. doi: 10.1016/j.pbb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, A., Prakash, A. & Dogra, S. Centella asiatica attenuates D-galactoseinduced cognitive impairment, oxidative and mitochondrial dysfunction in mice. International Journal of Alzheimer’s Disease. 347569 (2011). [DOI] [PMC free article] [PubMed]
- 29.Haider S, et al. A high dose of short term exogenous d-galactose administration in young male rats produces symptoms simulating the natural aging process. Life Sci. 2015;124:110–119. doi: 10.1016/j.lfs.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Lu J, et al. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem. Pharmacol. 2007;74:1078–1090. doi: 10.1016/j.bcp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Xian YF, et al. Isorhynchophylline improves learning and memory impairments induced by D-galactose in mice. Neurochem Int. 2014;76:42–49. doi: 10.1016/j.neuint.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Li XK, Cui X, Zuo PP. d-galactose injured neurogenesis in the hippocampus of adult mice. Neurol. Res. 2005;27:552–556. doi: 10.1179/016164105X25126. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, et al. Glutamine synthetase plays a role in D-galactose-induced astrocyte aging in vitro and in vivo. Experimental Gerontology. 2014;58:166–173. doi: 10.1016/j.exger.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Ullah F, Ali T, Ullah N, Kim MO. Caffeine prevents D-galactose-induced cognitive deficits, oxidative stress, neuroinflammation and neurodegeneration in the adult rat brain. Neurochemistry International. 2015;90:114–124. doi: 10.1016/j.neuint.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Anand KV, Jaabir MSM, Thomas PA, Geraldine P. Protective role of chrysin against oxidative stress in D-galactose-induced aging in an experimental rat model. Geriatr Gerontol Int. 2012;12:741–750. doi: 10.1111/j.1447-0594.2012.00843.x. [DOI] [PubMed] [Google Scholar]
- 36.Aydın AF, Küçükgergin C, Özdemirler-Erata G, Koçak-Toker N, Uysal M. The effect of carnosine treatment on prooxidantantioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology. 2010;11:103–109. doi: 10.1007/s10522-009-9232-4. [DOI] [PubMed] [Google Scholar]
- 37.Hsia CH, Wang CH, Kuo YW, Ho YJ, Chen HL. Fructooligosaccharide systemically diminished D-galactose-induced oxidative molecule damages in BALB/cJ mice. Br J Nutr. 2012;107:1787–1792. doi: 10.1017/S0007114511005150. [DOI] [PubMed] [Google Scholar]
- 38.Tsai SJ, Yin MC. Anti-oxidative, anti-glycative and anti-apoptotic effects of oleanolic acid in brain of mice treated by D-galactose. Eur J Pharmacol. 2012;689:81–88. doi: 10.1016/j.ejphar.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerativediseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular in flammatory process in aging. Antioxid. Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 41.Anthony C. Pyrroloquinoline quinone (PQQ) and quinoprotein enzymes. Antioxid Redox Signal. 2001;3:757–774. doi: 10.1089/15230860152664966. [DOI] [PubMed] [Google Scholar]
- 42.McIntire WS. Newly discovered redox cofactors: possible nutritional, medical, and pharmacological relevance to higher animals. Annu Rev Nutr. 1998;18:145–177. doi: 10.1146/annurev.nutr.18.1.145. [DOI] [PubMed] [Google Scholar]
- 43.Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J Nutr. 2000;130:719–727. doi: 10.1093/jn/130.4.719. [DOI] [PubMed] [Google Scholar]
- 44.Misra HS, et al. Pyrroloquinoline-quinone: a reactive oxygen species scavenger in bacteria. FEBS Lett. 2004;578:26–30. doi: 10.1016/j.febslet.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Prakash A, Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food and Chemical Toxicology. 2010;48(2):626–632. doi: 10.1016/j.fct.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, et al. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a at model of D-galactose-induced aging. PLoS One. 2014;9(6):e101291. doi: 10.1371/journal.pone.0101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan Q, et al. The anti-inflamm-aging and hepatoprotective effects of huperzine A in D-galactose-treated rats. Mechanisms of Ageing and Development. 2013;134:89–97. doi: 10.1016/j.mad.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Bondy SC, Lee DK. Oxidative stress induced by glutamate receptor agonists. Brain Res. 1993;610(2):229–233. doi: 10.1016/0006-8993(93)91405-H. [DOI] [PubMed] [Google Scholar]
- 49.Roy J, Minotti S, Dong LC, Figlewicz DA, Durham HD. Glutamate potentiates the toxicity of mutant Cu/Zn-superoxide dismutase in motor neurons by postsynaptic calcium-dependent mechanisms. J. Neurosci. 1998;18(23):9673–9684. doi: 10.1523/JNEUROSCI.18-23-09673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohwada K, et al. Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by oxidative stress in rats. J Clin Biochem Nutr. 2008;42(1):29–34. doi: 10.3164/jcbn.2008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou XQ, Qin XF, Cao GX, Zhang JK. Ion-Pair LC Analysis of Pyrroloquinoline Quinone in Neurotransmitter Amino Acid Incubations: Determination of Chemical Kinetics. Chromatographia. 2012;75:521–526. doi: 10.1007/s10337-012-2221-9. [DOI] [Google Scholar]
- 54.Zhou XQ, Chen QC, Hu XD, Mao SS, Kong YY. Pyrroloquinoline quinone prevents MK-801-induced stereotypical behavior and cognitive deficits in mice. Behavioural Brain Research. 2014;258:153–159. doi: 10.1016/j.bbr.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 55.Rucker R, Chowanadisai W, Nakano M. Potential Physiological Importance of Pyrroloquinoline Quinone. Altern Med Rev. 2009;14(3):268–277. [PubMed] [Google Scholar]
- 56.Ouchi A, Nakano M, Nagaoka S, Mukai K. Kinetic study of the antioxidant activity of pyrroloquinolinequinol (PQQH(2), a reduced form of pyrroloquinolinequinone) in micellar solution. J Agric Food Chem. 2009;57:450–456. doi: 10.1021/jf802197d. [DOI] [PubMed] [Google Scholar]
- 57.Zhang QB, et al. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol Res. 2003;48:151–155. doi: 10.1016/S1043-6618(03)00103-8. [DOI] [PubMed] [Google Scholar]
- 58.Iacobazzi D, et al. Increased antioxidant defense mechanism in human adventitia-derived progenitor cells is associated with therapeutic benefit in ischemia. Antioxid Redox Signal. 2014;21(11):1591–604. doi: 10.1089/ars.2013.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng YK, et al. Altered Expression Profile of Superoxide Dismutase Isoforms in Nasal Polyps from Nonallergic Patients. Laryngoscope. 2006;116(3):417–22. doi: 10.1097/01.MLG.0000199738.37455.55. [DOI] [PubMed] [Google Scholar]
- 60.Izuo N, et al. Brain-Specific Superoxide Dismutase 2 Deficiency Causes Perinatal Death with Spongiform Encephalopathy in Mice. Oxid Med Cell Longev. 2015;2015:238914. doi: 10.1155/2015/238914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westin K, et al. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLOS ONE. 2012;7:e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Echeverria V, Yarkov A, Aliev G. Positive modulators of the α7 nicotinic receptor against neuroinflammation and cognitive impairment in Alzheimer’s disease. Prog. Neurobiol. 2016;144:142–157. doi: 10.1016/j.pneurobio.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Laurent C, et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain. 2017;140:184–200. doi: 10.1093/brain/aww270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duan ZF, Wang DS, Shen YS. Study on IL-2 and IL-2 receptor of the aged. Chin J Geriatr. 1993;12:165–167. [Google Scholar]
- 65.Gola S, Gupta A, Keshri GK, Nath M, Velpandian T. Evaluation of hepatic metabolism and pharmacokinetics of ibuprofenin rats under chronic hypobaric hypoxia for targeted therapy at high altitude. Journal of Pharmaceutical and Biomedical Analysis. 2016;121:114–122. doi: 10.1016/j.jpba.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 66.Steinberg F, Stites TE, Anderson P, Storms D, Chan I. Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp Biol Med. 2003;228:160–166. doi: 10.1177/153537020322800205. [DOI] [PubMed] [Google Scholar]
- 67.Mizuno K, Kataoka H. Analysis of urinary 8-isoprostane as an oxidative stress biomarker by stable isotope dilution using automated online in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2015;112:36–42. doi: 10.1016/j.jpba.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 68.Milne GL, Dai Q, Roberts LJ., II. The isoprostanes-25 years later. Biochim. Biophys. Acta. 2015;1851:433–445. doi: 10.1016/j.bbalip.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Consilvio C, Vincent AM, Feldman EL. Neuroinflammation, COX-2, and ALS a dual role? Exp. Neurol. 2004;187:1–10. doi: 10.1016/j.expneurol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Donnelly MT, Hawkey CJ. Review article: COX-II inhibitors—a new generation of safer NSAIDs? Aliment. Pharmacol. Ther. 1997;11:227–235. doi: 10.1046/j.1365-2036.1997.154330000.x. [DOI] [PubMed] [Google Scholar]
- 71.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 72.Zhu KY, et al. The establishment of a sensitive method in determining different neurotransmitters simultaneously in rat brains by using liquid chromatography electrospray tandem mass spectrometry. Journal of Chromatography B. 2011;879:737–742. doi: 10.1016/j.jchromb.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 73.Headley PM, Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol. Sci. 1990;11:205–211. doi: 10.1016/0165-6147(90)90116-P. [DOI] [PubMed] [Google Scholar]
- 74.Liang LP, et al. Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: attenuation by a lipophilic metalloporphyrin. Neurobiology of Disease. 2012;45(3):1068–1076. doi: 10.1016/j.nbd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 76.Otis TS, Kavanaugh MP. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J. Neurosci. 2000;20:2749–2757. doi: 10.1523/JNEUROSCI.20-08-02749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glatz Z, Moravcova M, Janiczek O. Determination of pyrroloquinolinequinone by capillary zone electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 2000;739:101–107. doi: 10.1016/S0378-4347(99)00519-8. [DOI] [PubMed] [Google Scholar]
- 78.Srikanth V, Maczurek A, Phan T, Steele M, Westcott B. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiology of Aging. 2011;32(5):763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 79.Dewachter I, et al. GSK3beta, a centre-staged kinase in neuropsychiatric disorders, modulates long term memory by inhibitory phosphorylation at serine-9. Neurobiol Dis. 2009;35:193–200. doi: 10.1016/j.nbd.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Liu SJ, et al. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J. Neurochem. 2003;87:1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi-Yanaga F. Activator or inhibitor? GSK-3β as a new drug target. Biochem. Pharmacol. 2013;86:191e199. doi: 10.1016/j.bcp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 82.Papasozomenos S, Shanavas A. Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: implications for Alzheimer’s disease. Proc Natl Acad Sci USA. 2002;99:1140–1145. doi: 10.1073/pnas.032646799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leroy K, Yilmaz Z, Brion JP. Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilicgrains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 84.Sintoni S, Kurtys E, Scandaglia M, Contestabile A, Monti B. Chronic valproic acid administration impairs contextual memory and dysregulates hippocampal GSK-3β in rats. Pharmacology, Biochemistry and Behavior. 2013;106:8–15. doi: 10.1016/j.pbb.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 85.Rojo AI, Sagarra MR, Cuadrado A. GSK-3β down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J. Neurochem. 2008;105:192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 86.Liang MH, Chuang DM. Regulation and function of glycogen synthasekinase-3 isoforms in neuronal survival. J. Biol. Chem. 2007;282:3904–3917. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- 87.Woodgett JR. Molecular cloning and expression of glycogen synthasekinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou W, et al. Akt mediates GSK-3 beta phosphorylation in the rat prefrontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation. 2014;21(4):183–188. doi: 10.1159/000356517. [DOI] [PubMed] [Google Scholar]
- 89.Tao R, et al. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem Biophys Res Commun. 2007;363:257–262. doi: 10.1016/j.bbrc.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Q, Ding M, Gao XR, Ding F. Pyrroloquinoline quinone rescues hippocampal neurons from glutamate-induced cell death through activation of Nrf2 and up-regulation of antioxidant genes. Genet Mol Res. 2012;11:2652–2664. doi: 10.4238/2012.June.27.3. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Q, et al. Pyrroloquinoline quinine protects rat brain cortex against acute glutamate-induced neurotoxicity. Neurochem Res. 2013;38:1661–1671. doi: 10.1007/s11064-013-1068-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.