Abstract
Nanotechnology-based antitumor drug delivery systems, known as nanocarriers, have demonstrated their efficacy in recent years. Typically, the size of the nanocarriers is around 100 nm. It is imperative to achieve an optimum size of these nanocarriers which must be designed uniquely for each type of delivery process. For pH-responsive nanocarriers with programmable size, changes in pH (~6.5 for tumor tissue, ~5.5 for endosomes, and ~5.0 for lysosomes) may serve as an endogenous stimulus improving the safety and therapeutic efficacy of antitumor drugs. This review focuses on current advanced pH-responsive nanocarriers with programmable size changes for anticancer drug delivery. In particular, pH-responsive mechanisms for nanocarrier retention at tumor sites, size reduction for penetrating into tumor parenchyma, escaping from endo/lysosomes, and swelling or disassembly for drug release will be highlighted. Additional trends and challenges of employing these nanocarriers in future clinical applications are also addressed.
Keywords: nanocarriers, endogenous pH-responsive, size change, targeted drug delivery, tumor therapy
1. Introduction
Over the last decade, tremendous progress has been made in the development of nanocarriers as effective antitumor drug delivery agents. These carriers are highly attractive due to their nanometer size and versatile surface properties, which enhance their pharmacokinetics and bio-distribution while reducing systemic toxicity 1-5. To date, a broad range of nanocarriers has been designed and tested, including polymer nanoparticles, micelles, liposomes, dendrimers, star polymers, and inorganic nanoparticles made of iron oxide, quantum dots, silica, gold, and metal oxide frameworks 1, 6-18. Intravenously injected nanomedicine undergoes a multistep process before reaching solid tumors 19, 20. Therefore, for efficient drug delivery in the complex in vivo environment, it is important to develop multiple stimuli-responsive nanocarriers that can minimize nonspecific interactions with and uptake by non-targeted cells or the immune system 6, 21-24. Specifically designed intelligent stimuli-sensitive nanocarriers can increase the concentration of effective anticancer drugs by targeting their delivery to tumor locations, thus allowing for greater therapeutic efficacy and reducing undesired side effects.
Although much work has been carried out on the development of drug-delivery systems affected by external stimuli, various limitations have greatly hindered their biomedical application. For example, several temperature- and light-sensitive pharmaceuticals can damage normal cells and even tissues and organs. The magnetic-associated preparations (used as contrast agents in MRI) are limited by the stringent requirements of carrier materials. The ultrasound-associated formulations are usually used for synergistic therapy. Considering the limitations of external stimulant sources (e.g. light, ultrasound, magnetic fields), endogenous stimulations, such as enzymes 25-29, glucose concentrations 30, 31, redox reactions 32-38, temperatures 39-41 and pH differences 34, 42-51, are more useful when designing safe, efficient, and intelligent carriers. Among these endogenous stimuli, pH differences have been the most widely used control parameter for tumor-targeted drug delivery and controlled intracellular drug release. This is possible due to the pH differences between healthy and diseased tissues as well as between various cellular compartments 6, 52-56. Given the vulnerability of acid-base homeostasis, acidic microenvironment changes are associated with pathological tissues, such as in ischemia, inflammatory diseases, infections, rheumatoid arthritis, or solid tumors 6, 52, 57-60. Especially in solid tumor tissues, the substantial energy requirement for their intensive growth results in increased lactate and hydrogen ions (H+) produced by catabolism of glucose. Consequently, the tumor microenvironment becomes acidic with a pH ~6.5 in tumor tissues versus pH ~7.4 in normal tissues 57, 61-63. This difference can then be exploited for targeting the tumor tissue or triggering drug release in the tumor's extracellular matrix 60, 64-68. At the cellular level, the intracellular acidic components (pH ~5.5 for endosome, pH ~5.0 for lysosome) can also be used to trigger drug release and promote the escape of the carrier into the cytoplasm 6, 69-72. Therefore, these endogenous pH differences provide a strong option for targeted antitumor drug delivery and controllable release kinetics.
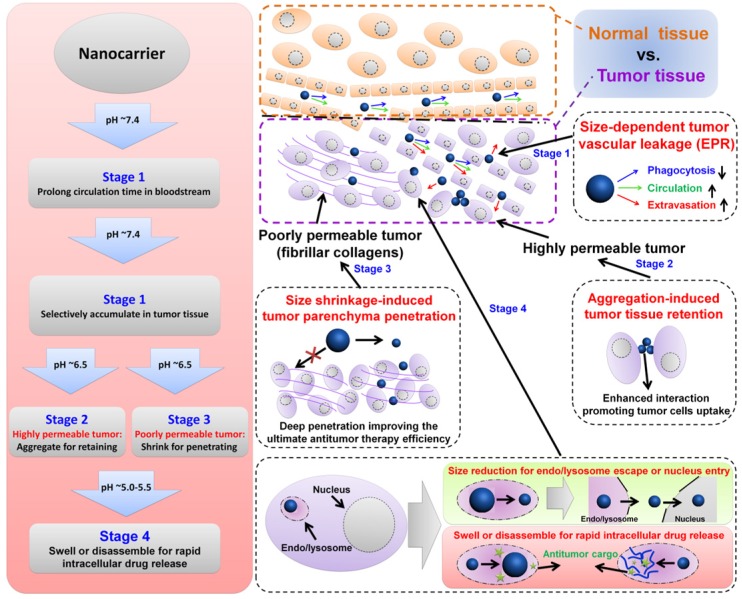
In general, the physicochemical characteristics of nanocarriers, including size, architecture, and surface properties determine their fate by affecting one or more of the above steps in the antitumor drug delivery process 1, 47, 49, 52, 60, 64, 73-75. Size-dependent enhanced antitumor findings have received much attention because of the variable and strict size requirements of the entire cascade processes, including their biodistribution, immune activation, blood circulation, tumor target accumulation/retention, tumor tissue penetration, tumor cell uptake, and final cargo release (Figure 1) 52, 76-81. For example, small nanocarriers (typically <10-20 nm) can spread into various organs, but they are also rapidly eliminated through the kidneys' glomeruli 1, 82. Large nanocarriers (typically >200 nm) tend to be rapidly taken up by the mononuclear phagocytic system (MPS) and subsequently accumulate in the liver, spleen, and to a lesser extent in the bone marrow 1, 83-88. Compared to the tight endothelial junctions of normal vessels (5-10 nm), the pores of tumor vascular walls have typical junction sizes ranging from 200 nm to 1200 nm allowing extravasation of appropriately sized nanocarriers into solid tumors 1, 19. Also, the lack of functional lymphatic drainage in tumor tissues prevented the reentry of accumulated nanocarriers into blood circulation. This phenomenon, termed the enhanced permeability and retention (EPR) effect, is the rational basis for passive targeting in antitumor drug delivery 1, 7, 89. However, proliferating tumor cells compress the intratumor blood and lymphatic vessels, leading to a uniformly elevated interstitial fluid pressure. Additionally, increased fibrillar collagens (such as type I and type III) in some tumor interstitial matrix can greatly hinder larger (>60 nm) nanocarriers from penetrating deeply into the tumor parenchyma 19, 90, 91. Therefore, pH-responsive nanocarriers with programmable size changes can be automatically altered during each stage of the anti-tumor drug delivery.
Figure 1.
Illustration of size-dependent antitumor drug delivery. stage 1: extravasation based on the tumor vascular pore size selection (Three arrows indicate that less phagocytosis by MPS, longer circulation time in bloodstream, and higher extraversion at tumor vasculature are beneficial for enhancing passive targeting of tumor by EPR effect); stage 2: antitumor drug delivery in the highly permeable tumor tissue; stage 3: antitumor drug delivery in the poorly permeable tumor tissue; stage 4: tumor cell uptake and subsequent intracellular drug release. pH ~7.4 for normal tumor tissue and blood stream, ~6.5 for tumor tissue, ~5.5 for endosome, ~5.0 for lysosome.
Recently, remarkable advances in size-dependent smart nanocarriers have attracted great interest for their advanced and efficient antitumor drug delivery as well as their reduced systematic side effects. Although pH-responsive 6, 92-102 and size-related 1, 20, 83, 103, 104 antitumor studies have been amply discussed in previous review articles, there is a need to review several aspects of endogenous pH-responsive nanocarriers for antitumor therapy. Considering the unique advantages of nanocarriers' size characteristics for antitumor drug delivery, this review focuses on pH-responsive nanocarriers with programmable size changes in antitumor applications, especially highlighting recently advanced design strategies.
2. Endogenous pH-responsive carriers with programmable size changes for antitumor drug delivery
To achieve efficient targeted payload delivery, nanocarriers must meet the following challenges: (a) long-term stability in blood circulation as well as retention within tumor lesions, (b) ability to accumulate at tumor tissue site and penetrate deeply into tumor parenchyma, and (c) avoiding the premature release of payload before reaching the target and then accelerating drug release once at the target 105. To achieve safe and efficient antitumor drug delivery, pH-responsive nanocarriers with programmable size changes based on the tumoral/intracellular phase-transition (protonation/deprotonation and cleavable bond) (Table 1) offer a feasible approach for overcoming many contradictory requirements during various phases.
Table 1.
The typical representative pH-responsive structures in the tumoral and intracellular endo/lysosomes.
| Phase-transition | Structure (Name) | pH | Examples | |
|---|---|---|---|---|
| Tumoral acid responsive structures (pH 6.5-7.2) |
Protonation |
 (PC6A) (PC7A) |
~ 7.2 ~ 6.9 |
99, 106-108 |
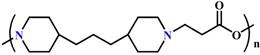 (PAE) |
~ 7.0 | 109-112 | ||
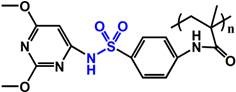 (PSD) |
~ 7.0 | 113-115 | ||
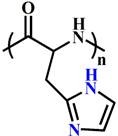 (PHis) |
~ 6.5 | 116-119 | ||
| Deprotonation |
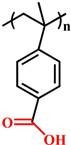 (PVBA) |
~ 7.1 | 120-123 | |
| Acid-labile bond |
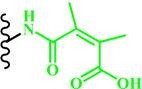 (DMMA) |
~ 6.8 | 53, 60, 124, 125 | |
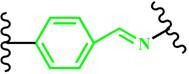 (Benzoic-imine) |
~ 6.5 | 126-129 | ||
| Endo/lysosome acid responsive structures (pH 4.5-6.5) |
Protonation |
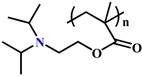 (PDPA) |
~ 6.3 | 49, 52, 64, 77, 130 |
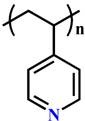 (P4VP) |
~ 5.6 | 131-134 | ||
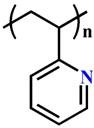 (P2VP) |
~ 5.0 | 135-137 | ||
| Deprotonation |
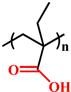 (PMAA) |
~ 6.3 | 138-141 | |
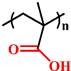 (PEAA) |
~ 5.6 | 142, 143 | ||
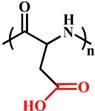 (PAsp) |
~ 5.0 | 144-147 | ||
| Acid-labile bond |
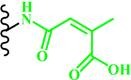 (Citraconic amide) |
~ 5.5 | 55, 148-150 | |
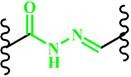 (Hydrazone) |
~ 6.0 | 117, 151-153 | ||
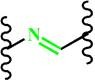 (Imine) |
~ 6.5 | 154-157 | ||
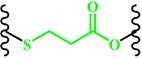 (β-Thiopropionate) |
~ 5.5 | 158-161 | ||
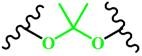 (Ketal) |
~ 5.0 | 162-165 | ||
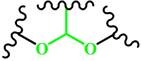 (Acetal) |
~ 5.0 | 166-169 | ||
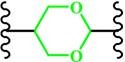 (Cyclic acetal) |
~ 5.0 | 170-172 | ||
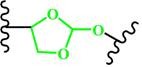 (Ortho ester) |
~ 5.0 | 72, 173-176 |
2.1. pH-responsive aggregation for tumor retention
2.1.1. Design principle
The long-term stability in the circulatory system necessitates nanocarriers with a different size than that required for uptake by tumor cells 8, 76, 90, 177-179. From previous anti-tumor studies, the well-accepted size for long-term blood circulation is around 100 nm due to the size-dependent balance between organ filtration and tumor vessel extravasation 8, 76, 83. After intravenous injection, carriers circulating for a longer time in the blood have a better chance of accumulating in the tumor tissue via EPR effects.
Therefore, for prolonging the blood circulation time, it is extremely important to avoid nonspecific interactions with blood components and other off-targeted cells. Also, nanocarriers undergo a series of screening events by the reticuloendothelial system (RES) and organs such as the liver, spleen, lung, and kidney. Improvement of their “stealth” capabilities is a key aspect for prolonging blood circulation time. Furthermore, once accumulation begins in tumor tissue, it is ideal for nanocarriers to “stick” to the tumor cells.
As a result of the time- and concentration-dependent internalization process caused by enhanced interactions between nanocarriers and cells, carriers can have increased chances of tumor cells uptake and retention in tumor tissue 1, 89, 180. Cytotoxicity of aggregated nanocarriers compared to non-aggregated ones has also been reported 179. All in all, nanocarriers must be “stealthy” in blood circulation to evade undesirable RES recognition or protein/cell adhesion but must be “sticky” to interact with tumor cells. To this end, nanoparticles with sizes of 20-200 nm are appropriate to avoid undesirable clearance through the nonspecific adsorption and urinary excretion. However, the design strategy employed may need to take into account the opposing requirements depending on the intercellular space. For the highly permeable tumor parenchyma with large intercellular space, the aggregated nanoparticles can be retained in the tumor tissue to enhance tumor cell endocytosis. This is one of the areas where the tunable properties of nanocarriers are advantageous as their surfaces can be functionalized appropriately to fulfill size requirement.
2.1.2. pH-responsive aggregation based on protonation/deprotonation
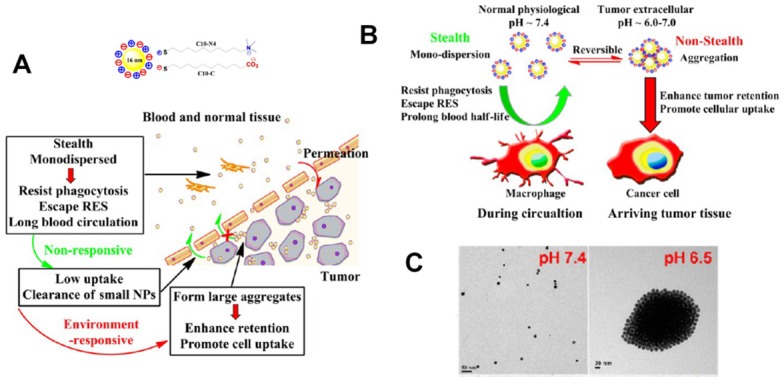
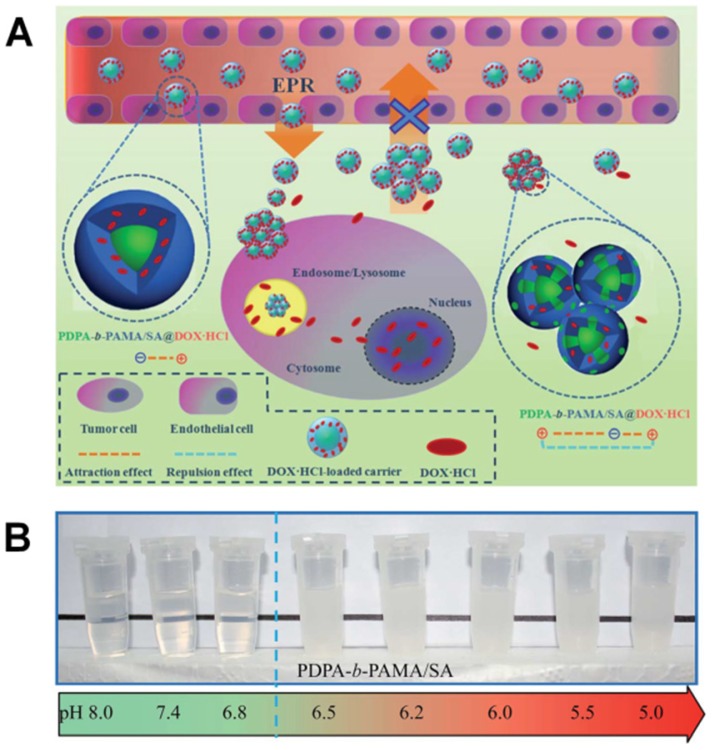
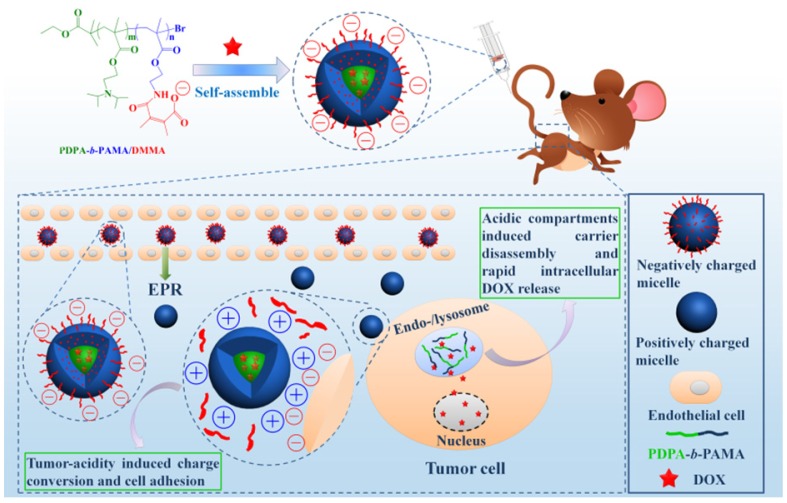
pH-responsive aggregation triggered by slightly acidic stimuli of the extracellular tumor matrix is a suitable mechanism for designing nanocarriers with low uptake by normal cells but high uptake by tumor cells. Based on the protonation/deprotonation transition triggered by mild acidic stimuli, Ji and coworkers prepared a series of mixed-charge zwitterionic monolayer-modified gold nanoparticles (Figure 2) 89, 181. Owing to zwitterionic monolayer protonation/deprotonation balance, the optimized nanoparticles were stable at the physiological pH of the bloodstream and normal tissues but rapidly aggregated within a few seconds in response to a slight pH change from 7.4 to 6.5. This work demonstrated that controllable aggregation at tumor sites could enhance retention efficiency and cellular uptake of nanoparticles in tumors for photo-thermal tumor-targeted diagnosis and therapy. Inspired by this study on the surface charge conversion resulting from ultra acid-labile amide bond breakage, we fabricated a pH-responsive polymeric nanocarrier with tumor-targeted aggregation properties for enhanced retention and endocytosis (Figure 3) 52. The doxorubicin-loaded smart nanocarrier was constructed from succinic anhydride-modified poly (2-diisopropylaminoethyl methacrylate)-block-poly(2-aminoethyl methacrylate hydrochloride) (PDPA-b-PAMA/SA@DOX) and was responsive to a slightly acidic stimulus. It exhibited size aggregation from ~154 nm to ~1517 nm due to the pH-responsive protonation of PDPA block-induced electrostatic attraction with its negatively charged corona. Importantly, cell uptake studies showed that the aggregated nanomedicine was endocytosed by tumor cells at pH 6.5 rather than pH 7. 4. Using the same mechanism of pH-sensitive surface charge transition, Chiu and coworkers constructed a nanocarrier using N-acetyl histidine-modified D-α-tocopheryl polyethylene glycol succinate on the surface of poly (lactic-co-glycolic acid) 182. The increased protonation of imidazole groups from histidine residues resulted in the lack of sufficient interparticle electrostatic repulsion for maintaining stable individual particles at pH 6.5, and the DOX-loaded nanoparticles with an almost neutral surface charge aggregated into large clusters. Both in vitro and in vivo results confirmed that tumor acidity-triggered surface charge neutralization and aggregation could significantly enhance tumor cell uptake. Ji's group also reported a pH-responsive supra-molecular prodrug micelle based on the host-guest inclusion 183. The size of this supra-assembly could be tuned by pH changes showing significant aggregation triggered by slight acidity of the tumor extracellular matrix. Benefitting from aggregation-induced accumulation in tumor tissues, this supra-assembly exhibited desirable antitumor effect by efficiently inhibiting tumor proliferation. In these design strategies, the pH-responsive size aggregation based on the protonation/deprotonation is reversible, so the nanoparticles can disperse as single nanoparticle once the pH condition recovers, which is convenient for its preparation, storage, and further functionalization.
Figure 2.
(A) Schematic illustration of pH-responsive aggregation of gold nanoparticlesenhancing targeted retention and cellular uptake in response to the tumor extracellular acidic stimuli. (B) Illustration of the size-dependent prolonging of blood circulation time and targeted tumor retention. (C) TEM images of pH-responsive aggregation. Reproduced with permission from 89, copyright 2013 American Chemical Society.
Figure 3.
(A) Scheme and (B) Digital image of the tumor-targeted aggregation of pH-responsive polymeric nanomicelles for enhanced retention at tumor tissue and rapid intracellular drug release in tumor cells. Reproduced with permission from 52, copyright 2014 Royal Society of Chemistry.
2.1.3. pH-responsive aggregation based on cleavable bond at acidic pH
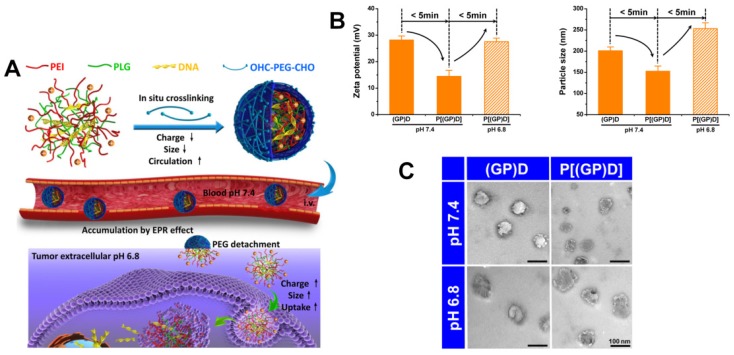
Recently, a nanocarrier with programmable size changes was designed to satisfy the different phases of the drug delivery process by Chen's groups 184. An ideal smart polymeric gene complex was prepared from pH-responsive cleavable polyethylene glycol (PEG) bonded in situ on the surface of a primary complex of polyethyleneimine (PEI), poly-L-glutamate and therapeutic genes via the Schiff base reaction (Figure 4). At the physiological pH 7. 4, the PEG cross-linked complex could shield the surface positive charges and enhance its stability to reduce side effects, prevent premature gene release, and prolong blood circulation time. However, once the complexes accumulated in tumor tissues by EPR, the ultra pH-sensitive Schiff base bonds were responsive to the slightly acidic environment of tumor tissue. The PEG surface layer rapidly detached from the complex. The complex without PEG corona exhibited a higher positive charge potential and larger size facilitating tumor cellular uptake and enhancing antitumor gene delivery. In this design, the cross-linking PEG corona could shield the surface positive charge and compress the nanoparticles tightly, exhibiting prolonged blood circulation, enhanced stability, and reduced cytotoxicity. The PEG layer could be rapidly detached by the acid labile cleavable bond in response to the slight acidity of the tumor tissue exposing the surface positive charge and inflated size to promote tumor cell endocytosis. However, compared to the protonation/deprotonation transition, pH-responsive size change induced by the acid labile cleavable bond is generally irreversible. Therefore, the acid labile nanoparticles have a potential risk of instability in their preparation, storage, and application.
Figure 4.
(A) Schematic of the ultrasensitive pH-triggered charge/size dual-rebound gene delivery system for efficient antitumor applications. (B) Fast hydrodynamic charge/size dual-rebound property and (C) TEM of the gene delivery system. (GP)D and P[(GP)D] represent (PLG/PEI)/DNA and PEG[(PLG/PEI)/DNA], respectively. Reproduced with permission from 184, copyright 2016 American Chemical Society.
2.2. pH-responsive size shrinkage for deep tumor penetration
2.2.1. Design principle
The size of nanocarriers is a significant factor in promoting their accumulation and penetration in tumor tissues 1, 76, 185. To sufficiently illustrate the important role of size in drug delivery, Tang et al. have fabricated micelles of the same chemical structures and physical properties from a single copolymer but ranging in size from 20 nm to 300 nm. Their results indicated that the optimal size of nanocarriers through the cascade processes was different at different stages, i.e. the optimal size range for suitable blood circulation time and tumor accumulation was between 100 and 160 nm. However, greater accumulation of the large micelles (100 nm) at tumor sites did not result in significantly improved therapeutic efficacy because of their poor tumor penetration compared to that of the smaller micelles (30 nm). A relatively large size (100 nm) is needed for accumulation at tumor sites and a smaller size (preferably <20 nm) is required for tumor penetration. Therefore, the two most commonly-used strategies are to construct slightly acidic pH-responsive nanocarriers with large aggregation for accumulation at the tumor site 52, 89, 186 or with a small size for deep-seated tumor penetration 25, 90, 187. In contrast to the pH-responsive aggregation in the highly permeable tumor parenchyma, the poorly permeable tumor parenchyma exhibits narrow intercellular spaces resulting from the increased fibrillar collagen crosslinking. For this reason, developing the ultra-pH-responsive nanoparticles with size shrinkage in response to mild acidic stimulus is beneficial for deep penetration into the tumor parenchyma to inhibit tumor proliferation.
2.2.2. pH-responsive size shrinkage based on cleavable bond at tumor extracellular acidity for tumor parenchyma penetration
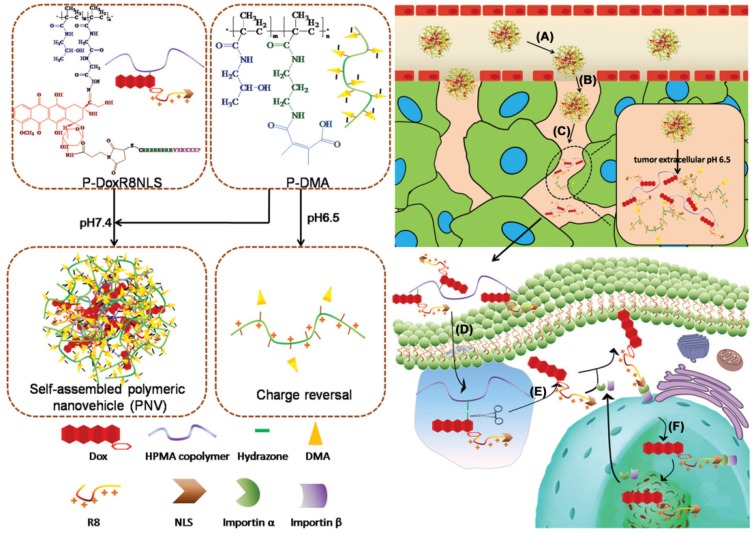
The pH-responsive size shrinkage due to the acidic environment of the extracellular tumor matrix provides a feasible strategy to facilitate deep penetration of nanocarriers into the tumor's dense collagen parenchyma. Thayumanavan and coworkers constructed a series of ultra pH-sensitive size-changeable nanogel clusters 42. Large nanoclusters were obtained by using a pH-sensitive dynamic covalent imine bond to cross-link individual nanogels. Under physiological pH conditions, nanoclusters showed a relatively large size and less positive surface charge to prolong the blood circulation time. Because of the breakage of cross-linked dynamic imine bonds triggered by the tumor-acidity at pH 6.5, nanoclusters transformed into the original nanogel, exhibiting small size and a more positive surface charge to promote deeper penetration into the tumor matrix. Wang's group reported pH-responsive clustered nanoparticles, comprising the platinum-poly(amidoamine) dendrimer prodrug-conjugated at the terminal of the amphiphilic poly(ethylene glycol)-b-poly(Ɛ-caprolactone) block polymer using the acid-labile bond 187. These smart clustered nanoparticles showed an initial diameter of ~100 nm, and discharged the small size (~5 nm) prodrug by cleaving its conjugation in the acidic environment within the tumor for deep penetration and enhanced endocytosis, followed by further intracellular reduction to kill tumor cells. Ge's group reported a supramolecular polymeric nanogel based on the host-guest interaction between adamantanine and β-cyclodextrin moieties 188. Due to the cleavage of the benzoic imine linkage in tumor-acidic conditions, this nanogel could reorganize into smaller nanoparticles from ~220 nm to ~25 nm. For targeted drug delivery into the tumor cell nucleus, Huang and colleagues synthesized a multistage nanovehicle with pH-responsive stepwise size reduction and charge reversal using an ultra pH-sensitive charge-reversal 2,3-dimethylmaleic amine modification and hydrazone linkage (Figure 5) 189. The neutrally charged nanovehicle with relatively large size (~55 nm) showed good blood persistence and excellent accumulation in tumor sites at pH 7.4. Responding to the acidic stimulus of tumor tissue, the nucleus-homing cell-penetrating peptide-modified drug conjugate was separated from the disassembled large nanovehicle, and had a size of ~10 nm in diameter, which allowed deeper tumor matrix penetration and better cellular internalization. Subsequently, the antitumor drug was cleaved from the conjugate in the acidic microenvironment of the endosome, and efficiently entered the nucleus via nuclear location signaling. Benefitting from the stepwise size reduction and on-demand target moiety exposure, this multistage nanovehicle greatly improved the antitumor drug efficiency inhibiting HeLa tumor growth in nude mice by 75%.
Figure 5.
Schematic illustration of a multistage pH-responsive stepwise size reduction and charge-reversal polymeric nanocarrier. (A) negatively charged nanocarrier at neutral pH to enhance blood retention, (B) passive accumulation of larger size nanocarriers in the tumor via EPR effect, (C) the first-stage size reduction into smaller linear copolymers for tumor tissue deep penetration, (D) increased nanocarrier uptake by tumor cells mediated by concomitant R8NLS, (E) the second-stage size reduction into therapeutic drug for intracellular release in the endo/lysosomal compartment, (F) endo/lysosome escape and nuclear targeted delivery. Reproduced with permission from 189, copyright 2015 Wiley-VCH.
2.2.3. pH-responsive size shrinkage based on protonation at tumor extracellular acidity for tumor parenchyma penetration
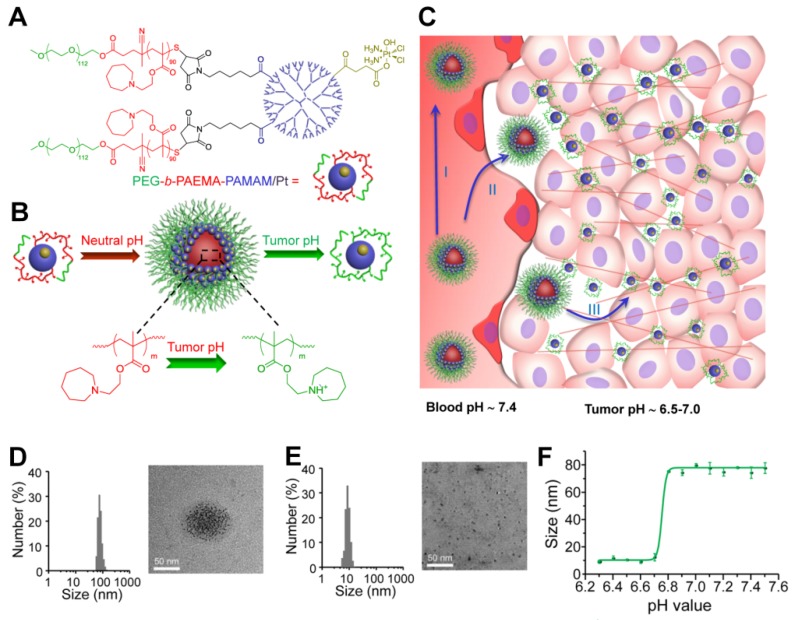
Another study by Ge and co-workers used ultra pH-sensitive charge reversal micelles with a large size (114 nm at pH 7.4) to introduce pH-responsive hydrophobicity to hydrophilicity transition of tertiary amine. Under slightly acidic condition (pH 6.8), this specifically accelerated the release of an encapsulated smaller poly(amidoamine) dendrimer-conjugated prodrug (several nanometers) for deep tumor penetration, and subsequently released the active drug by cleaving the conjugate in the intracellular reduction environment 190. Recently, Wang and colleagues have developed a class of pH-responsive nanoparticles superstructures with ultrasensitive size shrinkage for improving deep tumor penetration and efficient antitumor drug delivery (Figure 6) 90. Due to the rapid pH-responsive protonation of the ionizable tertiary amine group, this smart superstructure had an initial size of ~80 nm at the physiological pH of 7.4 during its circulation in the blood, but undergoes a dramatic and sharp size reduction to less than 10 nm in the slightly acidic microenvironment of the tumor. The ultra pH-sensitive size-changeable superstructures did not only facilitate enhanced targeted tumor accumulation by EPR by utilizing the long-circulation benefit of large nanoparticles but also promoted deeper tumor penetration by taking advantage of the increased penetration capability of smaller nanoparticles, which ultimately improved efficiency of antitumor therapy in the poorly permeable BxPC-3 pancreatic tumor model.
Figure 6.
(A) Structure of polymer-drug conjugate. (B) Schematic illustration of the pH-sensitive cluster nanobomb at neutral pH and the disintegration into small particles at tumor due to its acidic pH. (C) Schematic illustration of cluster nanobomb for in vivo drug delivery in the poorly permeable pancreatic tumor model. (I) large superstructure for prolonged blood circulation, (II) targeted tumor accumulation via EPR, (III) pH-responsive disintegration into small particles for deep tumor parenchyma penetration. Hydrodynamic diameter and TEM of polymer-drug conjugate at (D) pH 7.4 and (E) pH 6.7. (F) pH-dependent size change detected by DLS. Reproduced with permission from 90. Copyright 2016, American Chemical Society.
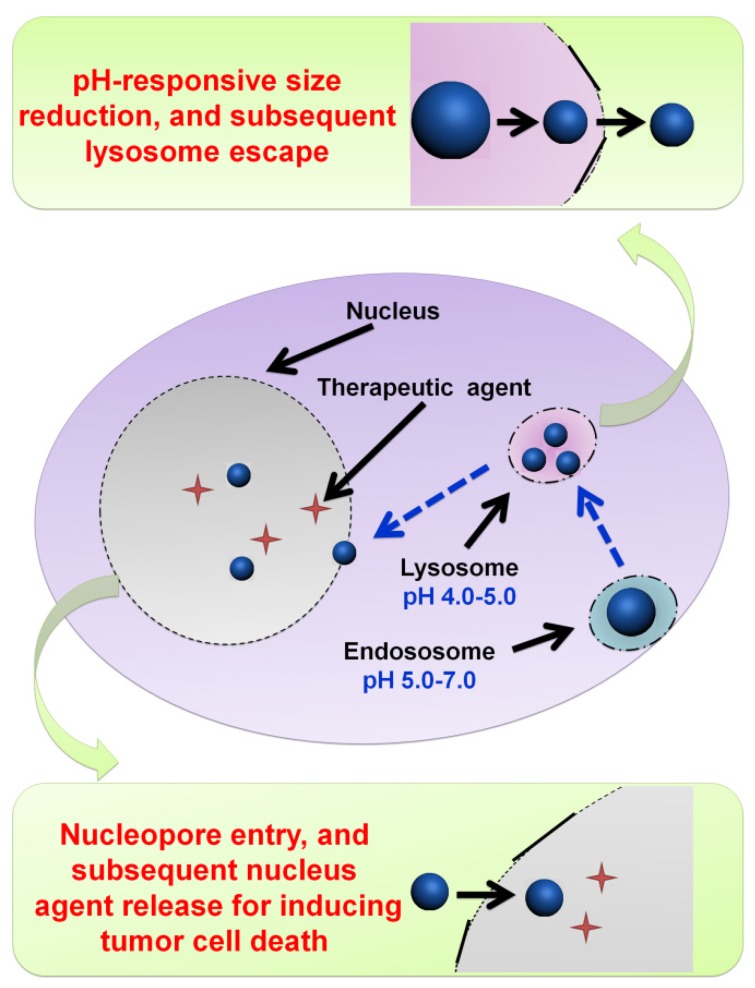
2.2.4. pH-responsive size shrinkage based on cleavable bond at tumor intracellular acidity for endo/lysosome escape
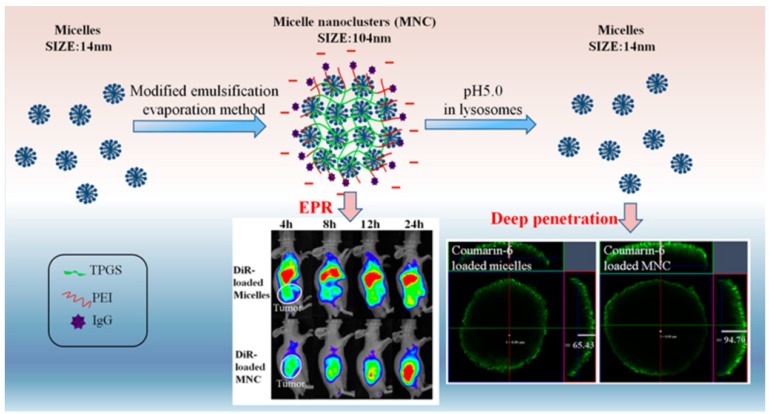
pH-responsive size shrinkage triggered by acidic stimuli of endo/lysosomes is helpful for facilitating the escape of nanocarriers from these subcellular compartments. Sha and colleagues prepared a size-shifting micelle nanocluster based on a cross-linked framework interspersed with small micelles using the emulsion-evaporation method (Figure 7) 91. The cross-linked nanocluster was stable with a size of around 104 nm and prolonged half-life for increased tumor accumulation by EPR. Due to the proton sponge effects of polyetherimide in an acidic environment, the cross-linked framework of nanoclusters was able to swell and disintegrate, thus accelerating the release of individual micelles (14 nm) and penetration into the cytoplasm. To ensure delivery of antitumor drug into the nucleus, Chen and coworkers designed a large compound nanoparticle with pH-activated size reduction for in vivo nucleus-targeted gene delivery (Figure 8) 191. This large compound nanoparticle with detachable PEG shell and folate decoration had a size of ~150 nm under neutral conditions. Once internalized by tumor cells, breakage of the benzoic imine bond of PEG conjugate in response to lower pH led to dissociation of the PEG shell resulting in the formation of smaller entities (~40 nm) exposing a previously shielded nuclear localization signal oligo-L-lysine. The resultant smaller nanoparticles could enter into the nucleus via the nucleopores, causing a 20-fold higher cytotoxicity in vitro and greater tumor suppression in animal models compared with the native drug. For endo/lysosome escape and nuclear entry, the size shrinkage in intracellular acidic conditions could greatly improve the therapeutic efficacy due to the reduced drug loss.
Figure 7.
pH-responsive size-shifting cross-linked micelle nanoclusters for enhanced tumor targeting and deep penetration. Reproduced with permission from 91, copyright 2016 American Chemical Society.
Figure 8.
Illustration of the large compound nanoparticles with pH-activated size reduction property for in vivo nucleus-targeted gene delivery.
2.3. pH-responsive swell or disassembly for burst drug release
2.3.1. Design principle
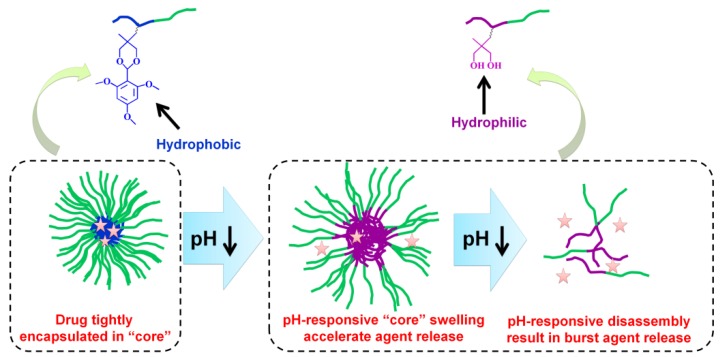
pH-responsive size change nanocarriers can also be constructed for encapsulated drug release intracellularly. After being endocytosed by tumor cells, the instantaneous pH-responsive swell accelerates release of encapsulated drugs from the loose nanocarriers and the pH-responsive disassembly results in the burst drug release 30, 49, 64, 77, 192. The controllable pH-responsive drug release determines the therapeutic efficacy and side effects of the drug 193-195. The ideal nanocarrier must tightly retain the encapsulated drug without premature release during transport but must show burst release once inside tumor cells to overcome multidrug resistance and subsequently efficiently induce tumor cells apoptosis 70, 105, 196-200. However, nanocarriers do not conform to the ideal drug release profile; there is burst release of up to 30% during the first several hours and sustained slow drug release over the following several days 196. The lost drug not only leads to reduced therapeutic efficiency but also increases toxicity to healthy cells and organs. Therefore, improvements in release kinetics are still a tremendous challenge. To match drug release as close to the ideal profile as possible, the tight encapsulation of the drug in the nanoparticles is required and their rapid disassembly to accelerate the release of the payload drug in response to the intracellular acidic environment. Many mechanisms including appropriate cross-linking, reversible conjugation, or specific physicochemical property transformations based on the pH are adapted for a viable strategy to modulate release kinetics and reaching an appropriate therapeutic window. The payload drug would be ineffective below a certain concentration 119, 201-204.
2.3.2. pH-responsive swell or disassembly based on protonation
pH-responsive protonation resulting in polymeric hydrophobic-hydrophilic conversion in the acidic microenvironment of endo/lysosomes is one of the most utilized strategies for controlling the size of nanocarriers. For example, we previously prepared a series of amphiphilic block copolymers based on the pH-sensitive block poly[2-(diisopropylamino)ethyl methacrylate] (pKa ∼6.2) 64, 77, 124. The pH-sensitive block copolymers could self-assemble into nano-micelles with a hydrophobic PDPA core for tightly encapsulating DOX during circulation and then rapidly released DOX intracellularly once transformed from amphiphilicity to double hydrophilicity due to protonation of PDPA block in the acidic environment of tumor cells (Figure 9) 64. Dong and colleagues prepared integrin-targeted zwitterionic polymeric nanoparticles based on the ultra pH-sensitive PDPA 205. In their study, 24% of the loaded drugs was released at pH 7.4 within 36 h, but the released drugs increased to 60% at pH 6.0 within 24 h and eventually 100% at pH 5.0 within 5 h. Shuai's group developed a highly packed interlayer-crosslinked nano-micelles for reduction- and pH-responsive burst drug release (Figure 10) 196. Benefitting from the hydrophobicity of the 2-(diisopropylamino) ethyl amine groups in copolymer at pH 7.4 and disulfide functional cross-linkage in a normal reduction environment, these micelles were capable of tightly encapsulating DOX to avoid drug leakage during storage and blood circulation. The pH-responsive hydrophilicity conversion and reduction-responsive cross-linker breakage in the lysosomes then led to the disassembly of nano-micelles into separate copolymers; Subsequently, there was a burst drug release (more than 95% of loaded DOX released in a lysosome-simulated aqueous solution of 10 mM dithiothreitol at pH 5.0) to induce cell apoptosis.
Figure 9.
Illustration of DOX-loaded micelles for rapid intracellular drug release in response to the acidic stimulus of endo/lysosome in anti-cancer therapy. Reproduced with permission from 64, copyright 2014 Royal Society of Chemistry.
Figure 10.
Highly packed interlayer-crosslinked nanomicelles for reduction- and pH-responsive collectively triggered burst drug release. Reproduced with permission from 196, copyright 2011 Wiley-VCH.
2.3.3. pH-responsive swell or disassembly based on acid-labile cleavable bond
Another pH-responsive intracellular release strategy is to use acid-labile bond cleavage. pH-responsive disassembled nanocarriers based on charge-reversal or degradation mechanisms are feasible for obtaining a burst release profile. Zhong's group improved a number of pH-responsive degradable polymeric nanocarriers using acid-labile polycarbonate modified for intracellular drug delivery (Figure 11) 3, 206-212. The polycarbonate modification provided the amphiphilic feature for a remarkably high drug loading content. Notably, these nanocarriers are stable at pH 7.4 but swell and eventually disassemble for fast hydrolysis of the polycarbonate in the acidic conditions of endo/lysosomes.
Figure 11.
Acid-labile polycarbonate-modified pH-responsive degradable polymeric nanocarriers showing swelling and eventual disassembly for controlling intracellular drug release.
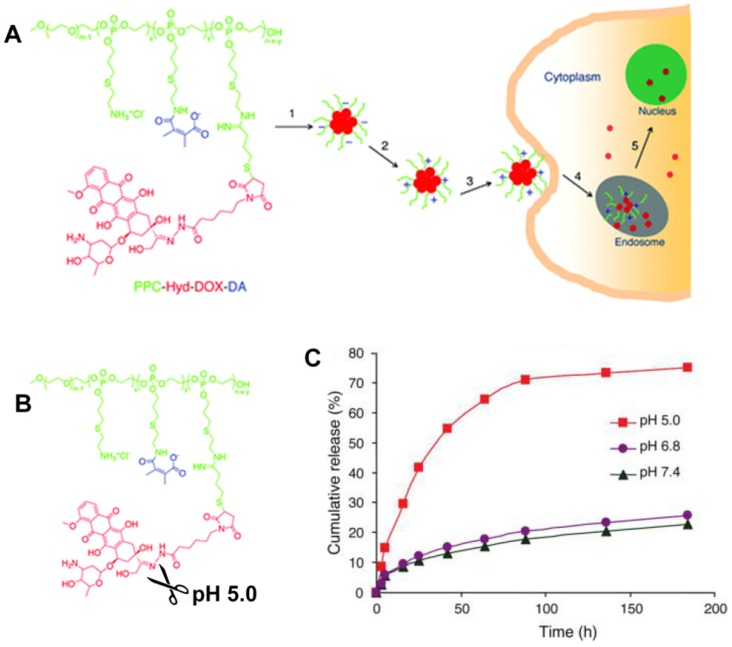
Given the high drug-carrying capacity and tunable drug-release kinetics, the pH-responsive prodrug conjugates provide a platform for further increasing drug delivery efficiency 69, 70, 213, 214. Zhong and coworkers developed a pH-sensitive nano-micelle based on the acid-labile acetal bridging polymer and drug conjugates for accelerated intracellular antitumor drug release 215. In release studies performed in vitro, the acetal-link breakage resulted in paclitaxel (PTX) monomer recovery and swelling of prodrug nanoparticles, showing that PTX release was highly pH-dependent, i.e. 86.9%, 66.4% and 29.0% in 48 h at pH 5.0, 6.0, and pH 7.4, respectively. Wang and colleagues developed a dual tailor-made pH-responsive polymer-drug conjugate to inhibit drug-resistant cancer stem cells (Figure 12) 53. This advanced polymeric prodrug could respond to both extracellular and intracellular acidic stimuli through chemically defined mechanisms. It was expected to have a prolonged blood circulation, accumulation in the tumor via EPR, and endocytosis through pH-responsive surface charge conversion from negative to positive in response to the mild acid conditions in tumor tissue. Interestingly, nanosized prodrug rapidly disassembled into the drug monomer due to the acid-labile hydrazone bond cleavage in endo/lysosomes. This multifunctional nanosized prodrug greatly enhanced antitumor efficacy providing an advanced guide for antitumor drug delivery research and clinical applications.
Figure 12.
(A) Illustration of the dual tailor-made pH-responsive polymer-drug conjugate to inhibit drug-resistant cancer stem cells. (B) Acid-labile breakage of the polymer-drug conjugate. (C) In vitro pH-sensitive drug release profiles. Reproduced with permission from 53, copyright 2011 American Chemical Society.
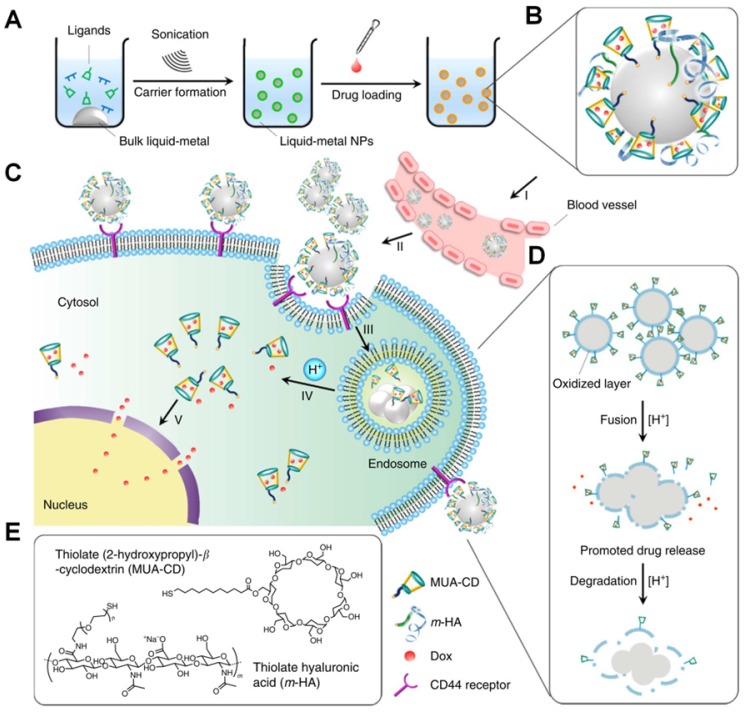
Gu et al. improved a potential theranostic reagent using a transformable liquid-metal nanomedicine with a polymeric shell for encapsulating DOX and a liquid inorganic core for fusion and subsequent degradation in a mildly acidic environment (Figure 13) 216. Within 4 h, the cumulative amount of drug released in the acidic buffer of pH 5.0 was significantly higher than that in the neutral condition of pH 7.4 (> 50% for DOX and 44% for paclitaxel). The increased drug release at pH 5.0 resulted from the liquid-metal core fusion mediating the disruption of the polymeric shell and subsequent dissociation of the drug-loaded surface ligand. Besides, this liquid-metal nanoformulation exhibited a contrast-enhancing capability for imaging applications, and eventually degraded under mildly acidic condition showing excellent biocompatibility.
Figure 13.
Illustration of the transformable liquid-metal nanomedicine for the antitumor application. (A) Preparation and (B) Main components of liquid-metal nanomedicine. (C) pH-responsive delivery of the DOX-loaded liquid-metal nanomedicine for targeted antitumor therapy. (D) Acid-triggered fusion and degradation process of liquid-metal nanomedicine. (E) Chemical structures of the main components. Reproduced with permission from 216, copyright 2015 Nature Publishing Group.
3. Advanced endogenous pH-responsive carriers with programmable size changes for tumor imaging
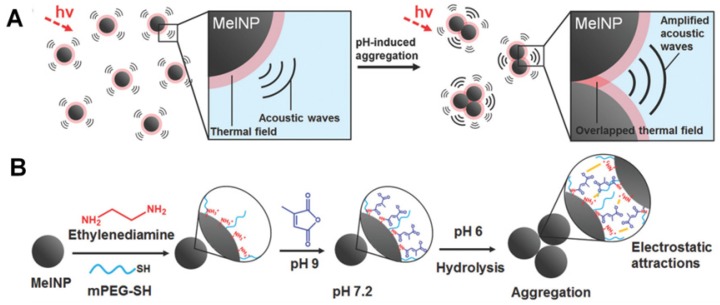
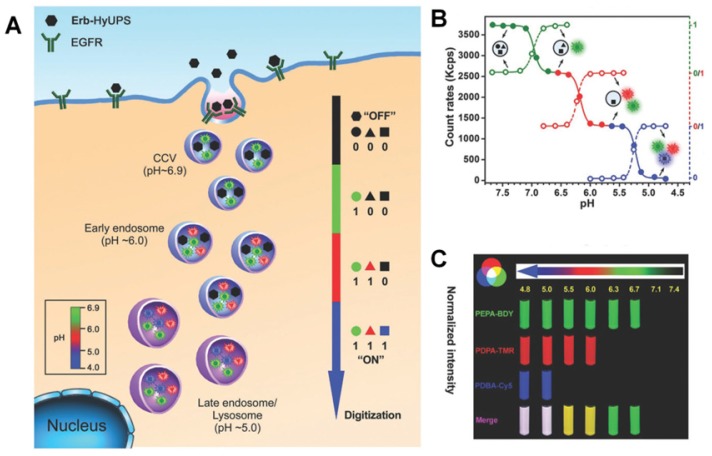
Besides being useful for antitumor therapy, the pH-responsive size-changeable design strategy has also been exploited for tumor diagnosis 217-219. For example, to overcome the low optical absorbance in biological tissues' near-infrared optical window, Chang et al. developed novel melanin-like nanoparticles by modifying the hydrolysis-susceptible surface citraconic amide for photoacoustic imaging (Figure 14) 55. These improved nanoparticles were able to aggregate within a mildly acidic environment, and, due to their overlapping thermal fields, resulted in the photoacoustic signal strength amplification (8.1 times at pH 6.0 than that at pH 7.4) in the near-infrared window of biological tissues without absorption tuning. The unique characteristic of photoacoustic amplification in melanin-like nanoparticle clusters might allow their use as a contrast agent for highly sensitive in vivo tumor target imaging. This innovative targeted tumor imaging based on the pH-responsiveness unveiled great opportunities for many basic research studies and clinical explorations. Recently, Gao and colleagues have fabricated a large number of the ultra-pH-responsive nanosensors for amplifying signals based on the positive cooperativity of the noncovalent selfassembly of the fluorescence-labeled functional block copolymers 66, 67, 71, 106-108, 114, 220-226. Typically, a hybrid nanotransistor probe, composed of a molecular mixture of three different ultra-pH-responsive block copolymers, served as a binary pH threshold sensor exhibiting dramatic signal amplification (>30-fold) and sharp (<0.25 pH) acidic response capabilities 220. The hybrid nanotransistor stayed “OFF” at pH 7.4, resulting from the dramatic fluorescence quenching through the hetero-/homo-molecular fluorescence energy transfer in micelle state. After endocytosis, the ultra-pH-responsive block copolymer components disassembled and fluoresced sequentially as the pH value decreased. After each pH transition encoding with a unique fluorescent reporter, the acidification kinetics at single-organelle resolution could be quantified using digitization paradigm by a series of barcodes with binary (0 and 1) output in each channel reporting the luminal pH of the individual endocytic organelle (Figure 15). This novel nanoprobe provided a feasible platform for imaging and new biological investigations in tumor pathology. The preliminary exploration of the pH-responsive size changeable nanoprobes showed exciting perspectives in tumor imaging diagnosis. For the same design mechanism, the pH-responsive nanoparticles with programmable size changes provided a feasible platform to integrate therapy and imaging for tumor theranostic improvement.
Figure 14.
(A) Schematic illustration of pH-responsive aggregation-induced amplification of the photoacoustic signal from Melanin-like nanoparticles and (B) Surface modification of the bare Melanin-like nanoparticles and their aggregation under mildly acidic condition. Reproduced with permission from 55, copyright 2016 Royal Society of Chemistry.
Figure 15.
(A) Schematic of the ultra-pH-responsive hybrid nanotransistor to digitize organelle pH after receptor-mediated endocytosis in tumor cells. (B) The count rates and normalized fluorescence intensity of the hybrid nanotransistor are plotted at different pH values. (C) Representative fluorescence images of multispectral ultra-pH-responsive hybrid nanotransistor at different pH values. Yellow is the merged color of green and red signals. White is the merged color of blue, green, and red signals. Reproduced with permission from 220, copyright 2017 Wiley-VCH.
4. Conclusions and perspectives
pH-responsive nanocarriers with programmable size changes have proven to be a powerful and flexible platform for designing more effective anticancer drug delivery systems with higher therapeutic efficacy and fewer clinical side effects. In this review, we focused on various advanced pH-responsive size-changeable design strategies: (a) Nanocarriers with appropriate size are necessary for avoiding undesirable clearance during circulation by nonspecific MPS and for enhancing accumulation and retention at tumor sites via the EPR effect. (b) For highly permeable tumors (such as C26 colon tumor), the slightly acidic environment in tumor tissues can promote uptake of nanocarriers with pH-responsive aggregation through concentration- and time-dependent interaction effects. (c) For poorly permeable tumors (such as the BXPC-3 pancreatic tumor), nanocarriers with pH-responsive size shrinkage are capable of accelerating nanocarrier penetration into the tumor parenchyma, escaping from endo/lysosomes, and delivering the drug into the nucleus. (d) After internalization by tumor cells, nanocarriers with pH-responsive swell and even disassembly can accelerate drug release to provide the required therapeutic dose for inducing tumor cell apoptosis. (e) pH-responsive nanoprobes with programmable size changes are expected to be useful for tumor imaging diagnosis.
Although considerable progress has been made with pH-responsive nanocarriers with programmable size changes, some challenges still exist. For example, rapid mutations of malignant tumors may result in unpredictable and unstable pathological environment including extracellular pH gradients, varying tumor vascular leakage by changing pore diameter, and tumor parenchyma collagen density. The synchronous development of real-time technology in tumor imaging and diagnosis is needed to detect the precise pathological changes for designing an accurate and targeted clinical approach. Furthermore, the strategy in the selection of sizes for nanocarrier aggregation or deep tumor penetration should be designed based on the permeability of various tumor types and location. For example, different size nanocarriers (with diameters of 30, 50, 70 and 100 nm) exhibit efficient penetration into the highly permeable tumor, but only small-sized nanocarriers with a diameter of 30 nm can penetrate the poorly permeable pancreatic tumors 227. For tumors with low blood supply and mesenchyme-enriched tumors, the size reduction strategy for deep tumor penetration is a better choice for enhancing antitumor therapeutic efficacy. On the other hand, the excessive pursuit of stimuli-responsiveness may come at the cost of loss of stability which may cause serious physiological toxicity. Therefore, it is necessary to achieve a rational balance between ultra sensitivity and stability. Although pH-responsive size variation is a major aspect of designing nanocarriers, it is not the only criterion that is worthy of consideration. Sophisticated novel antitumor nanocarriers must navigate a hostile and complex environment in vivo and, therefore, a variety of mechanisms are needed to integrate multiple stimuli (temperature, redox, enzyme, glucose, ion, etc.) as well as different factors (surface charged potential, hydrophilic-hydrophobic balance, shape etc.). Also, improving biocompatibility and degradability as well as functionality is important for the successful implementation of clinical nanomedicine. In summary, pH-responsive nanocarriers with programmable size changes provide an excellent platform for precise and personalized treatment for current antitumor therapy. However, a great number of challenges remain for the future clinical nanomedicine.
Acknowledgments
Financial support from the National Natural Science Foundation of China (51603023, 11332003), the National Key R&D Program (2016YFC1102305), the Fundamental Research Funds for the Central Universities (106112016CDJXY230002, 106112017CDJZRPY0012), the Chongqing Research Program of Basic research and Frontier Technology (cstc2017jcyjAX0186), the China Postdoctoral Science Foundation (2016M602656, 2017T100682) and the Chongqing Postdoctoral Scientific Research Foundation (Xm2016011) as well as the Chongqing Engineering Laboratory in Vascular Implants and the Public Experiment Center of State Bioindustrial Base (Chongqing) are gratefully acknowledged. We also greatly thank Dr. Xi Lu, Prof. Colm Durkan, Mr. Ali Maruf, Miss Deti Nurhidayah for editing language friendly.
Abbreviations
- EPR
enhanced permeability and retention
- DLS
dynamic light scattering
- DMMA
2,3-dimethylmaleic anhydride
- DOX
doxorubicin
- H+
hydrogen ion
- MPS
mononuclear phagocytic system
- P2VP
poly(2-vinylpyridine)
- P4VP
poly(4-vinylpyridine)
- PAE
poly(β-amino ester)
- PAMA
poly(2-aminoethyl methacrylate hydrochloride)
- PAsp
poly(aspartic acid)
- PC6A
poly[2-(pentamethyleneimino) ethyl methacrylate]
- PC7A
poly[2-(hexamethylene imino) ethyl methacrylate]
- PDPA
poly[2-(diisopropylamino) ethyl methacrylate]
- PEAA
poly(ethylacrylic acid)
- PEG
polyethylene glycol
- PEI
polyethylenimine
- PHis
poly(L-histidine)
- PMAA
poly(methacrylic acid)
- PLG
poly-L-glutamate
- PSD
poly(methacryloyl sulfadimethoxine)
- PTX
paclitaxel
- PVBA
poly(4-vinylbenzoic acid)
- RES
reticuloendothelial system
- SA
succinic anhydride
- TEM
transmission electron microscope.
Biographies
 Dr. Wei Wu received his Ph.D. in biomedical engineering at Sichuan University, China (2015). He is currently an associate professor at Chongqing University. He has broad experience in the fields of the stimuli-responsive polymeric nanocarriers for antitumor therapy, the biomembrane coating nanomedicine, and the cardiovascular bioresorbable scaffold.
Dr. Wei Wu received his Ph.D. in biomedical engineering at Sichuan University, China (2015). He is currently an associate professor at Chongqing University. He has broad experience in the fields of the stimuli-responsive polymeric nanocarriers for antitumor therapy, the biomembrane coating nanomedicine, and the cardiovascular bioresorbable scaffold.
 Dr. Li Luo entered Chongqing University in 2016 and as a candidate for his Ph.D. degree, major in bioengineering. His research interests include the multi-functional nanocarriers, the biomechanics of biomaterials, and the bioresorbable scaffold for the treatment of cardiovascular and cerebrovascular diseases.
Dr. Li Luo entered Chongqing University in 2016 and as a candidate for his Ph.D. degree, major in bioengineering. His research interests include the multi-functional nanocarriers, the biomechanics of biomaterials, and the bioresorbable scaffold for the treatment of cardiovascular and cerebrovascular diseases.
 Dr. Yi Wang received his BS degree from Chongqing Technology and Business University. He joined the Bio-engineering College of Chongqing University and entered Prof. Guixue Wang's group as a PhD student in 2012. The focus of his research is in surface modification of vascular implant materials and the biomimetic cell membrane-based drug delivery systems for vascular disease therapy.
Dr. Yi Wang received his BS degree from Chongqing Technology and Business University. He joined the Bio-engineering College of Chongqing University and entered Prof. Guixue Wang's group as a PhD student in 2012. The focus of his research is in surface modification of vascular implant materials and the biomimetic cell membrane-based drug delivery systems for vascular disease therapy.
 Prof. Gui-Xue Wang, dean of the Bioengineering College of Chongqing University, mainly engaged in biomedical engineering and biology field heart (brain) vascular biomechanics, biorheology, force-developmental biology, biological materials and tissue repair. Other job titles include: Executive director of intervention medical engineering branch of Chinese Society of Biomedical Engineering; vice director of the Chinese Atherosclerosis professional committee, director of the International Society of Atherosclerosis Chinese Branch, China Biomaterials Society, Chongqing Institute of biomedical engineering executive director.
Prof. Gui-Xue Wang, dean of the Bioengineering College of Chongqing University, mainly engaged in biomedical engineering and biology field heart (brain) vascular biomechanics, biorheology, force-developmental biology, biological materials and tissue repair. Other job titles include: Executive director of intervention medical engineering branch of Chinese Society of Biomedical Engineering; vice director of the Chinese Atherosclerosis professional committee, director of the International Society of Atherosclerosis Chinese Branch, China Biomaterials Society, Chongqing Institute of biomedical engineering executive director.
References
- 1.Duan XP, Li YP. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small. 2013;9:1521–32. doi: 10.1002/smll.201201390. [DOI] [PubMed] [Google Scholar]
- 2.King MR, Mohamed ZJ. Dual nanoparticle drug delivery: the future of anticancer therapies? Nanomedicine. 2017;12:95–8. doi: 10.2217/nnm-2016-0378. [DOI] [PubMed] [Google Scholar]
- 3.Du YF, Chen W, Zheng M, Meng FH, Zhong ZY. pH-sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials. 2012;33:7291–9. doi: 10.1016/j.biomaterials.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y. et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648–54. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xing H, Hwang K, Lu Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics. 2016;6:1336–52. doi: 10.7150/thno.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Li H, Jin Q, Ji J. Surface tailoring of nanoparticles via mixed-charge monolayers and their biomedical applications. Small. 2014;10:4230–42. doi: 10.1002/smll.201401440. [DOI] [PubMed] [Google Scholar]
- 8.Liang S, Yang XZ, Du XJ, Wang HX, Li HJ, Liu WW. et al. Optimizing the Size of Micellar Nanoparticles for Efficient siRNA Delivery. Adv Funct Mater. 2015;25:4778–87. [Google Scholar]
- 9.Wu W, Ye C, Xiao H, Sun X, Qu W, Li X. et al. Hierarchical mesoporous silica nanoparticles for tailorable drug release. Int J Pharm. 2016;511:65–72. doi: 10.1016/j.ijpharm.2016.06.133. [DOI] [PubMed] [Google Scholar]
- 10.Li S, He Q, Chen T, Wu W, Lang K, Li ZM, Controlled co-delivery nanocarriers based on mixed micelles formed from cyclodextrin-conjugated and cross-linked copolymers. Colloids Surf B Biointerfaces; 2014. p. 123. 486-92. [DOI] [PubMed] [Google Scholar]
- 11.Wu D, Yang J, Li J, Chen L, Tang B, Chen X. et al. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials. 2013;34:5036–47. doi: 10.1016/j.biomaterials.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Wang W, Li J. Star polymers: Advances in biomedical applications. Prog Polym Sci. 2015;46:55–85. [Google Scholar]
- 13.Sosnik A, Menaker Raskin M. Polymeric micelles in mucosal drug delivery: Challenges towards clinical translation. Biotechnol Adv. 2015;33:1380–92. doi: 10.1016/j.biotechadv.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Zhi X, Yang M, Zhang J, Lin L, Zhao X. et al. Tumor-triggered drug release from calcium carbonate-encapsulated gold nanostars for near-infrared photodynamic/photothermal combination antitumor therapy. Theranostics. 2017;7:1650–62. doi: 10.7150/thno.17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witting M, Obst K, Friess W, Hedtrich S. Recent advances in topical delivery of proteins and peptides mediated by soft matter nanocarriers. Biotechnol Adv. 2015;33:1355–69. doi: 10.1016/j.biotechadv.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Hajba L, Guttman A. The use of magnetic nanoparticles in cancer theranostics: Toward handheld diagnostic devices. Biotechnol Adv. 2016;34:354–61. doi: 10.1016/j.biotechadv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhai Y, Su J, Ran W, Zhang P, Yin Q, Zhang Z. et al. Preparation and Application of Cell Membrane-Camouflaged Nanoparticles for Cancer Therapy. Theranostics. 2017;7:2575–92. doi: 10.7150/thno.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Yang J, Li J, Chen L, Liang K, Wu W. et al. Bioinspired intrafibrillar mineralization of human dentine by PAMAM dendrimer. Biomaterials. 2013;34:6738–47. doi: 10.1016/j.biomaterials.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 19.Ruan S, Cao X, Cun X, Hu G, Zhou Y, Zhang Y. et al. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials. 2015;60:100–10. doi: 10.1016/j.biomaterials.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Stylianopoulos T, Wong C, Bawendi MG, Jain RK, Fukumura D. Multistage Nanoparticles for Improved Delivery into Tumor Tissue. Method Enzymol. 2012;508:109–30. doi: 10.1016/B978-0-12-391860-4.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karimi M, Ghasemi A, Zangabad PS, Rahighi R, Basri SMM, Mirshekari H. et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457–501. doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andresen TL, Jensen SS, Jorgensen K. Advanced strategies in liposomal cancer therapy: Problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv Drug Deliv Rev. 2012;64:866–84. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, Xie J, Chen H, Gu S, Zhao R, Shao J. et al. Nanotechnology-based intelligent drug design for cancer metastasis treatment. Biotechnol Adv. 2014;32:761–77. doi: 10.1016/j.biotechadv.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Wong C, Stylianopoulos T, Cui JA, Martin J, Chauhan VP, Jiang W. et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. P Natl Acad Sci U S A. 2011;108:2426–31. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen MM, Carlini AS, Chien MP, Sonnenberg S, Luo CL, Braden RL. et al. Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv Mater. 2015;27:5547–52. doi: 10.1002/adma.201502003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu GL, Chun XL, Wang Y, He Q, Gao HL. Peptide mediated active targeting and intelligent particle size reduction-mediated enhanced penetrating of fabricated nanoparticles for triple-negative breast cancer treatment. Oncotarget. 2015;6:41258–74. doi: 10.18632/oncotarget.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cun XL, Chen JT, Ruan SB, Zhang L, Wan JY, He Q. et al. A Novel Strategy through Combining iRGD Peptide with Tumor-Microenvironment-Responsive and Multistage Nanoparticles for Deep Tumor Penetration. ACS Appl Mater Interfaces. 2015;7:27458–66. doi: 10.1021/acsami.5b09391. [DOI] [PubMed] [Google Scholar]
- 29.Hu GL, Wang Y, He Q, Gao HL. Multistage drug delivery system based on microenvironment-responsive dendrimer-gelatin nanoparticles for deep tumor penetration. RSC Adv. 2015;5:85933–7. [Google Scholar]
- 30.Li X, Shang H, Wu W, Li S, Lin Z, Duan J. et al. Glucose-Responsive Micelles for Controlled Insulin Release Based on Transformation from Amphiphilic to Double Hydrophilic. J Nanosci Nanotechnol. 2016;16:5457–63. doi: 10.1166/jnn.2016.11655. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Wu W, Guo Z, Xin J, Li J. Controlled insulin release from glucose-sensitive self-assembled multilayer films based on 21-arm star polymer. Biomaterials. 2011;32:1759–66. doi: 10.1016/j.biomaterials.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Li RQ, Wu W, Song HQ, Ren Y, Yang M, Li J. et al. Well-defined reducible cationic nanogels based on functionalized low-molecular-weight PGMA for effective pDNA and siRNA delivery. Acta Biomater. 2016;41:282–92. doi: 10.1016/j.actbio.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Guo X, Wei X, Jing Y, Zhou S. Size Changeable Nanocarriers with Nuclear Targeting for Effectively Overcoming Multidrug Resistance in Cancer Therapy. Adv Mater. 2015;27:6450–6. doi: 10.1002/adma.201502865. [DOI] [PubMed] [Google Scholar]
- 34.Guo X, Shi CL, Yang G, Wang J, Cai ZH, Zhou SB. Dual-Responsive Polymer Micelles for Target-Cell-Specific Anticancer Drug Delivery. Chem Mater. 2014;26:4405–18. [Google Scholar]
- 35.Yang C, Li C, Zhang P, Wu W, Jiang X. Redox Responsive Hyaluronic Acid Nanogels for Treating RHAMM (CD168) Over-expressive Cancer, both Primary and Metastatic Tumors. Theranostics. 2017;7:1719–34. doi: 10.7150/thno.18340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang MM, Zhao KJ, Wang L, Lin SQ, Li JJ, Chen JB. et al. Dual Stimuli-Responsive Polymer Prodrugs Quantitatively Loaded by Nanoparticles for Enhanced Cellular Internalization and Triggered Drug Release. ACS Appl Mater Interfaces. 2016;8:11226–36. doi: 10.1021/acsami.5b12227. [DOI] [PubMed] [Google Scholar]
- 37.Patel A, Sant S. Hypoxic tumor microenvironment: Opportunities to develop targeted therapies. Biotechnol Adv. 2016;34:803–12. doi: 10.1016/j.biotechadv.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng X, Yang Y, Zhou L, Zhang L, Lv Y, Li S. et al. Dual-Responsive Molecular Probe for Tumor Targeted Imaging and Photodynamic Therapy. Theranostics. 2017;7:1781–94. doi: 10.7150/thno.18437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Z, Cao S, Chen X, Wu W, Li J. Thermoresponsive hydrogels from phosphorylated ABA triblock copolymers: a potential scaffold for bone tissue engineering. Biomacromolecules. 2013;14:2206–14. doi: 10.1021/bm4003442. [DOI] [PubMed] [Google Scholar]
- 40.Hossann M, Wang TT, Wiggenhorn M, Schmidt R, Zengerle A, Winter G. et al. Size of thermosensitive liposomes influences content release. J Control Release. 2010;147:436–43. doi: 10.1016/j.jconrel.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Wu W, Lin Z, Liu Y, Xu X, Ding C, Li J. Thermoresponsive hydrogels based on a phosphorylated star-shaped copolymer: mimicking the extracellular matrix for in situ bone repair. J Mater Chem B. 2017;5:428–34. doi: 10.1039/c6tb02657e. [DOI] [PubMed] [Google Scholar]
- 42.Raghupathi K, Li LY, Ventura J, Jennings M, Thayumanavan S. pH responsive soft nanoclusters with size and charge variation features. Polym Chem. 2014;5:1737–42. [Google Scholar]
- 43.Guan XG, Hu XL, Li ZH, Zhang H, Xie ZG. cRGD targeted and charge conversion-controlled release micelles for doxorubicin delivery. RSC Adv. 2015;5:22957–64. [Google Scholar]
- 44.Qu XZ, Yang ZZ. Benzoic-Imine-Based Physiological-pH-Responsive Materials for Biomedical Applications. Chem Asian J. 2016;11:2633–41. doi: 10.1002/asia.201600452. [DOI] [PubMed] [Google Scholar]
- 45.Ma S, Zhou J, Zhang Y, He Y, Jiang Q, Yue D. et al. Highly stable fluorinated nanocarriers with iRGD for overcoming the stability dilemma and enhancing tumor penetration in an orthotopic breast cancer. ACS Appl Mater Interfaces. 2016;8:28468–79. doi: 10.1021/acsami.6b09633. [DOI] [PubMed] [Google Scholar]
- 46.Wu W, Liu J, Cao S, Tan H, Li J, Xu F. et al. Drug release behaviors of a pH sensitive semi-interpenetrating polymer network hydrogel composed of poly(vinyl alcohol) and star poly[2-(dimethylamino)ethyl methacrylate] Int J Pharm. 2011;416:104–9. doi: 10.1016/j.ijpharm.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Chen T, Wu W, Xiao H, Chen Y, Chen M, Li J. Intelligent Drug Delivery System Based on Mesoporous Silica Nanoparticles Coated with an Ultra-pH-Sensitive Gatekeeper and Poly(ethylene glycol) ACS Macro Lett. 2016;5:55–8. doi: 10.1021/acsmacrolett.5b00765. [DOI] [PubMed] [Google Scholar]
- 48.Wu D, Chen X, Chen T, Ding C, Wu W, Li J. Substrate-anchored and degradation-sensitive anti-inflammatory coatings for implant materials. Sci Rep. 2015;5:11105. doi: 10.1038/srep11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu W, Wang W, Li S, Wang J, Zhang Q, Li X. et al. Physiological pH-triggered morphological transition of amphiphilic block copolymer self-assembly. J Polym Res. 2014;21:494. [Google Scholar]
- 50.Zhou Q, Hou Y, Zhang L, Wang J, Qiao Y, Guo S. et al. Dual-pH Sensitive Charge-reversal Nanocomplex for Tumor-targeted Drug Delivery with Enhanced Anticancer Activity. Theranostics. 2017;7:1806–19. doi: 10.7150/thno.18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C. et al. pH-Responsive Nanoparticle Vaccines for Dual-Delivery of Antigens and Immunostimulatory Oligonucleotides. ACS Nano. 2013;7:3912–25. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu W, Zhang Q, Wang J, Chen M, Li S, Lin Z. et al. Tumor-targeted aggregation of pH-sensitive nanocarriers for enhanced retention and rapid intracellular drug release. Polym Chem. 2014;5:5668–79. [Google Scholar]
- 53.Du JZ, Du XJ, Mao CQ, Wang J. Tailor-Made Dual pH-Sensitive Polymer-Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J Am Chem Soc. 2011;133:17560–3. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez-Piñeiro I, Badiola I, Sanchez A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnol Adv. 2017;35:350–60. doi: 10.1016/j.biotechadv.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Ju KY, Kang J, Pyo J, Lim J, Chang JH, Lee JK. pH-Induced aggregated melanin nanoparticles for photoacoustic signal amplification. Nanoscale. 2016;8:14448–56. doi: 10.1039/c6nr02294d. [DOI] [PubMed] [Google Scholar]
- 56.Li F, Lu J, Kong X, Hyeon T, Ling D. Dynamic Nanoparticle Assemblies for Biomedical Applications. Adv Mater. 2017;29:1605897. doi: 10.1002/adma.201605897. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. J Nucl Med. 2010;51:1167–70. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130099. doi: 10.1098/rstb.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linton SS, Sherwood SG, Drews KC, Kester M. Targeting cancer cells in the tumor microenvironment: opportunities and challenges in combinatorial nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:208–22. doi: 10.1002/wnan.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu W, Wang J, Lin Z, Li X, Li J. Tumor-acidity activated surface charge-conversion of polymeric nanocarriers for enhanced cell adhesion and targeted drug release. Macromol Rapid CommunMacromol Rapid Commun. 2014;35:1679–84. doi: 10.1002/marc.201400362. [DOI] [PubMed] [Google Scholar]
- 61.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 62.Baish JW, Gazit Y, Berk DA, Nozue M, Baxter LT, Jain RK. Role of tumor vascular architecture in nutrient and drug delivery: An invasion percolation-based network model. Microvasc Res. 1996;51:327–46. doi: 10.1006/mvre.1996.0031. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian A, Manigandan A, P.R S, Sethuraman S. Development of nanotheranostics against metastatic breast cancer-A focus on the biology & mechanistic approaches. Biotechnol Adv. 2015;33:1897–911. doi: 10.1016/j.biotechadv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Wu W, Chen M, Wang J, Zhang Q, Li S, Lin Z. et al. Nanocarriers with dual pH-sensitivity for enhanced tumor cell uptake and rapid intracellular drug release. RSC Advances. 2014;4:30780–3. [Google Scholar]
- 65.Li X, Zheng BY, Ke MR, Zhang Y, Huang JD, Yoon J. A Tumor-pH-Responsive Supramolecular Photosensitizer for Activatable Photodynamic Therapy with Minimal In Vivo Skin Phototoxicity. Theranostics. 2017;7:2746–56. doi: 10.7150/thno.18861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma XP, Wang YG, Zhao T, Li Y, Su LC, Wang ZH. et al. Ultra-pH-Sensitive Nanoprobe Library with Broad pH Tunability and Fluorescence Emissions. J Am Chem Soc. 2014;136:11085–92. doi: 10.1021/ja5053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang YG, Zhou KJ, Huang G, Hensley C, Huang XN, Ma XP. et al. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nature materials. 2014;13:204–12. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan F, Yu Y, Zhong F, Gao M, Sun T, Liu J. et al. Design of Tumor Acidity-Responsive Sheddable Nanoparticles for Fluorescence/Magnetic Resonance Imaging-Guided Photodynamic Therapy. Theranostics. 2017;7:1290–302. doi: 10.7150/thno.18557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J, Huang YR, Kumar A, Tan A, Jin SB, Mozhi A. et al. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. 2014;32:693–710. doi: 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials. 2016;85:152–67. doi: 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 71.Zhou KJ, Wang YG, Huang XN, Luby-Phelps K, Sumer BD, Gao JM. Tunable, Ultrasensitive pH-Responsive Nanoparticles Targeting Specific Endocytic Organelles in Living Cells. Angew Chem Int Ed. 2011;50:6109–14. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan B, Kang L, Chen L, Sun P, Jin M, Wang Q. et al. Systemic siRNA Delivery with a Dual pH-Responsive and Tumor-targeted Nanovector for Inhibiting Tumor Growth and Spontaneous Metastasis in Orthotopic Murine Model of Breast Carcinoma. Theranostics. 2017;7:357–76. doi: 10.7150/thno.16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu HL, Mao KL, Lu CT, Fan ZL, Yang JJ, Xu J. et al. An injectable acellular matrix scaffold with absorbable permeable nanoparticles improves the therapeutic effects of docetaxel on glioblastoma. Biomaterials. 2016;107:44–60. doi: 10.1016/j.biomaterials.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 74.Nowacek AS, Balkundi S, McMillan J, Roy U, Martinez-Skinner A, Mosley RL. et al. Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages. J Control Release. 2011;150:204–11. doi: 10.1016/j.jconrel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nam J, Won N, Jin H, Chung H, Kim S. pH-Induced Aggregation of Gold Nanoparticles for Photothermal Cancer Therapy. J Am Chem Soc. 2009;131:13639–45. doi: 10.1021/ja902062j. [DOI] [PubMed] [Google Scholar]
- 76.Wang JQ, Mao WW, Lock LL, Tang JB, Sui MH, Sun WL. et al. The Role of Micelle Size in Tumor Accumulation, Penetration, and Treatment. ACS Nano. 2015;9:7195–206. doi: 10.1021/acsnano.5b02017. [DOI] [PubMed] [Google Scholar]
- 77.Li S, Wu W, Xiu K, Xu F, Li Z, Li J. Doxorubicin Loaded pH-Responsive Micelles Capable of Rapid Intracellular Drug Release for Potential Tumor Therapy. J Biomed Nanotechnol. 2014;10:1480–9. doi: 10.1166/jbn.2014.1846. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Kong N, Zhang Y, Yang W, Yan F. Size-dependent Effects of Gold Nanoparticles on Osteogenic Differentiation of Human Periodontal Ligament Progenitor Cells. Theranostics. 2017;7:1214–24. doi: 10.7150/thno.17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen H, Tong X, Lang L, Jacobson O, Yung BC, Yang X. et al. Quantification of Tumor Vascular Permeability and Blood Volume by Positron Emission Tomography. Theranostics. 2017;7:2363–76. doi: 10.7150/thno.19898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S, Huang P, Chen X. Hierarchical Targeting Strategy for Enhanced Tumor Tissue Accumulation/Retention and Cellular Internalization. Adv Mater. 2016;28:7340–64. doi: 10.1002/adma.201601498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qiu L, Chen T, Ocsoy I, Yasun E, Wu C, Zhu G. et al. A cell-targeted, size-photocontrollable, nuclear-uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett. 2015;15:457–63. doi: 10.1021/nl503777s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI. et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–22. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 85.Moghimi SM. Mechanisms of Splenic Clearance of Blood-Cells and Particles-Towards Development of New Splenotropic Agents. Adv Drug Deliv Rev. 1995;17:103–15. [Google Scholar]
- 86.Jiang W, KimBetty YS, Rutka JT, ChanWarren CW. Nanoparticle-mediated cellular response is size-dependent. Nat Nano. 2008;3:145–50. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 87.Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S. Size-Dependent Endocytosis of Nanoparticles. Adv Mater. 2009;21:419–24. doi: 10.1002/adma.200801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang J, Bu L, Xie J, Chen K, Cheng Z, Li X. et al. Effects of Nanoparticle Size on Cellular Uptake and Liver MRI with Polyvinylpyrrolidone-Coated Iron Oxide Nanoparticles. ACS Nano. 2010;4:7151–60. doi: 10.1021/nn101643u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu XS, Chen YJ, Li H, Huang N, Jin Q, Ren KF. et al. Enhanced Retention and Cellular Uptake of Nanoparticles in Tumors by Controlling Their Aggregation Behavior. ACS Nano. 2013;7:6244–57. doi: 10.1021/nn402201w. [DOI] [PubMed] [Google Scholar]
- 90.Li HJ, Du JZ, Liu J, Du XJ, Shen S, Zhu YH. et al. Smart Superstructures with Ultrahigh pH-Sensitivity for Targeting Acidic Tumor Microenvironment: Instantaneous Size Switching and Improved Tumor Penetration. ACS Nano. 2016;10:6753–61. doi: 10.1021/acsnano.6b02326. [DOI] [PubMed] [Google Scholar]
- 91.Liang H, Ren X, Qian J, Zhang X, Meng L, Wang X. et al. Size-Shifting Micelle Nanoclusters Based on a Cross-Linked and pH-Sensitive Framework for Enhanced Tumor Targeting and Deep Penetration Features. ACS Appl Mater Interfaces. 2016;8:10136–46. doi: 10.1021/acsami.6b00668. [DOI] [PubMed] [Google Scholar]
- 92.Cheng R, Meng F, Deng C, Klok HA, Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–57. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 93.Maya S, Sarmento B, Nair A, Rejinold NS, Nair SV, Jayakumar R. Smart Stimuli Sensitive Nanogels in Cancer Drug Delivery and Imaging: A Review. Curr Pharm Design. 2013;19:7203–18. doi: 10.2174/138161281941131219124142. [DOI] [PubMed] [Google Scholar]
- 94.Yu J, Chu X, Hou Y. Stimuli-responsive cancer therapy based on nanoparticles. Chem Commun. 2014;50:11614–30. doi: 10.1039/c4cc03984j. [DOI] [PubMed] [Google Scholar]
- 95.Liechty WB, Peppas NA. Expert opinion: Responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm. 2012;80:241–6. doi: 10.1016/j.ejpb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhatnagar S, Venuganti VVK. Cancer Targeting: Responsive Polymers for Stimuli-Sensitive Drug Delivery. J Nanosci Nanotechnol. 2015;15:1925–45. doi: 10.1166/jnn.2015.10325. [DOI] [PubMed] [Google Scholar]
- 97.Nakayama M, Akimoto J, Okano T. Polymeric micelles with stimuli-triggering systems for advanced cancer drug targeting. J Drug Target. 2014;22:584–99. doi: 10.3109/1061186X.2014.936872. [DOI] [PubMed] [Google Scholar]
- 98.Jhaveri A, Deshpande P, Torchilin V. Stimuli-sensitive nanopreparations for combination cancer therapy. J Control Release. 2014;190:352–70. doi: 10.1016/j.jconrel.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 99.Ge Z, Liu S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem Soc Rev. 2013;42:7289–325. doi: 10.1039/c3cs60048c. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Gao GH, Lee DS. Stimulus-Sensitive Polymeric Nanoparticles and Their Applications as Drug and Gene Carriers. Adv Healthc Mater. 2013;2:388–417. doi: 10.1002/adhm.201200313. [DOI] [PubMed] [Google Scholar]
- 101.Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed. 2014;53:12320–64. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 102.Chen JJ, Ding JX, Xiao CS, Zhuang XL, Chen XS. Emerging antitumor applications of extracellularly reengineered polymeric nanocarriers. Biomater Sci. 2015;3:988–1001. doi: 10.1039/c5bm00044k. [DOI] [PubMed] [Google Scholar]
- 103.Nagayasu A, Uchiyama K, Kiwada H. The size of liposomes: a factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv Drug Deliv Rev. 1999;40:75–87. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 104.Sun Q, Zhou Z, Qiu N, Shen Y. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv Mater. 2017;29:1606628. doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 105.Sun QH, Radosz M, Shen YQ. Challenges in design of translational nanocarriers. J Control Release. 2012;164:156–69. doi: 10.1016/j.jconrel.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 106.Zhou K, Liu H, Zhang S, Huang X, Wang Y, Huang G. et al. Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J Am Chem Soc. 2012;134:7803–11. doi: 10.1021/ja300176w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang X, Huang G, Zhang S, Sagiyama K, Togao O, Ma X. et al. Multi-chromatic pH-activatable 19F-MRI nanoprobes with binary ON/OFF pH transitions and chemical-shift barcodes. Angewandte Chemie. 2013;52:8074–8. doi: 10.1002/anie.201301135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, Wang Z, Wei Q, Luo M, Huang G, Sumer BD. et al. Non-covalent interactions in controlling pH-responsive behaviors of self-assembled nanosystems. Polym Chem. 2016;7:5949–56. doi: 10.1039/C6PY01104G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang S, Yin Q, Su JH, Sun HP, Meng QS, Chen Y. et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly (β-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials. 2015;48:1–15. doi: 10.1016/j.biomaterials.2015.01.049. [DOI] [PubMed] [Google Scholar]
- 110.Zhang C, An T, Wang D, Wan GY, Zhang MM, Wang HM. et al. Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(β-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J Control Release. 2016;226:193–204. doi: 10.1016/j.jconrel.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 111.Han SS, Li ZY, Zhu JY, Han K, Zeng ZY, Hong W. et al. Dual-pH Sensitive Charge-Reversal Polypeptide Micelles for Tumor-Triggered Targeting Uptake and Nuclear Drug Delivery. Small. 2015;11:2543–54. doi: 10.1002/smll.201402865. [DOI] [PubMed] [Google Scholar]
- 112.Zhou DZ, Cutlar L, Gao YS, Wang W, O'Keeffe-Ahern J, McMahon S. et al. The transition from linear to highly branched poly(β-amino ester)s: Branching matters for gene delivery. Sci Adv. 2016;2:e1600102. doi: 10.1126/sciadv.1600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vila-Caballer M, Codolo G, Munari F, Malfanti A, Fassan M, Rugge M. et al. A pH-sensitive stearoyl-PEG-poly(methacryloyl sulfadimethoxine)-decorated liposome system for protein delivery: An application for bladder cancer treatment. J Control Release. 2016;238:31–42. doi: 10.1016/j.jconrel.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 114.Wang C, Zhao T, Li Y, Huang G, White MA, Gao J. Investigation of endosome and lysosome biology by ultra pH-sensitive nanoprobes. Adv Drug Deliv Rev. 2017;113:87–96. doi: 10.1016/j.addr.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zakeri-Milani P, Farkhani SM, Shirani A, Mohammadi S, Mojarrad JS, Akbari J. et al. Cellular Uptake and Anti-Tumor Activity of Gemcitabine Conjugated with New Amphiphilic Cell Penetrating Peptides. Excli J. 2017;16:650–62. doi: 10.17179/excli2017-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hung CC, Huang WC, Lin YW, Yu TW, Chen HH, Lin SC. et al. Active Tumor Permeation and Uptake of Surface Charge-Switchable Theranostic Nanoparticles for Imaging-Guided Photothermal/Chemo Combinatorial Therapy. Theranostics. 2016;6:302–17. doi: 10.7150/thno.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dong YQ, Yang J, Liu HM, Wang TY, Tang SQ, Zhang JC. et al. Site-Specific Drug-Releasing Polypeptide Nanocarriers Based on Dual-pH Response for Enhanced Therapeutic Efficacy against Drug-Resistant Tumors. Theranostics. 2015;5:890–904. doi: 10.7150/thno.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Z, Qiu LP, Chen Q, Hao TN, Qiao MX, Zhao HX. et al. pH-sensitive nanoparticles of poly(L-histidine)-poly(lactide-co-glycolide)-tocopheryl polyethylene glycol succinate for anti-tumor drug delivery. Acta Biomater. 2015;11:137–50. doi: 10.1016/j.actbio.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 119.Johnson RP, Uthaman S, John JV, Lee HR, Lee SJ, Park H. et al. Poly(PEGA)-b-poly(L-lysine)-b-poly(L-histidine) Hybrid Vesicles for Tumoral pH-Triggered Intracellular Delivery of Doxorubicin Hydrochloride. ACS Appl Mater Interfaces. 2015;7:21770–9. doi: 10.1021/acsami.5b05338. [DOI] [PubMed] [Google Scholar]
- 120.Wang CH, Wang CH, Hsiue GH. Polymeric micelles with a pH-responsive structure as intracellular drug carriers. J Control Release. 2005;108:140–9. doi: 10.1016/j.jconrel.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 121.Chen ML, Li YF, Besenbacher F. Electrospun Nanofibers-Mediated On-Demand Drug Release. Adv Healthc Mater. 2014;3:1721–32. doi: 10.1002/adhm.201400166. [DOI] [PubMed] [Google Scholar]
- 122.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed. 2003;42:4640–3. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 123.Yang XQ, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Matson VZ. et al. Multifunctional Stable and pH-Responsive Polymer Vesicles Formed by Heterofunctional Triblock Copolymer for Targeted Anticancer Drug Delivery and Ultrasensitive MR Imaging. ACS Nano. 2010;4:6805–17. doi: 10.1021/nn101670k. [DOI] [PubMed] [Google Scholar]
- 124.Li S, Zhao Z, Wu W, Ding C, Li J. Dual pH-responsive micelles with both charge-conversional property and hydrophobic-hydrophilic transition for effective cellular uptake and intracellular drug release. Polym Chem. 2016;7:2202–8. [Google Scholar]
- 125.Du JZ, Mao CQ, Yuan YY, Yang XZ, Wang J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol Adv. 2014;32:789–803. doi: 10.1016/j.biotechadv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 126.Xiao D, Jia HZ, Zhang J, Liu CW, Zhuo RX, Zhang XZ. A Dual-Responsive Mesoporous Silica Nanoparticle for Tumor-Triggered Targeting Drug Delivery. Small. 2014;10:591–8. doi: 10.1002/smll.201301926. [DOI] [PubMed] [Google Scholar]
- 127.Liu JJ, Luo Z, Zhang JX, Luo TT, Zhou J, Zhao XJ. et al. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials. 2016;83:51–65. doi: 10.1016/j.biomaterials.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 128.Feng T, Ai XZ, Ong HM, Zhao YL. Dual-Responsive Carbon Dots for Tumor Extracellular Microenvironment Triggered Targeting and Enhanced Anticancer Drug Delivery. ACS Appl Mater Interfaces. 2016;8:18732–40. doi: 10.1021/acsami.6b06695. [DOI] [PubMed] [Google Scholar]
- 129.Liu G, Tsai HI, Zeng XW, Zuo YX, Tao W, Han J. et al. Phosphorylcholine-based stealthy nanocapsules enabling tumor microenvironment-responsive doxorubicin release for tumor suppression. Theranostics. 2017;7:1192–203. doi: 10.7150/thno.17881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu HJ, Guo CY, Feng B, Liu JP, Chen XZ, Wang DG. et al. Triple-Layered pH-Responsive Micelleplexes Loaded with siRNA and Cisplatin Prodrug for NF-Kappa B Targeted Treatment of Metastatic Breast Cancer. Theranostics. 2016;6:14–27. doi: 10.7150/thno.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Woraphatphadung T, Sajomsang W, Gonil P, Saesoo S, Opanasopit P. Synthesis and characterization of pH-responsive N-naphthyl-N,O-succinyl chitosan micelles for oral meloxicam delivery. Carbohydr Polym. 2015;121:99–106. doi: 10.1016/j.carbpol.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 132.Ge ZS, Liu SY. Facile Fabrication of Multistimuli-Responsive Metallo-Supramolecular Core Cross-Linked Block Copolymer Micelles. Macromol Rapid Commun. 2013;34:922–30. doi: 10.1002/marc.201300072. [DOI] [PubMed] [Google Scholar]
- 133.Li CH, Liu SY. Polymeric assemblies and nanoparticles with stimuli-responsive fluorescence emission characteristics. Chem Commun. 2012;48:3262–78. doi: 10.1039/c2cc17695e. [DOI] [PubMed] [Google Scholar]
- 134.Zhang WQ, Shi LQ, Ma RJ, An YL, Xu YL, Wu K. Micellization of thermo- and pH-responsive triblock copolymer of poly(ethylene glycol)-b-poly(4-vinylpyridine)-b-poly(N-isopropylacrylamide) Macromolecules. 2005;38:8850–2. [Google Scholar]
- 135.Van Butsele K, Cajot S, Van Vlierberghe S, Dubruel P, Passirani C, Benoit JP. et al. pH-Responsive Flower-Type Micelles Formed by a Biotinylated Poly(2-vinylpyridine)-block-poly(ethylene oxide)-block-poly(ε-caprolactone) Triblock Copolymer. Adv Funct Mater. 2009;19:1416–25. [Google Scholar]
- 136.Zhang JC, Wu LL, Meng FH, Wang ZJ, Deng C, Liu HY. et al. pH and Reduction Dual-Bioresponsive Polymersomes for Efficient Intracellular Protein Delivery. Langmuir. 2012;28:2056–65. doi: 10.1021/la203843m. [DOI] [PubMed] [Google Scholar]
- 137.Popescu MT, Tsitsilianis C. Controlled Delivery of Functionalized Gold Nanoparticles by pH-Sensitive Polymersomes. Acs Macro Lett. 2013;2:222–5. doi: 10.1021/mz300637c. [DOI] [PubMed] [Google Scholar]
- 138.Yang P, Li D, Jin S, Ding J, Guo J, Shi WB. et al. Stimuli-responsive biodegradable poly(methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials. 2014;35:2079–88. doi: 10.1016/j.biomaterials.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 139.Jin S, Wan JX, Meng LZ, Huang XX, Guo J, Liu L. et al. Biodegradation and Toxicity of Protease/Redox/pH Stimuli-Responsive PEGlated PMAA Nanohydrogels for Targeting Drug delivery. ACS Appl Mater Interfaces. 2015;7:19843–52. doi: 10.1021/acsami.5b05984. [DOI] [PubMed] [Google Scholar]
- 140.Li RR, Feng FL, Wang YS, Yang XY, Yang XL, Yang VC. Folic acid-conjugated pH/temperature/redox multi-stimuli responsive polymer microspheres for delivery of anti-cancer drug. J Colloid Interf Sci. 2014;429:34–44. doi: 10.1016/j.jcis.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 141.Dai YL, Ma PA, Cheng ZY, Kang XJ, Zhang X, Hou ZY. et al. Up-Conversion Cell Imaging and pH-Induced Thermally Controlled Drug Release from NaYF4:Yb3+/Er3+@Hydrogel Core-Shell Hybrid Microspheres. ACS Nano. 2012;6:3327–38. doi: 10.1021/nn300303q. [DOI] [PubMed] [Google Scholar]
- 142.Shim MS, Kwon YJ. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Adv Drug Deliv Rev. 2012;64:1046–58. doi: 10.1016/j.addr.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 143.Kaur S, Prasad C, Balakrishnan B, Banerjee R. Trigger responsive polymeric nanocarriers for cancer therapy. Biomater Sci. 2015;3:955–87. doi: 10.1039/c5bm00002e. [DOI] [PubMed] [Google Scholar]
- 144.Li MQ, Song WT, Tang ZH, Lv SX, Lin L, Sun H. et al. Nanoscaled Poly(L-glutamic acid)/Doxorubicin-Amphiphile Complex as pH-responsive Drug Delivery System for Effective Treatment of Nonsmall Cell Lung Cancer. ACS Appl Mater Interfaces. 2013;5:1781–92. doi: 10.1021/am303073u. [DOI] [PubMed] [Google Scholar]
- 145.Yang H, Mao HJ, Wan ZH, Zhu AJ, Guo M, Li YL. et al. Micelles assembled with carbocyanine dyes for theranostic near-infrared fluorescent cancer imaging and photothermal therapy. Biomaterials. 2013;34:9124–33. doi: 10.1016/j.biomaterials.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 146.Li JG, Yu XS, Wang Y, Yuan YY, Xiao H, Cheng D. et al. A Reduction and pH Dual-Sensitive Polymeric Vector for Long-Circulating and Tumor-Targeted siRNA Delivery. Adv Mater. 2014;26:8217–24. doi: 10.1002/adma.201403877. [DOI] [PubMed] [Google Scholar]
- 147.Luo GF, Xu XD, Zhang J, Yang J, Gong YH, Lei Q. et al. Encapsulation of an Adamantane-Doxorubicin Prodrug in pH-Responsive Polysaccharide Capsules for Controlled Release. ACS Appl Mater Interfaces. 2012;4:5317–24. doi: 10.1021/am301258a. [DOI] [PubMed] [Google Scholar]
- 148.Zhou T, Zhou XM, Xing D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials. 2014;35:4185–94. doi: 10.1016/j.biomaterials.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 149.Chen GC, Xie YS, Peltier R, Lei HP, Wang P, Chen J. et al. Peptide-Decorated Gold Nanoparticles as Functional Nano-Capping Agent of Mesoporous Silica Container for Targeting Drug Delivery. ACS Appl Mater Interfaces. 2016;8:11204–9. doi: 10.1021/acsami.6b02594. [DOI] [PubMed] [Google Scholar]
- 150.Li ZH, Dong K, Huang S, Ju EG, Liu Z, Yin ML. et al. A Smart Nanoassembly for Multistage Targeted Drug Delivery and Magnetic Resonance Imaging. Adv Funct Mater. 2014;24:3612–20. [Google Scholar]
- 151.Xiao YL, Hong H, Matson VZ, Javadi A, Xu W, Yang YA. et al. Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and PET Imaging. Theranostics. 2012;2:757–68. doi: 10.7150/thno.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park S, Kim E, Kim WY, Kang C, Kim JS. Biotin-guided anticancer drug delivery with acidity-triggered drug release. Chem Commun. 2015;51:9343–5. doi: 10.1039/c5cc03003j. [DOI] [PubMed] [Google Scholar]
- 153.Lee CH, Cheng SH, Huang IP, Souris JS, Yang CS, Mou CY. et al. Intracellular pH-Responsive Mesoporous Silica Nanoparticles for the Controlled Release of Anticancer Chemotherapeutics. Angew Chem Int Ed. 2010;49:8214–9. doi: 10.1002/anie.201002639. [DOI] [PubMed] [Google Scholar]
- 154.Cai XJ, Dong CY, Dong HQ, Wang GM, Pauletti GM, Pan XJ. et al. Effective Gene Delivery Using Stimulus-Responsive Catiomer Designed with Redox-Sensitive Disulfide and Acid-Labile Imine Linkers. Biomacromolecules. 2012;13:1024–34. doi: 10.1021/bm2017355. [DOI] [PubMed] [Google Scholar]
- 155.Ding CX, Gu JX, Qu XZ, Yang ZZ. Preparation of Multifunctional Drug Carrier for Tumor-Specific Uptake and Enhanced Intracellular Delivery through the Conjugation of Weak Acid Labile Linker. Bioconjug Chem. 2009;20:1163–70. doi: 10.1021/bc800563g. [DOI] [PubMed] [Google Scholar]
- 156.Wang C, Wang GT, Wang ZQ, Zhang X. A pH-Responsive Superamphiphile Based on Dynamic Covalent Bonds. Chemistry. 2011;17:3322–5. doi: 10.1002/chem.201003502. [DOI] [PubMed] [Google Scholar]
- 157.Jain NK, Tare MS, Mishra V, Tripathi PK. The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomed-Nanotechnol. 2015;11:207–18. doi: 10.1016/j.nano.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 158.Zou J, Zhang FW, Zhang SY, Pollack SF, Elsabahy M, Fan JW. et al. Poly(ethylene oxide)-block-Polyphosphoester-graft-Paclitaxel Conjugates with Acid-Labile Linkages as a pH-Sensitive and Functional Nanoscopic Platform for Paclitaxel Delivery. Adv Healthc Mater. 2014;3:441–8. doi: 10.1002/adhm.201300235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi XX, Hou ML, Bai S, Ma XQ, Gao YE, Xiao B. et al. Acid-Activatable Theranostic Unimolecular Micelles Composed of Amphiphilic Star-like Polymeric Prodrug with High Drug Loading for Enhanced Cancer Therapy. Mol Pharm. 2017;14:4032–41. doi: 10.1021/acs.molpharmaceut.7b00704. [DOI] [PubMed] [Google Scholar]
- 160.Chen HC, Liu DY, Guo ZJ. Endogenous Stimuli-responsive Nanocarriers for Drug Delivery. Chem Lett. 2016;45:242–9. [Google Scholar]
- 161.Chen Y, Huang JS, Zhang SY, Gu ZW. Superamphiphile Based Cross-Linked Small-Molecule Micelles for pH-Triggered Release of Anticancer Drugs. Chem Mater. 2017;29:3083–91. [Google Scholar]
- 162.Heffernan MJ, Murthy N. Polyketal nanoparticles: A new pH-sensitive biodegradable drug delivery vehicle. Bioconjug Chem. 2005;16:1340–2. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 163.Shim MS, Kwon YJ. Ketalized poly(amino ester) for stimuli-responsive and biocompatible gene delivery. Polym Chem. 2012;3:2570–7. [Google Scholar]
- 164.Duong HTT, Marquis CP, Whittaker M, Davis TP, Boyer C. Acid Degradable and Biocornpatible Polymeric Nanoparticles for the Potential Codelivery of Therapeutic Agents. Macromolecules. 2011;44:8008–19. [Google Scholar]
- 165.Shim MS, Kwon YJ. Acid-transforming polypeptide micelles for targeted nonviral gene delivery. Biomaterials. 2010;31:3404–13. doi: 10.1016/j.biomaterials.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 166.Zhu AJ, Miao K, Deng YB, Ke HT, He H, Yang T. et al. Dually pH/Reduction-Responsive Vesicles for Ultrahigh-Contrast Fluorescence Imaging and Thermo-Chemotherapy-Synergized Tumor Ablation. ACS Nano. 2015;9:7874–85. doi: 10.1021/acsnano.5b02843. [DOI] [PubMed] [Google Scholar]
- 167.Oishi M, Sumitani S, Nagasaki Y. On-off regulation of 19F magnetic resonance signals based on pH-Sensitive PEGylated nanogels for potential tumor-specific smart 19F MRI probes. Bioconjug Chem. 2007;18:1379–82. doi: 10.1021/bc7002154. [DOI] [PubMed] [Google Scholar]
- 168.Chen Y, Ai KL, Liu JH, Sun GY, Yin Q, Lu LH. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials. 2015;60:111–20. doi: 10.1016/j.biomaterials.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 169.Du LL, Zhou JH, Meng LW, Wang XX, Wang CR, Huang YY. et al. The pH-Triggered Triblock Nanocarrier Enabled Highly Efficient siRNA Delivery for Cancer Therapy. Theranostics. 2017;7:3432–45. doi: 10.7150/thno.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lv YJ, Hao L, Hu WJ, Ran Y, Bai Y, Zhang LK. Novel multifunctional pH-sensitive nanoparticles loaded into microbubbles as drug delivery vehicles for enhanced tumor targeting. Sci Rep. 2016;6:29321. doi: 10.1038/srep29321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen ZH, Krishnamachary B, Penet MF, Bhujwalla ZM. Acid-degradable Dextran as an Image Guided siRNA Carrier for COX-2 Downregulation. Theranostics. 2018;8:1–12. doi: 10.7150/thno.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gillies ER, Jonsson TB, Frechet JMJ. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. J Am Chem Soc. 2004;126:11936–43. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- 173.Song CC, Ji R, Du FS, Liang DH, Li ZC. Oxidation-Accelerated Hydrolysis of the Ortho Ester-Containing Acid-Labile Polymers. Acs Macro Lett. 2013;2:273–7. doi: 10.1021/mz4000392. [DOI] [PubMed] [Google Scholar]
- 174.Fu SX, Yang GQ, Wang J, Wang X, Cheng X, Zha Q. et al. pH-sensitive poly(ortho ester urethanes) copolymers with controlled degradation kinetic: Synthesis, characterization, and in vitro evaluation as drug carriers. Eur Polym J. 2017;95:275–88. [Google Scholar]
- 175.Yang GQ, Wang X, Fu SX, Tang RP, Wang J. pH-triggered chitosan nanogels via an ortho ester-based linkage for efficient chemotherapy. Acta Biomater. 2017;60:232–43. doi: 10.1016/j.actbio.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 176.An JX, Dai XM, Wu ZM, Zhao Y, Lu ZT, Guo QQ. et al. An Acid-Triggered Degradable and Fluorescent Nanoscale Drug Delivery System with Enhanced Cytotoxicity to Cancer Cells. Biomacromolecules. 2015;16:2444–54. doi: 10.1021/acs.biomac.5b00693. [DOI] [PubMed] [Google Scholar]
- 177.Tasciotti E, Liu XW, Bhavane R, Plant K, Leonard AD, Price BK. et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3:151–7. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 178.Larsen EKU, Nielsen T, Wittenborn T, Birkedal H, Vorup-Jensen T, Jakobsen MH. et al. Size-Dependent Accumulation of PEGylated Silane-Coated Magnetic Iron Oxide Nanoparticles in Murine Tumors. ACS Nano. 2009;3:1947–51. doi: 10.1021/nn900330m. [DOI] [PubMed] [Google Scholar]
- 179.Yang JA, Lohse SE, Murphy CJ. Tuning Cellular Response to Nanoparticles via Surface Chemistry and Aggregation. Small. 2014;10:1642–51. doi: 10.1002/smll.201302835. [DOI] [PubMed] [Google Scholar]
- 180.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP. et al. Vascular-Permeability in a Human Tumor Xenograft-Molecular-Size Dependence and Cutoff Size. Cancer Res. 1995;55:3752–6. [PubMed] [Google Scholar]
- 181.Li H, Liu XS, Huang N, Ren KF, Jin Q, Ji J. "Mixed-charge Self-Assembled Monolayers" as A Facile Method to Design pH-induced Aggregation of Large Gold Nanoparticles for Near-Infrared Photothermal Cancer Therapy. ACS Appl Mater Interfaces. 2014;6:18930–7. doi: 10.1021/am504813f. [DOI] [PubMed] [Google Scholar]
- 182.Yu TW, Lu IL, Huang WC, Hu SH, Hung CC, Chiang WH. et al. Acidity-triggered surface charge neutralization and aggregation of functionalized nanoparticles for promoted tumor uptake. RSC Adv. 2016;6:36293–5. [Google Scholar]
- 183.Wang Y, Du JW, Wang YX, Jin Q, Ji J. Pillar[5]arene based supramolecular prodrug micelles with pH induced aggregate behavior for intracellular drug delivery. Chem Commun. 2015;51:2999–3002. doi: 10.1039/c4cc09274k. [DOI] [PubMed] [Google Scholar]
- 184.Guan XW, Guo ZP, Lin L, Chen J, Tian HY, Chen XS. Ultrasensitive pH Triggered Charge/Size Dual-Rebound Gene Delivery System. Nano Lett. 2016;16:6823–31. doi: 10.1021/acs.nanolett.6b02536. [DOI] [PubMed] [Google Scholar]
- 185.Gao N, Bozeman EN, Qian W, Wang L, Chen H, Lipowska M. et al. Tumor Penetrating Theranostic Nanoparticles for Enhancement of Targeted and Image-guided Drug Delivery into Peritoneal Tumors following Intraperitoneal Delivery. Theranostics. 2017;7:1689–704. doi: 10.7150/thno.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating Tumor Targeting Efficiency of Nanoparticles Through Design. Nano Lett. 2009;9:1909–15. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 187.Li HJ, Du JZ, Du XJ, Xu CF, Sun CY, Wang HX. et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. P Natl Acad Sci U S A. 2016;113:4164–9. doi: 10.1073/pnas.1522080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zan MH, Li JJ, Luo SZ, Ge ZS. Dual pH-triggered multistage drug delivery systems based on host-guest interaction-associated polymeric nanogels. Chem Commun. 2014;50:7824–7. doi: 10.1039/c4cc03120b. [DOI] [PubMed] [Google Scholar]
- 189.Li L, Sun W, Zhong JJ, Yang QQ, Zhu X, Zhou Z. et al. Multistage Nanovehicle Delivery System Based on Stepwise Size Reduction and Charge Reversal for Programmed Nuclear Targeting of Systemically Administered Anticancer Drugs. Adv Funct Mater. 2015;25:4101–13. [Google Scholar]
- 190.Li JJ, Ke WD, Li H, Zha ZS, Han Y, Ge ZS. Endogenous Stimuli-Sensitive Multistage Polymeric Micelleplex Anticancer Drug Delivery System for Efficient Tumor Penetration and Cellular Internalization. Adv Healthc Mater. 2015;4:2206–19. doi: 10.1002/adhm.201500379. [DOI] [PubMed] [Google Scholar]
- 191.Fan YB, Li CY, Li FY, Chen DY. pH-activated size reduction of large compound nanoparticles for in vivo nucleus-targeted drug delivery. Biomaterials. 2016;85:30–9. doi: 10.1016/j.biomaterials.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 192.Xu X, Saw PE, Tao W, Li Y, Ji X, Yu M. et al. Tumor Microenvironment-Responsive Multistaged Nanoplatform for Systemic RNAi and Cancer Therapy. Nano Lett. 2017;17:4427–35. doi: 10.1021/acs.nanolett.7b01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brys AK, Gowda R, Loriaux DB, Robertson GP, Mosca PJ. Nanotechnology-based strategies for combating toxicity and resistance in melanoma therapy. Biotechnol Adv. 2016;34:565–77. doi: 10.1016/j.biotechadv.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 194.Wang S, Qiu J, Shi Z, Wang Y, Chen M. Nanoscale drug delivery for taxanes based on the mechanism of multidrug resistance of cancer. Biotechnol Adv. 2015;33:224–41. doi: 10.1016/j.biotechadv.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 195.Xie J, Yang Z, Zhou C, Zhu J, Lee RJ, Teng L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol Adv. 2016;34:343–53. doi: 10.1016/j.biotechadv.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 196.Dai J, Lin SD, Cheng D, Zou SY, Shuai XT. Interlayer-Crosslinked Micelle with Partially Hydrated Core Showing Reduction and pH Dual Sensitivity for Pinpointed Intracellular Drug Release. Angew Chem Int Ed. 2011;50:9404–8. doi: 10.1002/anie.201103806. [DOI] [PubMed] [Google Scholar]
- 197.Lynn DM, Amiji MM, Langer R. pH-responsive polymer microspheres: Rapid release of encapsulated material within the range of intracellular pH. Angew Chem Int Ed. 2001;40:1707–10. [PubMed] [Google Scholar]
- 198.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–36. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 199.Meng FH, Zhong YA, Cheng R, Deng C, Zhong ZY. pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: concept and recent advances. Nanomedicine. 2014;9:487–99. doi: 10.2217/nnm.13.212. [DOI] [PubMed] [Google Scholar]
- 200.Liu JJ, Liu Q, Yang CH, Sun Y, Zhang YM, Huang PS. et al. cRGD-Modified Benzimidazole-based pH-Responsive Nanoparticles for Enhanced Tumor Targeted Doxorubicin Delivery. ACS Appl Mater Interfaces. 2016;8:10726–36. doi: 10.1021/acsami.6b01501. [DOI] [PubMed] [Google Scholar]
- 201.Chacko RT, Ventura J, Zhuang JM, Thayumanavan S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv Drug Deliv Rev. 2012;64:836–51. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Qiu L, Hu Q, Cheng L, Li L, Tian C, Chen W. et al. cRGDyK modified pH responsive nanoparticles for specific intracellular delivery of doxorubicin. Acta Biomater. 2016;30:285–98. doi: 10.1016/j.actbio.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 203.Wang L, Liu GH, Wang XR, Hu JM, Zhang GY, Liu SY. Acid-Disintegratable Polymersomes of pH-Responsive Amphiphilic Diblock Copolymers for Intracellular Drug Delivery. Macromolecules. 2015;48:7262–72. [Google Scholar]
- 204.Li S, Li WG, Khashab NM. Stimuli responsive nanomaterials for controlled release applications. Nanotechnol Rev. 2012;1:493–513. [Google Scholar]
- 205.Huang PS, Song HJ, Wang WW, Sun Y, Zhou JH, Wang X. et al. Integrin-Targeted Zwitterionic Polymeric Nanoparticles with Acid-Induced Disassembly Property for Enhanced Drug Accumulation and Release in Tumor. Biomacromolecules. 2014;15:3128–38. doi: 10.1021/bm500764p. [DOI] [PubMed] [Google Scholar]
- 206.Zheng M, Yang CM, Meng FH, Peng R, Zhong ZY. pH-sensitive degradable hydrophobe modified 1.8 kDa branched polyethylenimine as "artificial viruses" for safe and efficient intracellular gene transfection. Macromol Res. 2012;20:327–34. [Google Scholar]
- 207.Liu ZZ, Zheng M, Meng FH, Zhong ZY. Non-viral gene transfection in vitro using endosomal pH-sensitive reversibly hydrophobilized polyethylenimine. Biomaterials. 2011;32:9109–19. doi: 10.1016/j.biomaterials.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 208.Wu YL, Chen W, Meng FH, Wang ZJ, Cheng R, Deng C. et al. Core-crosslinked pH-sensitive degradable micelles: A promising approach to resolve the extracellular stability versus intracellular drug release dilemma. J Control Release. 2012;164:338–45. doi: 10.1016/j.jconrel.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 209.Chen W, Zhong P, Meng FH, Cheng R, Deng C, Feijen J. et al. Redox and pH-responsive degradable micelles for dually activated intracellular anticancer drug release. J Control Release. 2013;169:171–9. doi: 10.1016/j.jconrel.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 210.Chen W, Meng FH, Cheng R, Deng C, Feijen J, Zhong Z. Facile construction of dual-bioresponsive biodegradable micelles with superior extracellular stability and activated intracellular drug release. J Control Release. 2015;210:125–33. doi: 10.1016/j.jconrel.2015.05.273. [DOI] [PubMed] [Google Scholar]
- 211.Li X, Yang WJ, Zou Y, Meng FH, Deng C, Zhong ZY. Efficacious delivery of protein drugs to prostate cancer cells by PSMA-targeted pH-responsive chimaeric polymersomes. J Control Release. 2015;220:704–14. doi: 10.1016/j.jconrel.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 212.Li SK, Meng FH, Wang ZJ, Zhong YN, Zheng M, Liu HY. et al. Biodegradable polymersomes with an ionizable membrane: Facile preparation, superior protein loading, and endosomal pH-responsive protein release. Eur J Pharm Biopharm. 2012;82:103–11. doi: 10.1016/j.ejpb.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 213.Ulbrich K, Subr V. Polymeric anticancer drugs with pH-controlled activation. Adv Drug Deliv Rev. 2004;56:1023–50. doi: 10.1016/j.addr.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 214.Zhu LJ, Zhao LL, Qu XZ, Yang ZZ. pH-Sensitive Polymeric Vesicles from Coassembly of Amphiphilic Cholate Grafted Poly(L-lysine) and Acid-Cleavable Polymer-Drug Conjugate. Langmuir. 2012;28:11988–96. doi: 10.1021/la3015767. [DOI] [PubMed] [Google Scholar]
- 215.Gu YD, Zhong YN, Meng FH, Cheng R, Deng C, Zhong ZY. Acetal-Linked Paclitaxel Prodrug Micellar Nanoparticles as a Versatile and Potent Platform for Cancer Therapy. Biomacromolecules. 2013;14:2772–80. doi: 10.1021/bm400615n. [DOI] [PubMed] [Google Scholar]
- 216.Lu Y, Hu Q, Lin Y, Pacardo DB, Wang C, Sun W. et al. Transformable liquid-metal nanomedicine. Nat Commun. 2015;6:10066. doi: 10.1038/ncomms10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sharma S, Zapatero-Rodríguez J, O'Kennedy R. Prostate cancer diagnostics: Clinical challenges and the ongoing need for disruptive and effective diagnostic tools. Biotechnol Adv. 2017;35:135–49. doi: 10.1016/j.biotechadv.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 218.Wang TT, Wang DG, Yu HJ, Wang MW, Liu JP, Feng B. et al. Intracellularly Acid-Switchable Multifunctional Micelles for Combinational Photo/Chemotherapy of the Drug-Resistant Tumor. ACS Nano. 2016;10:3496–508. doi: 10.1021/acsnano.5b07706. [DOI] [PubMed] [Google Scholar]
- 219.Hou WX, Zhao X, Qian XQ, Pan F, Zhang CL, Yang YM. et al. pH-Sensitive self-assembling nanoparticles for tumor near-infrared fluorescence imaging and chemo-photodynamic combination therapy. Nanoscale. 2016;8:104–16. doi: 10.1039/c5nr06842h. [DOI] [PubMed] [Google Scholar]
- 220.Wang Y, Wang C, Li Y, Huang G, Zhao T, Ma X. et al. Digitization of Endocytic pH by Hybrid Ultra-pH-Sensitive Nanoprobes at Single-Organelle Resolution. Adv Mater. 2017;29:1603794. doi: 10.1002/adma.201603794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhao T, Huang G, Li Y, Yang S, Ramezani S, Lin Z. et al. A Transistor-like pH Nanoprobe for Tumour Detection and Image-guided Surgery. Nat Biomed Eng. 2016;1:0006. doi: 10.1038/s41551-016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li Y, Zhao T, Wang C, Lin Z, Huang G, Sumer BD. et al. Molecular basis of cooperativity in pH-triggered supramolecular self-assembly. Nat Commun. 2016;7:13214. doi: 10.1038/ncomms13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang Z, Luo M, Mao C, Wei Q, Zhao T, Li Y. et al. A Redox-Activatable Fluorescent Sensor for the High-Throughput Quantification of Cytosolic Delivery of Macromolecules. Angew Chem Int Ed. 2017;56:1319–23. doi: 10.1002/anie.201610302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang C, Wang Y, Li Y, Bodemann B, Zhao T, Ma X. et al. A nanobuffer reporter library for fine-scale imaging and perturbation of endocytic organelles. Nat Commun. 2015;6:8524. doi: 10.1038/ncomms9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang S, Zhou K, Huang G, Takahashi M, Sherry AD, Gao J. A novel class of polymeric pH-responsive MRI CEST agents. Chem Commun. 2013;49:6418–20. doi: 10.1039/c3cc42452a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yu H, Zou Y, Wang Y, Huang X, Huang G, Sumer BD. et al. Overcoming Endosomal Barrier by Amphotericin B-Loaded Dual pH-Responsive PDMA-b-PDPA Micelleplexes for siRNA Delivery. ACS Nano. 2011;5:9246–55. doi: 10.1021/nn203503h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M. et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–23. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]