Abstract
Background:
Vitamin D might be promising to serve as an adjunctive therapy for pulmonary tuberculosis (TB). However, the results remained controversial. We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of vitamin D in patients with pulmonary TB.
Methods:
Medline, SCOPUS, Google Scholar, EMBASE, Springer, and Science Direct were searched electronically from inception to Oct 2016. Randomized controlled trials (RCTs) and controlled clinical trials (CCTs) assessing the effect of vitamin D plus anti-tuberculosis treatment (ATT) versus placebo plus ATT on the treatment of pulmonary TB were included. Two investigators independently searched articles, extracted data, and assessed the quality of included studies. Data were analyzed using RevMan 5.3 software.
Results:
Five studies were included in this meta-analysis. Overall, compared with placebo intervention, vitamin D supplementation was found to have no significant effect on sputum smear negative conversion rates (RR=0.99; 95% CI=0.91 to 1.07; P=0.77), BMI (MD=0.11; 95% CI=−0.85 to 1.07; P=0.82) and ESR (MD=−2.29; 95% CI=−8.87 to 4.30; P=0.50).
Conclusion:
Vitamin D supplementation showed no influence on the improvement of sputum smear-negative conversion rates and BMI, as well as the decrease in ESR.
Keywords: Vitamin D, Adjunctive therapy, Pulmonary tuberculosis (TB), Efficacy, Meta-analysis
Introduction
There has been 9.4 million tuberculosis (TB) that could result in 1.8 million deaths (1). TB has become an increasingly serious global public health problem and was regarded as a major cause of illness and death. TB treatment was challenging with the emergence of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) (1–6). However, there was still lack of ideal and available chemotherapies for the treatment of TB. It was urgent and valuable to develop novel anti-tuberculosis treatment (ATT) to control the global tuberculosis epidemic.
Vitamin D deficiency was widespread in active tuberculosis. Vitamin D deficiency was associated with an increased susceptibility to tuberculosis infection and increased the rate of conversion from latent to active tuberculosis (7–12). Thus, vitamin D might have the capability to decrease the risk of TB infection, prevent the progression from latent to active TB, decrease the duration and to improve treatment effectiveness as an adjunct to antimicrobial therapy (13, 14). Vitamin D had some potential in improving the anti-mycobacterial activity in monocytes, and it was partly mediated by the upregulation of the antimicrobial peptide LL-37 (15, 16). Vitamin D was found to increase the response to antimicrobial therapy for pulmonary TB (17).
Accumulating relevant RCTs showed that vitamin D supplementation failed to significantly improve sputum smear-negative conversion rates, and to reduce erythrocyte sedimentation rate (ESR) (18–21). Considering these inconsistent effects, we conducted a systematic review and meta-analysis to evaluate the efficacy and safety of vitamin D supplementation for the treatment of pulmonary TB.
Methods
This review and meta-analysis was conducted according to the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement (22) and the Cochrane Handbook for Systematic Reviews of Interventions (23).
All analyses were based on previously published studies, and thus no ethical approval and patient consent were required.
Literature search and selection criteria
Medline, SCOPUS, Google Scholar, EMBASE, Springer, and Science Direct were systematically searched from inception to Oct 2016, with the following keywords: vitamin D and pulmonary tuberculosis or pulmonary TB. No limitation was enhanced. To include additional eligible studies, the reference lists of retrieved studies and relevant reviews were also hand-searched and the process above was performed repeatedly until no further article was identified. Conference abstracts meeting the inclusion criteria were also included.
The inclusion criteria were as follows: study population, patients with pulmonary TB; intervention, vitamin D plus ATT; control, placebo plus ATT; outcome measure, sputum smear-negative conversion rates; and study design, randomized controlled trials (RCT) or controlled clinical trials (CCT).
Data extraction and outcome measures
The following information was extracted for the included studies: first author, publication year, sample size, baseline characteristics of patients, intervention of vitamin D supplementation, intervention of control, study design, sputum smear-negative conversion rates, body mass index (BMI) and erythrocyte sedimentation rate (ESR). The author would be contacted to acquire the data when necessary.
The primary outcome was sputum smear-negative conversion rates. Secondary outcomes included BMI and ESR.
Quality assessment in individual studies
Two reviewers independently performed data extraction and quality assessment. Four items were used to assess the quality of included studies based on Cochrane Collaboration recommended criteria: Adequate sequence generation, Allocation concealment, Blinding, and addressing the problem of incomplete outcome data.
Statistical analysis
Mean differences (MDs) with 95% confidence intervals (CIs) for continuous outcomes (BMI, ESR, and serious adverse events) and relative risks (RRs) with 95% CIs for dichotomous outcomes (sputum smear-negative conversion rates) were used to estimate the pooled effects. The meta-analyses were performed using the fixed effect model or random effects model when appropriate. Heterogeneity was tested using the Cochran Q statistic (P<0.1) and quantified with the I2 statistic, which described the variation of effect size that was attributable to heterogeneity across studies. An I2 value greater than 50% indicated significant heterogeneity. Sensitivity analysis was performed to detect the influence of a single study on the overall estimate via omitting one study in turn when necessary. Owing to the limited number (<10) of included studies, publication bias was not assessed. P<0.05 in two-tailed tests was considered statistically significant. All statistical analyses were performed with Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Description of studies and quality assessment
Fig. 1 shows the search strategy and selection process of this meta-analysis. In all, 838 studies in the first search seemed to be potentially relevant. Overall, 215 duplicates were removed.
Fig. 1:
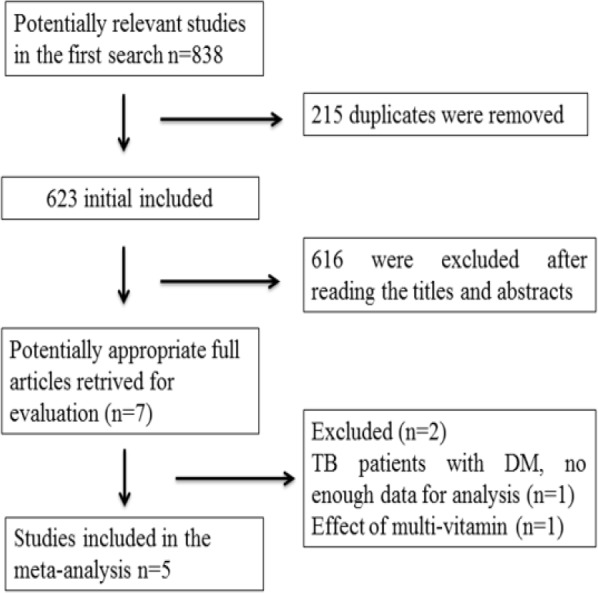
Flow diagram of study searching and selection process
A total of 616 studies were excluded (irrelevant subjects) on the basis of initial screening of the titles and/or abstracts. One study was removed for not providing enough data of TB patients with diabetes mellitus (DM) for analysis and one study regarding the effect of multi-vitamin on pulmonary TB was removed. The remaining 5 articles were included in the meta-analysis (18–21, 24).
Five trials were conducted and they were all RCTs (18–21, 24). In four trials, high dose of vitamin D (50,000U–60,000U or 2.5 mg every 1–2 wk) was administrated orally or intramuscularly (Table 1). After contacting the authors, four articles got “yes” in “Adequate sequence generation”, “Allocation concealment” and “Blinding” (18–21). But one trial only got one “yes” in “Adequate sequence generation” (24) (Table 2).
Table 1:
Characteristics of included studies
| NO. | Author | Vitamin D supplemented group | Vitamin D not supplemented group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age | Weight (kg)/BMI | Methods and Dosage | Follow up | No. | Age | Weight (kg)/BMI | Methods and Dosage | Follow up | ||
| 1 | Kota 2011 | 15 | 38.4±19.6 (mean±SD) | 49.1 ± 4.5 (mean±SD) | Oral calciferol (60,000 U/week) + ATT | 12 w | 15 | 40.2±17.7 (mean±SD) | 44.6±5.6 (mean±SD) | ATT | 12w |
| 2 | Martineau. 2011 | 62 | 30.7 (median) | 20.1±3.1 (mean±SD) | Four oral doses of 2.5 mg vitamin D at 1, 2, 4 and 6 wk after the start of antimicrobial treatment+ ATT | 8 w | 64 | 30.5 (median) | 20.2±2.7 (mean±SD) | placebo + ATT | 8 w |
| 3 | Ralph 2013 | 101 | 29 (15–65) (median/range) | 19.1 (13.3–32.5) (median/range) | Oral cholecalciferol 50,000 U (1250 mcg, 1 tablet) at baseline and on day 28+ ATT | 24 w | 99 | 26(15–73) (median/range) | 19.3 (12.0–26.3) (median/range) | placebo + ATT | 24 w |
| 4 | Salahuddin 2013 | 132 | 27.8±13.2 (mean±SD) | 17.2 (11–25) (median/rang) | Intramuscular calciferol (60,000U/twice/month) + ATT | 12 w | 127 | 28.1±14.1 (mean±SD) | 17.3 (11–27) (median/range) | placebo + ATT | 12 w |
| 5 | Tukvadze 2015 | 100 | 32.4±10.6 (mean±SD) | - | 50,000 U vitamin D3 orally 3 times for 8 consecutive weeks, followed by 50,000 IU vitamin D3 orally every 2 wk for an additional 8 wk+ ATT | 16 w | 99 | 34.1±12.4 (mean±SD) | - | placebo + ATT | 16 w |
Table 2:
Quality assessment of included studies
| NO. | Included studies | Type of study | Adequate sequence generation | Allocation concealment | Blinding | Incomplete outcome data addressed |
|---|---|---|---|---|---|---|
| 1 | Kota 2011 | RCT | Y | U | U | N |
| 2 | Martineau 2011 | RCT | Y | Y | Y | N |
| 3 | Ralph 2013 | RCT | Y | Y | Y | N |
| 4 | Salahuddin 2013 | RCT | Y | Y | Y | N |
| 5 | Tukvadze 2015 | RCT | Y | Y | Y | N |
RCT: randomized controlled trial, CCT: clinical controlled trial, Y: yes, N: no, U: unclear.
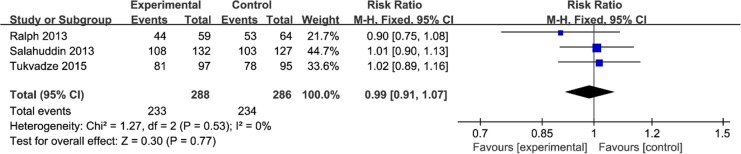
Primary outcomes: Sputum smears negative conversion rates
This outcome data were analyzed with the fixed-effect model, the pooled estimate of three included RCTs suggested that vitamin D supplementation had no influence on sputum smear-negative conversion rates (RR=0.99; 95% CI=0.91 to 1.07; P=0.77), with no heterogeneity among the studies (I2=0%, heterogeneity P=0.53, Fig. 2).
Fig. 2:
Forest plot for the meta-analysis of sputum smear-negative conversion rates
Sensitivity analysis
No heterogeneity was observed among the included studies for the sputum smear-negative conversion rates. Thus, we did not perform sensitivity analysis by omitting one study in each turn to detect the source of heterogeneity.
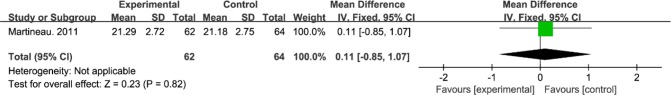
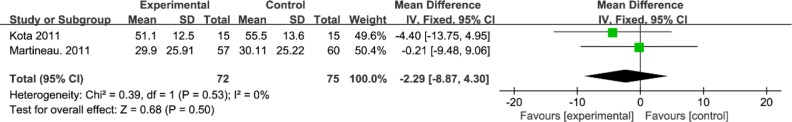
Secondary outcomes
Compared to control group, vitamin D supplementation showed no significant influence on BMI (MD=0.11; 95% CI=−0.85 to 1.07; P=0.82; Fig. 3) and ESR (MD=−2.29; 95% CI=−8.87 to 4.30; P=0.50; Fig. 4).
Fig. 3:
Forest plot for the meta-analysis of body mass index (BMI, kg/m2)
Fig. 4:
Forest plot for the meta-analysis of erythrocyte sedimentation rate (ESR, mm/h)
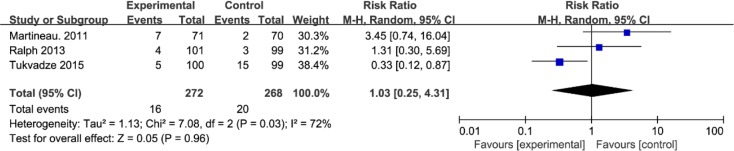
Adverse events
Serious adverse events were defined as any potentially life-threatening deterioration in health status within the study-monitoring period. They mainly included death, multi-organ failure, pneumonia, epistaxis and haemolytic anaemia, etc. There was no significant difference of serious adverse events between vitamin D supplementation group and placebo group (RR=1.03; 95% CI=0.25 to 4.31; P=0.96; Fig. 5).
Fig. 5:
Forest plot for the meta-analysis of serious adverse events in TB patients
Discussion
Our meta-analysis clearly suggested that compared to placebo intervention, vitamin D supplementation had no significant influence on sputum smear-negative conversion rates, BMI and ESR. There were similar serious adverse events between vitamin D supplementation group and placebo group. This was the first meta-analysis to study the treatment efficacy of vitamin D supplementation for pulmonary TB.
Vitamin D could enhance the containment and killing of Mycobacterium tuberculosis through activating 25-hydroxyvitamin D receptors (VDRs) of immune cells (25). Patients with low 25-hydroxyvitamin D levels were found to have increased susceptibility to TB infection and showed high risk of progression from TB infection to disease (13, 16). 25-hydroxyvitamin D demonstrated some capability to induce mycobacterial killing (26). However, our meta-analysis showed that vitamin D supplementation had no substantial effect on the treatment efficacy of pulmonary TB.
These negative findings regarding the effect of vitamin D on pulmonary TB might be explained as follows: firstly, the “vitamin D deficiency” in patients with active TB and possibly not TB but other factors caused the deficiency of vitamin D. Secondly, there might be just some patients obtain substantial effects from a supplementary dose of vitamin D (21, 27). Thirdly, vitamin D as an adjunctive therapy might show some potential in treating pulmonary TB, but the dose and time period of use may be insufficient in current clinical studies.
Supplementary vitamin D 60000 IU was administered intramuscularly in 199 Pakistani pulmonary TB patients and resulted in significantly greater weight gain (1.14 kg) and greater radiological improvements compared with patients receiving placebo, but it showed no impact on sputum smear clearance rates (19). It was almost not possible to cause hypercalcemia in TB patients after using Vitamin D (28). Future studies should focus on higher doses of vitamin D, and targeted participant selection for active TB (29).
Several limitations should be taken into account. Firstly, our analysis was based on only five RCTs and more clinical trials with larger sample were needed to explore this issue. The follow-up period of some studies was not long enough to obtain the results of long-term effects and the follow-up time points varied across studies. Next, the data regarding radiological appearances, cavity closure rates and immunological indicators (e.g. IL-2, IFN-γ and TNF-α) were not tested in the included trials. Finally, some unpublished and missing data might lead bias to the pooled effect.
Conclusion
Vitamin D supplementation showed no significantly favorable influence on improving outcome in patients with pulmonary TB, but more RCTs with higher dose of vitamin D and a longer time of use were needed to further confirm this issue for pulmonary TB.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Lienhardt C, Vernon A, Raviglione MC. (2010). New drugs and new regimens for the treatment of tuberculosis: review of the drug development pipeline and implications for national programmes. Curr Opin Pulm Med, 16(3):186–93. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins HE, Plesca V, Ciobanu A, et al. (2013). Assessing spatial heterogeneity of MDR-TB in a high burden country. Eur Respir J, 42(5):1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen T, Jenkins HE, Lu C, et al. (2014). On the spread and control of MDR-TB epidemics: an examination of trends in anti-tuberculosis drug resistance surveillance data. Drug Resist Updat, 17(4–6):105–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingfield T, Boccia D, Tovar M, et al. (2014). Defining Catastrophic Costs and Comparing Their Importance for Adverse Tuberculosis Outcome with Multi-Drug Resistance: A Prospective Cohort Study, Peru. PLoS Med, 11(7):e1001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks SM, Flood J, Seaworth B, et al. (2014). Treatment Practices, Outcomes, and Costs of Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis, United States, 2005–2007. Emerg Infect Dis, 20(5):812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naidoo P, van Niekerk M, du Toit E, et al. (2015). Pathways to multidrug-resistant tuberculosis diagnosis and treatment initiation: a qualitative comparison of patients’ experiences in the era of rapid molecular diagnostic tests. BMC Health Serv Res, 15:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wejse C, Olesen R, Rabna P, et al. (2007). Serum 25-hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr, 86(5):1376–83. [DOI] [PubMed] [Google Scholar]
- 8.Yamshchikov AV, Kurbatova EV, Kumari M, et al. (2010). Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr, 92(3):603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martineau AR, Nhamoyebonde S, Oni T, et al. (2011). Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A, 108(47):19013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai NS, Tukvadze N, Frediani JK, et al. (2012). Effects of sunlight and diet on vitamin D status of pulmonary tuberculosis patients in Tbilisi, Georgia. Nutrition, 28(4):362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Park JS, Cho YJ, et al. (2014). Low serum 25-hydroxyvitamin D level: An independent risk factor for tuberculosis? Clin Nutr, 33(6):1081–6. [DOI] [PubMed] [Google Scholar]
- 12.Mehta S, Mugusi FM, Bosch RJ, et al. (2013). Vitamin D status and TB treatment outcomes in adult patients in Tanzania: a cohort study. BMJ Open, 3(11):e003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibney KB, MacGregor L, Leder K, et al. (2008). Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis, 46(3):443–6. [DOI] [PubMed] [Google Scholar]
- 14.Chocano-Bedoya P, Ronnenberg AG. (2009). Vitamin D and tuberculosis. Nutr Rev, 67(5):289–93. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. (2007). Vitamin D deficiency. N Engl J Med, 357(3):266–81. [DOI] [PubMed] [Google Scholar]
- 16.Nnoaham KE, Clarke A. (2008). Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol, 37(1):113–9. [DOI] [PubMed] [Google Scholar]
- 17.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. (2007). Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol, 103(3–5):793–8. [DOI] [PubMed] [Google Scholar]
- 18.Ralph AP, Waramori G, Pontororing GJ, et al. (2013). L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS One, 8(8):e70032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salahuddin N, Ali F, Hasan Z, et al. (2013). Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis, 13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tukvadze N, Sanikidze E, Kipiani M, et al. (2015). High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr, 102(5):1059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martineau AR, Timms PM, Bothamley GH, et al. (2011). High-dose vitamin D 3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet, 377(9761):242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol, 62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S. (2011). Cochrane Handbook for Systematic Reviews of Interventions Version5.1 [updated March 2011]. http://handbook-5-1.cochrane.org
- 24.Kota SK, Jammula S, Kota SK, et al. (2011). Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diabetes Metab Syndr, 5(2):85–9. [DOI] [PubMed] [Google Scholar]
- 25.Liu PT, Stenger S, Tang DH, Modlin RL. (2007). Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol, 179(4):2060–3. [DOI] [PubMed] [Google Scholar]
- 26.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. (2007). A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med, 176(2):208–13. [DOI] [PubMed] [Google Scholar]
- 27.Lappe JM, Heaney RP. (2012). Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol, 4(2):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narang NK, Gupta RC, Jain MK. (1984). Role of vitamin D in pulmonary tuberculosis. J Assoc Physicians India, 32(2):185–8. [PubMed] [Google Scholar]
- 29.Vieth R. (2011). Vitamin D nutrient to treat TB begs the prevention question. Lancet, 377(9761):189–90. [DOI] [PubMed] [Google Scholar]