Abstract
Introduction: Data on the efficacy and risk of curative-intent chemoradiotherapy in patients with inoperable stage III non-small-cell lung cancer (NSCLC) and interstitial lung disease (ILD) are limited. The aim of this study was to explore the impact of ILD classification on acute exacerbation (AE) of ILD and prognosis in patients with stage III NSCLC and ILD treated with chemoradiotherapy.
Materials and methods: We retrospectively reviewed the medical records of patients with stage III NSCLC and ILD treated with curative-intent chemoradiotherapy as the first-line treatment at the Shizuoka Cancer Center between June 2009 and May 2014.
Results: Of 37 patients, 17 (46%) developed AE of ILD worse than grade 3 within 1 year after the last irradiation. In univariate analysis, the incidence rate of AE of ILD was lower in patients with a non-usual interstitial pneumonia (UIP) pattern than in those with a UIP pattern. Multivariate analysis showed that ILD classification was significantly associated with the incidence of AE of ILD. The median overall survival (OS) durations in patients with a non-UIP pattern and a UIP pattern were 16.5 and 9.3 months, respectively. In univariate analysis, patients with a non-UIP pattern showed better survival. Multivariate analysis showed that ILD classification was a significant independent prognostic factor.
Conclusion: The incidence of AE of ILD was high in patients with stage III NSCLC and ILD treated with chemoradiotherapy as the first-line treatment. However, diagnosis of a non-UIP pattern could predict lower risk of AE of ILD and longer OS durations.
Keywords: non-small cell lung cancer, interstitial lung disease, acute exacerbation, usual interstitial pneumonia, non-usual interstitial pneumonia
Introduction
In patients with inoperable localized stage I-III non-small-cell lung cancer (NSCLC), radiotherapy is required for achieving durable local control and prolonged survival 1-8. However, the propriety of radiotherapy should be discussed in the case of patients with interstitial lung disease (ILD) 9, which includes many different lung diseases that cause inflammation or fibrosis in the interstitial tissue of the lung 10, 11. Among patients with NSCLC and ILD, the most serious issue is acute exacerbation (AE) of ILD caused by anticancer treatment 9, 12-22. Pulmonary fibrosis identified on a plain chest radiograph was reported to be strongly associated with treatment-related death after thoracic radiotherapy 9. Moreover, some reports have shown that the presence of interstitial changes on chest computed tomography (CT) could be a risk factor for AE of ILD triggered by radiotherapy 14-20, 23. Therefore, patients with NSCLC and ILD are often excluded from clinical trials and even clinical settings concerned with radiotherapy for the treatment of lung cancer because they are considered to be at high risk for AE of ILD triggered by radiotherapy. Several risk factors, such as the volume of irradiated lung; mean lung dose; pretreatment serum KL-6, SP-D, lactate dehydrogenase (LDH), and C-reactive protein (CRP) levels; low partial pressure of arterial oxygen (PaO2) before radiotherapy; and pretreatment presence of ILD, have been reported for radiation pneumonitis in patients with early-stage or advanced lung cancer with or without ILD 9, 12, 14-20, 24-26. However, exploration of the risk factor for AE of ILD in patients with NSCLC and ILD treated with chemoradiotherapy remains limited. Thus, identifying patients at a low risk of the AE of ILD and long-term survival treated with radiotherapy among patients with NSCLC and ILD is imperative. The aim of this study was to explore the impact of ILD classification on the development of AE of ILD, including radiation pneumonitis, and prognosis in patients with stage III NSCLC and ILD after treatment with chemoradiotherapy at our institution.
Materials and Methods
We retrospectively reviewed the medical records of patients with stage III NSCLC and ILD treated with curative-intent chemoradiotherapy or curative-intent radiotherapy alone or chemotherapy alone as the first-line treatment at the Shizuoka Cancer Center between June 2009 and May 2014. Both concurrent and sequential chemoradiotherapy as the first-line treatment was included in the analysis. Patients treated with the first-line treatment who could not be followed up within 30 days after the last day of the first-line treatment were excluded from the analysis. We also reviewed the medical records of patients with stage III NSCLC without ILD treated with radiotherapy, with or without chemotherapy, in our institution at the same time.
A treatment planning CT study was performed to define gross tumor volume (GTV), clinical target volume, and planning target volume (scan thickness ≤ 5 mm). The primary tumor and clinically positive lymph nodes seen either on planning CT (short-axis diameter > 1 cm) or pretreatment positron emission tomography represented the GTV. The choice of whether or not to administer elective nodal irradiation depended on the physician. All patient treatment plans were designed on the basis of a 3-dimensional treatment planning system. X-rays at 6-10 MV were used in 2-Gy fractions 5 times weekly. All radiation doses were calculated with inhomogeneity corrections (superposition-convolution dose calculation algorithms or an analytical anisotropic algorithm). Planning for the total dose of curative-intent radiotherapy was defined as a dose over 56 Gy in 1.8 to 2.0 Gy fractions 27-29. Each dose was prescribed to the isocenter of possible cases. Lung V20 indicated the percentage of total lung volume receiving 20 Gy. We planned the lung V20 less than 35 percent 30.
ILD in this study was defined as chronic, progressive fibrosing interstitial pneumonia of unknown cause occurring primarily in older adults or associated with connective tissue disease and occupation. On the other hand, infectious lung disease and drug-induced pneumonia were excluded from this study. ILD was divided into an usual interstitial pneumonia (UIP) or non-UIP pattern using thoracic CT. The diagnosis of a UIP pattern was based on the following CT features, as defined by the International Consensus Statement of the American Thoracic Surgery and the European Respiratory Society: subpleural distribution, honeycombing, traction bronchiectasis, and architectural distortion 31. Thoracic CT images prior to treatment were evaluated by two radiologists (M.E. and H.H.) and two pulmonologists (H.K. and T.N.) without knowledge of the patient outcomes. The definition of AE of ILD was any acute respiratory event characterized by new bilateral ground glass-opacification/consolidation that was not fully explained by cardiac failure or fluid overload 32. The severity of AE of ILD, including radiation pneumonitis, was evaluated with reference to pneumonitis grading using the National Cancer Institute Common Terminology Criteria version 4.0 33. Grades 3, 4, and 5 of AE of ILD were defined as the administration of steroid therapy because of hypoxia, administration of intravenous pulse steroid therapy, and death, respectively.
Univariate and multivariate analyses of the incidence rate of AE of ILD, overall survival (OS), and 2-year survival were performed using a Cox proportional hazards approach. OS was defined as the time from the start of the first-line treatment to death. The parameters assessed with regard to the incidence of AE of ILD were sex, age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking history, ILD classification on CT, percentage of vital capacity (%VC), V20, and imaging findings of lung volume loss or honeycombing. Additionally, the parameters assessed with regard to OS were sex, age, ECOG-PS, smoking history, clinical staging, ILD classification on CT, %VC, and imaging findings of lung volume loss or honeycombing. All categorical variables were analyzed using Fisher's exact test and continuous variables were analyzed using the χ2 test. Event time was estimated using the Kaplan-Meier method. The log-rank test was used to compare cumulative survival in each group. The end date for survival analyses was March 1, 2017. All p-values are two-sided and values <0.05 were considered statistically significant. All statistical analyses were performed using JMP® 11.2.0 software (SAS Institute, Cary, NC). The study protocol was approved by the institutional review board of Shizuoka Cancer Center (IRB No. 28-J167-28-1-3).
Results
Patient characteristics
Among 79 patients with stage III NSCLC and ILD during the study period, 37 were treated with curative-intent chemoradiotherapy as the first-line treatment. The characteristics of these patients are shown in Table 1. ILD was classified as a UIP pattern in 11 patients (30%) and as a non-UIP pattern in 26 patients (70%) on pretreatment thoracic CT. The non-UIP pattern group in this study included 18 patients with non-specific interstitial pneumonia, 6 patients with airway centered fibrosis, and 2 patients with organizing pneumonia. Additionally, 4 patients had lung volume loss on thoracic radiography or honeycombing on thoracic CT.
Table 1.
Baseline characteristics of the patients in this study
| First-line treatment | Chemoradiotherapy | Radiotherapy | Chemotherapy |
|---|---|---|---|
| (N = 37) | (N = 17) | (N = 25) | |
| Variable | |||
| Age, median (range) | 73 (52-85) | 80 (59-89) | 69 (58-81) |
| Sex, male/female | 32 / 5 | 15 / 2 | 21 / 4 |
| PS, 0/1/2 | 15 / 22 / 0 | 6 / 7 / 4 | 10 / 15 / 0 |
| Clinical staging (the 7th edition of the TNM classification), IIIA/IIIB | 20 / 17 | 8 / 9 | 10 / 15 |
| Smoking history, yes/no | 33 / 4 | 16 / 1 | 24 / 1 |
| Histology, squamous/non-squamous | 19 / 18 | 7 / 10 | 13 / 12 |
| ILD classification on CT, UIP pattern/non-UIP pattern | 11 / 26 | 6 / 11 | 16 / 9 |
| %VC, median (range) | 96 (59-129) | 86 (62-112) | 85 (64-121) |
| V20, median (range) | 27 (12-35) | 25 (14-36) | |
| The number of patient with lung volume loss or honeycombing | 4 | 4 | 13 |
ILD, interstitial lung disease; CT, computed tomography; UIP, usual interstitial pneumonia; VC, vital capacity.
In comparison with patients treated with radiotherapy alone as the first-line treatment, patients treated with chemoradiotherapy had a significantly better %VC (p = 0.0159). Additionally, in comparison with patients treated with chemotherapy alone as the first-line treatment, patients treated with chemoradiotherapy had a significantly higher rate of diagnosis of a non-UIP pattern (p = 0.0099), had significantly less frequent findings of lung volume loss on thoracic radiography or honeycombing on thoracic CT (p = 0.0010), and had significantly better %VC (p = 0.0050). Therefore, patients with stage III NSCLC and ILD treated with chemoradiotherapy could be diagnosed with milder ILD compared to that in patients treated with radiotherapy alone or chemotherapy alone.
For patients treated with chemoradiotherapy, the frequency and composition of chemotherapy regimens administered during the study period are shown in the Supplementary Table 1. Daily low-dose carboplatin was the most frequently used first-line chemotherapy combined with radiotherapy in patients with NSCLC and ILD in this study. No patient received treatment with any currently used medicines for ILD (i.e., pirfenidone, nintedanib, and immunosuppressive agents).
The median follow-up duration for censored cases was 40.1 months (range, 9.3-93.8 months).
Acute exacerbation of interstitial lung disease
Of the 37 patients, 17 (46%; 95% CI, 31%-62%) developed AE of ILD worse than grade 3 within 1 year after the last irradiation. AEs of ILD of grades 3, 4, and 5 were experienced by 15, 1, and 1 patients, respectively. Additionally, the number of patients with AE of ILD that developed within 30 days after the last irradiation was 4, and these patients did not die within 90 days after the day of the onset of AE of ILD. On the other hand, among 273 patients with stage III NSCLC without ILD treated with radiotherapy with or without chemotherapy at the same time in our institution, radiation pneumonitis of grades 3, 4, and 5 was experienced by 23 (8.4%), 3 (1.1%), and 1 (0.4%) patients, respectively.
The findings of the univariate and multivariate analyses on the relationship between the incidence of AE of ILD and each individual factor in all patients with stage III NSCLC and ILD treated with chemoradiotherapy during the study period are shown in Table 2. In univariate analyses, patients with a non-UIP pattern showed a lower incidence rate of AE of ILD compared to that in those with a UIP pattern (31% vs. 82%, p = 0.0097). Moreover, patients with V20 of ≥25% showed a higher incidence rate of AE of ILD compared to that in those with V20 of <25% (59% vs. 23%, p = 0.0776). Multivariate analysis showed that ILD classification (non-UIP: OR, 0.11; 95% confidence interval [CI], 0.014-0.60; p = 0.0095) was significantly associated with the incidence of AE of ILD.
Table 2.
Univariate and multivariate analyses of acute exacerbation of interstitial lung disease (ILD) in patients with stage III non-small-cell lung cancer and ILD treated with chemoradiotherapy (n = 37)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | |
| Sex (female vs. male) | 1.92 | 0.28-13.2 | 0.6443 | |||
| Age (≥75 years vs. <75 years) | 0.31 | 0.07-1.28 | 0.1734 | |||
| ECOG-PS (1 vs. 0) | 1.50 | 0.40-5.66 | 0.7384 | |||
| Smoking history (never vs. current or former smoker) | 4.07 | 0.38-43.4 | 0.3153 | |||
| ILD classification on CT (non-UIP vs. UIP pattern) | 0.10 | 0.017-0.56 | 0.0097 | 0.11 | 0.014-0.60 | 0.0095 |
| %VC (≥80 vs. <80) (n = 34) | 0.76 | 0.09-6.17 | 1.0000 | |||
| V20 (≥25 vs. <25) (n = 35) | 4.81 | 1.03-22.6 | 0.0776 | 3.61 | 0.043-1.43 | 0.1270 |
| The finding of lung volume loss or honeycombing (either or both vs. none) | 4.07 | 0.38-43.4 | 0.3153 | |||
AE, acute exacerbation; ILD, interstitial lung disease; NSCLC, non-small-cell lung cancer; ECOG PS, Eastern Cooperative Oncology Group performance score; CT, computed tomography; UIP, usual interstitial pneumonia; VC, vital capacity; CI, confidence interval
Clinical outcome
The ratio of patients who could not be treated with a total dose of over 56 Gy was 4% (95% CI, 1-19) for the non-UIP pattern and 18% (95% CI, 5-48) for the UIP pattern (p = 0.2053), and the reason was AE of ILD in all these patients.
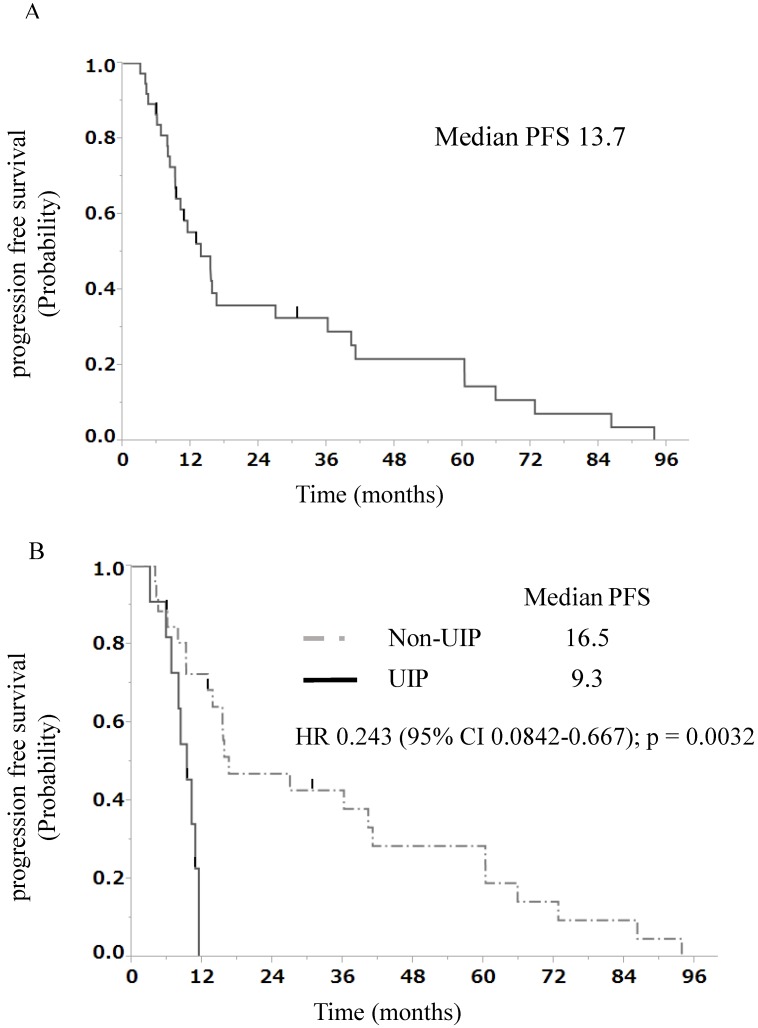
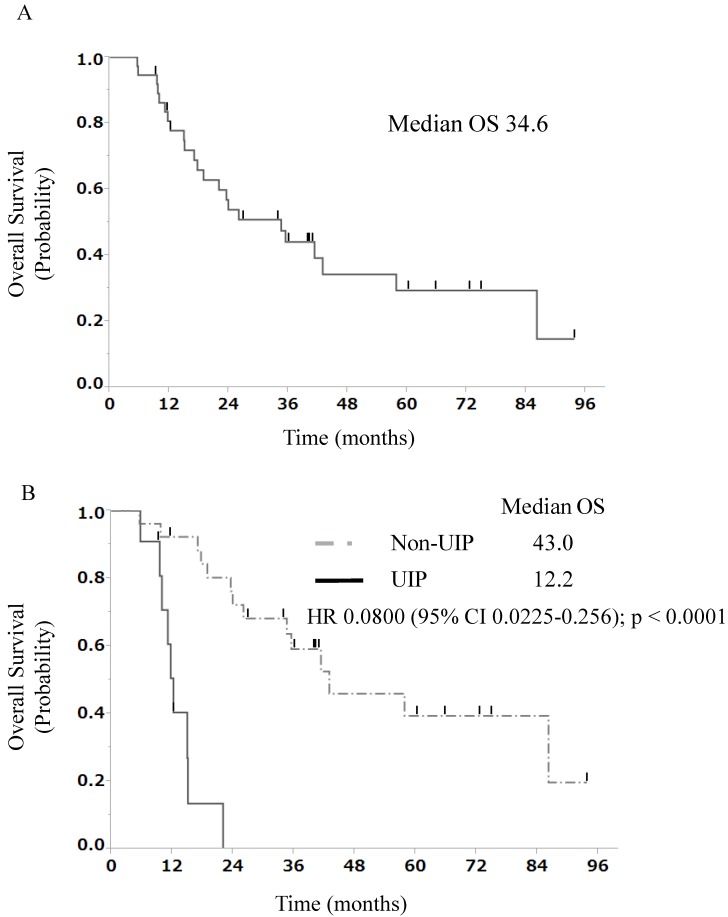
The median progression-free survival (PFS) duration at the first-line treatment was 13.7 months in all patients with stage III NSCLC and ILD treated with chemoradiotherapy (Fig. 1A). The findings of univariate and multivariate analyses with regard to PFS in all patients with stage III NSCLC and ILD treated with chemoradiotherapy are shown in Table 3. In univariate analysis, patients with a non-UIP pattern showed better progression-free survival than those with a UIP pattern (median OS, 16.5 vs. 9.3 months, p = 0.0032, Fig. 1B). Multivariate analysis of prognostic factors showed that ILD classification [non-UIP pattern: hazard ratio (HR), 0.27; 95% CI, 0.090-0.76; p = 0.0138] was a significant independent prognostic factor. The median OS duration at the first-line treatment was 34.6 months in all patients with stage III NSCLC and ILD treated with chemoradiotherapy (Fig. 2A). The findings of univariate and multivariate analyses with regard to OS in all patients with stage III NSCLC and ILD treated with chemoradiotherapy are shown in Table 4. In univariate analysis, patients with a non-UIP pattern showed better survival than those with a UIP pattern (median OS, 43.0 vs. 12.2 months, p < 0.0001, Fig. 2B). Multivariate analysis of prognostic factors showed that ILD classification (non-UIP pattern: hazard ratio [HR], 0.073; 95% CI, 0.019-0.25; p < 0.0001) was a significant independent prognostic factor.
Figure 1.
A) Progression-free survival curve at the first-line treatment for 37 patients with stage III NSCLC and ILD. B) Progression-free survival curve at the first-line treatment for 11 patients with stage III NSCLC and a UIP pattern and 26 patients with stage III NSCLC and a non-UIP pattern. PFS, progression-free survival; UIP, usual interstitial pneumonia; HR, hazard ratio; NSCLC, non-small-cell lung cancer; ILD, interstitial lung disease
Table 3.
Univariate and multivariate analyses of PFS in all patients with stage III NSCLC and ILD treated with chemoradiotherapy
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Relative risk | 95% CI | p value | Relative risk | 95% CI | p value | |
| Sex (Female vs Male) | 1.17 | 0.39-2.84 | 0.7520 | |||
| Age (≥75 years vs <75 years) | 1.30 | 0.61-2.68 | 0.4770 | 1.23 | 0.56-2.62 | 0.5866 |
| ECOG-PS (1vs 0) | 0.95 | 0.46-2.03 | 0.8903 | 0.87 | 0.40-1.93 | 0.7318 |
| Smoking history (never vs current or former smoker) | 2.65 | 0.75-7.37 | 0.0733 | 1.81 | 0.46-5.83 | 0.3670 |
| Clinical staging (IIIB VS IIIA) | 0.91 | 0.44-1.86 | 0.8030 | |||
| Histology (squamous vs non-squamous) | 1.51 | 0.73-3.19 | 0.2642 | |||
| ILD classification on CT (non-UIP vs UIP pattern) | 0.24 | 0.084-0.67 | 0.0032 | 0.27 | 0.090-0.76 | 0.0138 |
| %VC (≥80 vs <80) (N=34) | 0.80 | 0.27-3.41 | 0.7217 | |||
| The finding of lung volume loss or honeycombing (either or both vs nothing) | 2.25 | 0.51-7.01 | 0.1943 | |||
PFS, progression-free survival; NSCLC, non-small-cell lung cancer; ILD, interstitial lung disease; ECOG PS, Eastern Cooperative Oncology Group performance score; CT, computed tomography; UIP, usual interstitial pneumonia; VC, vital capacity; CI, confidence interval
Figure 2.
A) Overall survival curve at the first-line treatment for 37 patients with stage III NSCLC and ILD. B) Overall survival curve at the first-line treatment for 11 patients with stage III NSCLC and a UIP pattern and 26 patients with stage III NSCLC and a non-UIP pattern. OS, overall survival; UIP, usual interstitial pneumonia; HR, hazard ratio; NSCLC, non-small-cell lung cancer; ILD, interstitial lung disease
Table 4.
Univariate and multivariate analyses of overall survival in patients with stage III non-small-cell lung cancer and interstitial lung disease treated with chemoradiotherapy (n = 37)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Relative risk | 95% CI | p-value | Relative risk | 95% CI | p-value | |
| Sex (female vs. male) | 0.94 | 0.22-2.79 | 0.9329 | |||
| Age (≥75 years vs. <75 years) | 0.88 | 0.35-2.04 | 0.7718 | 0.75 | 0.29-1.81 | 0.5358 |
| ECOG-PS (1 vs. 0) | 0.90 | 0.39-2.14 | 0.8085 | 1.09 | 0.45-2.73 | 0.8412 |
| Smoking history (never vs. current or former smoker) | 1.79 | 0.41-5.50 | 0.3544 | |||
| Clinical staging (IIIB vs. IIIA) | 0.81 | 0.35-1.88 | 0.6140 | |||
| Histology (squamous vs. non-squamous) | 0.72 | 0.30-1.69 | 0.4499 | |||
| ILD classification on CT (non-UIP vs. UIP pattern) | 0.080 | 0.022-0.26 | <0.0001 | 0.073 | 0.019-0.25 | <0.0001 |
| %VC (≥80 vs. <80) (n = 34) | 2.66 | 0.55-47.8 | 0.3207 | |||
| The finding of lung volume loss or honeycombing (either or both vs. nothing) | 2.77 | 0.41-11.2 | 0.1835 | |||
OS, overall survival; NSCLC, non-small-cell lung cancer; ILD, interstitial lung disease; ECOG PS, Eastern Cooperative Oncology Group performance score; CT, computed tomography; UIP, usual interstitial pneumonia; VC, vital capacity; CI, confidence interval
Among all patients with stage III NSCLC and ILD treated with chemoradiotherapy, the 2-year survival rate was 54%. The 2-year survival rates in patients with a non-UIP pattern and those with a UIP pattern were 72% and 0%, respectively.
Discussion
The present study, to the best of our knowledge, is the first to report that ILD classification is significantly associated with the incidence of AE of ILD and is a prognostic factor in patients with stage III NSCLC and ILD treated with curative-intent chemoradiotherapy.
In this retrospective analysis, patients with NSCLC and ILD treated with chemoradiotherapy had a higher incidence of AE of ILD, including radiation pneumonitis worse than grade 3, within 1 year after the last irradiation when compared with the incidence in patients with NSCLC without ILD reported in pivotal trials 27-29 and in our clinical setting (46% vs. 0%-10%). Thus, the presence of ILD was a risk factor for AE of ILD, including radiation pneumonitis worse than grade 3, as in previous reports 9, 12-16, 18-20, 23. However, there is no report focusing on ILD classification for risk factor of AE of ILD in patients with NSCLC and ILD treated with radiotherapy. Thus, an important finding is that a UIP pattern on thoracic CT may be a major risk factor for AE of ILD in this study. In agreement with this result, several reports showed that a UIP pattern could increase the risk for AE of ILD when compared to the risk with a non-UIP pattern among non-cancer patients 34, 35. Furthermore, the incidence rate and severity of AE of ILD were significantly higher in those with a UIP pattern than in those with a non-UIP pattern among patients with advanced lung cancer and ILD treated with chemotherapy 36. Therefore, ILD classification could be a predictive factor for AE of ILD in patients with NSCLC and ILD treated with chemoradiotherapy.
Prospective phase III trials in Japan demonstrated the efficacies of paclitaxel in combination with carboplatin 28 and docetaxel in combination with cisplatin 29 for patients with unresectable stage III NSCLC and reported that the median OS duration and 2-year survival rate were 22.0 and 26.8 months and 46% and 60%, respectively. These findings are similar to the median OS duration and 2-year survival rate for patients with a non-UIP pattern in this study. On the other hand, the prognosis of patients with a UIP pattern in this study was worse than that of patients reported in the abovementioned trials. In agreement with these results, some reports have revealed that non-cancer patients with a non-UIP pattern had significantly longer OS than that in those with a UIP pattern 37, 38. Therefore, ILD classification could be a prognostic factor for patients with NSCLC and ILD treated with chemoradiotherapy. Additionally, these abovementioned trials demonstrated that the median PFS durations were 9.5 and 10.5 months, respectively. Based on the results, the antitumor effect of chemoradiotherapy as the first-line treatment in patients with stage III NSCLC and ILD in this study was similar to that reported in the previous trials. However, in this study, patients with a UIP pattern had a significantly shorter PFS duration compared to that in patients with a non-UIP pattern. The possible reason for this is that patients with a UIP pattern tended to be unable to complete curative-intent radiotherapy because of the occurrence of AE of ILD.
Several limitations of this study should be considered. First, chemotherapy regimens at the first-line treatment varied. However, none of the patients underwent regimens contraindicated for ILD. Therefore, the potential impact of chemotherapy regimens on the incidence of AE of ILD is expected to be minimal. Second, this was a retrospective, non-randomized study conducted at a single center. Nevertheless, this is the first report which stated that ILD classification is significantly associated with the incidence of AE of ILD, including radiation pneumonitis, and is a prognostic factor in patients with inoperable stage III NSCLC and ILD treated with curative-intent chemoradiotherapy.
In conclusion, this study showed that the incidence of AE of ILD was high in patients with stage III NSCLC and ILD treated with chemoradiotherapy as the first-line treatment. However, a non-UIP pattern, which does not exhibit lung volume loss on thoracic radiography and honeycombing on thoracic CT, significantly reduced the risk of ILD-AE, including radiation pneumonitis, and resulted in longer PFS and OS durations as well as a higher 2-year survival rate in this study. Therefore, radiotherapy might be effective in patients with NSCLC and milder ILD with a diagnosis of non-UIP pattern, which does not exhibit lung volume loss on thoracic radiography and honeycombing on thoracic CT, although there still remains a high risk for AE of ILD triggered by radiotherapy in this population. In the future, we need to analyze the risk and efficacy of chemoradiotherapy in patients with stage III NSCLC and a non-UIP pattern using multi-institutional data.
Supplementary Material
Supplementary table S1.
Abbreviations
- AE
acute exacerbation
- %VC
% vital capacity
- CI
confidence interval
- CRP
C-reactive protein
- CT
computed tomography
- ECOG
Eastern Cooperative Oncology Group
- GTV
gross tumor volume
- HR
hazard ratio
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- LDH
lactate dehydrogenase
- NSCLC
non-small-cell lung cancer
- OS
overall survival
- PaO2
low partial pressure of arterial oxygen
- PFS
progression-free survival
- PS
performance status
- UIP
usual interstitial pneumonia.
References
- 1.Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable): a systematic review. Thorax. 2001;56:628–638. doi: 10.1136/thorax.56.8.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibley GS. Radiotherapy for patients with medically inoperable Stage I nonsmall cell lung carcinoma: smaller volumes and higher doses-a review. Cancer. 1998;82:433–438. [PubMed] [Google Scholar]
- 3.Qiao X, Tullgren O, Lax I, Sirzen F, Lewensohn R. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung cancer. 2003;41:1–11. doi: 10.1016/s0169-5002(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 4.Morita K, Fuwa N, Suzuki Y. et al. Radical radiotherapy for medically inoperable non-small cell lung cancer in clinical stage I: a retrospective analysis of 149 patients. Radiother Oncol. 1997;42:31–36. doi: 10.1016/s0167-8140(96)01828-2. [DOI] [PubMed] [Google Scholar]
- 5.Tyldesley S, Boyd C, Schulze K, Walker H, Mackillop WJ. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973–985. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- 6.Chemotherapy in non-small cell lung cancer. a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 7.Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer. 1995;76:593–601. doi: 10.1002/1097-0142(19950815)76:4<593::aid-cncr2820760409>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med. 1996;125:723–729. doi: 10.7326/0003-4819-125-9-199611010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Ohe Y, Yamamoto S, Suzuki K. et al. Risk factors of treatment-related death in chemotherapy and thoracic radiotherapy for lung cancer. Eur J Cancer. 2001;37:54–63. doi: 10.1016/s0959-8049(00)00350-6. [DOI] [PubMed] [Google Scholar]
- 10.King TE Jr. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 2005;172:268–279. doi: 10.1164/rccm.200503-483OE. [DOI] [PubMed] [Google Scholar]
- 11.Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: A prospective study. Chest. 1999;116:1168–1174. doi: 10.1378/chest.116.5.1168. [DOI] [PubMed] [Google Scholar]
- 12.Kudoh S, Kato H, Nishiwaki Y. et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 13.Kenmotsu H, Naito T, Mori K. et al. Effect of platinum-based chemotherapy for non-small cell lung cancer patients with interstitial lung disease. Cancer Chemother Pharmacol. 2015;75:521–526. doi: 10.1007/s00280-014-2670-y. [DOI] [PubMed] [Google Scholar]
- 14.Makimoto T, Tsuchiya S, Hayakawa K, Saitoh R, Mori M. Risk factors for severe radiation pneumonitis in lung cancer. Jpn J Clin Oncol. 1999;29:192–197. doi: 10.1093/jjco/29.4.192. [DOI] [PubMed] [Google Scholar]
- 15.Sanuki N, Ono A, Komatsu E. et al. Association of computed tomography-detected pulmonary interstitial changes with severe radiation pneumonitis for patients treated with thoracic radiotherapy. J Radiat Res. 2012;53:110–116. doi: 10.1269/jrr.110142. [DOI] [PubMed] [Google Scholar]
- 16.Ueki N, Matsuo Y, Togashi Y. et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015;10:116–125. doi: 10.1097/JTO.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita H, Takahashi W, Haga A, Nakagawa K. Radiation pneumonitis after stereotactic radiation therapy for lung cancer. World J Radiol. 2014;6:708–715. doi: 10.4329/wjr.v6.i9.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi S, Ohguri T, Ide S. et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis. Lung cancer. 2013;82:260–265. doi: 10.1016/j.lungcan.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Ozawa Y, Abe T, Omae M. et al. Impact of Preexisting Interstitial Lung Disease on Acute, Extensive Radiation Pneumonitis: Retrospective Analysis of Patients with Lung Cancer. PLoS One. 2015;10:e0140437. doi: 10.1371/journal.pone.0140437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YH, Kim YS, Lee SN. et al. Interstitial Lung Change in Pre-radiation Therapy Computed Tomography Is a Risk Factor for Severe Radiation Pneumonitis. Cancer Res Treat. 2015;47:676–686. doi: 10.4143/crt.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YJ, Chen LX, Han MX, Zhang TS, Zhou ZR, Zhong DS. The Efficacy and Safety of Chemotherapy in Patients With Nonsmall Cell Lung Cancer and Interstitial Lung Disease: A PRISMA-Compliant Bayesian Meta-Analysis and Systematic Review. Medicine (Baltimore) 2015;94:e1451. doi: 10.1097/MD.0000000000001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita T, Azuma K, Sasada T. et al. Chemotherapy for non-small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol Lett. 2012;4:477–482. doi: 10.3892/ol.2012.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahig H, Filion E, Vu T. et al. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol. 2016;6:367–374. doi: 10.1016/j.prro.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Fay M, Tan A, Fisher R, Mac Manus M, Wirth A, Ball D. Dose-volume histogram analysis as predictor of radiation pneumonitis in primary lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1355–1363. doi: 10.1016/j.ijrobp.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol. 2003;67:275–283. doi: 10.1016/s0167-8140(03)00119-1. [DOI] [PubMed] [Google Scholar]
- 26.Inoue A, Kunitoh H, Sekine I, Sumi M, Tokuuye K, Saijo N. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49:649–655. doi: 10.1016/s0360-3016(00)00783-5. [DOI] [PubMed] [Google Scholar]
- 27.Furuse K, Fukuoka M, Kawahara M. et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto N, Nakagawa K, Nishimura Y. et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. [DOI] [PubMed] [Google Scholar]
- 29.Segawa Y, Kiura K, Takigawa N. et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3299–3306. doi: 10.1200/JCO.2009.24.7577. [DOI] [PubMed] [Google Scholar]
- 30.Ramella S, Trodella L, Mineo TC. et al. Adding ipsilateral V20 and V30 to conventional dosimetric constraints predicts radiation pneumonitis in stage IIIA-B NSCLC treated with combined-modality therapy. Int J Radiat Oncol Biol Phys. 2010;76:110–115. doi: 10.1016/j.ijrobp.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Lynch DA, Godwin JD, Safrin S. et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172:488–493. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 32.Collard HR, Ryerson CJ, Corte TJ. et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 33.Common Terminology Criteria for Adverse Events (CTCAE)
- 34.Miyazaki Y, Tateishi T, Akashi T, Ohtani Y, Inase N, Yoshizawa Y. Clinical predictors and histologic appearance of acute exacerbations in chronic hypersensitivity pneumonitis. Chest. 2008;134:1265–1270. doi: 10.1378/chest.08-0866. [DOI] [PubMed] [Google Scholar]
- 35.Park IN, Kim DS, Shim TS. et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–220. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 36.Kenmotsu H, Naito T, Kimura M. et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6:1242–1246. doi: 10.1097/JTO.0b013e318216ee6b. [DOI] [PubMed] [Google Scholar]
- 37.Latsi PI, du Bois RM, Nicholson AG. et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 38.Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol. 2000;24:19–33. doi: 10.1097/00000478-200001000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table S1.