Abstract
Objective
This study evaluated whether tobacco quitline telephone coaches can be trained to counsel hazardous-drinking smokers to improve smoking cessation success and to limit or abstain from alcohol use.
Method
Smokers (N=1,948) who called the New York State Smokers’ Quitline and reported hazardous drinking (exceeding sex-specific weekly limits [14 drinks for men, 7 drinks for women] or meeting/exceeding daily drinking limits [5 drinks for men, 4 drinks for women] at least once in the past year) were randomized to receive either brief motivational counseling to limit or abstain from alcohol plus an alcohol reduction booklet added to standard care (Alcohol + Tobacco Counseling; ATC), or only smoking cessation counseling plus a smoking cessation booklet added to standard care (Tobacco-Only Counseling; TOC).
Results
Acceptable coach adherence was achieved. The intention-to-treat (ITT) analysis showed that ATC was associated with a significantly higher rate of smoking abstinence at 7-month follow-up (13.5%) compared with TOC (10.3%; p = .03). The respondent analysis (ATC = 26.2%; TOC = 20.4%) paralleled the ITT findings. When controlling for treatment condition, participants who did not report any heavy drinking were significantly more likely to quit smoking than those who reported any heavy drinking (OR = 1.87, 95% CI [1.29, 2.71]; p = .001).
Conclusions
A brief alcohol intervention plus standard care via a telephone quitline resulted in significantly higher smoking cessation rates for hazardous-drinking callers. Given that quitline coaches were trained to provide the intervention with acceptable adherence, the potential to extend this intervention for wide-scale implementation and impact is promising.
Keywords: alcohol, heavy drinking, smoking, quitline
Telephone-based tobacco cessation services (“quitlines”) are an easily accessible and effective method of treatment delivery (Centers for Disease Control and Prevention, 2004; Stead, Perera, & Lancaster, 2006). Quitline services can be accessed by any smoker via a free telephone number in many countries in the world, including Australia, the United Kingdom, the United States, and parts of Asia (Abdullah, Lam, Chan, & Hedley, 2004; An et al., 2006; Borland, Balmford, Segan, Livingston, & Owen, 2003; Platt, Tannahill, Watson, & Fraser, 1997), and generally include counseling, referrals to local cessation services, and free or discounted pharmacotherapy (Centers for Disease Control and Prevention, 2004; Stead et al., 2006). Quitlines have been shown to be effective, with typical long-term (6- or 12-month) cessation rates of approximately 20% to 30%, depending on whether an intention-to-treat (ITT) or respondent analysis is reported (An et al., 2006; Hollis et al., 2007; N. Miller et al., 2005; Platt et al., 1997). One of the benefits of quitlines is that they can reach millions of smokers annually, including those with co-occurring alcohol use. Daily smokers are at heightened risk for hazardous drinking (i.e., exceeding sex-specific weekly limits [14 drinks for men, seven drinks for women] or meeting/exceeding daily drinking limits [five drinks for men, four drinks for women] at least once in the past year (National Institute on Alcohol Abuse and Alcoholism [NIAAA], 2005) and alcohol use diagnoses (McKee, Falba, O’Malley, Sindelar, & O’Connor, 2007). Thus, quitlines represent a unique opportunity to concurrently address both smoking abstinence and alcohol reduction.
The importance of addressing smoking and drinking concurrently is underscored by the fact that the relative risk of mortality increases with the combined versus singular abuse of alcohol and tobacco (Grucza, Abbacchi, Przybeck, & Gfroerer, 2007). In particular, the concurrent use of alcohol and tobacco increases the incidence of cirrhosis, pancreatitis, and pancreatic cancer (Klatsky & Armstrong, 1992; Talamini et al., 1999). In addition, the incidence of current tobacco and alcohol use is high among patients diagnosed with primary oral cancer, with heavy drinkers and smokers having 38 times the risk of abstainers (Warnakulasuriya, 2009). Indeed, the rate of tobacco and alcohol co-use is particularly high in patients diagnosed with oral and pharyngeal cancers, for whom use of tobacco products and excessive alcohol consumption is estimated to account for 75% to 80% of cases (Silverman, 2001; Warnakulasuriya, 2009). These statistics are especially troubling because cancers of the head and neck are lethal, with a 5-year survival rate of approximately 50%, and three quarters of these cancers could be prevented by smoking cessation and reduction of heavy drinking (Warnakulasuriya, 2009).
Numerous studies have shown a strong link between drinking and smoking behaviors (McKee et al., 2007; Shiffman & Balbanis, 1996), and smokers with current or past alcohol problems exhibit less likelihood of quitting (DiFranza & Guerrera, 1990; Kahler et al., 2009; Leeman et al., 2008). Indeed, evidence from clinical trials suggests that drinking during a smoking cessation attempt increases the likelihood of relapse (Humfleet, Muñoz, Sees, Reus, & Hall, 1999), and one study found that adding an alcohol intervention to smoking cessation treatment improved smoking quit rates among hazardous-drinking smokers (Kahler et al., 2008). The intervention tested in the clinical trial by Kahler et al. (2008) and the present study were based on motivational interviewing, a therapeutic approach that blends (a) a relational component in which counselors convey a highly empathic, collaborative, accepting, and compassionate stance toward individuals considering change, and (b) a technical component in which counselors intentionally elicit and reinforce client statements that favor change such that clients gradually talk themselves into changing the targeted behavior based on their unique self-motivations (W. Miller & Rollnick, 2012; W. R. Miller & Rose, 2009). Several meta-analyses have shown that this therapeutic style is associated with significant changes in heavy drinking compared with no intervention (Vasilaki, Hosier, & Cox, 2006; Wilk, Jensen, & Havighurst, 1997). Notably, this style is less explicitly directive than the standard smoking cessation treatment, which is practical counseling, a style that focuses on general problem solving and skills training (Fiore et al., 2008).
Evidence from a recent study of New York State Smokers’ Quitline (NYSSQL) callers found that over 20% met criteria for hazardous drinking, and hazardous drinkers had significantly less success quitting smoking (Toll et al., 2012). However, the effect of adding an alcohol intervention to smoking cessation treatment is untested in the context of a quitline. Given these extant data, we hypothesized that reductions in heavy drinking would promote smoking cessation success in this population.
The primary aims of the present research were (a) to train telephone counselors (i.e., quitline “coaches”) working for the NYSSQL to provide a brief alcohol intervention, and (b) to determine if this intervention resulted in improved smoking cessation outcomes. We hypothesized that we would be able to train quitline coaches to conduct brief alcohol counseling adherently. Further, we hypothesized that alcohol counseling plus standard care would result in improved smoking cessation outcomes for hazardous-drinking smokers. As secondary outcomes, we also examined changes in heavy drinking between groups and the association between smoking quit status and heavy drinking by group.
Method
Study Design, Participants, and Intervention
This investigation was a two-group randomized controlled study conducted with heavy-drinking adult smokers who contacted the NYSSQL for assistance from January 3, 2011, through October 7, 2011. Twenty-eight NYSSQL coaches (representing all full- and half-time coaches) participated. The institutional review boards of Roswell Park Cancer Institute and the Yale School of Medicine approved this study.
Training was initially conducted with a small group of senior coaches to test and refine all programming and flow of both the Alcohol + Tobacco Counseling (ATC) and Tobacco-Only Counseling (TOC) protocols. Five senior coaches were trained in the delivery of study interventions during a 3.5-hr classroom session conducted by one of the authors (BAT). The training included information on high-risk drinking, motivational interviewing skills, review of the format and content of counseling protocols as they would appear on computer screens, role-play practice, explicit instructions to limit study-specific counseling to 5 min (for both study conditions), discussion of written and verbal supervision, and discussion of how to implement the protocol in the NYSSQL setting. Once refined, the other 23 quitline coaches received a 3.5-hr training session and then were certified to perform the interventions in the trial. To be certified, coaches needed to exhibit at least adequate adherence to intervention strategies (an average of 4 or higher on a 7-point scale across all intervention adherence items) in at least two calls per intervention, as determined by doctoral-level supervisors from the research team. These call data were not included in the clinical trial data set.
Because all coaches delivered both counseling interventions, we implemented several procedures to ensure that there was little or no contamination between the conditions. First, online text screens guided the coach to adhere to the content of the counseling. Second, weekly supervision was conducted with review of adherence ratings. Last, consistent with previous studies (Carroll et al., 1998; Toll et al., 2010), “redlining” rules were implemented after coaches were certified. For either intervention condition, after an initial call was found to be below the certification standard, extra supervision was provided, and a new recording was coded. Of the 28 coaches who were trained to provide the study counseling interventions, 24 were certified and four failed to reach certification criterion. One coach was redlined following initial certification but returned to adequate performance with additional supervision. During the treatment phase of the study, the 24 coaches provided counseling, and all received weekly telephone supervision with written and verbal feedback on two audio tapes per week (one per condition) from a doctoral-level supervisor.
To be eligible, callers had to be (a) an adult (≥18 years old) smoker, (b) an English speaker, (c) calling the NYSSQL for assistance, (d) not enrolled in any cessation programs through another source (e.g., their insurance company), and (e) meet hazardous-drinking criteria as defined by NIAAA (i.e., exceeding sex-specific weekly limits [14 drinks for men, seven drinks for women] or meeting/exceeding daily drinking limits [five drinks for men, four drinks for women] at least once in the past year (NIAAA, 2005). Callers receiving extra cessation services were excluded to ensure the same standard of cessation assistance for all participants.
To ensure translatability, this study conformed to the NYSSQL’s standard practices. All callers received an initial intake call that included medical screening for a 2-week starter package of nicotine patches or gum and a free mailing that included the NYSSQL’s stop smoking booklet “Break Loose!” and smoking cessation tip sheets. This call included a secure, web-based structured interview and counseling session. All callers also received a quit-day follow-up call, which was scheduled to occur within 30 days of the initial call. The quit-day telephone call included protocol specific counseling and assessed smoking status and receipt of nicotine replacement therapy (NRT) medication. These calls began approximately 7 to 10 days from the NRT shipping date, which shipped 24 hr from the initial call, or 72 hr if ordered on a Friday. Participants were called up to four times, with calls generally spaced every other day (Monday through Thursday, 9:00 a.m. to 9:00 p.m.; Friday, Saturday, and Sunday, 9:00 a.m. to 5:00 p.m.).
After final determination of eligibility, callers were consented, enrolled, randomized by a computer program without stratification (created by author SS), and electronically assigned to their treatment condition. Enrolled callers received additional assessment questions in their interviews. After the caller completed the additional assessment items, the coach delivered the condition-specific counseling protocol, which is described briefly in Table 1. The ATC protocol focused initially on having the caller examine how smoking and drinking are paired and the ways in which drinking may have inhibited previous smoking cessation attempts. Caller statements that supported changes in drinking behavior were reflected and amplified (e.g., if the caller stated, “Yeah, when I drank before it sometimes led to smoking even though I really didn’t want to smoke,” the coach might say, “Drinking led to smoking in the past for you, so now you wonder if it might be important to not drink when you try to quit smoking this time around”). After asking permission, research findings were shared with callers, showing that hazardous alcohol use can undermine efforts at smoking cessation and that abstaining from drinking for 1 month could increase their chances for successful smoking cessation (Kahler et al., 2008). After that month, it was recommended that callers never drink more than three (for women) or four (for men) drinks (i.e., below NIAAA guidelines) at a single sitting, both to maintain good health and to avoid having a slip and smoking when intoxicated. Coaches were trained to elicit from ATC callers their goal for reduced drinking. Callers in the ATC condition also received an additional NIAAA booklet, “Rethinking Drinking.” The TOC protocol focused on standard smoking cessation advice (i.e., practical counseling—general problem solving and skills training), which is shown to have a strong base of evidence for its efficacy (Fiore et al., 2008). Because the ATC condition received the “Rethinking Drinking” booklet, the TOC condition received “Clearing the Air” to balance the groups on literature received.
Table 1.
Representative Alcohol Tobacco Counseling Content and Tobacco-Only Counseling Content
| Time point | Alcohol + Tobacco Counseling | Tobacco-Only Counseling |
|---|---|---|
| Intake | How do you see your drinking and smoking going together? | We suggest that you work on staying busy on your quit date and beyond by spending as much time as you can in nonsmoking places like… |
| Describe your personal experiences with drinking during past smoking quit attempts. | We also suggest that you work on being active, doing things like… | |
| Tell me how you think drinking alcohol might affect your effort to quit smoking? | In the quitting process, you will experience urges. We suggest that you cope in specific ways like… | |
| Based on the research we just discussed, we recommend not drinking for the first month that you are quitting smoking, if that is possible for you. After that month, we recommend never drinking more than (3 for women/4 for men) drinks at a single sitting, both to maintain good health and to avoid having a slip and smoking when you are intoxicated. Of course, you have to choose the goal that will work best for you. What are your thoughts? | To make your quit attempt go as smoothly as possible, we suggest that you make some lifestyle changes that minimize your level of stress. What are some changes you can make to reduce stress in your life? | |
| Follow-up | At our last call we talked about not drinking or drinking less. How has it gone? | At your last call, we discussed several coping skills to help with your quit attempt (for instance, going to nonsmoking places, staying busy, and coping with urges). Which of these were you able to do? |
| What is your current goal regarding drinking over the next month? | What were the specific things you did to stay busy (e.g., going to nonsmoking places like gyms and being active like walking or biking)? |
Blinded independent surveyors assessed smoking cessation during 7-month follow-up calls, as advised by the North American Quitline Consortium (NAQC, 2009). Callers provided verbal informed consent to participate in all telephone-based interviews (i.e., intake and quit day and 7-month follow-ups) and received a $10 payment for completion of the 7-month follow-up. The protocol for the 7-month follow-up call was a 15-callback design (meaning all participants were called up to 15 times), with the first three calls placed at the same time as the initial call to the NYSSQL. The timeframe for these calls usually fell within approximately two weeks of the 7-month follow-up date. Calls to complete the follow-up interviews were placed 7 days a week between 9:00 a.m. and 9:00 p.m., and the specific callback times to individual cases was tailored so that calls were placed on both evenings and days as well as weekends and weekdays. Procedures were also in place to trace participants who changed their phone number using commercially available services.
Protocol Adherence
Adherence by coaches to the counseling protocol was tested through audiotape ratings of study calls. Seven blind, independent tape raters rated a random sample of approximately 20% (n = 400) of all tapes. The raters used a validated adherence system that evaluated discriminability between the two types of counseling in the use of ATC variables, TOC variables, and standard care variables (Carroll et al., 2000). TOC variables were variables specific to the counseling protocol for that condition (e.g., statements about staying busy and active), whereas standard care variables refer to variables that were standard in all interviews (e.g., statements about assessment of smoking). Raters assessed these items using a 7-point scale regarding adherence from 1 (not at all) to 7 (extensively). These ratings reflected how often a coach used specific strategies within a session. Consistent with previous research (Carroll et al., 1998; Martino, Carroll, Nich, & Rounsaville, 2006; Toll et al., 2010), a reliability sample of 10 tapes was randomly selected for coding (i.e., all 10 tapes were coded by all seven raters) and revealed a high level of interrater reliability by use of Shrout and Fleiss’s (1979) model for random effects (range of mean intraclass correlation coefficient estimates for nine representative items from Table 2 = .88 to .99). After deletion of one outlier because of error (i.e., related to the time recorded going over the actual call time after a call was interrupted), time spent per call was not significantly different between the groups (ATC, n = 200, M = 823.03 s; TOC, n = 199, M = 845.08 s; t [df = 397] = −.58, p = .05).
Table 2.
Mean Adherence Ratings of Coaches in the Alcohol Tobacco Counseling and Tobacco-Only Counseling Groups
| Mean score (±SD)
|
Statisticsa
|
||||
|---|---|---|---|---|---|
| Type of counseling and representative items | Alcohol + Tobacco Counseling group | Tobacco-Only Counseling group | 95% CI of mean difference | t | p |
| Alcohol + Tobacco Counseling | |||||
| Linking alcohol and smoking | 4.09 (1.69) | 1.94 (0.80) | [−2.4, −1.90] | −16.24 | <.001 |
| Goals for drinking behavior | 3.07 (1.49) | 1.13 (0.45) | [−2.17, −1.73] | −17.64 | <.001 |
| Strategies to reduce drinking | 2.67 (1.49) | 1.11 (0.42) | [−1.77, −1.34] | −14.18 | <.001 |
| Tobacco-Only Counseling | |||||
| Staying busy and active | 1.31 (0.70) | 2.78 (1.07) | [1.29, 1.65] | 16.21 | <.001 |
| Coping with urges | 1.40 (0.78) | 2.49 (0.99) | [0.92, 1.27] | 12.20 | <.001 |
| Coping with negative moods and stress | 1.31 (0.66) | 3.02 (1.08) | [1.53, 1.88] | 19.06 | <.001 |
| Standard care | |||||
| Goals for smoking behavior | 3.15 (1.39) | 2.97 (1.26) | [−0.44, 0.09] | −1.32 | .19 |
| Assess smoking | 4.57 (1.57) | 4.52 (1.57) | [−0.36, 0.26] | −0.32 | .75 |
| Medication assessment | 5.23 (1.62) | 5.22 (1.50) | [−0.32, 0.30] | −0.06 | .95 |
Note. Adherence ratings are on a 7-point Likert scale: 1 = not at all, 2 = a little (once), 3 = infrequently (twice), 4 = somewhat (3–4 times), 5 = quite a bit (5–6 times), 6 = considerably (>6 times/more depth in interventions), 7 = extensively (high frequency/characterizes entire session). CI = confidence interval.
All independent samples t tests had between 395 and 398 degrees of freedom. All statistical tests were two-sided. The Bonferroni-corrected significance level for these nine tests is p = .005.
Participant Measurement
Outcome measures
We analyzed counseling protocol adherence and smoking cessation as primary outcomes. The treatment adherence outcome examined was the difference in ratings of frequency of ATC, TOC, and standard care statements between the two experimental groups. The smoking cessation outcome examined was 7-day point prevalence abstinence at 7-month follow-up. Abstinence from smoking was defined as self-reported abstinence (no smoking, not even a puff) during the specified treatment period after quitting. Point prevalence abstinence was assessed with two items, based on the standard interview already in place at the NYSSQL. Item 1 was “Are you smoking now?” with a yes–no answer format. Participants who answered “no” to this question were given Item 2, which was “Have you smoked a cigarette even a puff in the past 7 days?” An answer of “no” to both of these items was considered point prevalence abstinence. We examined both an ITT population as our primary outcome and survey respondents as a secondary outcome. For all ITT analyses, enrolled callers who dropped out were considered to be smoking, which is the standard in the field of nicotine and tobacco research (Ahluwalia, Harris, Catley, Okuyemi, & Mayo, 2002; Gonzales et al., 2006; Hurt et al., 1997). For the alcohol outcomes, we used the timeline followback (TLFB) method of collecting alcohol data on a day-by-day basis for the 7 days before the date of measurement, which is a validated tool for collection of alcohol use by telephone (Sobell & Sobell, 2003).
Descriptive measures
Descriptive data were collected on participants, including age, race, gender, education, number of cigarettes smoked per day, and number of years smoking.
Statistical Analysis
Caller characteristics
Baseline characteristics of callers were analyzed by use of chi-square tests for categorical variables and a general linear model for continuous variables (conducted with SAS 9.3). All statistical tests were two-sided.
Primary adherence outcome
Independent sample t tests were used to analyze differences in adherence between the ATC and the TOC groups (conducted with SPSS 19). The Bonferroni corrected significance level for these nine tests was set at p = .005.
Primary smoking cessation outcome
To examine primary treatment effects on tobacco, differences between groups in self-reported 7-day point prevalence abstinence from smoking at the 7-month follow-up were analyzed by use of logistic regression, with the ATC group compared with the TOC group in simple regression models (conducted with SAS). For the primary analysis, all tests were evaluated at the 0.05 level and participants with missing data were counted as smokers (Ahluwalia et al., 2002; Gonzales et al., 2006; Hays et al., 2001; Hurt et al., 1997); and in accordance with the field of quitline studies, we relied on self-reported abstinence from smoking (An et al., 2006; Hollis et al., 2007; N. Miller et al., 2005). We planned to obtain n = 974 per group to achieve a standard error of 0.02 and a reliable effect size estimate.
Secondary outcome: Heavy drinking
For effects on heavy drinking, we analyzed the effect of treatment condition on the binary outcome of any heavy drinking in the past 7 days at the 7-month follow-up as follows: 0 = no heavy drinking in the past 7 days, 1 = any heavy drinking in the past 7 days using logistic regression (conducted with SPSS).
Secondary outcome: Association between smoking status and heavy drinking
We examined the likelihood of reporting any heavy-drinking days over the last 7 days at 7-month follow-up as a predictor of smoking quit status, controlling for treatment condition using logistic regression (conducted with SPSS).
Results
Caller Characteristics
Of the 32,328 callers screened, 28,324 were excluded for not meeting inclusion criteria, 2,056 declined enrollment, and 1,948 were enrolled (see Figure 1). As presented in Table 3, callers who declined enrollment were different from those who were enrolled on racial background, sex, caller education, number of cigarettes smoked per day, and years smoked. Age was similar between these groups.
Figure 1.
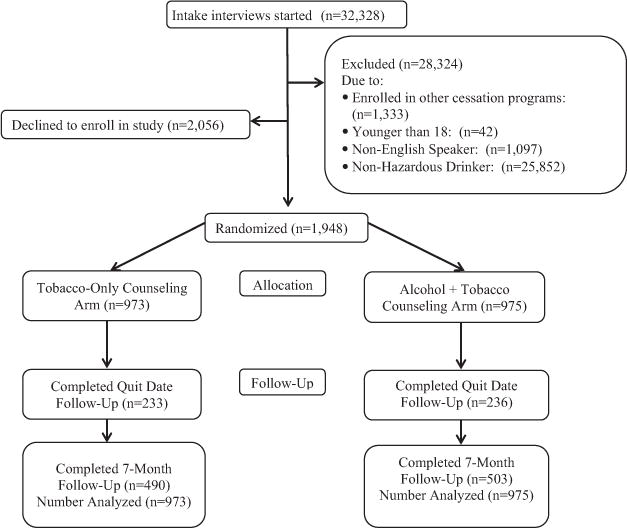
Flow of callers for smoking cessation treatment study.
Table 3.
Baseline Characteristics of Callers by Enrollment Status
| Variables | Enrolled (n = 1,948) |
Declined (n = 2,056) |
pa |
|---|---|---|---|
| Race | <.001 | ||
| % White | 69.3 | 73.8 | |
| % African American | 25.6 | 20.1 | |
| % Native American | 1.2 | 0.9 | |
| % Asian/Pacific Islander | 1.0 | 2.8 | |
| % Other/Multiracial | 2.9 | 2.4 | |
| % Hispanicb | 13.6 | — | |
| % Women | 46.4 | 42.0 | .005 |
| % With a college degree | 16.4 | 19.6 | .01 |
| No. of cigarettes smoked per day (±SD) | 20.3 (10.5) | 19.6 (9.8) | .03 |
| No. of years smoking cigarettes (±SD) | 21.3 (12.2) | 20.2 (12.9) | .004 |
| Age in years (±SD) | 41.5 (12.6) | 41.0 (13.1) | .29 |
Variables were analyzed with chi-square tests for categorical variables and a general linear model for continuous variables. All statistical tests were two-sided.
These data were not collected for participants who declined.
The 1,948 callers who composed the ITT sample consented to and enrolled in the study at the initial treatment telephone call and were randomly assigned to either the ATC (n = 975) or TOC (n = 973) group. There were no differences on demographic and smoking or drinking variables between the treatment conditions (see Table 4).
Table 4.
Baseline Characteristics of Callers by Condition
| Variable | Alcohol + Tobacco Counseling (n = 975 |
Tobacco-Only Counseling (n = 973) |
pa |
|---|---|---|---|
| Race | .65 | ||
| % White | 69.8 | 68.8 | |
| % African American | 24.7 | 26.5 | |
| % Native American | 1.6 | 0.9 | |
| % Asian/Pacific Islander | 1.0 | 1.1 | |
| % Other/Multiracial | 3.0 | 2.8 | |
| % Hispanic | 12.9 | 14.4 | .34 |
| % Women | 47.7 | 45.1 | .26 |
| % With a college degree | 15.9 | 16.7 | .66 |
| No. of cigarettes smoked per day (SD) | 20.4 (10.6) | 20.2 (10.5) | .69 |
| No. of years smoking cigarettes (SD) | 21.5 (12.4) | 21.2 (12.1) | .61 |
| Age in years (SD) | 41.6 (12.8) | 41.3 (12.4) | .59 |
| % No heavy drinking daysb | 57.5 | 57.0 | .54 |
Variables were analyzed with chi-square tests for categorical variables and a general linear model for continuous variables. All statistical tests were two-sided.
This variable was defined as the percentage of participants reporting any National Institute on Alcohol Abuse and Alcoholism defined heavy drinking (for men >4 a day; for women >3 a day) in the week before the baseline assessment was conducted.
Primary Outcome: Adherence
As shown in Table 2, adherence outcomes differed significantly by group in the expected directions. When coaches provided ATC, they used alcohol-focused statements significantly more frequently than when providing TOC, and when they provided TOC, coaches used smoking cessation statements significantly more frequently than when providing ATC. As expected, standard care items were not significantly different between the two groups.
Primary Outcome: Smoking Cessation
As presented in Table 5, in the ITT analysis, the participants who received the ATC intervention displayed a statistically significant higher rate of 7-day point prevalence abstinence from smoking at the 7-month follow-up interview (13.5%) compared with participants who received the TOC intervention (10.3%; odds ratio [OR] = 1.37, 95% confidence interval [CI] [1.04, 1.80], p = .03). The respondent analysis (ATC = 26.2% vs. TOC = 20.4%; OR = 1.39, 95% CI [1.03, 1.87], p = .03) paralleled the ITT findings. Of note, the difference between dropout rates was not significant (ATC = 48.4% [472/975] vs. TOC = 49.6% [483/973], p = .59). We analyzed differences between participants retained for follow-ups and dropouts and found that callers who dropped out were different from those who were retained with regard to age (retained M [SD] = 43.2 [12.7] vs. dropout = 39.6 [12.2], p = <.0001) and number of years smoking (retained M [SD] = 22.7 [12.9] vs. dropout = 19.9 [11.4], p = <.0001). The racial background, sex, caller education, number of cigarettes smoked per day, years smoking, and heavy-drinking days variables were similar between survey responders and dropouts. These results were comparable when the analyses were conducted within condition.
Table 5.
Rates of Smoking Cessation and Heavy Drinking Reduction for Alcohol + Tobacco Counseling and Tobacco-Only Counseling Groups at the 7-Month Follow-Up
| Outcome | Sample size | % Alcohol + Tobacco Counseling group (No. abstinent/No. in sample) | % Tobacco-Only Counseling group (No. abstinent/No. in sample) | OR [95% CI] |
|---|---|---|---|---|
| 7-day abstinence from tobacco (ITT) | 1,948 | 13.5 (132/975) | 10.3 (100/973) | 1.37*** [1.04, 1.80] |
| 7-day abstinence from tobacco (respondents) | 993 | 26.2 (132/503) | 20.4 (100/490) | 1.39*** [1.03, 1.87] |
| 7-day abstinence from heavy drinking days (respondents) | 955 | 74.6 (361/484) | 70.3 (331/471) | 1.29*[.98, 1.69] |
Note. Callers in the Alcohol + Tobacco Counseling group received counseling and a booklet with messages regarding smoking cessation and reducing drinking. Callers in the Tobacco-Only Counseling group received counseling and a booklet with messages regarding smoking cessation. All statistical tests were two-sided. OR = odds ratio; CI = confidence interval; ITT = intention to treat.
p = .07.
p = .05.
Secondary Outcomes
Any heavy drinking
As shown in Table 5, participants in the ATC group reported fewer heavy-drinking days in the week prior to the 7-month follow-up (74.6% with no heavy-drinking days) compared with the TOC group (70.3% with no heavy-drinking days), but this effect was not significant (OR = 1.29, 95% CI [.98, 1.69], p = .07).
Association between smoking status and heavy drinking
To evaluate whether tobacco cessation rates were associated with heavy drinking, we evaluated the likelihood of achieving smoking abstinence by heavy-drinking status at the 7-month follow-up. When controlling for treatment condition, heavy-drinking status was a significant predictor of smoking abstinence (OR = 1.77, 95% CI [1.25, 2.71], p = .001); participants who did not report any heavy drinking were more likely to quit smoking. Furthermore, the effect of treatment condition on smoking abstinence was no longer significant when heavy-drinking status was included in the model (OR = .76, 95% CI [.56, 1.02], p = .07; reference group ATC). We explored the interaction between heavy-drinking days and treatment condition, and this interaction was not significant.
Discussion
The present study is the first quitline investigation to offer an alcohol intervention to hazardous-drinking smokers. This randomized trial showed that telephone quitline coaches can be trained to provide brief alcohol reduction counseling in addition to a standard tobacco intervention, and that the provision of such counseling significantly improved smoking cessation rates (13.5%) compared with those who received tobacco counseling only (10.3%). This difference was paralleled when survey respondents were compared (ATC = 26.2% vs. TOC = 20.4%).
This clinical trial was not designed to answer the question of why these quit rates are lower than the typical quit rate of 20% to 30% for quitline studies, but the lower quit rates we found with smokers who drink at hazardous levels may be related to the fact that these individuals have more problems than the general population because of heavy drinking (Dawson, Li, & Grant, 2008). Moreover, drinking is a known smoking relapse precipitant (Shiffman, 1986), which may help to explain these lowered quit rates as well. The sample in this study is composed entirely of smokers who have the additional trigger of alcohol use, whereas this group of smokers who drink at hazardous levels only make up a subgroup of most other smoking cessation clinical trials.
Among respondents at follow-up, the percentage of participants who reported recent heavy drinking was lower in both groups. A smaller percentage of ATC participants reported recent heavy drinking than TOC participants, but this difference was not significant. However, when controlling for treatment condition, participants who did not report any heavy drinking were significantly more likely to quit smoking than those who reported any heavy drinking. Moreover, the effect of treatment condition on smoking abstinence was no longer significant when heavy-drinking status was included in this analytic model. Thus, the smoking cessation effect may be attributed to greater reductions in heavy drinking, but future research using prospective data is needed to test this mediation hypothesis.
These findings are important, given that over 20% of all quitline callers drink at hazardous levels, which provides an opportunity to reach millions of such smokers who call quitlines annually (Toll et al., 2012). Hazardous-drinking smokers also have more difficulty quitting smoking (DiFranza & Guerrera, 1990; Humfleet et al., 1999; Kahler et al., 2009), and the relative risk of mortality (Grucza et al., 2007) and other major health problems (e.g., oral and pancreatic cancer; Silverman, 2001; Talamini et al., 1999; Warnakulasuriya, 2009) increases with the combined use of alcohol and tobacco.
In this sample of adult smokers who also reported a pattern of hazardous drinking, a brief alcohol intervention significantly reduced the reported level of cigarette smoking and had small, but potentially clinically meaningful, effects on high-risk drinking at 7-month follow-up. Prior research evaluating the benefits of very brief counseling (i.e., 5 min) for hazardous drinking among patients seen in health care settings has also demonstrated a similar reduction in drinking behavior (WHO Brief Intervention Study Group, 1996).
There are multiple reasons why our alcohol intervention might have translated to significant effects for smoking cessation but nonsignificant differences for heavy alcohol use at the 7-month follow-up interview. An important feature is that the alcohol intervention recommended abstaining from alcohol for a 1-month period to minimize the risk of smoking relapse related to alcohol consumption. We did not, however, assess alcohol consumption until the 7-month follow-up, and so perhaps may have missed a critical period in which alcohol use might have been significantly decreased. Although abstinence was not proposed as a long-term change, it was suggested that participants should not drink at levels above NIAAA hazardous-drinking guidelines. Future studies might attempt to engage callers in longer-term changes in their heavy alcohol use. Another factor may be that an assessment of 1 week of drinking, although practical within the quitline setting, may not be sensitive enough to detect changes in heavy drinking. Perhaps if we had assessed alcohol consumption for a longer duration either with the TLFB or another quantity frequency index (Sobell & Sobell, 2003), we might have been able to demonstrate a significant difference in rates of heavy drinking.
We chose to not analyze the quit-date callback follow-up data for several reasons. First, the response rate was deemed to be unacceptably low. Second, we felt that the most rigorous test of smoking cessation would be the later 7-month follow-up. Last, the 7-month follow-up is consistent with the NAQC guidelines (NAQC, 2009).
There have been several smoking cessation studies examining various aspects of quitlines (e.g., the number or length of calls; An et al., 2006; Hollis et al., 2007), but there have been fewer studies that investigate novel counseling strategies. This is the second study (Toll et al., 2010) to show that quitline coaches can be trained to provide a novel counseling intervention with good adherence, which results in improved smoking cessation outcomes. Future research could examine the degree to which quitline coach counseling adherence directly affects smoking cessation outcomes, as well as the feasibility, acceptability, and effectiveness of training coaches in other counseling approaches to maximize the efficacy of quitlines.
A large proportion of eligible callers (51.3%; 2,056/4,004) refused to participate in the study intervention. This is consistent with our previous research, which has shown high refusal rates for research conducted in a real-world state quitline (for example, 58.6%; Toll et al., 2010). It is unfortunate that participation in quitline research studies is not appealing to quitline callers, given that the present study and others have shown the treatments tested to be efficacious among those willing to participate. Now that the present study has demonstrated efficacy for hazardous-drinking smokers, one possibility is to explicitly state the treatment’s efficacy to callers in an effort to encourage them to participate. Other options to promote participation are to provide incentives (e.g., money, gift cards, free medications) or a lottery to win an incentive.
After the coaches were certified, there appears to have been some drift backward in adherence to the counseling protocol (e.g., the average of strategies to reduce drinking was below certification level). Thus, for future studies, it seems prudent to increase the adherence promotion activities. For instance, the number of calls monitored per week could be increased and the threshold for certification could also be increased.
Strengths of the study include random assignment, coach supervision, large sample size, and independent adherence assessment. Limitations include some baseline demographic and smoking pattern differences between callers who enrolled in this study compared with those who declined, as well between callers who completed the 7-month follow-up and those who dropped out. However, although these differences were significant, they were not numerically large, and therefore they are likely not clinically meaningful. In addition, we did not collect drinking data on individuals who declined to participate, so we could not characterize their alcohol use. These issues reduce the generalizability of study findings. Our 50% response rate at the 7-month follow-up is a limitation of the study, although it is consistent with other published quitline studies (An et al., 2006; Cummings et al., 2006), falling somewhat below the rate of 57.8% from An et al. and slightly above the rate of 46.2% from Cummings et al. Notably, the results were maintained using an ITT analysis in which those lost to follow-up were treated as smokers. Nonetheless, it will be important for future quitline studies to strive to improve follow-up rates using methods such as a more extensive follow-up protocol (e.g., a 20-callback design), larger incentives (e.g., $20), and novel methods of data capture (e.g., SMS text, interactive voice response).
Although it is beyond the scope of this article to analyze the counseling data in a fine-grained way, it is worth noting that discussing a behavior intimately related to smoking (i.e., drinking) may have allowed for a fuller discussion of cues and cue related coping skills that would also affect smoking. Indeed, smoking and drinking behaviors are often interwoven (Shiffman & Balbanis, 1996). For example, previous research indicates that heavy-drinking smokers report more smoking cues in their environment and a greater impact of those cues on their motivation to smoke (Cook et al., 2012). Thus, in order to change their smoking, it is likely that smokers had to change their environments or social contacts. These environmental changes may also support changes in drinking because they reduced exposure to smokers. This possibility may account for the observed association between smoking quit status and drinking patterns and the reduced effect of treatment condition on smoking when accounting for drinking in the model. The integrated intervention condition may have better addressed cues and coping strategies that are relevant to heavy-drinking smokers than the other condition. Clearly, this is an area in which further research is warranted, as a better understanding of mechanisms of change might allow for strengthened impact of future related interventions.
In conclusion, brief integrated alcohol and tobacco counseling provided via a telephone smoking cessation quitline provides a means to improve smoking cessation among smokers who report heavy drinking. Quitline coaches were trained to provide this brief intervention with acceptable adherence, suggesting that the approach could be disseminated to other quitlines across the country. Because hazardous-drinking smokers are at higher risk for multiple health problems, the potential benefit of providing this low-cost, easily accessible treatment may be substantial.
What is the public health significance of this article?
Brief alcohol and tobacco counseling provided via a telephone smoking cessation quitline service improves smoking cessation among smokers who also report heavy drinking. This approach could be implemented with quitlines across the country, and the potential benefit of providing this low-cost, easily accessible treatment may be substantial.
Acknowledgments
This research was supported by National Institutes of Health grants (R01-CA140256 [to Benjamin A. Toll], K05-AA014715 [to Stephanie S. O’Malley], K23-AA020000 [to Lisa M. Fucito], and T32-DA007238 [to Alana M. Rojewski] from the National Institute on Drug Abuse), the National Cancer Institute, and the National Institute on Alcohol Abuse and Alcoholism, by a contract from the New York State Department of Health, and by the State of Connecticut, Department of Mental Health and Addictions Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institute on Alcohol Abuse and Alcoholism, or the National Institutes of Health. Portions of these data were presented at the Society for Research on Nicotine and Tobacco annual convention in March 2013. We thank Elaine LaVelle for assistance with data management. The study is publicly registered at www.clinicaltrials.gov (Identifier: NCT01120080). The corresponding author (Benjamin A. Toll) had full access to all data in this study and had final responsibility for the decision to submit the article for publication. Dr. Toll reports the following activities for the past 3 years: Recipient of a grant for medicine only from Pfizer Pharmaceuticals; Dr. Cummings reports the following activities for the past 3 years: Consultant to Pfizer Pharmaceuticals for a study on the economic benefits of smoking cessation; Contract as a site for a multisite study, NABI Pharmaceuticals; payment as an expert witness on behalf of plaintiffs in litigation against the tobacco industry. Dr. O’Malley reports the following activities for the past 3 years: Member of the ACNP/ASCP workgroup, the Alcohol Clinical Trials Initiative, supported with funding from Abbott Laboratories, Alkermes, Eli Lilly, GlaxoSmith Kline, Janssen, Lundbeck, Pfizer, Schering Plough, Ethypharm; Consultant/advisory board member, Pfizer Pharmaceuticals, Alkermes, Arkeo Pharmaceuticals; Contracts from Eli Lilly and NABI Pharmaceuticals as a site for multisite studies, NABI Pharmaceuticals; Medication supplies, Pfizer Pharmaceuticals; Partner, Applied Behavior Research; Scientific Panel Member, Hazelden Betty Ford Foundation; Alkermes, Inc Consultant, Arkeo Consultant, Pfizer Advisory Board, Donated medications, NABI Pharmaceuticals Contract for multisite study, Eli Lilly Contract for multisite study, Applied Behavior Research Partner, Hazelden Betty Ford Foundation Honorarium, Scientific Panel Member; Workgroup member in the American College of Neuropsychopharmacology (ACNP)/American Society of Clinical Psychopharmacology (ASCP)’s Alcohol Clinical Trials Initiative supported with funding from Abbott Laboratories, Alkermes, Eli Lilly, GlaxoSmith Kline, Janssen, Lundbeck, Pfizer, Schering Plough, Ethypharm. Interest: honorarium.
Contributor Information
Benjamin A. Toll, Department of Psychiatry, Yale University School of Medicine, Yale Cancer Center, New Haven, Connecticut
Stephanie S. O’Malley, Department of Psychiatry, Yale University School of Medicine, Yale Cancer Center, New Haven, Connecticut
Christopher W. Kahler, Center for Alcohol and Addiction Studies, Department of Behavior and Social Sciences, Brown University School of Public Health
Martin C. Mahoney, Department of Health Behavior, Roswell Park Cancer Institute, Buffalo, New York
Paula Celestino, Department of Health Behavior, Roswell Park Cancer Institute.
Srinivasa Seshadri, Department of Health Behavior, Roswell Park Cancer Institute.
James Koutsky, Department of Health Behavior, Roswell Park Cancer Institute.
Andrew Hyland, Department of Health Behavior, Roswell Park Cancer Institute.
Steve Martino, Department of Psychiatry, Yale University School of Medicine, VA Connecticut Healthcare Center, New Haven, Connecticut.
Lisa M. Fucito, Department of Psychiatry, Yale University School of Medicine
Sherry A. McKee, Department of Psychiatry, Yale University School of Medicine
Alana M. Rojewski, Department of Psychiatry, Yale University School of Medicine
Ran Wu, Department of Psychiatry, Yale University School of Medicine.
K. Michael Cummings, Department of Psychiatry & Behavioral Sciences, Medical University of South Carolina.
References
- Abdullah AS, Lam TH, Chan SS, Hedley AJ. Which smokers use the smoking cessation Quitline in Hong Kong, and how effective is the Quitline? Tobacco Control. 2004;13:415–421. doi: 10.1136/tc.2003.006460. http://dx.doi.org/10.1136/tc.2003.006460xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: A randomized controlled trial. JAMA: Journal of the American Medical Association. 2002;288:468–474. doi: 10.1001/jama.288.4.468. http://dx.doi.org/10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- An LC, Schillo BA, Kavanaugh AM, Lachter RB, Luxenberg MG, Wendling AH, Joseph AM. Increased reach and effectiveness of a statewide tobacco quitline after the addition of access to free nicotine replacement therapy. Tobacco Control. 2006;15:286–293. doi: 10.1136/tc.2005.014555. http://dx.doi.org/10.1136/tc.2005.014555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Balmford J, Segan C, Livingston P, Owen N. The effectiveness of personalized smoking cessation strategies for callers to a Quitline service. Addiction. 2003;98:837–846. doi: 10.1046/j.1360-0443.2003.00389.x. http://dx.doi.org/10.1046/j.1360-0443.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Connors GJ, Cooney NL, DiClemente CC, Donovan DM, Kadden RR, Zweben A. Internal validity of Project MATCH treatments: Discriminability and integrity. Journal of Consulting and Clinical Psychology. 1998;66:290–303. doi: 10.1037//0022-006x.66.2.290. http://dx.doi.org/10.1037/0022-006X.66.2.290. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. http://dx.doi.org/10.1016/S0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Edition F, editor. Centers for Disease Control and Prevention. Telephone quitlines: A resource for development, implementation, and evaluation. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- Cook JW, Fucito LM, Piasecki TM, Piper ME, Schlam TR, Berg KM, Baker TB. Relations of alcohol consumption with smoking cessation milestones and tobacco dependence. Journal of Consulting and Clinical Psychology. 2012;80:1075–1085. doi: 10.1037/a0029931. http://dx.doi.org/10.1037/a0029931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KM, Hyland A, Fix B, Bauer U, Celestino P, Carlin-Menter S, Frieden TR. Free nicotine patch giveaway program 12-month follow-up of participants. American Journal of Preventive Medicine. 2006;31:181–184. doi: 10.1016/j.amepre.2006.03.027. http://dx.doi.org/10.1016/j.amepre.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: At risk for what? Drug and Alcohol Dependence. 2008;95:62–72. doi: 10.1016/j.drugalcdep.2007.12.00. http://dx.doi.org/10.1016/j.drugalcdep.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. Journal of Studies on Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaén C, Baker T, Bailey W, Benowitz N, Curry S, et al. Treating tobacco use and dependence 2008 update: Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Varenicline Phase 3 Study Group Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. http://dx.doi.org/10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Abbacchi AM, Przybeck TR, Gfroerer JC. Discrepancies in estimates of prevalence and correlates of substance use and disorders between two national surveys. Addiction. 2007;102:623–629. doi: 10.1111/j.1360-0443.2007.01745.x. http://dx.doi.org/10.1111/j.1360-0443.2007.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, White JD. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Annals of Internal Medicine. 2001;135:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. http://dx.doi.org/10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tobacco Control. 2007;16(Suppl 1):i53–i59. doi: 10.1136/tc.2006.019794. http://dx.doi.org/10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humfleet G, Muñoz R, Sees K, Reus V, Hall S. History of alcohol or drug problems, current use of alcohol or marijuana, and success in quitting smoking. Addictive Behaviors. 1999;24:149–154. doi: 10.1016/s0306-4603(98)00057-4. http://dx.doi.org/10.1016/S0306-4603(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. The New England Journal of Medicine. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. http://dx.doi.org/10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Borland R, Hyland A, McKee SA, Thompson ME, Cummings KM. Alcohol consumption and quitting smoking in the International Tobacco Control (ITC) Four Country Survey. Drug and Alcohol Dependence. 2009;100:214–220. doi: 10.1016/j.drugalcdep.2008.10.006. http://dx.doi.org/10.1016/j.drugalcdep.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, LaChance HR, Ramsey SE, Abrams DB, Monti PM, Brown RA. Addressing heavy drinking in smoking cessation treatment: A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2008;76:852–862. doi: 10.1037/a0012717. http://dx.doi.org/10.1037/a0012717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. American Journal of Epidemiology. 1992;136:1248–1257. doi: 10.1093/oxfordjournals.aje.a116433. [DOI] [PubMed] [Google Scholar]
- Leeman RF, McKee SA, Toll BA, Krishnan-Sarin S, Cooney JL, Makuch RW, O’Malley SS. Risk factors for treatment failure in smokers: Relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine & Tobacco Research. 2008;10:1793–1809. doi: 10.1080/14622200802443742. http://dx.doi.org/10.1080/14622200802443742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino S, Carroll KM, Nich C, Rounsaville BJ. A randomized controlled pilot study of motivational interviewing for patients with psychotic and drug use disorders. Addiction. 2006;101:1479–1492. doi: 10.1111/j.1360-0443.2006.01554.x. http://dx.doi.org/10.1111/j.1360-0443.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status as a clinical indicator for alcohol misuse in US adults. Archives of Internal Medicine. 2007;167:716–721. doi: 10.1001/archinte.167.7.716. http://dx.doi.org/10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Frieden TR, Liu SY, Matte TD, Mostashari F, Deitcher DR, Bassett MT. Effectiveness of a large-scale distribution programme of free nicotine patches: A prospective evaluation. The Lancet. 2005;365:1849–1854. doi: 10.1016/S0140-6736(05)66615-9. http://dx.doi.org/10.1016/S0140-6736(05)66615-9. [DOI] [PubMed] [Google Scholar]
- Miller W, Rollnick S. Motivational interviewing: Helping people change. New York, NY: Guilford Press; 2012. [Google Scholar]
- Miller WR, Rose GS. Toward a theory of motivational interviewing. American Psychologist. 2009;64:527–537. doi: 10.1037/a0016830. http://dx.doi.org/10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L, Betzner A, Luxenberg ML, Rainey J, Capesius T, Subialka E, editors. NAQC. Measuring quit rates. Quality improvement initiative. Phoenix, AZ: North American Quitline Consortium; 2009. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. Bethesda, MD: Author; 2005. (NIH Publication No. 05-3769). [Google Scholar]
- Platt S, Tannahill A, Watson J, Fraser E. Effectiveness of antismoking telephone helpline: Follow up survey. British Medical Journal (Clinical Research Ed) 1997;314:1371–1375. doi: 10.1136/bmj.314.7091.1371. http://dx.doi.org/10.1136/bmj.314.7091.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. A cluster-analytic classification of smoking relapse episodes. Addictive Behaviors. 1986;11:295–307. doi: 10.1016/0306-4603(86)90057-2. http://dx.doi.org/10.1016/0306-4603(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balbanis M. Do drinking and smoking go together? Alcohol Health and Research World. 1996;20:107–110. [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. http://dx.doi.org/10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Silverman S., Jr Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. The Journal of the American Dental Association. 2001;132(Suppl):7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Alcohol consumption measures. In: Allen J, Wilson V, editors. Assessing alcohol problems: A guide for clinicians and researchers. 2nd. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. pp. 75–99. [Google Scholar]
- Stead L, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD002850.pub2. Art. No.: CD002850. http://dx.doi.org/10.1002/14651858.CD002850.pub2. [DOI] [PubMed]
- Talamini G, Bassi C, Falconi M, Sartori N, Salvia R, Rigo L, Pederzoli P. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Digestive Diseases and Sciences. 1999;44:1303–1311. doi: 10.1023/a:1026670911955. http://dx.doi.org/10.1023/A:1026670911955. [DOI] [PubMed] [Google Scholar]
- Toll BA, Cummings KM, O’Malley SS, Carlin-Menter S, McKee SA, Hyland A, Celestino P. Tobacco quitlines need to assess and intervene with callers’ hazardous drinking. Alcoholism: Clinical and Experimental Research. 2012;36:1653–1658. doi: 10.1111/j.1530-0277.2012.01767.x. http://dx.doi.org/10.1111/j.1530-0277.2012.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, Martino S, Latimer A, Salovey P, O’Malley S, Carlin-Menter S, Cummings KM. Randomized trial: Quitline specialist training in gain-framed vs standard-care messages for smoking cessation. Journal of the National Cancer Institute. 2010;102:96–106. doi: 10.1093/jnci/djp468. http://dx.doi.org/10.1093/jnci/djp468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: A meta-analytic review. Alcohol and Alcoholism. 2006;41:328–335. doi: 10.1093/alcalc/agl016. http://dx.doi.org/10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncology. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. http://dx.doi.org/10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- WHO Brief Intervention Study Group. A cross-national trial of brief interventions with heavy drinkers. American Journal of Public Health. 1996;86:948–955. doi: 10.2105/ajph.86.7.948. http://dx.doi.org/10.2105/AJPH.86.7.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. Journal of General Internal Medicine. 1997;12:274–283. doi: 10.1046/j.1525-1497.1997.012005274.x. http://dx.doi.org/10.1007/s11606-006-5063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


