Abstract
Non-invasive drug and gene delivery to the brain to treat central nervous system pathologies has long been inhibited by the blood-brain barrier. The activation of microbubbles with focused ultrasound has emerged as a promising non-invasive approach to circumvent this obstacle, by transiently disrupting the blood-brain barrier and permitting passage of systemically administered therapeutics into the tissue. Clinical trials are underway to evaluate the safety of this technique; however, concerns remain regarding the potential for the treatment to induce sterile inflammation or petechiae. In this issue of Theranostics, Jones et al.[1] address these concerns through the development of an advanced three-dimensional imaging system for monitoring acoustic emissions from oscillating microbubbles. When subharmonic emissions are detected with this system, focused ultrasound pressure is reduced by 50% for the remainder of the treatment. This serves to transiently open the blood-brain barrier without generating adverse effects. While the ideal configuration of the transducer array for treatment and monitoring still presents an area for further optimization, the approach indicates that the acoustic signature of microbubble behavior within the skull can be used to ensure safe and effective blood-brain barrier opening using focused ultrasound.
Keywords: focused ultrasound, blood-brain barrier, acoustic emissions, acoustic monitoring, targeted drug and gene delivery
Many diseases of the central nervous system (CNS) have limited treatment options. Despite significant advancements in understanding these diseases, development of effective CNS therapeutics is greatly hindered by the blood brain barrier (BBB), which prevents penetration of most systemically administered therapeutics into brain tissue 2. The BBB consists of tight junctions between capillary endothelial cells, preventing paracellular transport of agents into the tissue. Barrier function is further supported by a thick basement membrane, pericytes, and astrocyte endfeet, which together form the neurovascular unit. While some small, lipophilic molecules can pass through the barrier, 98% of small molecule drugs and 100% of large molecule drugs are unable to enter brain tissue from systemic circulation 2. One method to overcome the BBB is to engineer drugs for carrier-mediated or receptor-mediated transport across the BBB, taking advantage of endogenous transport mechanisms. Alternatively, the BBB can be bypassed by direct injection methods such as convection enhanced delivery (CED), which are very invasive. Agents such as mannitol can permeabilize the blood brain barrier, however this induces BBB disruption throughout the brain 3, 4.
Focused ultrasound (FUS) is a non-invasive method for targeted and reversible blood brain barrier disruption with sub-millimeter precision that holds great promise for revolutionizing the treatment of CNS diseases. In the presence of circulating microbubbles (MBs), FUS yields transient tight junction opening, vascular endothelial sonoporation, and enhanced transcytosis spanning an estimated 4-6 hour period over which the BBB is open 5,6. The bioeffects exerted by circulating MBs vary as a function of acoustic pressure. In stable cavitation regimens, MBs steadily oscillate in size within an acoustic field to produce mechanical shear forces and circumferential stresses on microvessel walls. Inertial cavitation occurs when these oscillations lose stability in a manner that generates rapid, violent bubble collapse; this can impose irreversible damage on vasculature and surrounding tissues through highly localized temperature rise, acoustic streaming, and shock wave formation 6. BBB disruption with FUS and MBs has played a pivotal role in enabling a vast array of pre-clinical applications exploring the delivery of drug- or gene-bearing nanocarriers, chemotherapeutics, antibodies, bioactive compounds, and even cells to the CNS across a breadth of pathologies 7-16. Moreover, emerging studies suggest that the physical perturbation of tissues with FUS is capable of generating immune modulatory effects in both normal and diseased tissues, a capacity that can be exploited for the treatment of CNS pathologies 17. Taken together, these preclinical studies have established FUS-mediated BBB opening as a pervasive platform for alleviating the bottleneck on therapeutic interventions that are able to effectively target the brain.
Beyond the pre-clinical setting, the last three years have seen significant momentum in the clinical advancement of FUS as a technique for safe, transient BBB opening 18. To date, two separate clinical trials have evaluated the safety of non-invasive BBB opening using a commercially available extracorporeal MRI-guided FUS system (ExAblate Neuro; Insightec). These trials have explored the use of FUS BBB opening with i.v. contrast agents for enhanced chemotherapy delivery to malignant brain tumors (NCT02343991) and in early Alzheimer's disease (NCT02986932). Separately, an implantable ultrasound device (SonoCloud; CarThera) capable of repeated BBB opening in the presence of MBs has been demonstrated as safe and well-tolerated in patients with recurrent glioblastoma 19.
Nonetheless, despite these promising early successes and opportunities for clinical applications of FUS, it is also well-understood that driving microbubbles in the inertial cavitation regime can elicit neuroinflammation and petechiae. In a recent pre-clinical study, blood-brain barrier disruption using FUS and MBs induced a sterile inflammatory response in the brain parenchyma, as evidenced by damage-associated molecular patterns, activation of microglia and astrocytes, and tissue infiltration by CD68+ macrophages (indicative of an innate immune response) 20. However, it has been noted that this study involved a dose of MBs roughly ten times higher than the accepted clinical imaging dose. Ensuing work indicates that higher doses of MBs are associated with higher rates of edema and petechiae, as well as elevated expression of inflammatory genes 21. Animals treated with lower, clinically relevant doses of MBs did not display such responses, suggesting that the parameters of FUS-mediated BBB opening can be optimized in order to enhance drug and gene delivery without causing injury and inflammation 21. Another concern that has been raised is variability in bioeffect response and treatment efficacy between FUS-treated regions, due in part to skull shape/thickness variability across and within animals. This variability is relevant to human clinical applications as well, and has motivated further study to better optimize BBB opening on a case-by-case basis.
In order to better address the risks raised by such preclinical studies, several groups have sought to develop systems for monitoring MB behavior within the brain, and to use these observations to inform treatments. These monitoring systems hinge on the principle of passive cavitation detection, which involves recording the acoustic emissions generated by oscillating MBs within the skull upon exposure to FUS. These acoustic signatures correlate with the cavitation activity of the MBs, as well as the biological effects of the associated FUS exposures 22,23. Previous work by the Hynynen group obviated adverse events by identifying the FUS pressure at which subharmonic signals begin to be detected and then conducting all following sonications at a fixed percentage of this pressure. They identified that sonications conducted at 50% of the threshold pressure (where subharmonic activity was detected) resulted in safe BBB opening without gross tissue damage (Figure 1) 24. In this issue of Theranostics, Jones et al. have iterated upon this technique by using multi-element arrays of sensors to produce a 3-dimensional map of the detected acoustic emissions, in order to monitor MB behavior within and beyond the targeted volume of brain, and identify locations of potentially damaging cavitation behavior1. Theirs is the first system of its kind to be used for active calibration of FUS pressures during treatment (as opposed to offline acoustic reconstructions after treatment has concluded).
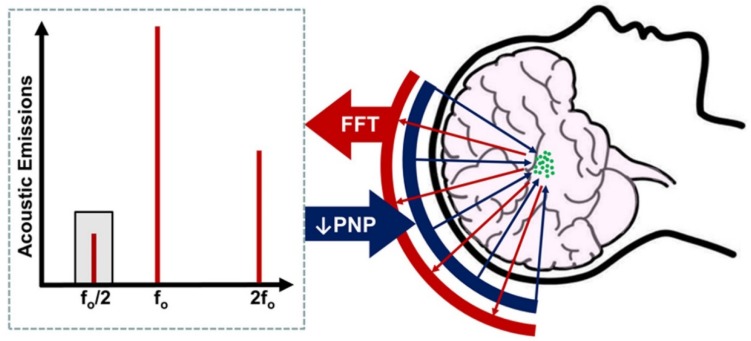
Figure 1.
Basic principle of acoustic emissions monitoring for control of blood-brain barrier opening with focused ultrasound. After intravenous microbubble injection, focused ultrasound (blue arrows) is applied from a phased-array transducer, activating microbubbles (green circles) in the treatment volume. Acoustic emissions from the oscillating microbubbles (red arrows) are monitored by a separate set of elements within the array. The peak-negative pressure (PNP) of applied focused ultrasound is steadily increased until subharmonic (i.e. f0/2; grey shading) emissions are detected. PNP is then reduced by a fixed amount, typically 50%, to ensure safe and reversible BBB opening in the treatment volume.
The FUS phased array designed by Jones et al. for both treatment sonications and cavitation monitoring features 256 individual transducer modules arranged sparsely in a hemispherical dome. Each module consists of three elements in concentric circles, with driving frequencies of 306, 612, or 1224 kHz (from outer to inner). The 612 kHz elements are used to deliver treatment sonications, while the 306 kHz elements detect subharmonic frequency emissions and the 1224 kHz elements pick up ultraharmonic signals. The subharmonic activity detected is filtered and beamformed to identify the spatial source of the microbubble emissions. Jones et al. found that increasing the number of elements in the system improved sensitivity, but that different array configurations resulted in highly variable read-outs. As in previous studies, the peak negative pressure of the ultrasound sonications was increased until the system detected spatially-coherent microbubble activity in the subharmonic frequency (with an average non-derated pressure of 0.68 MPa required to achieve subharmonic activity).
After calibrating the system to a particular point in space, and identifying the threshold pressure, a grid of points around the calibration point were sonicated at fixed percentages of the threshold pressure. Consistent with previous findings 24, brain regions treated at 75-95% of the threshold pressure displayed extravasated red blood cells upon histological examination and by T2*w MR imaging. In comparison, tissues treated at 50% of threshold showed no signs of damage, with the exception of one individual focus within one grid, which showed minor erythrocyte extravasation that was not detectable by T2*w imaging. All volumes treated at 50% of the threshold pressure displayed increased blood-brain barrier permeability, though to variable degrees. The authors hypothesize that this could be due to differences in cerebral vascularity, skull thickness, or other variability between subjects and across locations within the same subject. This finding suggests the need to calibrate treatment pressures to each and every sonication point for optimal safety in the clinic.
In summary, this study1 supports the growing body of promising evidence for using the acoustic emissions of oscillating microbubbles to control the efficacy and safety of focused ultrasound-mediated blood-brain barrier disruption 25,26. While further study is needed to continue to optimize the sensitivity of acoustic emissions detection and rapidly calibrate sonications on a point-by-point basis, the groundwork laid by these investigators will help to inform the development and use of novel systems for improved monitoring of the safety of FUS-mediated drug and gene delivery in the clinic.
Acknowledgments
Supported by National Institutes of Health Grants R01CA197111, R01EB020147, and R01CA164789.
References
- 1.Jones RM, Deng L, Leung K, McMahon D, O'Reilly MA, Hynynen K. Three-dimensional transcranial microbubble imaging for guiding volumetric ultrasound-mediated blood-brain barrier opening. Theranostics. 2018;8(11):2909–2926. doi: 10.7150/thno.24911. doi:10.7150/thno.24911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8:1481–93. doi: 10.7150/thno.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release. 2015;219:61–75. doi: 10.1016/j.jconrel.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konofagou EE. Optimization of the Ultrasound-Induced Blood-Brain Barrier Opening. Theranostics. 2012;2:1223–37. doi: 10.7150/thno.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–7. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340:1085–90. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–23. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etame AB, Diaz RJ, O'Reilly MA, Smith CA, Mainprize TG, Hynynen K. et al. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine Nanotechnology, Biol Med. 2012;8:1133–42. doi: 10.1016/j.nano.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samiotaki G, Acosta C, Wang S, Konofagou EE. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood-brain barrier opening in vivo. J Cereb Blood Flow Metab. 2015;35:611–22. doi: 10.1038/jcbfm.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Olumolade OO, Sun T, Samiotaki G, Konofagou EE. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 2015;22:104–10. doi: 10.1038/gt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thévenot E, Jordão JF, O'Reilly M a, Markham K, Weng Y-Q, Foust KD. et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther. 2012;23:1144–55. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nance E, Timbie K, Miller GW, Song J, Louttit C, Klibanov AL. et al. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood - Brain barrier using MRI-guided focused ultrasound. J Control Release. 2014;189:123–32. doi: 10.1016/j.jconrel.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mead BP, Mastorakos P, Suk JS, Klibanov AL, Hanes J, Price RJ. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J Control Release. 2016;223:109–17. doi: 10.1016/j.jconrel.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H-L, Fan C-H, Ting C-Y, Yeh C-K. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4:432–44. doi: 10.7150/thno.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curley CT, Sheybani ND, Bullock TN, Price RJ. Focused ultrasound immunotherapy for central nervous system pathologies: challenges and opportunities. Theranostics. 2017;7:3608–23. doi: 10.7150/thno.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Y, Suppiah S, Mithani K, Solomon B, Schwartz ML, Lipsman N. Current and emerging brain applications of MR-guided focused ultrasound. J Ther Ultrasound. 2017;5:26. doi: 10.1186/s40349-017-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C. et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8:343re2. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK. et al. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A. 2017;114:E75–84. doi: 10.1073/pnas.1614777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon D, Hynynen K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound is Dependent on Microbubble Dose. Theranostics. 2017;7:3989–4000. doi: 10.7150/thno.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 23.Tung Y-S, Vlachos F, Choi JJ, Deffieux T, Selert K, Konofagou EE. In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys Med Biol. 2010;55:6141–55. doi: 10.1088/0031-9155/55/20/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Reilly MA, Hynynen K. Blood-Brain Barrier: Real-time Feedback-controlled Focused Ultrasound Disruption by Using an Acoustic Emissions-based Controller. Radiology. 2012;263:96–106. doi: 10.1148/radiol.11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun T, Zhang Y, Power C, Alexander PM, Sutton JT, Aryal M. et al. Closed-loop control of targeted ultrasound drug delivery across the blood-brain/tumor barriers in a rat glioma model. Proc Natl Acad Sci U S A. 2017;114:E10281–90. doi: 10.1073/pnas.1713328114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Reilly MA, Jones RM, Barrett E, Schwab A, Head E, Hynynen K. Investigation of the Safety of Focused Ultrasound-Induced Blood-Brain Barrier Opening in a Natural Canine Model of Aging. Theranostics. 2017;7:3573–84. doi: 10.7150/thno.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]