Abstract
The objective of this study is to synthesize and evaluate the current body of sleep research among long-term care (LTC) residents in China and provide insights for future research. Systematic searches identified 15 studies that examined sleep in LTC residents in China. Sleep disturbances and poor sleep quality were prevalent in Chinese LTC residents. Eight cross-sectional studies reported that demographics, comorbidities, lifestyle, and environment were associated with sleep quality in Chinese LTC residents. Seven intervention studies, including exercise, traditional Chinese medicine, light therapy, and behavioral interventions resulted in improved sleep quality. Only subjective sleep measures were used in all 15 studies. Some methodological issues were identified in studies, especially those conducted in Mainland China. Sleep research in LTC residents in China is still at the beginning stages. Future studies should consider more rigorous designs and objective sleep measures, and develop target interventions based on factors associated with sleep disturbances.
Keywords: sleep, long-term care, China
Background
The aging population in China has been growing rapidly over the past two decades. At the end of 2012, the population aged 65 years and older reached 127 million, accounting for 9.4% of the total Chinese population (National Health and Family Planning Commission, 2013). The increasing aging statistics and the Chinese nuclear family structure call for a shift in elder care from traditional family care at home to institutions (Chen, 2015; Chu & Chi, 2008). The Chinese government has increased financial and policy support to promote the construction of senior housing, residential institutions for the aged, and nursing homes (Feng, Liu, Guan, & Mor, 2012). The use of long-term care (LTC) service has boomed in China in recent years, yet the health issues of older adults living in LTC settings have not been well studied (Wang, Hou, Xu, & Zhang, 2011).
Sleep is an essential aspect of health promotion in older adults (Townsend-Roccichelli, Sanford, & VandeWaa, 2010). Sleep architecture changes with advanced age, resulting in a decrease in deep, restorative sleep and sleep duration, and an increase in light sleep (Rodriguez, Dzierzewski, & Alessi, 2015). Sleep disturbances, such as difficulty initiating sleep, sleep fragmentation, and early morning awakenings, are more commonly reported in older adults than their younger counterparts and may result in consequences such as poor perceived sleep quality and excessive daytime sleepiness (Gleason & McCall, 2015; Rodriguez et al., 2015). Sleep quality reflects a subjective perception and experience of sleep and excessive daytime sleepiness refers to difficulty maintaining wakefulness (Harvey, Stinson, Whitaker, Moskovitz, & Virk, 2008; Young, 2004). Although sleep architecture changes with age, one study reported that sleep disturbances in older adults were more dependent on physical, environmental, and health factors than on age-dependent sleep changes (Martin, Sforza, Barthelemy, Thomas-Anterion, & Roche, 2014). Therefore, it is imperative to address sleep disturbances in older adults and not simply view them as a normal consequence of aging.
Multiple factors can contribute to sleep disturbances, and sleep disturbances often co-occur with physical and psychological disorders in older adults (Rodriguez et al., 2015). Physical and cognitive decline, multiple comorbidities, psychosocial status, and sedentary lifestyle are a few factors that may negatively affect sleep (Gong, Chen, Yao, Yin, & Han, 2012; Li, Chang, & Porock, 2015; Valenza et al., 2013). Furthermore, sleep disturbances and poor sleep quality are associated with multiple negative health consequences, including increased risk of falls, neurodegenerative disease, and decreased functional status (Helbig et al., 2013; Isaia et al., 2011). Sleep disturbances are even more common in LTC residents and are often attributed to a high burden of comorbidities, lifestyle, and environmental factors (Lorenz, Harris, & Richards, 2011). For example, older adults living in LTC settings often have decreased physical activity, limited social interaction, minimal daylight exposure, and poor sleep hygiene (Li, Chang, & Porock, 2015).
Despite the high prevalence and negative effects of sleep disturbances on health, only a few studies examined sleep in LTC residents in China. The purpose of this review is to synthesize and evaluate the current body of sleep research in older adults living in LTC settings in China. Specifically, this review will (a) provide an understanding of sleep in LTC residents in China, including the prevalence of sleep disturbances and poor sleep quality and factors associated with poor sleep quality, and (b) examine effects of existing interventions on sleep disturbances and sleep quality in LTC residents in China.
Method
Search strategies were used to examine all sleep-related research on LTC residents in China, including Mainland China, Taiwan, and Hong Kong. MEDLINE, EMBASE, Wan Fang, and China National Knowledge Infrastructure (CNKI) databases were searched using variations of the following keywords (in English and Chinese): sleep, insomnia, sleepiness, nursing home, LTC, assisted living, institutionalized, elders, older adults, residents, elderly, China, and Chinese. Research articles that met the following inclusion criteria were included: (a) reported sleep disturbances or sleep quality, factors associated with sleep, or effects of sleep-promoting interventions in LTC residents in China; (b) written in Chinese or English; (c) published from 2000 to January 2016 (LTC institutions in China started to emerge in early 2000); and (d) conducted in Mainland China, Taiwan, and Hong Kong. Exclusion criteria were (a) case reports, (b) letters, and (c) articles without full text.
All potential articles were retrieved using database searches and hand searching (see Figure 1). The authors assessed the titles and abstracts of all articles for relevance based on the inclusion and exclusion criteria. The full texts of relevant studies were obtained and evaluated independently by two authors, and discrepancies were discussed.
Figure 1.
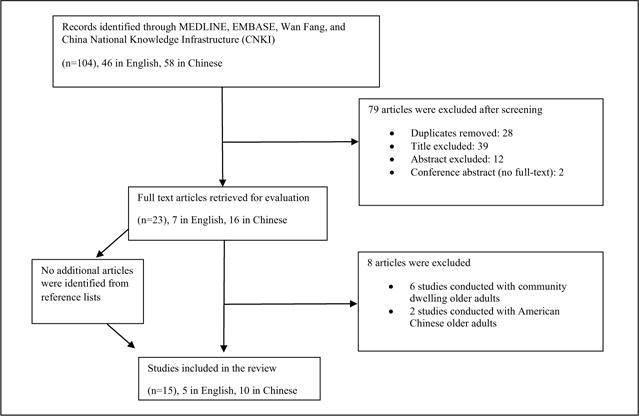
Process of article identification and selection.
Basic information including author, publication year, location, sample demographics, and sleep measures were extracted from all reviewed articles. In addition, we extracted the results on prevalence of sleep disturbances and sleep quality and all possible determinants of sleep quality from each cross-sectional study. The prevalence of overall poor sleep quality and each type of sleep disturbance (short or long nocturnal sleep duration, difficulty initiating and maintaining sleep, early morning awakening, long sleep latency, low sleep efficiency, sleep medication use, and daytime dysfunction) were synthesized and compared across cross-sectional studies. Possible factors associated with sleep quality were categorized into nonsignificant or significant factors based on the type of statistical analysis (bivariate/multiple regression) and synthesized across the studies. For intervention studies, we extracted information on study design, details of intervention, and study results on sleep outcomes. The effects of the interventions were synthesized based on the study design and type of intervention provided. We summarized the strengths and limitations of each study according to all data extracted (see Tables 1 and 2).
Table 1.
Characteristics of Cross-Sectional Studies.
| Outcome: Sleep disturbances and sleep quality
|
||||||
|---|---|---|---|---|---|---|
| Authors* year Location |
Factors associated with sleep quality
|
|||||
| Sample characteristics | Sleep measure | Type and prevalence | Significant | Nonsignificant | Strengths and limitations | |
| Chen, Zhang, Zhao, & Dong, 2014 Mainland China | 151 residents from 4 nursing homes Age (M ± SD): 79.4 ±7.4 62.9% female 51% low level of education 75.5% widower |
PSQI | Overall poor sleep quality (Global PSQI Score > 7): 64.9% Long sleep latency: 58.9% Short sleep duration (≤6 hr): 58.9% Low sleep efficiency (<75%): 61.6% Sleep disturbances: 42.4% Sleep medication use: 18.5% Daytime dysfunction: 42.4% M PSQI (SE) = 9.70 (0.376) |
Bivariate analysis (t tests) Married + Healthy spouse + Chronic diseases − Anxiety and loneliness + Worrying about future − Life satisfaction + Hypnotic use − Environmental factors + Multiple regression Life satisfaction + (β = −1.0, p = .01) Hypnotic use − (β = 1.01 ; p = .006) |
Gender Age BMI Occupation Income Length of stay Facility type |
Limitations:
|
| Yao et al., 2012 Mainland China | 409 residents from 8 nursing homes M age: 79.29 52.6% aged 80 and above 70.7% low level of education |
PSQI | Overall poor sleep quality (Global PSQI score > 7): 51.6% M PSQI (SD) 6= 8.24 (4.35) |
Bivariate analysis (chi-square) Female − (χ2 = 13.35, p < .001) Depression − (χ2 = 19.99, p < .001) Social relationship + (χ2 = 7.68, p = .006) Foot bath before bed + (χ2 = 7.53, p = .008) Excitement before bed − (χ2 = 24.54, p < .001) |
N/A | Strength:
Limitations:
|
|
Lin, Lu, & Ma, 2012 Mainland China |
97 residents from 4 nursing homes M age: 74.5, range (60-88) |
PSQI Sleep status questionnaires |
Difficulty fall asleep after awakening: 50% Frequent awakening and early morning awakening: 32% M PSQI: not reported |
Bivariate analysis (ANOVA) Female − (F = 6.15; p < .01) Age − (F = 5.18; p < .01) Environment + (living environment): (F = 4.53; p < .01) |
N/A | Limitations:
|
|
Li, Wang, & Yin, 2012 Mainland China |
107 residents from one nursing home Age (M ± SD): 82.5 ± 6.9; range (68-98) 60.7% female 64.4% widower |
PSQI | Overall poor sleep quality (Global PSQI score > 7): 32.7% Long sleep latency: 33.64% Short sleep duration (≤6 hr): 30.0% Low sleep efficiency (<75%): 20.6% Sleep disturbances: 13.1% Sleep medication use: 34.7% Daytime dysfunction: 18.6% M PSQI : not reported |
Bivariate analysis: (Spearman correlation) Depression − (r = .405; p < .01) |
N/A | Limitations:
|
|
Fang, & Lin, 2010 Mainland China |
97 nursing home residents in three communities; Age > 60 57.7% aged 80 and above |
Self-designed sleep evaluation questionnaire | Difficulty initiating and maintaining sleep: 59.8% Long nocturnal sleep duration (>9 hr): 69.1% M PSQI: not reported |
Not reported | N/A | Limitations:
|
|
Shi, 2009 Mainland China |
179 nursing home residents Age (M ± SD): 79.8 ± 0.5 45.3% female 70.9% low level of education 62.5% widower |
PSQI | Overall poor sleep quality (Global PSQI score > 7): 41.8% Long sleep latency: 45.8% Short sleep duration (≤6 hr): 23.5% Low sleep efficiency (<75%): 21.2% Sleep disturbances: 16.2% Sleep medication use: 4.4% Daytime dysfunction: 27.3% M PSQI (SD) = 6.99 (0.26) |
Bivariate analysis (ANOVA) Female − Income (more than 800 RMB) + Chronic diseases − Hypnotic use − Exercise frequency + Anxiety/Worrying − Social involvement + Loneliness − Drinking water before bedtime − Chatting before bedtime − Foot bath before bedtime + Comfortable Environment + Multiple regression analysis Income (more than 800 RMB) +(β = −0.823, p < .001) Chronic diseases − (β = 0.705, p = .002) Hypnotic use − (β = 1.771, p = .031) Worrying − (β = 0.752, p = .044) Exercise frequency + (β = −0.640, p = .004) Drinking water before bedtime − (β = 1.169, p = .011) Comfortable Environment + (β = 1.020, p = .020) |
Age BMI Facility type Drinking Smoking Life satisfaction |
Strength:
Limitation:
|
| Zhang, Yang, & Zeng, 2008 Mainland China |
80 nursing home residents and 80 community-dwelling elders Age > 65 42.5% aged 80 and above |
PSQI | Overall poor sleep quality (global PSQI score > 7): In nursing home residents: 73%; In community-dwelling elders: 40% Nursing home residents had significant higher global score and subscale scores of PSQI than elders living in community M PSQI (SD) = 11.47 (2.33) in nursing home residents versus 7.35 (3.06) community-dwelling older adults. |
Adjusted logistic regression Age − (OR = 1.45, p = .02) Education + (OR = 0.78, p < .01) Widower − (OR = 1.54, p = .04) Chronic disease − (OR = 1.75, p < .01) Inverted personality − (OR = 3.03, p < .01) |
Gender Occupation |
Limitation:
|
| Tsai, Wong, & Ku, 2008 Taiwan |
196 residents without severe cognitive impairment from 9 nursing homes in Taiwan M age: 79 65.3% low level of education 53.1 % widower |
PSQI | Overall poor sleep quality (global PSQI score > 5): 46.4% Only 48.5% of the participants used self-care strategies to manage sleep disturbances M PSQI : not reported |
Bivariate analysis (T test/chi-square test) Education + Widower − Depression − Life satisfaction + Perceived health status + Perceived income adequacy + Multiple regression Adjusted logistic regression Widower − (OR = 3.18, p < .01, 95% Cl = [1.63, 6.20]) Education level + (OR = 0.50, p < .05, 95% Cl = [0.27, 0.99]) |
Age Gender Number of diseases Length of stay Cognitive/functional status |
Strengths:
Limitation:
|
Note. PSQI = Pittsburgh Sleep Quality Index; BMI = body mass index; OR = odds ratio; Cl = confidence interval.
The first three authors of articles are listed.
Table 2.
Characteristics of Intervention Studies.
| Authors* year Location |
Study design | Sample characteristics | Intervention | Sleep measures | Outcomes | Strengths and limitations |
|---|---|---|---|---|---|---|
| Chen, Huang, Cheng, Li, & Chang, 2015 Taiwan | A cluster RCT with 114 elders from 10 nursing homes. Intervention group (5 nursing homes, n = 59) and control group (5 nursing homes, n = 55) Data were collected at baseline (t0), 3-month (t1), and the end of 6-month (t2) intervention |
Age (M ± SD): 79.15 ±7.03 57.9% widower 74.6% low level of education 100% using wheelchairs for mobility, cognitively intact, heavily/moderately dependent on others to perform activities of daily living |
Intervention group (six months): Group exercise using wheelchair-bound senior elastic band exercise Program (warm-up, aerobic motion, harmonic stretching) Three 40-min sessions weekly for 6 months (Months 1-3 basic level; Months 4-6 advanced level) Control group: routine daily activity |
Overall sleep quality: PSQI global score Sleep efficiency, sleep latency, and sleep duration were measured by PSQI subscales |
1. Within group (compare with t0) Intervention Longer sleep duration at t1 & t2 (F = 7.12; p = .002); Larger sleep efficiency at t1 (F = 4.27; p = .025) Control Worse overall sleep quality at t2 (F = 5.02; p = .015).2. Between group (adjusted for t0): compare with control group, intervention group had longer sleep durations at t1 (F = 11.09; p = .001) & t2 (F = 10.07; p = .002); Larger sleep efficiency at t1 (F = 7.46; p = .007); and better overall sleep quality at t2 (F = 4.83; p = .03) |
Strengths:
Limitation:
|
| Wu, M.Sung, Lee, & Smith, 2015 Taiwan | A quasi-experimental pretest–posttest study with control group (cluster randomization of 4 units within on facility) Intervention group (2 units, n = 34) and control group (2 units, n = 31) Data were collected at baseline (t0) and post-intervention (t1) |
Age (M ± SD): intervention: 81.0 ±9.0 vs. control:79.0 ± 7.0 >76.5% low level of education > 74% using wheelchair for mobility |
Intervention group (4 weeks): Group bright light therapy— sitting as a group in front of a 10,000-lux light box 30 min in the morning 3 times a week for 4 weeks Control group: usual standard care |
Sleep disruption at night: self-designed daily sleep recording sheet |
|
Limitations:
|
| Chen et al., 2013 Mainland China | Single-group pretest– posttest design 21 residents from one nursing home Data were collected at baseline and after 6-week intervention |
Age (M ± SD): 81.95 ±5.18 57.1% female 57.1% low level of education Poor sleep quality (PSQI global score > 7) No sleep medication use |
6-week multicomponent intervention
|
Overall sleep quality: PSQI global score Subjective sleep quality, sleep efficiency, sleep latency, sleep duration, and daytime function were measured by PSQI subscales |
Overall sleep quality (F = 29.48; p < .001) and most of the sleep parameters measured by PSQI subscales were significantly improved (FSubjective sleep quality = 18.06, Fsleep duration = 8.70, FSleep efficiency 4.95, Fsleep disturbances = 27.98; FDaytime functioning = 24.90, All ps < .04) | Limitations:
|
|
Yi & Zhang, 2012 Mainland China |
A quasi-experimental pretest–posttest study with control group Data were collected at baseline and post-intervention Intervention group (n = 38) and control group (n = 38) from one nursing home |
Have insomnia | Intervention group
Control group: usual care |
Overall sleep quality: PSQI global score Subjective sleep quality, sleep efficiency, sleep latency, sleep duration, sleep disturbances, use of sleep medications, and daytime function were measured by PSQI subscales |
Compared with control group, the overall sleep quality (F = 24.47; p < .001) and all sleep parameters measured by PSQI subscales (FSubjective sleep quality = 15.83; Fsleep latency = 11.86; FSleep duration = 7.13, Fsleep efficiency = 8.90, FSleep disturbances = 6.64, FSleep medication = 11.67; FDaytime functioning = 36.20, All ps ≤ .01) in the intervention group were significantly improved. | Limitations:
|
| Sun, Sung, Huang, Cheng, & Lin, 2010 Taiwan | A RCT 50 elders from two nursing homes were randomly assigned to intervention (n = 25) and control (n = 25) groups Data were collected at baseline and every week for 7 weeks, starting from the first week of intervention until 2 weeks after intervention |
Insomnia: Athens Insomnia Scale–Taiwan form (AIS) > 6 and PSQI > 5. 84% with low level of education 12% with dementia diagnosis |
Intervention group (4 weeks): HT7 (Shenmen point) acupressure on both wrists with an interval of 5-s pressure followed by l-s rest for 5 min at bedtime every night Control group: slightly touch on the same position with on pressure |
Sleep quality was measured by PSQI Insomnia severity was measured by AIS |
During the course of intervention and 2-week after intervention, the AIS score decreased significantly (less severity of insomnia) in intervention group, compared with control group (adjusted for time and group effects; Week 2-5: β ranged from −18.28 to 16.38, all ps < .001 ; 2-week post-intervention; Week 6: β = −11.40, p < .001 ; Week 7: β = −4.84, p < .001) During the 2 weeks after intervention, AIS returned to its original status in intervention group (AIS [M ± SD] Week 1 vs. Week 7: 16.60 ± 6.04 vs. 15.72 ±4.01) |
Strengths:
Limitations:
|
| Chen, et al., 2010 Taiwan | A quasi-experimental pretest–posttest design with control group. Data were collected at baseline t0 3-month t1 and 6-month t2 of the study 69 residents from 2 assisted living facilities were divided randomly into a yoga exercise (n = 38) and control group (n = 31), and 55 participants completed the study |
Aged (M ± SD): 75.4 ± 6.7 52.7% widower; 81.8% lower level of education Cognitively intact residents who were mild functionally dependent and able to walk without assistance |
Intervention (6 months) 70-min group silver yoga exercise practice session, 3 times a week for 6 months (12-13 participants/group) Control group: usual daily activities |
Overall sleep quality: PSQI global score Subjective sleep quality, sleep efficiency, sleep latency, sleep duration, sleep disturbances, use of sleep medications, and daytime function were measured by PSQI subscales |
Intervention Overall sleep quality was significantly improved (F = 4.13; p = .03) and sleep disturbances (F = 3.64; p = .04), daytime Dysfunction (F = 6.68, p = .007) were significantly decreased Control Overall sleep quality (F = 5.93; p = .005) and sleep efficiency decreased (F = 3.82; p = .037) and sleep disturbances (F = 7.00; p = .002) increased significantly.
|
Strengths:
Limitations:
|
| Song, 2009 Mainland China | Single-group pretest–posttest design with 50 residents from one nursing home Data were collected at baseline and after 1-year intervention |
Aged 70-90 | 1-year intervention
|
Overall sleep quality was measured by questions asking sleep latency, sleep duration, awakening, nocturia, and daytime functions | The percentage of elders with good sleep quality increased significantly after the 1-year intervention (F = 12.98, p = .001) The percentages of elders with not good or very poor sleep quality decreased significantly after the 1-year intervention (F = 9.25, p < .01) |
Strength:
Limitations:
|
Note. PSQI = Pittsburgh Sleep Quality Index; RCT = randomized controlled trial; AIS = Athens Insomnia Scale.
The first three authors of articles are listed.
Results
Overview of the Studies
Fifteen articles met the inclusion criteria for this review. Ten studies were conducted in Mainland China (written in Chinese) and five in Taiwan (written in English). Eight studies used a cross-sectional design (Table 1) and reported subjective sleep quality of LTC residents in China as well as factors associated with sleep (Table 1; Chen, Zhang, Zhao, & Dong, 2014; Fang & Lin, 2010; Li, Wang, & Yin, 2012; Lin, Lu, & Ma, 2012; Shi, 2009; Tsai, Wong, & Ku, 2008; Yao et al., 2012; Zhang, Yang, & Zeng, 2008), and seven studies evaluated the effects of interventions on sleep outcomes in LTC residents (Table 2).
Across the 15 studies, participant’s mean age ranged from 74 to 80 years. The majority of the participants were widows (52.7%-75.5%) with low levels of education (51.0%-84.0%). Participants’ cognitive status was mentioned in four studies, but not described in detail. For example, one cross-sectional study excluded residents with severe cognitive impairment; however, the number of participants with mild or moderate cognitive impairment was not reported (Tsai et al., 2008). One intervention study specified that 12% of the participants had dementia (Sun, Sung, Huang, Cheng, & Lin, 2010), and two intervention studies described that all participants were cognitively intact (Chen et al., 2010; Chen, Huang, Cheng, Li, & Chang, 2015).
Measurement
All 15 studies measured sleep disturbances and sleep quality subjectively, using questionnaires such as the Chinese/Taiwanese version of Pittsburgh Sleep Quality Index (PSQI), Athens Insomnia Scale (AIS), and researcher-designed sleep questionnaires. Eleven studies used PSQI, one study applied both PSQI and AIS (Sun et al., 2010), and the other three studies used researcher-designed sleep questionnaires (Fang & Lin, 2010; Song, 2009; Wu, Sung, Lee, & Smith, 2015). No detailed information was presented on the researcher-designed sleep questionnaires, and therefore, the psychometric properties of these tools are unknown (Fang & Lin, 2010; Song, 2009; Wu et al., 2015).
The PSQI is a 19-item self-report measure of sleep quality and sleep disturbances over the past month. The PSQI captures seven domains including subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, the use of sleep medications, and daytime dysfunction. Each domain is rated from 0 to 3, and the total score ranges from 0 to 21, with higher scores representing worse sleep quality (Chen et al., 2010). The PSQI has been extensively used in populations across the world. It is a valid and reliable tool with reported Cronbach’s α of .84, sensitivity of 98.3%, and specificity of 90.2% in the Chinese population (Liu et al., 1996). A global score greater than 7 has been found to be associated with clinically significant sleep disorders in Mainland China. The Chinese Version PSQI was tested and a cut point of greater than 5 was determined to discriminate poor from good sleepers in Taiwanese (Tsai et al., 2005). The Chinese Version PSQI has been widely used in Taiwan (Chen et al., 2015; Lu, Lin, Chen, Tsang, & Su, 2013). To be consistent with the cut point greater than 5, some recent studies in Mainland China also adopted global score greater than 5 as the cutoff to define good and poor sleepers (Li et al., 2012; Luo et al., 2013). In this review, greater than 7 was used as the cutoff value for all studies in Mainland China and a cutoff of greater than 5 was adopted by studies in Taiwan.
The AIS is an eight-item self-report measure of insomnia, which assesses people’s nocturnal sleep (sleep latency, early morning awakening, nighttime awakenings, sleep duration, overall sleep quality) and daytime dysfunction (Sun, Chiou, & Lin, 2011). Conceptually, AIS overlaps with PSQI in certain domains such as sleep duration, overall sleep quality, and daytime function. The AIS, however, focuses more on insomnia symptoms, such as nocturnal awakening and early morning awakenings, and fails to assess for sleep medication use (Okajima, Nakajima, Kobayashi, & Inoue, 2013). Each item can be scored 0 to 3, and the total score ranges between 0 and 24. Higher scores represent worse insomnia severity, and a score of greater than or equal to 6 indicates insomnia (Soldatos, Dikeos, & Paparrigopoulos, 2000). One interventional study in this review used AIS in conjunction with PSQI to screen for insomnia and measure insomnia severity. This study reported that the AIS score was strongly correlated with the PSQI (r = .75, p < 0.001; Sun et al., 2010).
Cross-Sectional Studies (Table 1)
Prevalence of sleep disturbances and poor sleep quality
Sleep disturbances were reported in all eight cross-sectional studies. Six studies assessed residents’ sleep disturbances and sleep quality using PSQI and its seven subscales. The prevalence of poor sleep quality ranged from 33% to 73% in six studies. Three studies (Chen et al., 2014; Li et al., 2012; Shi, 2009) reported the prevalence of each PSQI subscale with combined ranges of the following: prolonged sleep latency 33.6% to 48.9%, poor sleep efficiency 20.6% to 61.6%, sleep disturbances 13.1% to 42.4%, sleep medication use 4.4% to 34.7%, and daytime dysfunction 18.6% to 42.4%. The means of PSQI were reported in four studies and ranged between 6.99 and 11.47 (Chen et al., 2014; Yao et al., 2012; Shi, 2009; Zhang et al., 2008). Other sleep disturbances reported in studies include long nocturnal sleep duration (>9 hr/night; 69.1%), difficulty falling asleep after awakening (50%), difficulty initiating and maintaining sleep (59.8%), and early morning awakening (32%; Fang & Lin, 2010; Lin et al., 2012).
Factors associated with sleep disturbances and sleep quality
Residents’ demographic characteristics, physical and psychosocial status, life habits, and living environment were associated with sleep disturbances or sleep quality in all studies except one (Fang & Lin, 2010). The global PSQI score was used to represent residents’ sleep quality in the seven studies.
Demographic characteristics
The associations of age, gender, occupation, marital status, and income with sleep quality were evaluated in six studies. After controlling for covariates, widower (odds ratios [ORs] ranged from 1.54 to 3.18, ps ≤ .04) and low level of education (ORs ranged from 0.50 to 0.78, ps < .05) were significantly associated with poor sleep quality (PSQI global score; Tsai et al., 2008; Zhang et al., 2008). Occupation was not associated with sleep quality (Chen et al., 2014; Zhang et al., 2008). The associations of age, gender, and income with sleep quality were inconsistent across studies. One (Zhang et al., 2008) out of five studies that examined the associations between age and sleep quality found that increased age (OR = 1.45; p = .02) was significantly associated with poor sleep quality. In the bivariate analyses, males had better sleep quality than females in two (Lin et al., 2012; Shi, 2009) of four studies that examine gender differences in sleep quality. However, these associations were no longer significant in the multivariate analysis (Shi, 2009). Two studies found that adequate income or perceived adequate was associated with better sleep quality in bivariate analyses (Shi, 2009; Tsai et al., 2008) and the association remained significant (β = −0.82, p < .001) after adjusting for covariates (Shi, 2009).
Physical and psychological conditions
Chronic disease, hypnotic medication use, and psychological conditions were all associated with poor sleep quality in LTC residents in China (Chen et al., 2014; Fang & Lin, 2010; Shi, 2009; Tsai et al., 2008). After adjusting for covariates, having a chronic disease was found to be associated with worse sleep quality (β = 0.705, p = .002; Shi, 2009) and increased risk of sleep disturbances (OR = 1.75; p < .001; Zhang, Yang, & Zeng, 2008). In addition, good sleepers reported better perceived health status than poor sleepers (t = −3.31; p < .01; Tsai et al., 2008). Residents with regular hypnotic medication use reported significantly worse sleep quality than those who did not use hypnotics (βs ranged from 1.01 to 1.77, ps ≤ .031; Chen et al., 2014; Shi, 2009). Psychological symptoms, including anxiety, loneliness, worrying (Chen et al., 2014; Shi, 2009), dissatisfaction with life (Chen et al., 2014; Tsai et al., 2008), and depression (Li, Wang, & Yin, 2012; Yao et al., 2012), were associated with residents’ worse sleep quality. Cognitive status was not significantly different between good and poor sleepers (Tsai et al., 2008).
Lifestyle and environmental
Lifestyle factors and environmental factors were associated with residents’ sleep quality (Lin et al., 2012; Shi, 2009; Yao et al., 2012). The prevalence of poor sleep quality was significantly less in residents who had good social relationships compared with those who did not (F = 7.68; p = .002; Yao et al., 2012). In addition, residents who participated more frequently in leisure activities (F = 3.12; p = .04) and physical exercises (F = 3.26; p = .04) had better sleep quality than those who did not participate (Shi, 2009). Drinking water (F = 11.89; p = .001) and excitement before bedtime (F = 24.54; p < .001) were associated with worse sleep quality, whereas foot bath before bedtime was associated with better sleep quality (F values ranged from 4.52 to 7.53; Shi, 2009; Yao et al., 2012). Comfortable bedroom environment (e.g., temperature, humidity, light, and noise, and bed; Shi, 2009; Yao et al., 2012) and overall better facility environment (F = 4.53; p = .01; Lin et al., 2012) were associated with better sleep quality.
Intervention Studies (Table 2)
Characteristics of intervention studies
Among the seven sleep intervention studies, there were two randomized controlled trials (RCTs): One was randomized at subject level (Sun et al., 2010), and the other was randomized at facility level (Chen et al., 2015), three pretest–posttest controlled trials (Song, 2009; Wu et al., 2015; Yi & Zhang, 2012), and two single-group pretest–posttest studies (Chen et al., 2013; Song, 2009). In general, the sample sizes of studies were relatively small. Four of the five RCTs and pretest–posttest trials had 25 to 36 residents in each group, and only one study had more than 50 residents in each group (Chen et al., 2015). The two studies without control groups had 25 and 50 residents, respectively (Chen et al., 2013; Song, 2009).
The type and length of the intervention varied among studies: two 6-month exercise interventions (Chen et al., 2010; Chen et al., 2015), one 4-week light therapy (Wu et al., 2015), one 5-week traditional Chinese medicine treatment (Sun et al., 2010), and three behavioral interventions lasted from 4 weeks to a year (Chen et al., 2013; Song, 2009; Yi & Zhang, 2012). Three of the seven total intervention studies collected data at baseline, during the intervention and post-intervention (Chen et al., 2010; Chen et al., 2015; Sun et al., 2010). One of the three studies followed up with participants 2 weeks after the intervention ended (Sun et al., 2010).
Three of the seven studies screened residents’ sleep at baseline, and only included residents with sleep disturbances (i.e., insomnia) or poor sleep quality (Chen et al., 2013; Sun et al., 2010; Yi & Zhang, 2012). Having a sleep disturbance or poor sleep quality was not part of the inclusion criteria for the other four studies. Four studies used PSQI as sleep outcome measure (Chen et al., 2010; Chen et al., 2015; Chen et al., 2013; Yi & Zhang, 2012), one study used PSQI as screening tool and used AIS as the outcome measure for insomnia severity (Sun et al., 2010), whereas the other two studies used researcher-designed questionnaires to measure nocturnal sleep disturbances (Wu et al., 2015) and sleep quality (Song, 2009).
Effects of interventions on sleep
In general, the interventions showed beneficial effects on sleep in LTC residents in China. In the first of the two studies testing an exercise intervention in Taiwan, a 6-month silver Yoga program was implemented by residents who were mildly dependent in activities of daily livings (ADLs; Chen et al., 2010). After the 6-month Yoga intervention, participants in the intervention group had significantly better overall sleep quality (F = 19.91; p = .001), less sleep disturbances (F = 16.12; p = .001), and higher habitual sleep efficiency (F = 8.81; p = .005) compared with the control group. In the second study, a 6-month elastic band exercise intervention was implemented in wheelchair-bound residents who were heavily or moderately dependent in ADLs. Participants in the intervention group had longer sleep duration (F = 11.09; p = .001) and better habitual sleep efficiency (F = 7.46; p = .007) than the control group at 3 months, and these results were maintained at the end of the 6 months (Chen et al., 2015). Therefore, these two exercise interventions improved residents’ overall sleep quality and sleep efficiency (Chen et al., 2010; Chen et al., 2015).
The 4-week light exposure intervention failed to reduce sleep disruption in the intervention group compared with the control group (Wu et al., 2015). As sleep disruption was the only sleep measure in the study, the effects of this light intervention on other sleep parameters (i.e., sleep duration) are unknown. One RCT utilized 5-week traditional Chinese medicine treatment (Acupressure on Shenmen points) to decrease residents’ sleep disturbances (i.e., insomnia; Sun et al., 2010). The severity of participants’ insomnia significantly decreased in the intervention group both during ([Weeks 2-5] βs ranged from −18.28 to 16.38, all ps < .001) and at post-intervention ([Week 6] β= −11.40, p < .001; [Week 7] β = −4.84, p < .001) and was significantly lower than the control group. However, insomnia severity bounced back to baseline status at 2 weeks after completion of the intervention (Sun et al., 2010).
Multi-component behavioral interventions using education for healthy sleep, daytime physical and social engagement, self-relaxation, and bedtime foot bath were used in three studies in the Mainland China and increased the participants’ overall sleep quality (F values ranged from 12.98 to 29.48; ps < .001), sleep efficiency (F values ranged from 4.97 to 8.9; ps < .04), and daytime function (F values ranged from 24.90 to 36.20; ps < .001; Chen et al., 2013; Song, 2009; Yi & Zhang, 2012).
Discussion
Sleep is a critical but overlooked health outcome in older adults’ health care management, especially for those living in institutions. Although there is a fair amount of sleep research in China during the last two decades, sleep in LTC residents had not been well studied in China. We only found 15 studies that met our relatively broad inclusion criteria, sleep research in LTC residents in China. The heterogeneity of the study designs, statistical approaches, and types of interventions across studies makes the comparison of results difficult. Nevertheless, this literature review can provide a first overview on the existing body of sleep research in LTC residents in China and offer helpful directions for future studies.
There were some variations in the prevalence of poor sleep quality and sleep disturbances reported in the reviewed studies, despite the fact that demographic characteristics (age, gender, education, and marital status [widower]) were similar in these studies. These variations could be related to clinical characteristics of samples (e.g., some may have higher incidence of comorbidities associated with insomnia) and/or differences in the cutoff scores of the PSQI applied across studies. The variation may also be partially due to the varied economic status in different regions in China and participants’ cognitive function. Overall, more than 40% residents in five of the six cross-sectional studies reported poor sleep quality. One epidemiological study reported that the prevalence of sleep disturbances in community-dwelling Chinese older adults was 33.7% (Niu et al., 2016). We were not able to find any large-scale epidemiological study that examined the prevalence of sleep disturbances in Chinese LTC residents. Taken together, these results suggest that sleep disturbances are more prevalent in institutionalized Chinese older adults than those living in the community.
Our review revealed that multiple factors were associated with sleep disturbances and poor sleep quality in LTC residents in China. These factors include widower, low level of education, comorbidities, psychological symptoms (e.g., anxiety, depression, and loneliness), lack of physical and social activities, bedtime habits (e.g., drinking water, bedtime excitement, etc.), and bedroom and institutional environment. The lack of association between occupation and sleep quality may be explained by the fact that participants were no longer working at the time of data collection. Our findings are consistent with the results from studies conducted in LTC residents in the United States (Ancoli-Israel, Ayalon, & Salzman, 2008; Li, Grandner, Chang, Jungquist, & Porock, 2015; Martin & Ancoli-Israel, 2008).
Chinese culture may be a unique factor that affects residents’ sleep in LTC settings in China. Chinese older adults are usually reluctant to transit into LTC from home, because taking care of older adults is an obligation of their adult children (filial piety) in China and moving to an LTC setting means they do not have children or their children do not have filial piety. These culture-related negative thoughts of living in LTC may affect sleep.
There are some methodological limitations in the reviewed cross-sectional studies that may have affected the results and warrant further discussion. First, the description of study methods and results was very brief and often vague (Chen et al., 2014; Fang & Lin, 2010; Li, Wang, & Yin, 2012; Lin et al., 2012; Yao et al., 2012). Second, the statistical approaches may not have been sufficient to answer the research questions. For example, bivariate analyses were used without adjusting for any possible confounders, such as, age, gender, body mass index (BMI), physical and psychological conditions, and medication use (Lin et al., 2012; Yao et al., 2012), and associations between sleep and possible factors were claimed based on results from univariate descriptive statistics, rather than appropriate statistical methods better suited to examine associations between variables (Fang & Lin, 2010), despite adequate sample sizes. In addition, cognitive impairment and dementia are well-established factors associated with sleep disturbances (Yaffe, Falvey, & Hoang, 2014), yet seven of the eight cross-sectional studies did not provide information on participants’ cognitive function. We recommend that at a minimum, cognitive function needs to be assessed to determine participants’ capacity to complete subjective study instruments, such as PSQI.
Furthermore, sleep disturbances and sleep quality were assessed mostly using the Chinese version of the PSQI. Different cut points for poor sleep quality were used in studies conducted in different regions in China (Mainland vs. Taiwan). The inconsistent use of and scoring of subjective sleep measures raise doubts on the validity of the data and make the results difficult to interpret. No objectively measured sleep parameters were assessed in any of the studies included in this review. Actigraphy has been widely utilized as an objective sleep measure in the larger body of sleep research conducted in LTC settings in the United States and other countries (Li, Grandner, et al., 2015; Martin, Marler, Harker, Josephson, & Alessi, 2007; Ouslander et al., 2006; Richards et al., 2011). Future research should consider adding actigraphy to comprehensively assess sleep by capturing both subjective sleep quality and objective sleep parameters in LTC residents in China. In addition, the cut point of PSQI needs to be more mainstream and should not vary by region.
All seven intervention studies used nonpharmacological interventions. Overall, the reviewed exercise, traditional Chinese acupressure, and behavioral interventions showed beneficial effects on residents’ sleep. The 4-week light therapy study failed to show differences between intervention and control groups.
The study designs, interventions, and durations of intervention varied greatly between studies. Overall, the sample sizes of these studies were relatively small and may not have been sufficiently powered to detect changes. In addition, four studies did not screen sleep disturbances or report the proportion of good sleepers at baseline, which may confound the study results (Chen et al., 2010; Chen et al., 2015; Song, 2009; Wu et al., 2015). In general, the four studies conducted in Taiwan had more rigorous study designs than the three studies from Mainland China. The methods and results were clearly described in the studies from Taiwan, with stronger designs (e.g., RCT and clustered RCT), longer intervention periods, mid-intervention assessment, and post-intervention follow-up. Among the three Mainland China studies, two of the studies used a single-group design (Chen et al., 2013; Song, 2009) whereas the other used a pretest–posttest with control group design. All participants were recruited from one single LTC facility, and the studies lacked an in-depth description of the intervention and statistical analyses (Yi & Zhang, 2012).
Improving sleep hygiene, stimulus control therapy, sleep restriction, relaxation training, and cognitive behavioral therapy for insomnia (CBT-I) in older adults are among the most widely used nonpharmacological therapies to improve sleep (Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008). Other therapies include exercise, light therapy, and environmental modifications, as found in this review. In community-dwelling older adults, there is evidence to suggest that exercise may be an effective way to improve sleep (Varrasse, Li, & Gooneratne, 2015). A combination of approaches may be best to improve sleep in LTC residents. First, determining the cause(s) of the sleep disturbance(s) is an important step to be able to alleviate the disturbance and negative consequences. Modifying the daytime and nighttime environment will promote sleep in LTC residents. Environmental modifications include keeping the nighttime environment dark, quiet, and at a comfortable temperature; keeping the daytime environment bright; increasing physical activity; and maintaining a consistent mealtime schedule (Neikrug & Ancoli-Israel, 2010). Martin and colleagues (2007) found that increasing daytime activities, maintaining a routine bedtime, and a reducing nighttime noise and light all decreased daytime sleepiness in nursing home residents. Nonpharmacological treatments have advantages over pharmacological treatment as they can be effective at improving sleep in both the short and long terms with no serious consequences (Morin, Colecchi, Stone, Sood, & Brink, 1999).
Sleep research in LTC residents in China seems to be in its infancy. Future intervention studies in LTC China need to have more rigorous designs, larger sample sizes, and sufficient statistical approach, and to determine dose response. Increasing data collection time periods will help increase power and clustered randomization should be considered if randomization at the individual level is not feasible. As we know there are promising nonpharmacological treatments for sleep disturbances in LTC residents, these methodological suggestions may guide future studies.
There are a few limitations of this review. First, the 15 heterogeneous articles reviewed may be a small number of studies to represent sleep disturbances and sleep quality in LTC residents in China. However, our search criteria were broad, and these articles represent the state of the science of sleep in LTC residents in China. Second, our extraction and synthesis of material is subjective, but was discussed among multiple authors. The strength of this review is that we provide the first overview of current body of sleep research in the growing LTC population in China and suggest directions for future research.
Conclusion
This review presents the current body of sleep research in LTC residents in China and provides suggestions for future research. Sleep disturbances and poor sleep quality are prevalent in LTC residents in China. There are physical, psychosocial, and environmental factors that are associated with sleep disturbances and poor sleep quality. The nonpharmacological sleep interventions were beneficial for residents’ sleep. However, the sleep research in LTC residents in China is still at the beginning stages, especially in Mainland China. Future studies should consider more rigorous designs and adding objective sleep measures (e.g., actigraphy).
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by an NIH nursing/neuroscience training grant at the University of Pennsylvania (T32 HL07953).
Biographies
Junxin Li, PhD, RN, is a postdoctoral research fellow at the University of Pennsylvania and a visiting scholar at Jilin University.
Binbin Yang, RN, is a graduate student at the School of Nursing, Jilin University.
Miranda Varrasse, MSN, RN, is a PhD candidate at the School of Nursing, University of Pennsylvania.
Kun Li, PhD, RN, is an associate professor at the School of Nursing, Jilin University.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Ancoli-Israel S, Ayalon L, Salzman C. Sleep in the elderly: Normal variations and common sleep disorders. Harvard Review of Psychiatry. 2008;16:279–286. doi: 10.1080/10673220802432210. [DOI] [PubMed] [Google Scholar]
- Chen KM, Chen MH, Lin MH, Fan JT, Lin HS, Li CH. Effects of yoga on sleep quality and depression in elders in assisted living facilities. The Journal of Nursing Research. 2010;18:53–61. doi: 10.1097/JNR.0b013e3181ce5189. [DOI] [PubMed] [Google Scholar]
- Chen KM, Huang HT, Cheng YY, Li CH, Chang YH. Sleep quality and depression of nursing home older adults in wheelchairs after exercises. Nursing Outlook. 2015;63:357–365. doi: 10.1016/j.outlook.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Chen L. Decisions for institutionalization among nursing home residents and their children in Shanghai. Qualitative Health Research. 2015;25:458–469. doi: 10.1177/1049732314551990. [DOI] [PubMed] [Google Scholar]
- Chen QX, Zhang Q, Zhao Y, Dong LN. Investigation on the sleep quality of old people in nursing home and its influencing factors. Journal of Qilu Nursing. 2014;20(5):6–9. [Google Scholar]
- Chen QX, Zhang Q, Zhao Y, Dong LN, Luo YY, Wang XX, Du L. Influence of comprehensive nursing intervention on sleep quality of the elderly in nursing home. Chinese Nursing Research. 2013;27:1352–1353. [Google Scholar]
- Chu LW, Chi I. Nursing homes in China. Journal of the American Medical Directors Association. 2008;9:237–243. doi: 10.1016/j.jamda.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Fang J, Lin H. Investigation on the sleep quality of old people in community nursing home and its strategies. Journal of Community Medicine. 2010;8(11):8–9. [Google Scholar]
- Feng Z, Liu C, Guan X, Mor V. China’s rapidly aging population creates policy challenges in shaping a viable long-term care system. Health Affairs. 2012;31:2764–2773. doi: 10.1377/hlthaff.2012.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason K, McCall WV. Current concepts in the diagnosis and treatment of sleep disorders in the elderly. Current Psychiatry Reports. 2015;17(6):45. doi: 10.1007/s11920-015-0583-z. [DOI] [PubMed] [Google Scholar]
- Gong LC, Chen CX, Yao XJ, Yin YN, Han WT. Relationship between sleep disorders and depression among old people in elderly apartments. Modern Preventive Medicine. 2012;39(16):4175–4178. [Google Scholar]
- Harvey AG, Stinson K, Whitaker KL, Moskovitz D, Virk H. The subjective meaning of sleep quality: A comparison of individuals with and without insomnia. Sleep. 2008;31(3):383–393. doi: 10.1093/sleep/31.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig AK, Doring A, Heier M, Emeny RT, Zimmermann AK, Autenrieth CS, Meisinger C. Association between sleep disturbances and falls among the elderly: Results from the German cooperative health research in the region of Augsburg-age study. Sleep Medicine. 2013;14:1356–1363. doi: 10.1016/j.sleep.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Isaia G, Corsinovi L, Bo M, Santos-Pereira P, Michelis G, Aimonino N, Zanocchi M. Insomnia among hospitalized elderly patients: Prevalence, clinical characteristics and risk factors. Archives of Gerontology and Geriatrics. 2011;52:133–137. doi: 10.1016/j.archger.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Li J, Chang YP, Porock D. Factors associated with daytime sleep in nursing home residents. Research on Aging. 2015;37:103–117. doi: 10.1177/0164027514537081. [DOI] [PubMed] [Google Scholar]
- Li J, Grandner MA, Chang YP, Jungquist C, Porock D. Personcentered dementia care and sleep in assisted living residents with dementia: A pilot study. Behavioral Sleep Medicine. 2015;15:97–113. doi: 10.1080/15402002.2015.1104686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Guo Q, Ye X, Lin J, Yi C, Mao H, Yang X, Yu X. Prevalence and risk factors of sleep disturbance in continuous ambulatory peritoneal dialysis patients in Guangzhou, southern China. International Urology and Nephrology. 2012;44:929–936. doi: 10.1007/s11255-011-0060-5. [DOI] [PubMed] [Google Scholar]
- Li YK, Wang ZW, Yin X. The relationship between sleep quality and depression among old people in the elderly home. Journal of Nursing Administration. 2012;12:697–699. [Google Scholar]
- Lin J, Lu HF, Ma S. Sleep quality of senior people in nursing home of Wuhan. Journal of Nursing. 2012;19(4):23–26. [Google Scholar]
- Liu XC, Tang MQ, Hu L, Wang AZ, Wu HX, Zhao GF, Li WS. The reliability and validity of the Pittsburgh Sleep Quality Index. Chinese Journal of Psychiatry. 1996;29:103–107. [Google Scholar]
- Lorenz RA, Harris M, Richards KC. Sleep in adult long-term care. In: Redeker NS, McEnany GP, editors. Sleep disorders and sleep promotion in nursing practice. Springer; 2011. pp. 339–354. [Google Scholar]
- Lu MJ, Lin ST, Chen KM, Tsang HY, Su SF. Acupressure improves sleep quality of psychogeriatric inpatients. Nursing Research. 2013;62:130–137. doi: 10.1097/NNR.0b013e3182781524. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhu G, Zhao Q, Guo Q, Meng H, Hong Z, Ding D. Prevalence and risk factors of poor sleep quality among Chinese elderly in an urban community: Results from the Shanghai aging study. PLoS ONE. 2013;8(11):e81261. doi: 10.1371/journal.pone.0081261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Ancoli-Israel S. Sleep disturbances in long-term care. Clinics in Geriatric Medicine. 2008;24:39–50. doi: 10.1016/j.cger.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Marler MR, Harker JO, Josephson KR, Alessi CA. A multicomponent nonpharmacological intervention improves activity rhythms among nursing home residents with disrupted sleep/wake patterns. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62:67–72. doi: 10.1093/gerona/62.1.67. [DOI] [PubMed] [Google Scholar]
- Martin MS, Sforza E, Barthelemy JC, Thomas-Anterion C, Roche F. Sleep perception in non-insomniac healthy elderly: A 3-year longitudinal study. Rejuvenation Research. 2014;17:11–18. doi: 10.1089/rej.2013.1457. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. The Journal of the American Medical Association. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- National Health and Family Planning Commission. China health statistics yearbook 2012. 2013 Retrieved from http://www.nhfpc.gov.cn/htmlfiles/zwgkzt/ptjnj/year2012/index2012.html.
- Neikrug AB, Ancoli-Israel S. Sleep disturbances in nursing homes. The Journal of Nutrition, Health & Aging. 2010;14:207–211. doi: 10.1007/s12603-010-0051-8. [DOI] [PubMed] [Google Scholar]
- Niu J, Han H, Wang Y, Wang L, Gao X, Liao S. Sleep quality and cognitive decline in a community of older adults in Daqing City, China. Sleep Medicine. 2016;17:69–74. doi: 10.1016/j.sleep.2015.07.033. [DOI] [PubMed] [Google Scholar]
- Okajima I, Nakajima S, Kobayashi M, Inoue Y. Development and validation of the Japanese version of the Athens Insomnia Scale. Psychiatry and Clinical Neurosciences. 2013;67:420–425. doi: 10.1111/pcn.12073. [DOI] [PubMed] [Google Scholar]
- Ouslander JG, Connell BR, Bliwise DL, Endeshaw Y, Griffiths P, Schnelle JF. A nonpharmacological intervention to improve sleep in nursing home patients: Results of a controlled clinical trial. Journal of the American Geriatrics Society. 2006;54:38–47. doi: 10.1111/j.1532-5415.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- Richards KC, Lambert C, Beck CK, Bliwise DL, Evans WJ, Kalra GK, Sullivan DH. Strength training, walking, and social activity improve sleep in nursing home and assisted living residents: Randomized controlled trial. Journal of the American Geriatrics Society. 2011;59:214–223. doi: 10.1111/j.1532-5415.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JC, Dzierzewski JM, Alessi CA. Sleep problems in the elderly. Medical Clinics of North America. 2015;99:431–439. doi: 10.1016/j.mcna.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of Clinical Sleep Medicine. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- Shi YH. The research on status and impact factors of sleep quality in the senior citizen in elderly apartments. China Medical University; Shenyang, Liaoning, China: 2009. (Master thesis) [Google Scholar]
- Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. Journal of Psychosomatic Research. 2000;48:555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Song LJ. Study on quality of life of the aged receiving nursing care in nursing home. Chinese Journal of Nursing. 2009;44:324–327. [Google Scholar]
- Sun JL, Chiou JF, Lin CC. Validation of the Taiwanese version of the Athens Insomnia Scale and assessment of insomnia in Taiwanese cancer patients. Journal of Pain and Symptom Management. 2011;41:904–914. doi: 10.1016/j.jpainsymman.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Sun JL, Sung MS, Huang MY, Cheng GC, Lin CC. Effectiveness of acupressure for residents of long-term care facilities with insomnia: A randomized controlled trial. International Journal of Nursing Studies. 2010;47:798–805. doi: 10.1016/j.ijnurstu.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Townsend-Roccichelli J, Sanford JT, VandeWaa E. Managing sleep disorders in the elderly. Nurse Practitioner. 2010;35(5):30–37. doi: 10.1097/01.NPR.0000371296.98371.7e. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Wang SY, Wang MY, Su CT, Yang TT, Huang CJ, Fang SC. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Quality of Life Research. 2005;14:1943–1952. doi: 10.1007/s11136-005-4346-x. [DOI] [PubMed] [Google Scholar]
- Tsai YF, Wong TK, Ku YC. Self-care management of sleep disturbances and risk factors for poor sleep among older residents of Taiwanese nursing homes. Journal of Clinical Nursing. 2008;17:1219–1226. doi: 10.1111/j.1365-2702.2007.02020.x. [DOI] [PubMed] [Google Scholar]
- Valenza MC, Cabrera-Martos I, Martin-Martin L, Perez-Garzon VM, Velarde C, Valenza-Demet G. Nursing homes: Impact of sleep disturbances on functionality. Archives of Gerontology and Geriatrics. 2013;56:432–436. doi: 10.1016/j.archger.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Varrasse M, Li J, Gooneratne N. Exercise and sleep in community-dwelling older adults. Current Sleep Medicine Reports. 2015;1:232–240. doi: 10.1007/s40675-015-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RJ, Hou WX, Xu CE, Zhang XJ. Survey of elderly health condition in Wenzhou city nursing home. Chinese Journal of Gerontology. 2011;31:1400–1402. [Google Scholar]
- Wu MC, Sung HC, Lee WL, Smith GD. The effects of light therapy on depression and sleep disruption in older adults in a long-term care facility. International Journal of Nursing Practice. 2015;21:653–659. doi: 10.1111/ijn.12307. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. The Lancet Neurology. 2014;13:1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- Yao XJ, Chen CX, Chen XY, Su YS, Liu Y, Li J. Sleep quality of nursing home older adults. Chinese Journal of Gerontology. 2012;32:5571–5572. [Google Scholar]
- Yi N, Zhang HJ. The effect of psychological intervention on patients with insomnia in nursing home. Shandong Medical Journal. 2012;52(1):100–101. [Google Scholar]
- Young TB. Epidemiology of daytime sleepiness: Definitions, symptomatology, and prevalence. The Journal of Clinical Psychiatry. 2004;65(Suppl. 16):12–16. [PubMed] [Google Scholar]
- Zhang KP, Yang DL, Zeng XX. Investigation on the sleep quality of 80 elderly people in a nursing home in Chongqing. Chinese Journal of Gerontology. 2008;28(20):2043–2045. [Google Scholar]


