Abstract
Sepsis is a serious life threatening medical emergency which, if not treated properly, oftentimes results in organ failure and death. Current sepsis treatment protocols are largely centered on the use of antibiotics and supportive care. Recent studies have suggested that antibiotics fail to be effective for sepsis treatment when administered during hypo-dynamic phase of sepsis that is usually characterized by the presence of a cytokine storm. As such, there is an urgent need to develop novel therapeutic drugs that target the inflammatory cytokines that are secreted as a result of increased reactive oxygen species. Cerium oxide nanoparticles (CeO2) have been shown to act as anti-inflammatory and anti-oxidant agent. More recently, they have been shown to attenuate polymicrobial insult-induced mortality in Sprague Dawley rats. Here, we investigated whether CeO2 nanoparticles can attenuate splenic damage in this animal model of sepsis. A single intravenous dose (0.5 mg/kg) of CeO2 nanoparticles attenuated the sepsis-induced loss in splenic cell structural integrity. These improvements in splenic structure were accompanied by a decrease in expression of late phase pro-inflammatory cytokine high mobility group box 1 (HMGB1) along with reduced bacterial load in the blood and peritoneal fluid of septic animals. Taken together these findings suggest that CeO2 nanoparticles can be used to attenuate polymicrobial insult-induced splenic damage in Sprague dawley rats.
Keywords: Sepsis, CeO2 nanoparticles, Spleen
Specifications Table
| Subject area | Biology |
|---|---|
| More specific subject area | Nanomedicine |
| Type of data | Graph, figure |
| How data was acquired | Immunoblotting and histochemical analysis |
| Data format | Analyzed |
| Experimental factors | Rodent based intervention study examining the effects of cerium oxide nanoparticle treatment on sepsis. |
| Experimental features | Balanced design that encompasses both in vivo and in vitro observations. |
| Data source location | Huntington, WV USA |
| Data accessibility | Data is with this article and is related to articles published and in review [1], [2], [3], [4], [5], [6] |
Value of the data
-
•
The data can be referenced by other scientists investigating the effects of the cecal inoculum induced sepsis on splenic morphology and sepsis-related signaling.
-
•
The data can provide comprehensive analysis of the effect of cerium oxide nanoparticles on cecal inoculum-induced sepsis.
-
•
These data provides a more thorough understanding of the splenic involvement in cecal inoculum-induced sepsis.
1. Data
1.1. Cerium oxide nanoparticles attenuate the splenic damage induced by polymicrobial sepsis
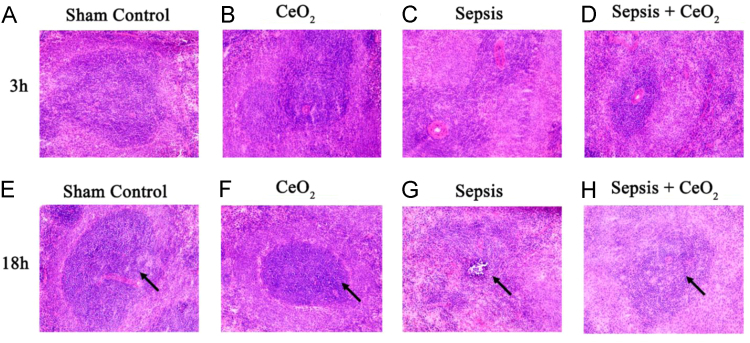
Compared to that observed in the controls, polymicrobial sepsis did not result in extensive splenic damage/necrosis in early stages (3 h) of sepsis (Fig. 1, A–D). Conversely, in the latter stages of sepsis (18 h) polymicrobial insult appeared to result in the loss of lymphocyte rich white pulp (Fig. 1, E, and G). Cerium oxide nanoparticle treatment appeared to attenuate the loss of white pulp at the 18 h time point (Fig. 1, G and H).
Fig. 1.
CeO2 nanoparticles attenuate severe sepsis induced inflammatory damage in spleen. A-D) Hematoxylin and eosin (H&E) staining of 3 h time point spleen sections imaged at 200× magnification - Control, CeO2, Sepsis, and Sepsis + CeO2. E-H) Hematoxylin and eosin (H&E) staining of 18 h time point spleen sections imaged at 200× magnification - Control, CeO2, Sepsis, and Sepsis + CeO2 (n = 3/group). Note: loss of white pulp in sepsis 18 h (arrow).
1.2. Cerium oxide nanoparticle treatment attenuates sepsis associated increases in splenic HMGB1 expression
Previous studies have shown sepsis is associated with a massive increase in the splenic levels of HMGB1 [7]. Consistent with this finding, HMGB1 expression was increased with sepsis at 3 h and 18 h after induction (Fig. 2A). Consistent with our histological findings of improved tissue splenic structure, nanoparticle treatment decreased HMGB1 levels at 18 h time point (Fig. 2A).
Fig. 2.
CeO2 nanoparticles attenuate severe sepsis induced increase in expression of pro-inflammatory cytokine HMGB1. Levels of HMGB1 at 3 h and 18 h in Control, CeO2, Sepsis, and Sepsis + CeO2 groups (n = 6/group).
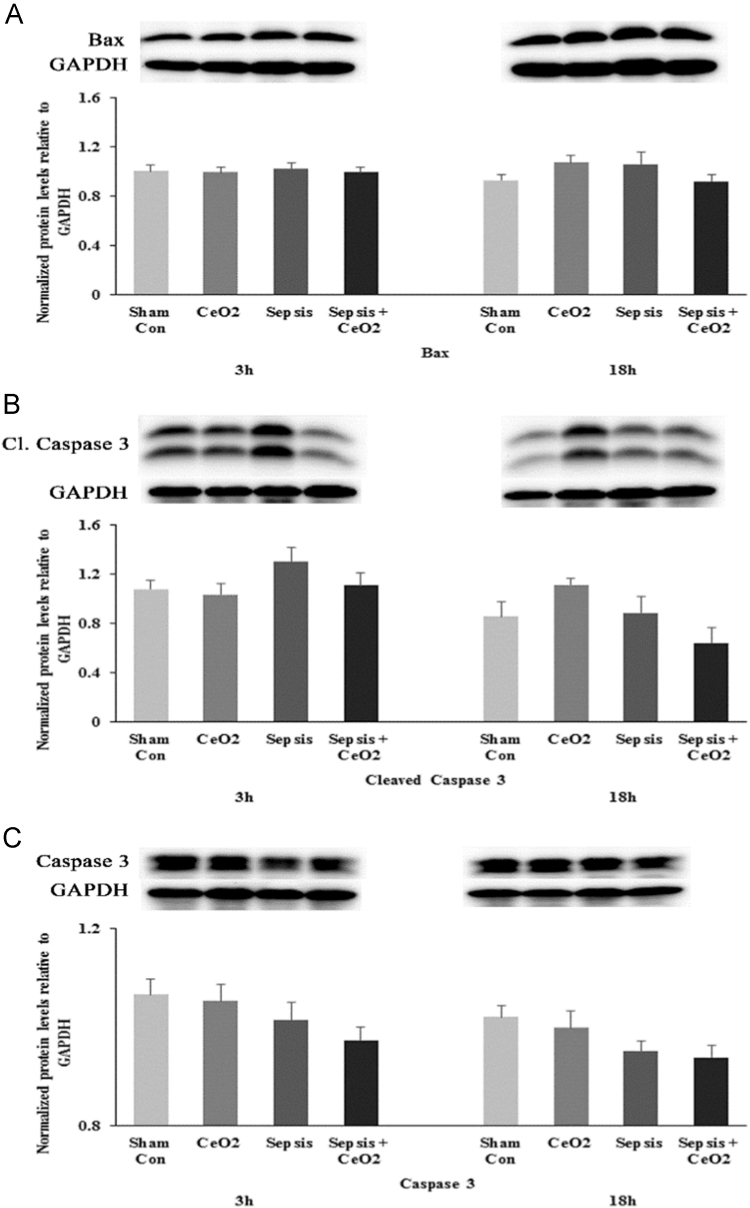
1.3. Apoptosis is not a major pathway for splenic cell death in the polymicrobial inoculum sepsis model
Although recent data has suggested that lymphocyte apoptosis in the spleen is increased in the cecal ligation and puncture sepsis model [8] we did not find similar evidence in the CI model. Specifically, sepsis did not appear to increase the levels of apoptotic proteins Bax, cleaved caspase-3 and caspase-3 in the spleen (Fig. 3A, B, and C). Similarly, treatment with CeO2 nanoparticles did not appear to affect splenocyte death.
Fig. 3.
Cecal inoculum method of polymicrobial sepsis does not induce caspase 3 mediated apoptosis in spleen. A) Total protein levels of Bax at 3 h and 18 h in Control, CeO2, Sepsis, and Sepsis + CeO2 groups. B) Levels of cleaved caspase 3 at 3 h and 18 h in Control, CeO2, Sepsis, and Sepsis + CeO2 groups. C) Total levels of caspase 3 at 3 h and 18 h in Control, CeO2, Sepsis, and Sepsis + CeO2 groups. (n = 6/group).
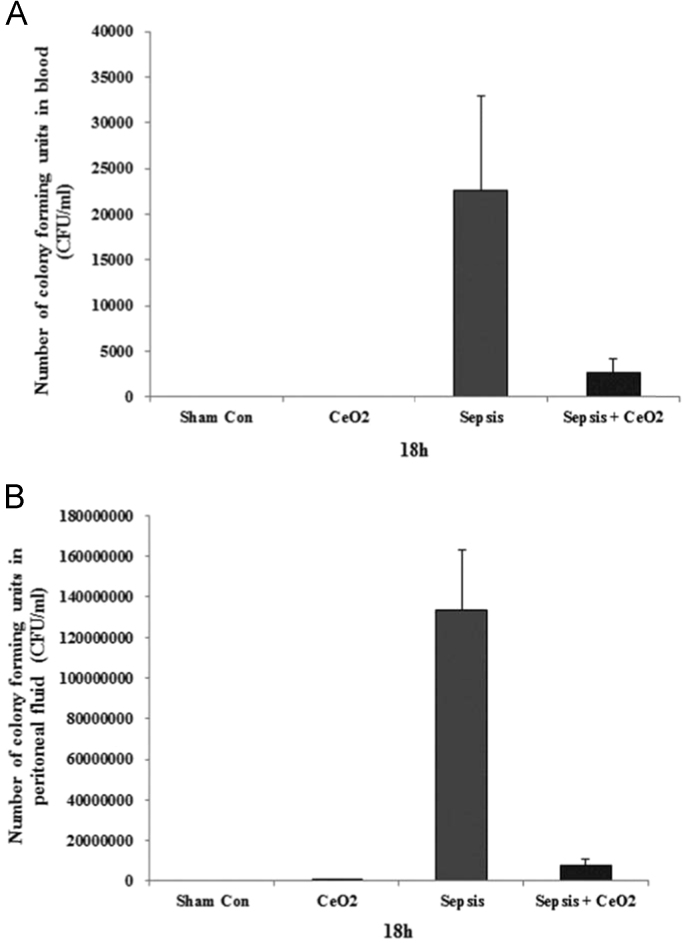
1.4. Cerium oxide nanoparticles attenuate bacterial load in the blood and peritoneal fluid of septic animals
The number of CFU in the blood and peritoneal fluid were significantly increased at 18 h in the septic animals which were decreased with nanoparticle treatment (Fig. 4, A-B).
Fig. 4.
CeO2 nanoparticles attenuate bacterial load in blood and peritoneal fluid of septic animals. A) Bacterial load in blood. B) Bacterial load in peritoneal fluid.
2. Experimental design and methods
This data articles contains data related to the research articles entitled “Cerium oxide nanoparticles inhibit lipopolysaccharide induced MAP kinase/NF-kB mediated severe sepsis”” in Biomaterials [3]. In the present study we have confirmed how the administration of a single dose (0.5 mg/kg) of CeO2NPs intravenously into septic rats prevent the cecal inoculum-induced sepsis alternation in the spleen. These changes are an indicator of splenic involvement in the systemic inflammatory response to sepsis [9]. The data presented here is of importance to the understanding of sepsis and the potential therapeutic use of CeO2-NP as an adjuvant treatment.
2.1. Animal model of polymicrobial sepsis and therapeutic intervention
Ten week old male Sprague Dawley rats were purchased from Hill-Top laboratories and housed two-per cage at 22 ± 2 ° C with a 12:12 light-dark cycle. Animals were allowed to acclimate to their surroundings for two weeks before any experimentation. Animals were fed with standard rodent chow and had access to food and water ad libitum during the entire study. All surgical procedures were performed in accordance with the guidelines provided by the Marshall University Institutional Animal Care and Use Committee (IACUC) and The Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Septis was induced in these animals according to the protocol outlines in the Biomaterials [3] article and as described previously by our center [10]. Animals were randomly assigned to one of the four groups and were sacrificed at 3 h and 18 h after sepsis induction to study the molecular events during early and late phases of sepsis.
2.2. Collection and preservation of spleen
Animals from different groups were sacrificed under anesthesia at 3 h and 18 h after sepsis induction. Peritoneal fluid was collected with a sterile syringe while whole blood was collected through cardiac puncture. Spleen was collected and washed with Krebs–Ringer bicarbonate buffer (KRB) to remove any blood, blot dried and frozen in liquid nitrogen. Spleens were stored at −80 °C for further experiments.
2.3. Histology
Frozen spleens were sectioned (8μm) using Leica CM1950 cryostat onto poly-L-lysine coated slides at -18 °C and then stored at −80 °C. Hematoxylin and eosin staining was performed using BBC Biochemical H & E staining kit (Cat No. NC9960321, Fisher Scientific, Pittsburgh, PA) on frozen spleen sections to visualize the change in red pulp and white pulp morphology with sepsis and CeO2 nanoparticle treatment according to manufactures guidelines. After alcohol dehydration and xylene clearing the splenic morphology was visualized using an Evos XL microscope (Life technologies, Grand Island, NY).
2.4. SDS-PAGE and immunoblotting
Approximately 50 mg of frozen spleen tissue was measured and pulverized in liquid nitrogen. Four hundred and fifty microliters of T-PER (Pierce, Rockford, IL, USA) containing 1% protease and phosphatase inhibitors (P8340 and P5726, Sigma- Aldrich, St. Louis, MO, USA) was added to the sample, the samples homogenized and then centrifuged at 5000 g for 15 min at 4 °C to collect the supernatant. The 660 nm assay (Pierce, Rockford, IL, USA) was performed to determine the protein content in the supernatant and the samples were normalized with T-PER and 4× Laemlli buffer to obtain equal protein concentration across all samples. Forty micrograms of protein were loaded in 10% PAGEr Gold Precast gel (Lonza, Rockland, ME) and transferred to nitrocellulose membranes as described elsewhere [5]. Nitrocellulose membranes were then blocked with 5% milk in Tris-Buffered saline with 0.05% Tween-20 (TBST) for 1 h at room temperature, washed three times with TBST and probed with antibodies specific for Bax, cleaved caspase 3, caspase 3, HMGB1, and GAPDH (Cell Signaling Technology, Danvers, MA). Membranes were incubated with primary antibody for 16 h at 4 °C and washed three times with TBST for 5 min each. After incubation with a secondary anti-rabbit antibody (Cell Signaling Technology, Danvers, MA) for 1 h at room temperature, immunoreactivity was visualized using Super signal West Pico Chemiluminescent substrate (Pierce, Rockford, IL, USA) and quantified using Fluorchem 9900 software (Protein Simple, Santa Clara, CA). Protein expression relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to normalize protein expression.
2.5. Bacterial cultures
Whole blood was collected via cardiac puncture and peritoneal lavage fluid was obtained using a sterile syringe. Blood and peritoneal lavage samples were serially diluted in sterile saline and plated on sheep's blood agar/ nutrient agar plates. Plates were incubated overnight at 37 °C and colony counts were determined 24 h later. Colony counts were expressed as CFU/ml of fluid and then converted to a logarithmic scale for statistical analysis.
Statistical analysis
Results are presented as mean ± standard error of mean.
Acknowledgements
This work was supported in part from DOE grant (DE-PS02–09ER-01 to E.R.B) and by the National Science Foundation under Award no. OIA-1458952.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.dib.2018.03.073.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Asano S., Arvapalli R., Manne N.D., Maheshwari M., Ma B., Rice K.M., Selvaraj V., Blough E.R. Cerium oxide nanoparticle treatment ameliorates peritonitis-induced diaphragm dysfunction. Int. J. Nanomed. 2015;10:6215–6225. doi: 10.2147/IJN.S89783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano S., Manne N.D., Nandyala G., Ma B., Selvaraj V., Arvapalli R., Rice K.M., Blough E.R. Cecal inoculum peritonitis: an alternative model for sepsis vascular dysfunction study. Life Sci. 2015;141:108–118. doi: 10.1016/j.lfs.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Selvaraj V., Nepal N., Rogers S., Manne N.D., Arvapalli R., Rice K.M., Asano S., Fankenhanel E., Ma J.Y., Shokuhfar T., Maheshwari M., Blough E.R. Cerium oxide nanoparticles inhibit lipopolysaccharide induced MAP kinase/NF-kB mediated severe sepsis. Data Brief. 2015;4:105–115. doi: 10.1016/j.dib.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selvaraj V., Nepal N., Rogers S., Manne N.D., Arvapalli R., Rice K.M., Asano S., Fankenhanel E., Ma J.Y., Shokuhfar T., Maheshwari M., Blough E.R. Lipopolysaccharide induced MAP kinase activation in RAW 264.7 cells attenuated by cerium oxide nanoparticles. Data Brief. 2015;4:96–99. doi: 10.1016/j.dib.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvaraj V., Nepal N., Rogers S., Manne N.D., Arvapalli R., Rice K.M., Asano S., Fankhanel E., Ma J.J., Shokuhfar T., Maheshwari M., Blough E.R. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials. 2015;59:160–171. doi: 10.1016/j.biomaterials.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvaraj V., Manne N.D., Arvapalli R., Rice K.M., Nandyala G., Fankenhanel E., Blough E.R. Effect of cerium oxide nanoparticles on sepsis induced mortality and NF-kappaB signaling in cultured macrophages. Nanomedicine. 2015;10:1275–1288. doi: 10.2217/nnm.14.205. [DOI] [PubMed] [Google Scholar]
- 7.Valdes-Ferrer S.I., Rosas-Ballina M., Olofsson P.S., Lu B., Dancho M.E., Ochani M., Li J.H., Scheinerman J.A., Katz D.A., Levine Y.A., Hudson L.K., Yang H., Pavlov V.A., Roth J., Blanc L., Antoine D.J., Chavan S.S., Andersson U., Diamond B., Tracey K.J. HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6C(high) inflammatory monocytes in murine sepsis survivors. J. Intern. Med. 2013;274:381–390. doi: 10.1111/joim.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss R.S., Coopersmith C.M., Karl I.E. Prevention of lymphocyte apoptosis--a potential treatment of sepsis? Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2005;41(Suppl 7):S465–S469. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 9.Swan R., Chung C.S., Albina J., Cioffi W., Perl M., Ayala A. Polymicrobial sepsis enhances clearance of apoptotic immune cells by splenic macrophages. Surgery. 2007;142:253–261. doi: 10.1016/j.surg.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manne N.D., Arvapalli R., Nepal N., Thulluri S., Selvaraj V., Shokuhfar T., He K., Rice K.M., Asano S., Maheshwari M., Blough E.R. Therapeutic potential of cerium oxide nanoparticles for the treatment of peritonitis induced by polymicrobial insult in Sprague-Dawley rats. Crit. Care Med. 2015;43:e477–e489. doi: 10.1097/CCM.0000000000001258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material