Abstract
Pigeon pea is an important legume infested by a plethora of insect pests amongst which gram pod borer Helicoverpa armigera is very prominent. Imparting resistance to this insect herbivore is of global importance in attaining food security. Expression of insecticidal crystal proteins (ICP) in diverse crops has led to increased resistance to several pests. We report in this paper, expression of Cry2Aa in transgenic pigeon pea and its effectiveness towards H. armigera by employing Agrobacterium-mediated in planta transformation approach. Approximately 0.8% of T1 generation plants were identified as putative transformants based on screening in the presence of 70 ppm kanamycin as the selection agent. Promising events were further recognized in advanced generations based on integration, expression and bioefficacy of the transgenes. Seven T3 lines (11.8% of the selected T1 events) were categorized as superior as these events demonstrated 80–100% mortality of the challenged larvae and improved ability to prevent damage caused by the larvae. The selected transgenic plants accumulated Cry2Aa in the range of 25–80 µg/g FW. The transgenic events developed in the study can be used in pigeon pea improvement programmes for pod borer resistance.
Introduction
Pigeon pea is an important grain legume in Asia, Africa and parts of Latin America1,2. In India, pigeon pea is grown in 3.8 million hectares3,4 contributing to 90% of global production. High level of protein in pigeon pea makes it an important component of diet especially amongst the Indian vegetarian population. Despite improved production of pigeon pea to 4.85 million tonnes over the last decade, there has been stagnation in the yield per hectare2. One of the major reasons for this is its susceptibility to a lepidopteran pest, gram pod borer (H. armigera)5. It is the most difficult pest to control with high fecundity and strong migratory behaviour resulting in approximately 85% of yield loss. The herbivore apparently is responsible to cause losses up to or more than US $300 million annually6. The wide host range, high degree of migration, indiscriminate pesticide application by farmers and innate ability of the insect to quickly develop resistance to insecticides have made it attain the status of a key pest7,8. Furthermore, screening of more than 14,000 accessions of cultivated pigeon pea has revealed moderate or low levels of resistance towards the insect9,10. Therefore, incorporating the trait for pod borer resistance is an integral part of pigeon pea crop improvement.
One of the effective strategies to manage gram pod borer is transgenic expression of Bacillus thuringiensis insecticidal crystal proteins (ICPs), also known as delta-endotoxins11,12. A number of cry genes with effectiveness against insect pests are incessantly being identified by scientists worldwide for their introgression into crop plants through transgenesis13. Individual Cry toxins are usually toxic to only a few insect species within an order and receptors on midgut epithelial cells have been shown to be critical determinants of Cry specificity. The increasing acreage of the transgenic crops harboring these Bt ICPs is demonstration enough for the acceptance and success of the technology14. There have been several reports about the use of various Bt ICPs in pigeon pea to manage pod borer15–18. However, several transgenic events harboring a range of effective ICP genes need to be developed so that superior events with commercialization potential and sustainability under field conditions can be identified.
In addition to Cry1 series of ICPs, it is pertinent to assess the ability of another ICP, Cry2Aa, for its efficacy against H. armigera in pigeon pea. In addition, utilizing a non-tissue culture-based in planta approach19 for the development of stable and effective events can be an added advantage due to the recalcitrance of pigeon pea to tissue culture. Earlier, cry2Aa has been tested and proven effective against H. armigera in transgenic rice, chickpea and pigeon pea17,20–22. Transgenic plants with cry2Aa can consequently be used as an additional strategy for the development of insect resistant pigeon pea17,23–25 to tackle the devastating herbivore. Our group is also actively involved in the development of pigeon pea transgenics with other novel Bt ICPs26. Since the mode of action of cry2Aa is different when compared to cry1 series of Bt ICPs17, stacking transgenic crops expressing both the genes can be a viable approach for delaying evolution of resistance to cry toxins by Helicoverpa armigera. In this direction, the purpose of the present study was to demonstrate the utility of cry2Aa gene in transgenic pigeon pea against H. armigera and also reiterate the applicability of an apical meristem-targeted tissue culture-independent in planta transformation approach for pigeon pea improvement.
Results
Development and selection of transformants in pigeon pea carrying cry2Aa gene
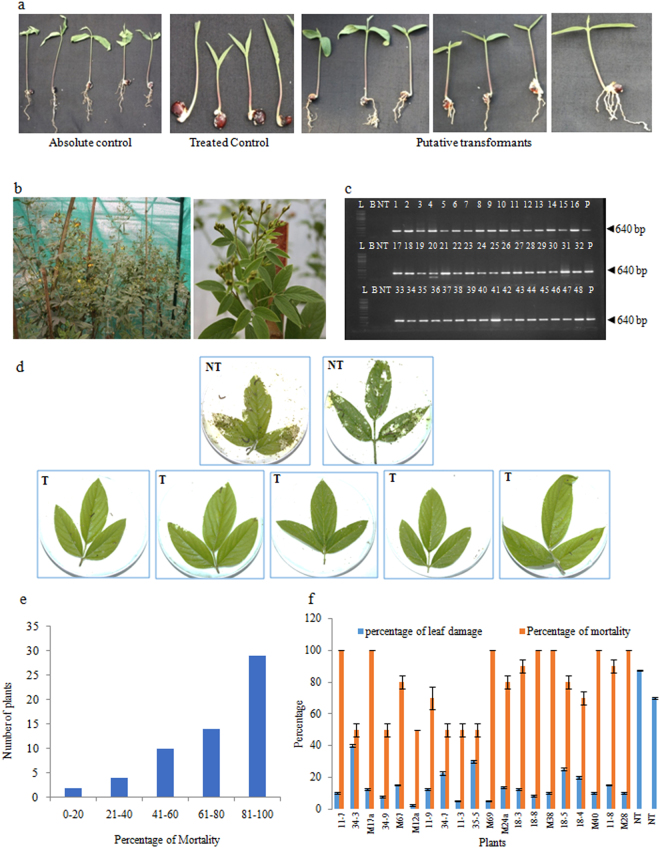
Primary transformants of pigeon pea cv. Pusa 992 were developed using the apical meristem-targeted Agrobacterium-mediated in planta transformation strategy19. About 50 seedlings were subjected to in planta transformation of which 28 individual T0 plants (primary transformants) established in the green house that grew normally, flowered and set seeds. Since the transformants developed by in planta transformation strategy are chimeras in T0 generation, screening of T1 generation plants for the identification of putative transformants27 is essential. In the present study, preliminary screening of the seedlings on 70 ppm kanamycin identified 59 putative transformants in T1 generation (Fig. 1a) that grew normally and established in the greenhouse (Fig. 1b). This accounted for 0.8% of the total number of seeds that were subjected to screening. Further to confirmation of the presence of T-DNA by PCR analysis, (Fig. 1c), leaf bioassays against neonate larvae of H. armigera revealed significant variability in larval mortality and the extent of damage to the leaves (Fig. 1d). There was diversity in the response of T1 generation plants to the larval challenge which varied between 0–100% larval mortality (Fig. 1e); moreover, the plants that showed high mortality exhibited less leaf damage (Fig. 1f). Based on the stringent efficacy evaluation, 29 T1 generation plants (about 49% of the total putative transformants selected on kanamycin) were selected as promising. These experimental evidences provided comprehensible evidence about the transgenic nature of the plants. The selected plants were advanced to T2 generation for the analysis of stability in T-DNA integration, inheritance as well as sustained efficacy.
Figure 1.
Analysis of T1 generation plants. (a) Screening for the selection of putative transformants grown in quartz sand under the selection pressure of 70 ppm kanamycin; (b) putative transgenic plants established in the greenhouse; (c) PCR analysis of T1 generation plants for the amplification of a 640 bp cry2Aa gene fragment (L: 1 kb ladder, B: template blank, NT: non transgenic, lane 1–48 = representative samples of putative transformants, P: plasmid); (d) Performance of putative transformants vis- á- vis non transgenic plants in the in vitro leaf bioassay against neonate larvae of Helicoverpa armigera (NT: non transgenic plants; T: putative transformants); (e) frequency distribution of the plants based on percentage mortality of H. armigera in the bioassay; (f) a representative histogram depicting the variation in the performance of T1 generation plants in the in vitro bioassay.
Analysis of the transgenic plants for stable integration and inheritance of T-DNA
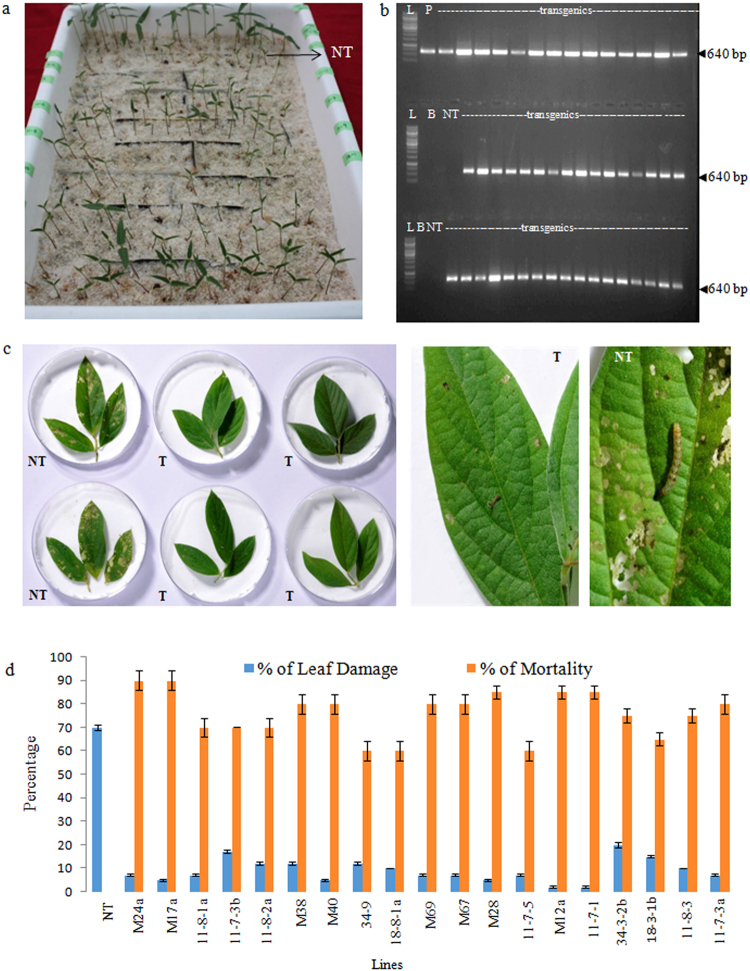
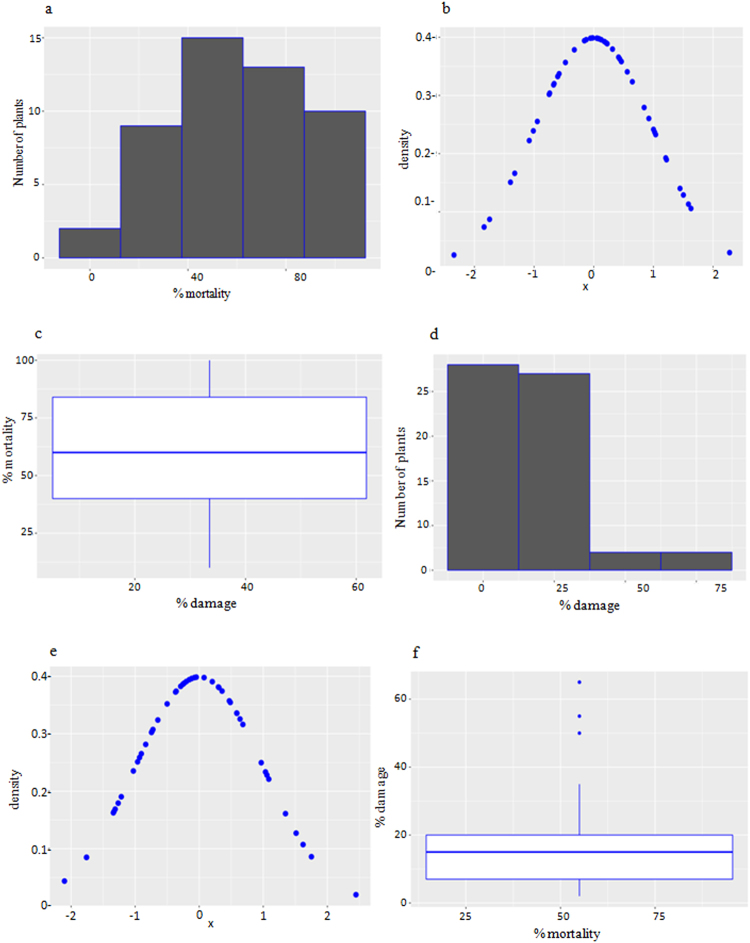
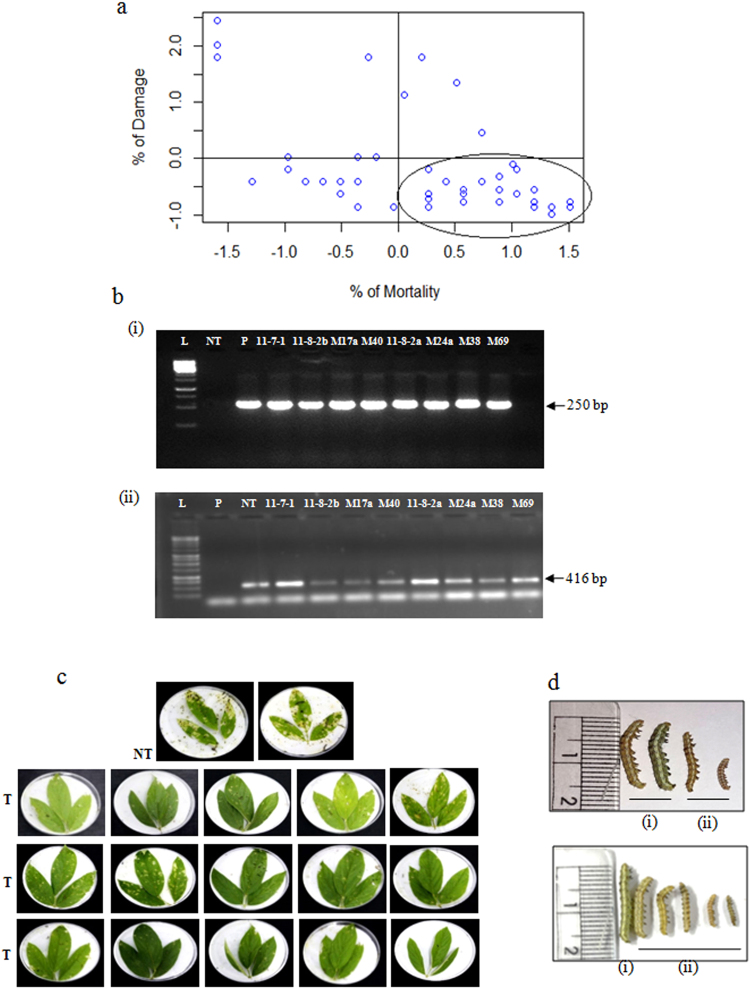
Ability of the progeny of the selected 29 T1 generation plants to survive and grow on 70 ppm kanamycin (Fig. 2a) demonstrated that the T-DNA was stably integrated in the genome of the transgenic plants. Further molecular and bioefficacy analysis to confirm the inheritance of T-DNA was carried out with these plants that survived on kanamycin selection. The first molecular evidence for the stability of T-DNA was demonstrated by PCR analysis for the gene of interest cry2Aa and the expected amplicon of 640 bp was observed in all the surviving transgenics (Fig. 2b). In vitro leaf bioassays of the established T2 generation plants against deliberate challenging with neonate larvae of H. armigera demonstrated their superior bioefficacy (Fig. 2c-representative picture) in terms of higher larval mortality and lesser leaf damage (Fig. 2d). The bioassays conclusively demonstrated that the selected transformants not only had the cry2Aa gene integrated stably in their genome (Fig. 3a,d) but also their greater ability to resist the insect herbivory as depicted explicitly by the bell curve for two parameters viz., insect mortality and leaf damage (Fig. 3b,e). Further, it was observed that, >75% of the plants in the T2 generation demonstrated <20% leaf damage with synchronised mortality (Fig. 3c,f). However, based on Z distribution analysis (Fig. 4a) for the two parameters, larval mortality and extent of leaf damage, 15 events (in the 1st quadrant) were identified as superior. Further, randomly selected superior events concomitantly confirmed accumulation of cry2Aa gene transcripts (Fig. 4b) implying that the performance of the events in the bioassay was because of the expression of the integrated cry2Aa gene. Ability of the selected plants to combat the herbivore was reiterated by their performance after challenging the leaves with H. armigera (Fig. 4c-representative photograph). Variation in larval morphology in terms of drastic reduction and mortality upon feeding on some of the transgenics was clearly evident at the end of leaf bioassay (Fig. 4d). However, larvae that fed on the non transgenic plants depicted normal morphology and development (Fig. 4d). Based on the molecular and bioefficacy analysis, 15 events, i.e., approximately 25% of the earlier identified T1 generation plants (M24a, M17a, 11-8-1a, 11-7-3b, 11-8-2a, M38, M40, M69, M67, M28, M12a, 11-7-1, 34-3-2b, 11-8-3, 11-7-3a) were advanced for further analysis in T3 generation.
Figure 2.
Analysis of transgenic plants in T2 generation. (a) Stability in the resistance response of the progeny of selected T1 plants on 70 ppm kanamycin (NT: non transgenic plants); (b) PCR analysis of T2 generation plants for the amplification of a 640 bp cry2Aa gene fragment (L: 1 kb ladder, B: template blank, NT: non transgenic; P: plasmid); (c) in vitro leaf bioassay to depict the performance of transgenic plants vis- á- vis non transgenic plants (NT: non transgenic plants; T: putative transformants); (d) histogram portraying the performance of individual transgenic lines to deliberate challenging of the larvae of H. armigera in terms of percent mortality of the larvae and extent of leaf damage caused by the larvae.
Figure 3.
Analysis of the performance of T2 generation plants against H. armigera in the leaf bioassays. (a) Histogram depicting the performance of T2 generation plants in the in vitro leaf bioassay with respect to mortality of H. armigera larvae; (b) bell curve demonstrating the skewed distribution of percent mortality; (c) comparative box whisker plot elucidating the performance spread of transgenic plants depicting improved mortality of the larvae (Min. 10.00; 1st Qu. 40.00; Median 60.00; Mean 59.82; 3rd Qu. 84.00; Max. 100.00); (d) histogram depicting the percent damage caused by H. armigera on the leaves; (e) bell curve demonstrating the skewed distribution of percent damage; (f) Comparative box whisker plot depicting the extent of damage caused by the larvae (Min. 2.00; 1st Qu. 7.00; Median 15.00; Mean 17.12; 3rd Qu. 20.00; Max. 65.00).
Figure 4.
(a)Z distribution analysis for two parameters, percent mortality of H. armigera larvae and % damage caused by the larvae in the in vitro leaf bioassay; (b) sqRT-PCR analysis in the selected T2 generation transgenic plants for transcript accumulation of (i) cry2Aa gene (ii) 18s rRNA gene as an internal control (L: ladder; NT: non transgenic; P: plasmid); (c) depiction of the performance of selected transgenic plants of T2 generation in the in vitro leaf bioassay against deliberate challenging of H. armigera; (d) Larval morphology at the end of in vitro leaf bioassay after feeding on (i) non transgenic plants; (ii) transgenic plants (event, M40).
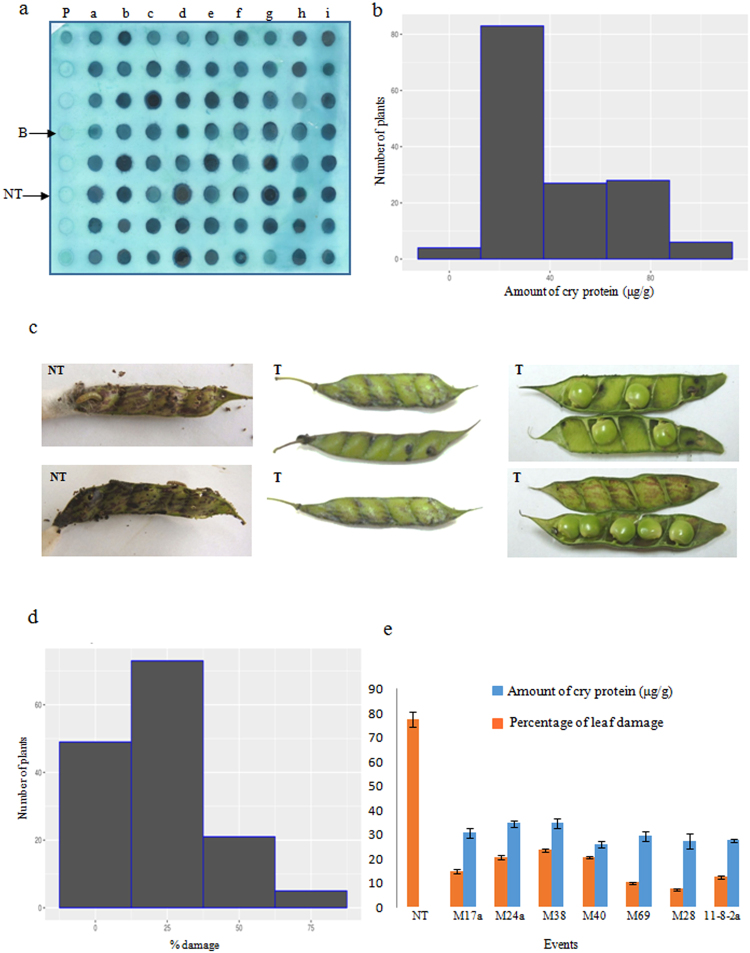
In the advanced T3 generation, dot blot analysis for Cry2Aa expression was used as an initial proof to assess the stability in the transgene expression in the selected events. The selected events demonstrated strong signals not only indicating high levels of protein but also uniformity in expression across the progeny of the selected transformants (Fig. 5a). Quantitative analysis of the dot blot showed that Cry2Aa expression varied between ~7 µg/g FW and ~96 µg/g FW of leaf tissue. Maximum number of transgenic plants expressed in the range of 25–50 µg/g FW of the Cry2Aa protein (Fig. 5b). Based on this observation, stability in efficacy of these plants against Helicoverpa was analysed in well-developed pods and the extent of damage caused by the 2nd instar larvae was considered as the parameter for analysis. Study of the performance of selected transgenic plants demonstrated a clear constancy in the ability of the plants to resist the attack of the larvae with reduced damage (Fig. 5c). Damage was seen to be skewed to the left as maximum number of plants demonstrated 0 to 25% leaf damage clearly indicating that the damage was well under control (Fig. 5d). Segregation analysis carried out with the selected events provided additional proof for the stable integration and inheritance of the T-DNA (Table 1). The analysis depicted that majority of the selected events followed the expected 3:1 segregation ratio indicating T-DNA stability. However, among the various promising events that were subjected to evaluation, 7 events (M17a, M24a, M38, M40, M69, M28, 11-8-2a) with consistent Cry2Aa protein expression and less damage when challenged with H. armigera were identified as promising. The selected events showed coherence in both the parameters under analysis as they not only showed higher protein expression in the range of 26–34 µg/g FW but also efficiently resisted the attack of the pest by demonstrating reduced damage (7.5–23%) (Fig. 5e). Comparison of means by two tailed ‘t’ test with unequal sample sizes, indicated that the selected transgenic plants were significantly superior with respect to the accumulation of Cry2Aa protein (t = 35.778; P < 2.2e−16). Similarly, significant difference was also seen with respect to leaf damage by H. armigera (t = −30.883; P 5.54e−14) demonstrating the ability of the transgenic plants to resist the herbivore attack (Table 2).
Figure 5.
Analysis of T3 generation plants. (a) High throughput dot blot analysis (representative blot) showing the stable expression of Cry2Aa in different transgenic plants vis- á- vis non transgenic (P: 20 ng purified Cry2Aa protein; B: blank; NT: non transgenic; a-i: total protein extracts from different transgenic lines comprising of 8 plants per line); (b) Histogram illustrating the expression levels of Cry2Aa protein in µg/g FW across various transgenic plants; (c) Performance of the transformants vis- a- vis non transgenic plants against deliberate challenging of H. armigera in an in vitro pod bioassay (NT: non transgenic plants; T: transformants); (d) Histogram illustrating the percent damage across various transgenic plants following pod bioassay; (e) Performance of the selected plants in pod bioassay vis- á- vis Cry2Aa protein accumulation (µg/g FW).
Table 1.
Transgene inheritance analysis in the selected transgenic events based on bioassay against H. armigera.
| Sl. No | Event identity | Total number of plants | Positive plants | Negative plants | χ2 | P |
|---|---|---|---|---|---|---|
| 1 | 11-7-3a | 13 | 9 | 4 | 0.23 | 0.632 |
| 2 | 11-8-2a* | 17 | 11 | 6 | 0.96 | 0.327 |
| 3 | M40a | 6 | 1 | 5 | 10.89 | <0.001 |
| 4 | M17a* | 4 | 0 | 4 | 12.00 | <0.001 |
| 5 | M17b | 5 | 0 | 5 | 15.00 | <0.001 |
| 6 | M24a* | 4 | 3 | 1 | 0.00 | 1 |
| 7 | M27 | 8 | 2 | 6 | 10.67 | <0.001 |
| 8 | M40* | 12 | 7 | 5 | 1.78 | 0.182 |
| 9 | M40b | 8 | 6 | 2 | 0.00 | 1 |
| 10 | M69* | 23 | 18 | 5 | 0.13 | 0.718 |
| 11 | 11-8-1 | 14 | 6 | 8 | 7.71 | 0.006 |
| 12 | 11-8-2b | 11 | 6 | 5 | 2.45 | 0.116 |
| 13 | 11-7-3b | 2 | 0 | 2 | 6.00 | 0.014 |
| 14 | M38* | 2 | 0 | 2 | 6.00 | 0.014 |
| 15 | M28* | 7 | 7 | 0 | 2.33 | 0.127 |
*Seven selected superior events.
Segregation analysis was calculated based on the performance of transgenic plants in the pod bioassay. The plants that showed <25% pod damage were considered as positive.
Table 2.
Comparison between the transgenic plants of T3 generation vis- á- vis non-transgenic plants with respect to Cry2Aa protein content and pod damage in the insect bioassay.
| n | Protein Content | % Leaf Damage | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| Transgenics | 50 | 29.34 ± 5.79 | 18.35–44.28 | 9.49 ± 4.96 | 1.25–20 |
| Non-Transgenic plants | 12 | 0 ± 0 | 0 | 77.5 ± 7.22 | 70–90 |
| T-test | 35.778 | −30.883 | |||
| P-values | <2.2e−16 | 5.54e−14 | |||
Molecular characterization of the transgenic events for transgene integration and inheritance
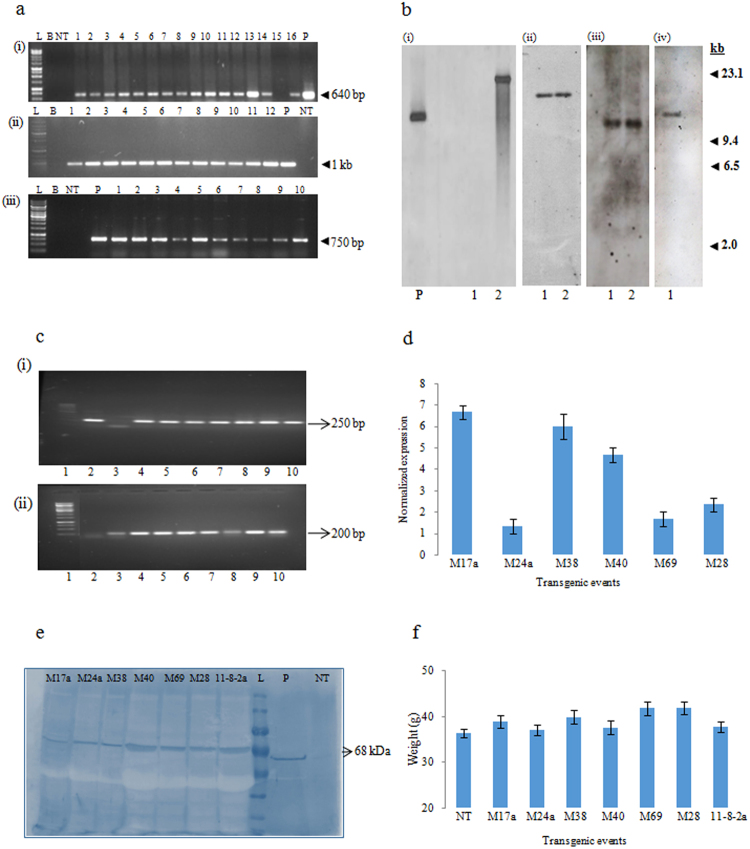
Comprehensive molecular characterization of the 7 superior events was carried out for unambiguous demonstration of stable integration and inheritance of T-DNA. PCR analysis using primers specific for both cry2Aa and nptII (Fig. 6a) demonstrated their presence in the genomic DNA of the selected events. Further, genomic Southern analysis revealed that the T-DNA was integrated as a single copy in four of the superior events, M17a (Fig. 6b[i]), M40 (Fig. 6b [ii]), M69 (Fig. 6b[iii], and 11-8-2a (Fig. 6b [iv]). To corroborate integration, expression and efficacy analyses, all the selected transgenic plants when further assessed for transcript accumulation by both sqRT-PCR (Fig. 6c; Supplementary fig. S2) and qPCR (Fig. 6d) showed varied levels of cry2Aa expression in different events. The conclusive evidence for the expression of Cry2Aa in all the selected transgenic events was further supported by western blot analysis (Fig. 6e) using Cry2Aa-specific antibody reiterating that the observed superior bioefficacy in these events against H. armigera was due to the integrated cry2Aa transgene. Further, absence of variation in the pod yield in selected transgenic plants vis- á- vis non transgenic plants at maturity demonstrated that the integrated T-DNA did not result in any unintended effects either due to the position of the T-DNA or due to the accumulation of Cry2Aa protein (Fig. 6f).
Figure 6.
Molecular analysis of the selected T3 generation transgenic plants. (a) PCR analysis for the amplification of cry2Aa (i and ii) and (iii) nptII gene fragments in the transgenic plants vis- á- vis non transgenics; (b) Genomic Southern analysis of transgenic pigeon pea events: 15 µg of genomic DNA was digested with HindIII and probed with a 640 bp DIG labelled cry2Aa gene fragment (i) M17a (Lane P: pBinAR plasmid-250 pg; Lane 1: DNA from non transgenic plant; Lane 2: DNA from event M17a); (ii) M40 (Lane 1: DNA from M40–1 and Lane 2: DNA from 40–2); (iii) M69 (Lane 1: DNA from M69-1and Lane 2: DNA from M69-2); (iv) 11-8-2a; (c) sqRT-PCR analysis in the selected T3 generation transgenic plants for transcript accumulation (i) amplification of 250 bp cry2Aa gene fragment; (ii) amplification of a 200 bp actin gene fragment as an internal control (Lane 1: ladder; Lane 2: plasmid; Lane 3: non transgenic plant; Lanes 4–10: selected transgenic plants (M17a, M24a, M38, M40, M69, M28, 11-8-2a); (d) qRT-PCR analysis of six selected events for the expression of cry2Aa gene. Initiation Factor 4α gene was used as an internal control and ∆Ct values were calculated using difference in the Ct mean of the target gene and the reference gene. X axis represents different transgenic events and Y axis represents normalized expression of the transgene; (e) Western blot analysis for the expression of Cry2Aa in the selected T3 generation plants (Lane L: ladder; Lane NT: total protein extract from the non transgenic plant; Lane P: purified Cry2Aa [30 ng]); (f) pod yield at maturity in different selected transgenic plants vis- á- vis non transgenic plants.
Discussion
Development of insect resistance in crop species using biotechnological tools has gained prominence over the past three decades28,29. Since the advent of genetic engineering, the major candidate proteins for achieving insect resistance have been the ICPs (insecticidal crystal proteins) and Bt ICPs in particular30. Of the very many insect pests, H. armigera, a lepidopteran pest is one of the most serious and widespread pests of pigeon pea31. Increasing use of toxic pesticides32 as well as development of resistance by pests29 has led to the continued quest for alternate approaches to tackle H. armigera. Modern genomic tools such as molecular markers and candidate genes associated with resistance offer the possibility of facilitating pigeon pea breeding towards improved biotic stress resistance. However, absence of known sources of resistance and limited genetic variability in the cultivated germplasm1 has minimized the utility of conventional breeding approaches to address this problem. Insect resistant transgenic plants, therefore, are considered to be an ideal seed borne solution32,33.
Effectiveness of Bt ICPs in the control of pod borer has been demonstrated earlier in pigeon pea15–18,34,35. However, potential events arising from various strategies need to be assessed to identify a prospective product with an ability to sustain the devastating herbivore under field conditions. This necessitates the use of a variety of genes and strategies for the development of viable transgenic events. The recalcitrance of pigeon pea to tissue culture and reduced ability in the development of transformants has resulted in researchers adopting non-conventional approaches for the development of transformants in this crop15,16,18,19. In the present study, a tissue culture independent apical meristem-targeted in planta transformation that was standardized earlier by our group was used to develop transformants in pigeon pea19. The strategy mainly deals with the in planta inoculation of the shoot apical meristem and allowing them to grow ex vitro. The progeny of the chimeric primary transformants are required to be screened for the identification of putative transformants in the T1 generation. The major advantage of this transformation strategy as demonstrated in our earlier studies is the ability to develop a large number of primary transformants27. This necessitates the need of a high throughput yet stringent selectable marker-based screening for the initial identification of putative transformants. In this study, quartz sand-based screening of a large number of T1 generation seedlings in the presence of the selection agent kanamycin (70 ppm)27 resulted in the identification of 59 putative transformants. This selection strategy not only allowed the identification of putative transformants but also contributed towards selection of transgenics expressing the selectable marker gene in higher levels27 under the given selection pressure. The putative transformants were advanced with an assumption that correlation would exist between the expression of selectable marker gene as well as the target gene. The major emphasis in the present study was in the identification of promising pigeon pea events depicting increased Cry2Aa expression and superiority in their efficacy against pod borer, H. armigera. Both leaf and pod-level rigorous in vitro bioassays performed in successive generations aided in the identification and selection of events with 80–100% larval mortality and <5–10% leaf or pod damage. This was substantiated by in-depth analysis of the bioefficacy studies carried out in the respective generations. Based on this, 29 and 15 events were selected at the end of T1 and T2 generation with these events demonstrating categorical evidences of T-DNA integration.
It was observed that, with the advancement of generations, the performance of the overall population of plants in T2 and T3 generations improved towards resisting H. armigera. Further proof of the stability of the transgene was obtained by segregation analysis which showed that most of the selected plants followed the Mendelian 3:1 ratio. Additionally, majority of the plants accumulated Cry2Aa protein that was effective against the pest reiterating the stability of the integrated T-DNA and promising events expressing in the range of 26–34 µg/g FW. Though the expression levels of Bt ICPs can vary across crops and genes15,17, the quantity estimated in the study was on par with other studies where Cry protein expression has been provided17. Meticulous analysis for the confirmation of integration, expression and efficacy resulted in the identification of 7 superior events, which are now being advanced to identify a viable commercial event through event selection trials. Though studies have demonstrated the ability of different Bt ICPs to combat H. armigera, very few depict the stability of the transgene in advanced generations15,34. Moreover, since utility of cry2Aa in pigeon pea has not been widely proved, except for a single study17, this study is the first of its kind to prove the stability in the expression and performance of the transgenic pigeon pea plants up to T3 generation.
We provide evidence for the usefulness of the apical meristem-targeted in planta protocol not only in successful transformation of an economically important crop like pigeon pea but also the ability to develop a large number of superior events. Stable events with high expression and efficacy of Cry2Aa have been identified and advanced till T3 generation. The utilized approach as well as the set of promising events can be an effective contribution to the fraternity trying to effectively mitigate the devastating herbivore through transgenesis.
Materials and Methods
Development of primary transformants and identification of putative transformants
Primary transformants in pigeon pea were developed following the apical meristem-targeted tissue culture independent in planta transformation protocol19 in the cv. Pusa 992, which is a susceptible genotype to H. armigera. The 1.8 kb cry2Aa gene used in the study was synthesized and validated at ICAR-NRCPB. The gene was sub-cloned in pBinAR (Supplementary Fig. S1a), mobilized into Agrobacterium strain EHA105 and used for transformation. The T1 seeds from the primary transformants were harvested and analysed subsequently for the selection of putative transformants.
Screening of T1 transformants was carried out in the presence of 70 ppm kanamycin27. Four day old germinated seedlings were soaked in 70 ppm kanamycin for 4 h at room temperature and transferred to quartz sand. These seedlings were grown under greenhouse conditions for 10 days and supplemented with ¼th strength Hoagland solution36. Well-established plants were transferred to pots and further analysed for transgene integration, expression and insecticidal efficacy.
Molecular analyses for T-DNA integration
Total genomic DNA was isolated from tender leaves of transgenic and non transgenic plants37. PCR analysis was carried out to amplify gene fragments of transgenes using standardized primers and conditions (Supplementary Fig. S1b). The PCR reaction mixture (25 µl) contained 1 U Taq DNA polymerase (Bangalore Genei, Bangalore), 1 × assay buffer (10 mM pH 9.0 Tris HCl, 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin), 150 µM of each dNTPs, 0.5 µl of each forward and reverse primer at a final concentration of 0.25 µM and 100 ng template DNA. The DNA extracted from non transgenic plant was used as a negative control, while pBinAR vector was used as a positive control and the reaction mix without DNA as water blank. The PCR reaction profile comprised of 30 cycles, with strand separation at 94 °C for 1 min, annealing at 58 °C for 1 min and extension at 72 °C for 1 min. The program was extended at 72 °C for 10 min. The products were electrophoresed on a 0.8% agarose gel, stained with ethidium bromide and visualized under ultraviolet light38.
Southern analysis
For analysis of copy number by genomic Southern analysis, purified genomic DNA (15 µg) from transgenic and non transgenic plants was digested overnight with HindIII (NEB high fidelity, NEB), that has a single restriction site in the T-DNA. The digested DNA samples were electrophoresed on a 0.8% agarose gel in TAE buffer and blotted onto a positively charged nylon membrane (Millipore India private Ltd.). A 640 bp PCR product of cry2Aa was labeled using DIG PCR labeling kit. Hybridization and washing was carried according to manufacturer’s instructions (Roche Holding AG) and the membrane was incubated for 18 h in dark, wrapped and exposed to an X-ray film for 30 min in dark.
Analysis of transgenic plants for cry2Aa transcript accumulation
Sq-PCR
Total RNA39 was isolated from the leaves of both transgenic and non transgenic plants and 1 µg was reverse transcribed to single stranded cDNA according to the manufacturer’s instructions (Agilant Accuscript high fidelity kit; www.agilant.com). To evaluate transcript accumulation, 1 µl of the cDNA mix was used as a template for the amplification of 250 bp cry2Aa fragment, 416 bp 18srRNA gene fragment and 200 bp actin gene fragment, following initial denaturation at 95 °C for 5 min and 35 cycles of 95 °C for 30 sec, 55 °C for 30 sec, 72 °C for 30 sec and a final extension of 72 °C for 5 min. The products were later visualized on a 0.8% agarose gel.
qPCR
Total RNA was isolated from leaves of both transgenic and non transgenic plants (Spectrum™, Sigma-Aldrich). Yield and integrity of RNA was assessed using a Nanodrop Micro Photometer (Thermo Scientific) and by agarose gel electrophoresis respectively. cDNA was synthesized from 1 µg total RNA according to manufacturer’s instructions (SuperScript®VILO™, Invitrogen). Primers to amplify cry2Aa gene and Initiation factor 4α40 (IF4α-a house keeping gene to normalize the data) were used to set up qPCR reaction (Supplementary fig. S1b). PCR program consisted of 3 min initial denaturation at 95 °C followed by 40 cycles of 95 °C for 30 sec, 55 °C for 30 sec and 72 °C for 20 sec. Melting curve was analysed at 95 °C for 30 sec followed by 60 °C for 30 sec and 95 °C for 30 sec and used for ascertaining the variations in gene expression among the transgenic events vis-à-vis non-transgenic.
Analysis of Cry2Aa expression in the transgenic events
Dot blot analysis
A high throughput protein dot blot analysis was performed to analyze the expression of Cry2Aa protein in the transgenic vis-à-vis non transgenic plants. About 100 mg of fresh leaf tissue from the third fully expanded leaf was used for extraction in 50 mM carbonate buffer (pH 9.0). Following quantification using Bradford assay41, 10–15 µg total protein (transgenic as well as non transgenic plants) was mixed with 25 µl of 0.4% SDS, volume made up to 200 µl with carbonate buffer and loaded onto an activated nitrocellulose membrane (activated by dipping in carbinol for 30 sec). Dot blot set up was prepared by first placing 4 wet Whatmann paper no. 3 followed by the charged nitrocellulose membrane. Samples were loaded in wells and 20 Kpa suction pressure was applied until protein solution drained out of the wells onto the membrane. The blot was later dried and stored at 4 °C until further development.
Western blot analysis
Total protein was isolated from 100 mg of leaf tissue from both transgenic and non transgenic plants using 50 mM phosphate buffer (pH 7.0). Protein was quantified by Bradford’s assay and 20–25 µg was subjected to PAGE and blotted onto nitrocellulose membrane by using transfer buffer (1.5 g Tris, 7.2 g glycine and 100 ml methanol made up to 500 ml with distilled water).
Development of both western and dot blots was initiated by blocking non-specific binding using NAP blocker (Non-animal protein blocker, G Biosciences) followed by initial hybridization with 1:3000 dilution of primary antibody (specific to Cry2Aa; procured from Amar immunodiagnostics, Hyderabad, India) and subsequently with 1:6000 dilution of HRP conjugated secondary antibody. The blots were washed thrice with 1 × PBST after each hybridization followed by addition of TMB (3,3′,5,5′-Tetramethylbenzidine, Promega) substrate for colour development. Dot blots were analyzed and quantified via ImageJ (http://imagej.nih.gov/ij) software following online instructions.
Bioefficacy of the cry2Aa gene in pigeon pea pods and leaves
Efficacy of the transgenic vis-à-vis non transgenic plants against H. armigera was assessed in both leaves and pods. In vitro bioassays with the leaves were conducted in all the generations (T1 to T3) whereas pod bioassay was conducted in T3 generation. Fully expanded trifoliate leaves from 45–60 days old plants and 2 week old tender pods were excised and the leaf petioles/the pod stalks were covered with cotton to maintain moisture in the bioassay plates. Over each trifoliate leaf, 10 neonate larvae were released whereas three 2nd instar larvae were released on the pods. Two replicates from each plant were maintained for each treatment. Larval mortality, growth and extent of damage on the plant tissue were recorded at 24, 48, 72, and 96 h after release of larvae.
The experiments were conducted in a completely randomized design (CRD). For statistical analyses, the resultant bioefficacy data was subjected to analysis of variance (ANOVA) followed by mean separation by the Student–Newman–Keuls’ test (P = 0.05). Graphs and regression models were built using R language in R studio version 1.0.136. For regression model, the observations were split into train and test sets with 2/3 ratio. Chi-square (χ2) test was performed in the T3 generation to assess the observed segregation ratio (3:1) for the transgene42 based on the performance in the pod bioassay.
Electronic supplementary material
Acknowledgements
The authors acknowledge financial support from ICAR-National Agricultural Science Fund (NFBSFARA/PB-2010) and ICAR-Network Project on Transgenic Crops (NPTC). Thanks are due to Mr. P. T. Raghunandan for assisting us with the statistical analyses.
Author Contributions
P.A.K. and R.S. conceived and designed the experiments. S.S., N.R.K. and M.R. conducted the experiments. L.K.R., N.B.S.B. and K.Y.S.R. developed the transformants. N.M. and T.A. helped in bioassays. K.K. performed genomic Southern analysis. V.T. helped in qPCR. P.K.D. helped in data analysis and critically revised the manuscript. The manuscript was drafted by N.R.K., S.S., M.R. and R.S. and all authors contributed in writing the draft. P.A.K., P.K.D. and D.P. critically edited the manuscript and overall data presentation. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Shweta Singh, Nikhil Ram Kumar and R. Maniraj contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26358-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nene, Y. L. & Sheila, V. K. Pigeon pea: Geography and Importance, In: Nene, Y. L., Hall, S. D. & Sheila, V. K. The Pigeon pea, CAB International, Wallingford, pp. 1–14 (1990).
- 2.FAO. FAOSTAT data in 2014. Available at: http://faostaT3.fao.org (2014).
- 3.FAOSTAT Food and Agricultural organization (http://faostaT3.fao.org/) (2013).
- 4.Srivastava SK, Sivaramane N, Mathur VC. Diagnosis of Pulses Performance of India. Agri. Econ. Res. Rev. 2010;23:137–148. [Google Scholar]
- 5.Shanower TG, Romeis JMEM, Minja EM. Insect pests of pigeon pea and their management. Ann. Rev. Entomol. 1999;44:77–96. doi: 10.1146/annurev.ento.44.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Choudhary AK, Raje RS, Datta S, Sultana R, Ontagodi T. Conventional and molecular approaches towards genetic improvement in pigeonpea for insects resistance. Amer. J. Plant Sci. 2013;4:372–385. doi: 10.4236/ajps.2013.42A049. [DOI] [Google Scholar]
- 7.Tripathi AK, Prajapati V, Aggarwal KK, Kumar S. Toxicity, feeding deterrence, and effect of activity of 1, 8-cineole from Artemisia annua on progeny production of Tribolium castanaeum (Coleoptera: Tenebrionidae) J. Eco. Entomol. 2001;94:979–983. doi: 10.1603/0022-0493-94.4.979. [DOI] [PubMed] [Google Scholar]
- 8.Vishwadhar SK, et al. Forecasting of Helicoverpa armigera infestation on long duration pigeon pea in central Uttar Pradesh. J. Food Leg. 2008;21:189–192. [Google Scholar]
- 9.Reed, W. & Lateef, S. S. Pest management. pp. 349-374. In The Pigeon pea (Edited by Nene, Y. L., Hall, S. D. & Sheila, V. K.). CAB International. Wallingford, UK (1990).
- 10.Rana N, Ganguli J, Agale SV. Screening of Pigeon pea genotypes under field conditions against pod borer complex. J. Entonol. Zool. Studies. 2017;5:1914–1920. [Google Scholar]
- 11.Kumar PA, Sharma RP, Malik VS. The insecticidal proteins of Bacillus thuringiensis. Adv. Appl. Microbiol. 1996;42:1–43. doi: 10.1016/S0065-2164(08)70371-X. [DOI] [PubMed] [Google Scholar]
- 12.Sanahuja G, Banakar R, Twyman RM, Capell T, Christou P. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol J. 2011;9:283–300. doi: 10.1111/j.1467-7652.2011.00595.x. [DOI] [PubMed] [Google Scholar]
- 13.Bravo A., et al. Bacillus thuringiensis and Lysinibacillus sphaericus. Insecticidal Proteins from Bacillus thuringiensis and Their Mechanism of Action (eds Fiuza, L., Polanczyk, R. & Crickmore, N.) (Springer, 2017).
- 14.James, C. Executive Summary: Global Status of Commercialized Biotech/GM Crops: 2014, Brief 52. International Service for the Acquisition of Agri-Biotech Applications. (2016).
- 15.Ramu SV, et al. Expression of a synthetic cry1AcF gene in transgenic Pigeon pea confers resistance to Helicoverpa armigera. J. App.Entomol. 2012;136:675–687. doi: 10.1111/j.1439-0418.2011.01703.x. [DOI] [Google Scholar]
- 16.Kaur A, et al. Pod borer resistant transgenic pigeon pea (Cajanus cajan L.) expressing cry1Ac transgene generated through simplified Agrobacterium transformation of pricked embryo axes. Plant Cell Tiss. Organ Cult. 2016;127:717–727. doi: 10.1007/s11240-016-1055-9. [DOI] [Google Scholar]
- 17.Ghosh G, et al. Transgenic pigeon pea events expressing Cry1Ac and Cry2Aa exhibit resistance to Helicoverpa armigera. Plant Cell Rep. 2017;36:1037–1051. doi: 10.1007/s00299-017-2133-0. [DOI] [PubMed] [Google Scholar]
- 18.Das A, et al. Expression of chimeric Bt gene, Cry1Aabc in transgenic pigeon pea (cv. Asha) confers resistance to gram pod borer (Helicoverpa armigera Hubner.) . Plant Cell Tiss. Org. Cult. 2016;127:705–715. doi: 10.1007/s11240-016-1131-1. [DOI] [Google Scholar]
- 19.Rao KS, Sreevathsa R, Sharma PD, Keshamma E, Kumar MU. In planta transformation of pigeon pea: a method to overcome recalcitrancy of the crop to regeneration in vitro. Physiol. Mol. Biol. Plants. 2008;14:321–328. doi: 10.1007/s12298-008-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bashir K, et al. Field evaluation and risk assessment of transgenic indica basmati rice. Mol Breeding. 2004;13:301–312. doi: 10.1023/B:MOLB.0000034078.54872.25. [DOI] [Google Scholar]
- 21.Chen H, et al. Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theo. App. Genet. 2005;111:1330. doi: 10.1007/s00122-005-0062-8. [DOI] [PubMed] [Google Scholar]
- 22.Acharjee S, et al. Transgenic chickpeas (Cicer arietinum L.) expressing a sequence-modified cry2Aa gene. Plant Sci. 2010;178:333–339. doi: 10.1016/j.plantsci.2010.02.001. [DOI] [Google Scholar]
- 23.Morse RJ, Yamamoto T, Stroud RM. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure. 2001;9:409–417. doi: 10.1016/S0969-2126(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 24.Tabashnik BE, et al. Control of resistant pink bollworm (Pectinophora gossypiella) by transgenic cotton that produces Bacillus thuringiensis toxin Cry2Ab. Appl. Environ. Micro. 2002;68:3790–3794. doi: 10.1128/AEM.68.8.3790-3794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Rodriguez CS, Vliet AV, Bautsoens N, Rie JV, Ferre J. Specific Binding of Bacillus thuringiensis Cry2A Insecticidal proteins to a common site in the midgut of Helicoverpa species. App. Env. Microbiol. 2008;74:7654–7659. doi: 10.1128/AEM.01373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra P, et al. Comparative Proteomic and Nutritional Composition Analysis of Independent Transgenic Pigeon Pea Seeds Harboring cry1AcF and cry2Aa Genes and Their Nontransgenic Counterparts. J.Agri. Food Chem. 2017;65:395–1400. doi: 10.1021/acs.jafc.6b05301. [DOI] [PubMed] [Google Scholar]
- 27.Shivakumara, T. N., et al Overexpression of Pea DNA Helicase 45 (PDH45) imparts tolerance to multiple abiotic stresses in chili (Capsicum annuum L.) Sci. Rep.7 (2017). [DOI] [PMC free article] [PubMed]
- 28.Kumar, P. A. & Bambawale, O. M. Advances in Microbial toxin Research: Insecticidal proteins of Bacillus thuringiensis and their applications in Agriculture. Plenum Publishers, New York (2002).
- 29.Tabashnik BE, Brévault T, Carriere Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotech. 2013;31:510–521. doi: 10.1038/nbt.2597. [DOI] [PubMed] [Google Scholar]
- 30.Sharma, H. C. Heliothis/Helicoverpa Management: Emerging Trends and Strategies for Future Research. (Oxford & IBH, and Science Publishers, 2005).
- 31.Sison MLJ, Shanower TG. Development and survival of Helicoverpa armigera (Lepidoptera: Noctuidae) on short-duration pigeon pea. J. Econ. Entomol. 1994;87:1749–1753. doi: 10.1093/jee/87.6.1749. [DOI] [Google Scholar]
- 32.Sharma KK, Lavanya M, Anjaiah V. Agrobacterium mediated production of transgenic pigeon pea (Cajanus cajan L. Millsp.) expressing the synthetic Bt cry1AB gene. In vitro Cell Dev. Biol. Plant. 2006;42:165–173. doi: 10.1079/IVP2005730. [DOI] [Google Scholar]
- 33.Chakrabarti SK, Mandaokar A, Kumar PA, Sharma RP. Toxicity of lepidopteran specific delta endotoxins of Bacillus thuringiensis towards neonate larvae of Helicoverpa armigera. J. Invert. Pathol. 1998;72:336–337. doi: 10.1006/jipa.1998.4786. [DOI] [PubMed] [Google Scholar]
- 34.Surekha C, et al. Agrobacterium-mediated genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) using embryonal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci. 2005;169:1074–1080. doi: 10.1016/j.plantsci.2005.07.011. [DOI] [Google Scholar]
- 35.Krishna G, et al. Agrobacterium-mediated genetic transformation of pigeon pea [Cajanus cajan (L.) Millsp.] for resistance to legume pod borer Helicoverpa armigera. J. Crop Sci. Biotech. 2011;14:197–204. doi: 10.1007/s12892-010-0063-2. [DOI] [Google Scholar]
- 36.Hoagland, D. R. Mineral nutrition. In Laboratory Experiments in Plant Physiology. (eds Kaufman, P. B., Labavitch, L., Anderson-Prouty, A. & Ghosheh, N. S.) 129–134. (Macmillan, 1975).
- 37.Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol. Biol. Rep. 1983;1:19–21. doi: 10.1007/BF02712670. [DOI] [Google Scholar]
- 38.Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular cloning: a laboratory manual (No. Ed. 2), (Cold spring harbor laboratory press, 1989).
- 39.Datta K, Schmidt A, Marcus A. Characterization of two soybean repetitive proline-rich proteins and a cognate cDNA from germinated axes. Plant Cell. 1989;1:945–952. doi: 10.1105/tpc.1.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha P, et al. Evaluation and validation of housekeeping genes as reference for gene expression studies in pigeonpea (Cajanus cajan) under drought stress conditions. PloS one. 2015;10:e0122847. doi: 10.1371/journal.pone.0122847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Greenwood, P.E. & Nikulin, M. S. A Guide to Chi-Squared Testing. pp. 280. Wiley, New York, USA (1996).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.