Abstract
Purpose
Summarise survival of patients with resected lung cancers manifesting as part-solid nodules (PSNs).
Methods
PubMed/MEDLINE and EMBASE databases were searched for all studies/clinical trials on CT-detected lung cancer in English before 21 December 2015 to identify surgically resected lung cancers manifesting as PSNs. Outcome measures were lung cancer-specific survival (LCS), overall survival (OS), or disease-free survival (DFS). All PSNs were classified by the percentage of solid component to the entire nodule diameter into category PSNs <80% or category PSNs ≥80%.
Results
Twenty studies reported on PSNs <80%: 7 reported DFS and 2 OS of 100%, 6 DFS 96.3-98.7%, and 11 OS 94.7-98.9% (median DFS 100% and OS 97.5%). Twenty-seven studies reported on PSNs ≥80%: 1 DFS and 2 OS of 100%, 19 DFS 48.0%-98.0% (median 82.6%), and 16 reported OS 43.0%-98.0% (median DFS 82.6%, OS 85.5%). Both DFS and OS were always higher for PSNs <80%.
Conclusion
A clear definition of the upper limit of solid component of a PSN is needed to avoid misclassification because cell-types and outcomes are different for PSN and solid nodules. The workup should be based on the size of the solid component.
Keywords: Subsolid nodules, Ground-glass, Survival, Staging, Lymph node metastases
Introduction
CT screening for lung cancer is now being reimbursed in the USA. As a consequence, the workup of nodules identified on CT scans is important to maximise the benefits of screening and minimise potential harms, including overdiagnosis and overtreatment of lung cancers. Questions have been raised about the appropriate treatment of lung cancers manifesting as subsolid nodules [nonsolid nodules (NSNs) and part-solid nodules (PSNs)], as these have very high reported survival rates and have been observed in up to 10% of screening participants [1–14]. Slow growth of such cancers has been documented in pathology reviews as well [6–14]. A multidisciplinary group headed by Travis [8–10] led to revision of the pathology classification of adenocarcinomas and to recommendations that the focus should be on the invasive component, which typically is the solid component of PSNs rather than their overall size. This consensus is also reflected in the latest recommendations of the Fleischner Society [15, 16].
Our goal in this report is to summarise the publications on survival of patients with resected lung cancers manifesting as PSNs and to further the development of consensus definitions of the CT appearance and the workup of such nodules.
Methods
Search strategy
The PubMed/MEDLINE and EMBASE databases were searched for all studies and clinical trials on CT-detected lung cancer published in English on or before 21 December 2015. Search strategies are listed in Appendix A. Furthermore, reference lists of all identified relevant articles and important reviews on this topic were manually searched. Titles and abstracts (and in ambiguous cases, full text) of the articles were reviewed by three independent reviewers for eligibility of studies. Lung cancer patients whose cancer manifested as a PSN on CT scans detected by either screening or clinical work-up were included. Only surgically resected cases were considered in this report.
Survival rates
All studies that evaluated survival of patients with lung cancers manifesting as PSNs were included. Survival measures included: (1) disease-free or relapse-free survival (DFS), (2) lung-cancer-specific survival (LCS), and (3) overall survival (OS). Survival rates were extracted directly when reported in the publications. We also included publications in which the survival rates were not reported but could be extracted from reported Kaplan-Meier survival graphs (see footnotes of the relevant tables). Follow-up time was defined as time from surgery to the final event (disease recurrence, lung cancer-related death, or last follow-up visit), whichever came first.
Studies that provided no survival information but reported only total numbers of recurrences and/or deaths due to lung cancer or other causes were excluded, as the survival rate could not be determined for these studies.
Definitions of nodules based on the CT scans
For this report, the following definitions were used:
Nonsolid nodules (NSNs): Nodules without a solid component that obscures the underlying lung parenchyma other than blood vessels [1, 2, 12] on thin-section CT scans (less than 1 mm) viewed on CT lung window settings. NSNs have also been called “ground-glass opacities (GGOs)”, “pure GGOs”, and “pure ground-glass nodules” [15–18].
Part-solid nodules (PSNs): Nodules with a solid component obscuring the underlying lung parenchyma other than blood vessels on thin-section CT scans [4, 5, 12] viewed on CT lung window settings. PSNs initially manifest as NSNs and later progress to develop internal solid components [1, 2, 4, 5]. PSNs have also been called “GGOs”, “mixed GGOs”,” mixed tumour with GGO”, “ground-glass nodules,” “mixed nodules,” “part-solid GGO”, “part-solid GGN”, and “partly solid or semisolid” [15–18].
Thin-section (less than 1.0-mm slice thickness) CT scans have been recognised as being important to avoid misclassification of NSNs and PSNs based on prior publication and society recommendations [1–5, 15, 16].
Measurements of NSNs and PSNs
Three different measurement approaches were used in the studies:
The first approach was to measure the diameter of the entire nodule (E) and of the solid component (S) of a PSN using lung window settings and calculate R1 = S/E × 100. When there is no solid component R1 = 0% (i.e., the nodule is an NSN) and when the nodule is totally solid, R1 = 100%. PSNs have R1 values between 1% and 99%.
A second approach was to measure the percentage of ground-glass component (GGO) instead of the solid component and use R2 = GGO% = (E - S)/E × 100. Clearly R2 = 1 - R1.
A third approach used both lung (L) and mediastinal (M) window settings to measure the nodule. The CT mediastinal to lung ratio (CT M/L) = tumour area measurement (M)/tumour measurement (L). The other measure, called the tumour disappearance ratio (TDR), is defined as TDR = [1- CT M/L].
PSNs categories
We focused on PSNs, so studies reporting only on NSNs or solid nodules were excluded. Distinguishing between PSNs and solid nodules with only one CT scan may be difficult, particularly for solid nodules as they may be surrounded by a thin rim of haziness (i.e., nonsolid component). Thus, when progression from an NSN to PSN or solid nodule cannot be confirmed on thin-section CT scans; upper limits of the solid component of PSNs should be used to avoid misclassifying a solid nodule as a PSN [4, 5, 19, 20]. Examples of such misclassifications have been reported, leading to misunderstanding of the cell types that manifest as PSNs and their survival rates [5]. Distinguishing between NSNs and PSNs with small solid components may also be difficult, but the management for both is essentially the same and, when diagnosed as lung cancer, both are adenocarcinomas.
Since the survival rates reported by studies were stratified into groups of PSNs defined by different cut-offs for the proportion of solid components, we classified each group into one of the following two PSN categories:
– Category R1<80%: groups with R1<80% (including NSNs only if they could not be separated from PSNs) and
– Category PSN≥80%: groups with R1≥80% (here solid nodules were included if they could not be separated from PSNs).
When the distinction between PSNs with R1<80% and R1≥80% in a particular group could not be made, or a group included solid nodules, the group was included in the PSN≥80% category.
Data extraction
Two independent reviewers examined the full text of each article identified for inclusion. Data extraction was then performed independently using a standardised data extraction form. Study characteristics, measurement method, definition of a part-solid nodule, percentage of solid component, tumour size, duration of follow-up, survival rate, and recurrence rate were extracted. If the same data were reported in more than one relevant article, the information in the most recently published study was used. Disagreements were resolved by a third reviewer according to a predefined protocol.
Quality of the study and risk of bias assessment
Study quality assessment was done independently by two reviewers using a systematic, standardised quality assessment tool for evaluation of internal and external validity of each included study. Each study was evaluated on these domains: presence of a clearly stated research question or objective, presence of a clearly defined study population, presence of pre-specified inclusion and exclusion criteria, sample size justification, sufficient (≥3 years) duration of follow-up, reliability of nodule measurement and outcome ascertainment, blinding of radiologists and outcome assessors, missing data, loss to follow-up, consideration of potential confounding variables, and other biases.
Data synthesis and analysis
We anticipated considerable diversity in the included studies; most importantly, most of the relevant studies either did not report on the proportion of solid component at all or did not report in the same way. This makes performing any meta-analysis of the data very challenging. This review was the first attempt to summarise the relationship between the proportion (size) of solid component and lung cancer survival using the existing literature. Thus, we thought that a narrative synthesis approach allowed us to take a first look at the data and offer a simple solution to our question. Descriptive summaries are tabulated by the two categories: Table 2: category PSN<80% and Table 3: category PSN≥80%.
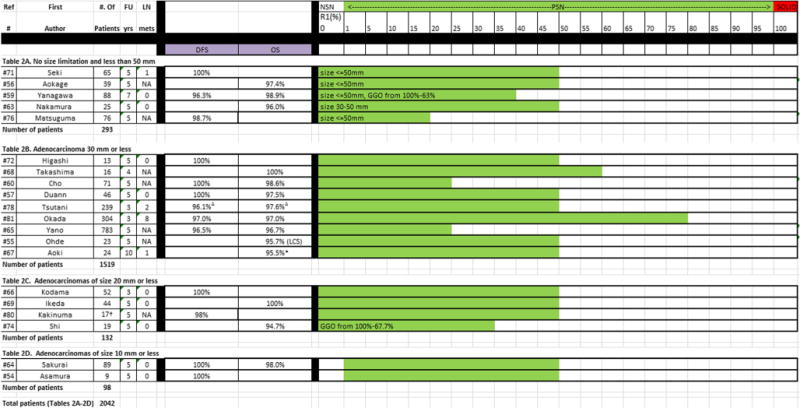
Table 2.
Publications on nodule category of PSN < 80%, listed by the different groups reported in each study. For each group, the number of patients, follow-up time, lymph node metastases, disease-free survival (DFS), overall survival (OS), and R1 (ratio of solid component to the overall nodule size) is given
|
NSN = Nonsolid nodule, defined as R1 = 0% or GGO =100%; PSN = part-solid nodule, defined as R1 = 1-79%; SN = solid nodule, defined as R1 = 100% (alternatively R2 = 0%), and PSN≥80%
Study did not report on survival rate, rates estimated from figure.
Numbers based on length method reported in the study. Mean 5-year relapse-free survival and mean number of patients having a GGO extent of 50% or more among seven institutions
DFS: 96.4% lobectomy (n = 90), 96.1% segmentectomy (n = 56), 98.7% wedge (n = 93); OS: 97.6% lobectomy, 98.2% segmentectomy, 98.7% wedge. For calculation of median survival rates, the lowest DFS and OS among the different types of surgery were used to provide conservative estimates
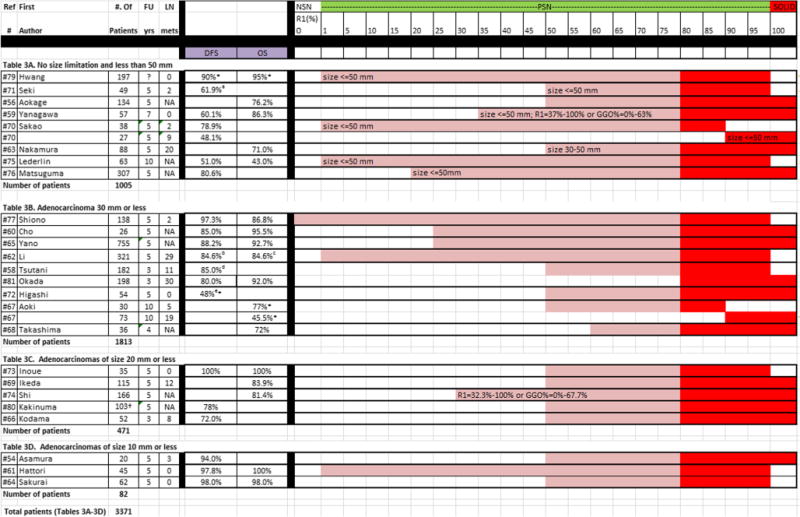
Table 3.
Publications on nodule category of PSN≥80%, listed by the different groups reported in each study. For each group, the number of patients, follow-up time, lymph node metastases, disease-free survival (DFS), overall survival (OS), and R1 (ratio of solid component to the overall nodule size) is given
|
NSN = Nonsolid nodule, defined as R1 = 0% or GGO = 100%; PSN = part-solid nodule, defined as R1 = 1-79%; SN = solid nodule, defined as R1 = 100% (alternatively R2 = 0%), and PSN≥80%
Study did not report on survival rate, rates estimated from figure.
DFS was 95.5% for p-stage IA (n = 35) and 61.9% for IB (n = 14). For calculation of median survival rates, the lowest DFS in stage IA and IB was used to provide conservative estimates.
DFS for subgroups of different path subtypes, 100% for AAH, 100% for AIS, 100% for MIA, 95.2% for LPA, 95.2% for PPA, 100% for IMA, 93.1% for MPA, 93.3% for APA and 84.6% for SPA. For calculation of median survival rates, the lowest DFS among the different subtypes was used to provide conservative estimates.
OS for subgroups of different path subtypes, 100% for AAH, 100% for AIS, 96.7% for MIA, 95.2% for LPA, 93.0% for PPA, 100% for IMA, 88.0% for MPA, 90.2% for APA and 84.6% for SPA. For calculation of median survival rates, the lowest OS among the different subtypes was used to provide conservative estimates.
DFS was 91.0% for lobectomy (n = 154) and 85.0% for segmentectomy (n = 28). For calculation of median survival rates, the lowest DFS and OS among the different types of surgery were used to provide conservative estimates.
DFS was 82% for low FDG (n = 33) and 48% for high FDG and CEA <20 (n = 21). For calculation of median survival rates, the lowest DFS in the two subgroups was used to provide conservative estimates
Numbers based on length method reported in the study. Mean 5-year relapse-free survival and mean number of patients having a GGO extent of less than 50% among seven institutions
Results
Study selection
The PubMed/MEDLINE and EMBASE search identified 828 potential articles on surgically resected lung cancers manifesting as part-solid nodules (Fig. 1) of which 81 fit the inclusion and exclusion criteria. Among the 81 articles, 11 articles did not report on PSNs [19, 21–30], 2 did not include surgically resected cases [31, 32], 13 did not report DFS, lung cancer-specific-survival, or overall survival [33–45], 9 articles reported only on recurrence/death [13, 46–53], and 10 articles were conference abstracts with insufficient data. This left 36 articles reporting on 31 unique studies (Table 1).
Fig. 1.
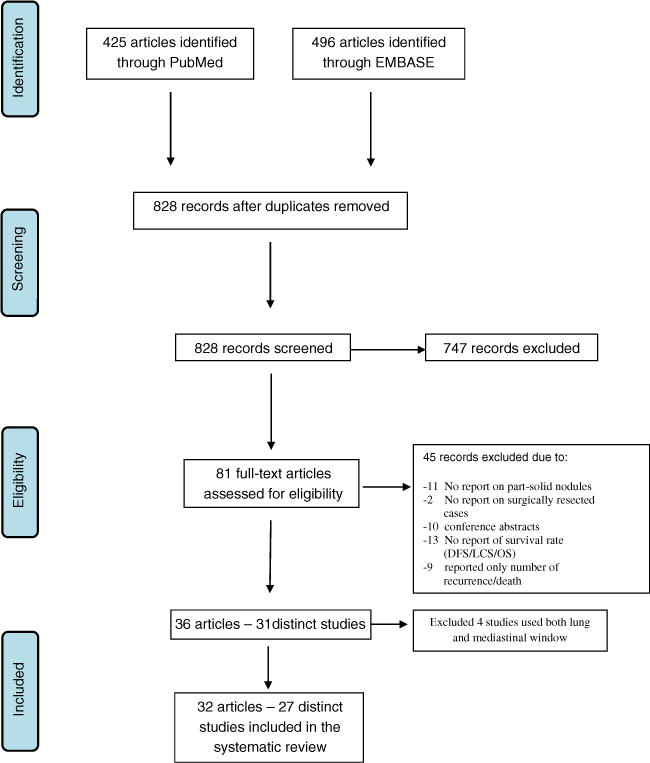
Summary of search and selection strategy
Table 1.
Listing of the 28 publications that met the inclusion criteria detailing age, male-to-female distribution, and groups within the two categories of PSN<80% and PSN≥80% by year of publication
| Category
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author/publication year | Ref no. | Country | Blinded | Age median (range) | M:F | PSN < 80% groups | N | PSN > 80% groups | N |
| Aoki 2001 | 67 | Japan | N | 64 (32-84) | 68:59 | R1 < 50% | 24 | R1 > 50%-90% | 30 |
| R1 > 90% | 73 | ||||||||
| Kodama 2001 | 66 | Japan | N | 60 (34-78) | 55:49 | R1 < 50% | 52 | R1 > 50% | 52 |
| Asamura 2003 | 54 | Japan | N | 61 (43-77) | 23:25 | R1 ≤ 50% | 9 | R1 > 50% | 20 |
| Ohde 2003 | 55 | Japan | Y | NA | NA | R1 < 50% | 23 | ||
| Takashima 2003 | 68 | Japan | N | 66 (40-82) | 20:32 | R1 < 60% | 16 | R1 > 60% | 36 |
| Sakao 2004 | 70 | Japan | N | 66 (42-81) | 5:24 | R1 > 1-90% | 38 | ||
| R1 > 90% | 27 | ||||||||
| Ikeda 2004 | 69 | Japan | N | 63 (40-84) | 67:92 | R1 < 50% | 44 | R1 > 50% | 115 |
| Kakinuma 2008 | 80 | Japan | Y | 63 (26-83) | 69:51 | R1 < 50% | 17 | R1 > 50% | 103 |
| Seki 2008 | 71 | Japan | N | Mean = 66 (SD = 11) | 336:156 | R1 = 0-50% | 65 | R1 = 51-99% | 49 |
| Higashi 2009 | 72 | Japan | Y | 64 (42-84) | 40:47 | R1 < 50% | 13 | R1 > 50% | 54 |
| Inoue 2010 | 73 | Japan | N | 65 (38-83) | 41:77 | R1 > 50% | 35 | ||
| Okada 2011 | 81 | Japan | N | 65.3 ± 9.6 | 223:279 | R1 ≤ 80% | 304 | R1 > 80% | 198 |
| Shi 2011 | 74 | China | N | 54 (39-76) | 127:58 | R1 ≤ 2/3 | 19 | R1 > 2/3 | 166 |
| Aokage 2013 | 56 | Japan | Y | <70Y 111; ≥70Y 62 | 75:98 | R1 < 50% | 39 | R1 > 50% | 134 |
| Duann 2013 | 57 | Taiwan | N | 60.3 (40-83) | 23:23 | R1 ≤ 50% | 46 | ||
| Lederlin 2013 | 75 | Europe | Y | N/A | N/A | R1 = 1%-99% | 63 | ||
| Matsuguma 2013 | 76 | Japan | N | 66 (34-85) | 187:196 | R1 < = 20% | 76 | R1 > 20% | 307 |
| Tsutani 2014 | 58 | Japan | N | 66 (37-86) | 74:108 | R1 = 50%-99% | 182 | ||
| Tsutani 2014 | 78 | Japan | N | 65 (31-89) | 94:145 | R1 ≤ 50% | 239 | ||
| Shiono 2014 | 77 | Japan | N | N/A | N/A | R1 = 0-99% | 138 | ||
| Yanagawa 2014 | 59 | Japan | N | 63.6 | 68:77 | R1 < 63% | 88 | R1 > 63% | 57 |
| Cho 2015 | 60 | Korea | N | 60.3 (31-81) | 43:54 | R1 ≤ 25% | 71 | R1 > 25% | 26 |
| Hattori 2015 | 61 | Japan | N | N/A | N/A | R1 = 1%-99% | 45 | ||
| Hwang 2015 | 79 | Korea | N | 61.3 (35-86) | 76:121 | R1 = 1%-99% | 197 | ||
| Li 2015 | 62 | China | Y | 53.9 (28-76) | 161:160 | R1 = 1%-99% | 321 | ||
| Nakamura 2015 | 63 | Japan | N | 71 (56-79) for R1 ≤ 50%; | 64:49 | R1 ≤ 50% | 25 | R1 > 50% | 88 |
| 68 (26-85) for R1 > 50% | |||||||||
| Sakurai 2015 | 64 | Japan | N | 61.8 (33-78) | 64:87 | R1 = 1%-50% | 89 | R1 = 50%-99% | 62 |
| Yano 2015 | 65 | Japan | N | NA | NA | R1 < = 25% | 783 | R1 > 25% | 755 |
| Totals | 20 groups | 2042 | 27 groups | 3371 | |||||
Among these 31 unique studies, 25 categorised the PSN using lung windows only, reporting either (R1) or (1-R1) [48, 49, 54–79]. Two articles measured PSNs, one time using lung window settings only and the other time using both mediastinal and lung window settings [80, 81]; these two articles are included. Another four articles used only the approach using mediastinal and lung window settings and these four articles were excluded [46, 82–84] because the mediastinal settings were not standardised and varied widely. In summary, 4 of the 31 studies were excluded, leaving 27 studies.
The 27 studies are listed according to year of publication, outcome measures, and PSN category (Table 1). The 27 studies were performed between 2001 and 2015, 26 in Asia and 1 in Europe. Of the 5,309 patients, 5,246 (98.8%) were from Asia.
Biases
All 27 studies were retrospective in nature. Radiologists were blinded as to the pathology diagnoses in six studies [55, 56, 62, 72, 75, 80]; this should minimise bias in assessing the nodule consistency and GGO percentage prior to resection. Management of PSNs may be different across countries, institutions, and surgeons and this may have played a role in the decision to perform surgery.
No standard measurement methods or CT acquisition parameters were reported in the 27 studies. Six studies did not provide CT slice thickness while the remaining 21 studies reported CT slices from 0.5 to 3.0 mm. Time from the initial identification of the PSN to diagnosis and resection was given in only 1 of the 27 studies [57]. Tumour size was an inclusion criterion in all but two articles [56, 61]. The year of publication may be important, as CT scanner and surgical technologies may have changed over time, but no statistically significant relationship was found when we examined the association between year of publication and survival outcomes; thus year of publication did not have an effect on lung cancer survival.
Results on Category PSNs<80%
Twenty of the 27 studies each contributed one group of PSNs (2042 patients) to category PSNs<80% (Table 2). Of the 20 listed groups, 13 reported DFS/RFS [54, 57, 59, 60, 64–66, 71, 72, 76, 78, 80, 81], 100% for 7 groups (345 patients) and ranged from 96.3% to 98.7% for another 6 groups (1507 patients). Among the 13 groups reporting OS [55–57, 59, 60, 63–65, 68, 69, 74, 78, 81], 2 (60 patients) reported an OS of 100% and 11 (1727 patients) reported OS rates ranging from 94.7% to 98.9%. One group of 23 patients had an LCS of 95.7% [55]. The median reported DFS rate was 100% and median OS was 97.5%.
Tumour size ranged up to 50 mm in 5 studies (Table 2A), was less than 30 mm in 9 studies (Table 2B), less than 20 mm in 4 (Table 2C), and less than 10 mm in 2 (Table 2D). Median survival showed an overall increasing trend with decreasing size, with median DFS values of 98.7%, 98.5%, 99%, and 100% for the decreasing size categories, respectively. The median OS was 97.4%, 97.3 %, 97.4%, and 98.0% for these size categories, respectively.
Frequency of lymph node (LN) involvement was reported in 13 studies (1017 patients) [54, 57, 59, 63, 64, 66, 67, 69, 71, 72, 74, 78, 81]. No LN metastases were present in nine studies (385 patients), and in the remaining four studies LN metastases ranged from 1% to 4%. Thus, the median percentage of LN metastases among the 1017 patients on whom they were reported was 0%. When considering the overall size of the nodule, the median frequencies of LN involvement were 0%, 0%, 1%, and 0% for studies with adenocarcinomas ≤10 mm, ≤20 mm, ≤30 mm, and ≤50 mm. No LN metastases were reported in the studies that reported on adenocarcinomas ≤20 mm (Table 2C) or ≤10 mm (Table 2D).
Results on Category PSNs≥80%
Twenty-three of the 27 studies contributed one group and 2 studies contributed 2 groups each, accounting for a total of 27 groups (3371 patients) to category PSNs≥80% (Table 3). Of these, 20 reported DFS [54, 58–62, 64–66, 70–73, 75–77, 79–81]. DFS was 100% in 1 (35 patients) group and it ranged from 48.0% to 98.0% in the remaining 19 groups (2694 patients). Among the 18 groups reporting on OS [56, 59–65, 67–69, 73–75, 77, 79, 81], OS was 100% in 2 groups (80 patients) and ranged from 43.0% to 98.0% in 16 groups (2459 patients). The median DFS was 82.6% and the median OS was 85.5%.
In Table 3, consisting of 27 groups, tumour size was not specified or ranged up to 50 mm in 9 groups. In the remaining 18 groups, size was less than 30 mm in 10, less than 20 mm in 5, and less than 10 mm in 3 groups. Median survival showed an overall increasing trend with decreasing size; median DFS values were 61.9%, 85%, 78%, and 97.8%, respectively, and the median OS values were 76.2%, 80.8%, 83.9%, and 99.0% for these size categories, respectively.
Frequency of LN involvement was reported in 19 of the 27 groups (1781 patients) [54, 58, 59, 61–64, 66, 67, 69–73, 77, 79, 81]. In 6 of the 19 groups, no LN metastases were found (450 patients), and in the remaining 13 groups, LN metastases ranged from 1%-33%. Thus, the median number of LN metastases among the 1781 patients on whom they reported was 6%.
When considering the overall size of the nodule, the median frequency of LN involvement was 0%, 10%, 9%, and 4.5% for groups with adenocarcinomas ≤10 mm, ≤20 mm, ≤30 mm, and ≤50 mm, respectively. This risk, however, is most likely distorted by the proportion of solid component of the included groups. There were more PSNs with 50% or more solid component included for smaller adenocarcinomas [Table 3C (≤20 mm), Table 3D (≤10 mm] while nonsolid or PSNs with as little as 1% solid component were included in the groups with larger adenocarcinomas [Table 3A (3/6 groups with adenocarcinomas ≤50 mm), Table 3B (2/7 groups with adenocarcinomas ≤30 mm)].
Comparison of lung cancer patients manifesting in category PSNs<80% and category PSNs≥80%
The median DFS or OS rates were high for all reported groups, but were always higher for category PSN<80% than for category PSN≥80%. The median DFS was 100% and the median OS was 97.5% for category PSN<80%, whereas the median DFS and OS for category PSN≥80% was lower, at 82.6% and 85.5%, respectively.
Median DFS increased with decreasing size for both categories. For category PSN<80%, median DFS increased from 98.7% to 100% when tumour size decreased from 50 mm to less than 10 mm. The median OS, however, was about the same across tumour size categories. For category PSN≥80%, median DFS increased from 61.9% to 97.8% when tumour size decreased from 50 mm to less than 10 mm. Similarly, median OS increased from 76.2% to 99.0%.
Sensitivity analysis
To further focus on PSNs, we considered only those studies that did not included NSN (R1 = 0%) or solid nodules (R1 = 100%). Only two studies (82 patients) reported survival rates for category PSN<80% [54, 64], both studies included only tumours that measured 10 mm or less, and both reported rates for DFS of 100% and no lymph node metastases. OS was only reported by one of the two studies and it was 98% [64]. For the ten studies (1022 patients) that reported on rates for category PSN≥80% [58, 61, 62, 64, 67, 70, 71, 73, 75, 79] after excluding those that also had solid nodules, the median DFS was 85% in nine groups (992 patients) and median OS was 95% in seven groups (753 patients). The frequency of lymph node involvement was reported in nine of the ten groups (959 patients); four of the nine groups (339 patients) reported no lymph node metastases, and the remaining five groups (620 patients) reported lymph node metastases at rates ranging from 4%-17%.
Discussion
Review of 27 retrospective studies on 5309 patients, published between 2001 and 2015, clearly showed that survival was very high for patients with lung cancers manifesting as PSN, and particularly for those whose solid component was less than 80% of the entire nodule diameter (Table 2). For the 20 groups reporting on lung cancers manifesting as PSN<80%, the median DFS and OS rates were above 97%, whereas in the 27 groups with lung cancers manifesting as PSN≥80%, median rates were never above 86% − ten per cent below the rates for PSN<80%. As 6 of the 27 groups [61, 62, 70, 75, 77, 79] reporting on PSN≥80% included PSNs with very small solid components (as low as 1%) as well as solid nodules, these rates may be somewhat inaccurate because of these extreme inclusions. However, survival rates remained very high when articles that included NSNs and solid nodules were excluded, further corroborating our main finding that patients with lung cancers manifesting as PSN had very good survival, particularly when the solid component was less than 80% of the entire nodule diameter. While the studies listed in this review were predominantly performed in Asia, perhaps due to the widespread screening being performed there, survival rates do not appear to be different in Asia as compared with the two large screening studies performed in North America [4, 5].
The results of this review of the literature between 2001 and 2015 support the results of two large, prospectively collected screening studies, published in 2016, which found 100% lung-cancer-specific survival of resected cancers for PSNs<80% [4, 5]. Both of these reports used the same definition of PSN and did not include any patients with lung cancers manifesting as NSNs or solid nodules. Both confirmed that all lung cancers manifesting as PSNs on repeat screenings started as NSNs, and no patient diagnosed with lung cancer manifesting as a PSN with a solid component of less than 10 mm in diameter had lymph node metastases on pathology review. In this review of the 27 studies, lymph node metastases were unlikely among the groups in category PSNs<80%, specifically in cancers less than 20 mm in size (Tables 2C, 2D). The emerging consensus by several multi-disciplinary groups recommends that the focus should be on the solid component [8–10, 13, 15, 17].
Since 1995 when Noguchi et al. [6, 7] reported 100% survival of certain subtypes of adenocarcinoma, questions have been raised as to whether and when diagnosis and treatment need to be pursued in NSNs or even PSNs. When diagnosed as lung cancer, these cancers have been shown to be slow growing [13–15, 34, 85–87] and thus plausible candidates for overdiagnosis. Further evidence of slow growth comes from the fact that all lung cancers manifesting as PSNs in the NLST were identified on the initial baseline CT scan, but diagnosis was not pursued for some time [2, 5]. All these NLST cases were still stage I at time of diagnosis, and none of the patients died of lung cancer according to the NLST endpoint verification process. Other studies of lung cancers manifesting as NSNs and PSNs have shown that survival of adenocarcinoma decreases as the lepidic (previously called bronchioloalveolar) percentage of the cancer decreases [6–14, 25, 88]. Therefore, it is important to correctly identify nodule consistency, distinguishing NSNs and PSNs from solid nodules, so that the management and subsequent treatment can be tailored to reflect their biologic behaviour.
The need to specify the upper limit of the solid component has also been recognised as most studies reported here used an upper limit to define groups of PSNs and provided outcomes for the different groups. Hopefully, consensus will develop over time about the most appropriate limit to use as a cutoff. We urge primary researchers to report the results for finer grained categories of consistency and size to aid in determining the best cut-off percentage. The cut-off is important in the identification and thus management of PSNs, especially when only a single CT is available as illustrated in prior publications [2, 5] the progression from an NSN to a PSN or solid nodule cannot be documented. An upper limit of 80% appeared to distinguish cases of lung cancer manifesting as PSNs with essentially 100% survival rates from those that had lower survival rates [4, 5] and this cut-off has been used in the I-ELCAP protocol [89].
This review revealed several limitations of the current literature. The main limitation arose from the varied and imprecise definitions of PSN used across studies. The studies used both different definitions (with different cut-off criteria) and different measurement approaches for determining PSNs. Consensus has been reached that the focus should be on the solid component of the PSN, but most studies did not provide the size of the solid component. Also, earlier articles did not use thin-section CT scans of less than 1.0 mm thickness, which are important to properly classify nodule subtypes and measure the solid component, and this slice thickness is now preferred for screening [15, 16, 89]. This review is limited to the outcome of surgically resected lung cancers manifesting as PSNs, which often represent a selected group of PSNs that demonstrate more aggressive behaviours. Long-term follow-ups of PSNs that were under surveillance without intervention have been reported [4]. Therefore, our observed survival outcomes may be lower than what they would have been if all PSNs regardless of treatment were considered. Another limitation is that the time from the initial identification of the PSN to diagnosis and resection was given in only 1 of 27 studies. Thus, whether these nodules were observed for a period of time before resection is unknown. No statistically significant relationship was found when we examined the association between year of publication and survival outcomes; thus year of publication did not have an effect on lung cancer survival.
Large lung cancer screening databases provided valuable empirical evidence using standardised definitions [4, 5]. Retrospective reviews of such databases are now driving management protocols. This is exemplified by the shift in the size threshold for a positive result for solid nodules first to 5 mm [90] and later to 6 mm on baseline screening [91, 92]. This cut-off is now also used by NCCN [93], LungRads [94–96], and the Fleischner [15, 16] and British Thoracic Society [17] guidelines.
In conclusion, it is important to develop a consensus definition of PSNs. Such a definition is important for the management and treatment, especially when the solid component is small, as already reflected in the I-ELCAP [89] and LungRads [96] reporting system as well as the Fleischner [15, 16] and British Thoracic Society [17] guidelines.
Key points.
Lung cancers manifesting as PSNs are slow growing with high cure rates.
Upper limits of the solid component are important for correct interpretation.
Consensus definition is important for the management of PSNs.
Median disease-free-survival (DFS) increased with decreasing size of the nodule.
Acknowledgments
We would also like to thank Ms. Camille Chan, who provided assistance with the literature search and identification of relevant studies to be included in this review.
Funding
Funding for this study was in part by the Flight Attendants Medical Research Institute.
Abbreviations
- AIS
Adenocarcinoma-in-situ
- DFS
Disease-free or relapse-free survival
- GGO
Ground-glass opacities
- I-ELCAP
International Early Lung Cancer Action Program
- LCS
Lung cancer-specific survival
- MIA
Minimally invasive adenocarcinoma
- NLST
National Lung Screening Trial
- NSN
Nonsolid nodules
- OS
Overall survival
- PSN
Part-solid nodules
- TDR
Disappearance rate
Appendix A
Part Solid - All Concept
Embase
part-solid.mp.
partsolid.mp.
semi-solid.mp.
semisolid.mp.
subsolid.mp.
ground glass.mp.
1 or 2 or 3 or 4 or 5 or 6
lung cancer/
lung tumor/
((lung or pulmonary) and (cancer or cancers or tumor or tumors or carcinoma or carcinomas or neoplasm or neoplasms)).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
8 or 9 or 10
mortality/or cancer mortality/
disease free survival/or cancer survival/or disease specific survival/or event free survival/or cancer specific survival/or long term survival/or metastasis free survival/or survival/or overall survival/
recurrent disease/
metastasis/or lung metastasis/
(mortality or survival or recurrence or metastasis or metastases or death rate or relapsing disease or relapse).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
12 or 13 or 14 or 15 or 16
computer assisted tomography/
(CT scan or CT scans or CAT scan or cat scans).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
(Comput$ adj3 Tomography).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
18 or 19 or 20
7 and 11 and 17 and 21
PS all concept
Medline
Solitary Pulmonary Nodule/or part-solid.mp.
part solid.mp.
semi-solid.mp.
semisolid.mp.
subsolid.mp. or Solitary Pulmonary Nodule/
ground glass.mp.
ground-glass.mp.
1 or 2 or 3 or 4 or 5 or 6 or 7
Disease-Free Survival/or Survival Analysis/or Survival/or Survival Rate/or survival.mp.
Hospital Mortality/or Mortality/or mortality.mp.
Recurrence/or Neoplasm Recurrence, Local/or recurrence.mp.
metastasis.mp. or Neoplasm Metastasis/
metastases.mp. or Neoplasm Metastasis/
fatality.mp.
fatalities.mp.
9 or 10 or 11 or 12 or 13 or 14 or 15
cancer.mp. or Neoplasms/
cancers.mp. or Neoplasms/
carcinoma.mp. or Carcinoma/or Carcinoma, Small Cell/or Carcinoma, Non-Small-Cell Lung/or Carcinoma, Squamous Cell/or Carcinoma, Adenosquamous/
carcinomas.mp. or Carcinoma/
tumor.mp. or Neoplasms/
tumors.mp. or Neoplasms/
17 or 18 or 19 or 20 or 21 or 22
lung.mp. or Lung/
pulmonary.mp.
24 or 25
23 and 26
Lung Neoplasms.mp. or Lung Neoplasms/
27 or 28
CT scan.mp.
CT scans.mp.
CAT scan.mp.
CAT scans.mp.
Tomography, X-Ray Computed/or computed scan.mp.
Tomography, X-Ray Computed/or computed scans.mp.
Tomography, X-Ray Computed/or computed assisted tomography.mp.
computerized scan.mp.
computerized scans.mp.
30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
8 and 16 and 29 and 39
Footnotes
Compliance with ethical standards
Guarantor
The scientific guarantors of this publication are Dr Claudia Henschke and Ms. Rowena Yip.
Conflict of interest
Dr Yankelevitz is a named inventor on a number of patents and patent applications relating to the evaluation of diseases of the chest including measurement of nodules. Some of these, which are owned by Cornell Research Foundation (CRF), are non-exclusively licensed to General Electric. As an inventor of these patents, Dr Yankelevitz is entitled to a share of any compensation that CRF may receive from its commercialisation of these patents. He is also an equity owner in Accumetra, a privately held technology company committed to improving the science and practice of image-based decision-making. Dr Yankelevitz also serves on the advisory board of GRAIL.
Dr Henschke is the President and serves on the board of the Early Diagnosis and Treatment Research Foundation. She receives no compensation from the Foundation. The Foundation is established to provide grants for projects, conferences, and public databases for research on early diagnosis and treatment of diseases. Dr Claudia Henschke is also a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on CT scans of the chest that are owned by Cornell Research Foundation (CRF). Since 2009, Dr Henschke has not accepted any financial benefit from these patents including royalties and any other proceeds related to the patents or patent applications owned by CRF.
Other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Dr. Claudia Henschke, Ms. Rowena Yip, Dr Betsy Becker and Dr Emanuela Taioli kindly provided statistical advice for this manuscript.
At least one of the authors has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because this study is a review of existing literature and no human subjects were involved.
Ethical approval
Institutional Review Board approval was not required because this study is a review of existing literature and no human subjects were involved.
Methodology
• prospective
• observational
• multi-centre study
References
- 1.Yankelevitz DF, Yip R, Smith JP, Liang M, Liu Y, Xu DM, et al. CT screening for lung cancer: nonsolid nodules in baseline and annual repeat rounds. Radiology. 2015;277(2):555–564. doi: 10.1148/radiol.2015142554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yip R, Yankelevitz DF, Hu M, Li K, Xu DM, Jirapatnakul A, et al. Lung cancer deaths in the National Lung Screening Trial attributed to nonsolid nodules. Radiology. 2016;281(2):589–596. doi: 10.1148/radiol.2016152333. [DOI] [PubMed] [Google Scholar]
- 3.Yip R, Wolf A, Tam K, Taioli E, Olkin I, Flores R, et al. Outcomes of lung cancers manifesting as nonsolid nodules. Lung Cancer. 2016;97:35–42. doi: 10.1016/j.lungcan.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Henschke CI, Yip R, Smith JP, Wolf AS, Flores RM, Liang M, et al. CT screening for Lung Cancer: part-solid nodules in baseline and annual repeat rounds. AJR Am J Roentgenol. 2016;207(6):1176–1184. doi: 10.2214/AJR.16.16043. [DOI] [PubMed] [Google Scholar]
- 5.Yip R, Yankelevitz D, Li K, Xu D, Jirapatnakul A, Henschke C. Lung Cancer deaths in the National Lung Screening Trial attributed to cancers manifesting as part-solid nodules. AJR Am J Roentgenol. 2016 doi: 10.2214/ajr.16.16930. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M, Morikawa A, Kawasaki M, Matsuno Y, Yamada T, Hirohashi S, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75(12):2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi M, Shimosato Y. The development and progression of adenocarcinoma of the lung. Cancer Treat Res. 1995;72:131–142. doi: 10.1007/978-1-4615-2630-8_6. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8(5):381–385. doi: 10.1513/pats.201107-042ST. [DOI] [PubMed] [Google Scholar]
- 9.Travis W, Brambilla E, Burke A, Marx A, Nicholson A. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th. International Agency for Research on Cancer; Lyon: 2015. [DOI] [PubMed] [Google Scholar]
- 10.Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, et al. The IASLC Lung Cancer Staging Project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2016;11(8):1204–1223. doi: 10.1016/j.jtho.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Kuriyama K, Seto M, Kasugai T, Higashiyama M, Kido S, Sawai Y, et al. Ground-glass opacity on thin-section CT: value in differentiating subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol. 1999;173(2):465–469. doi: 10.2214/ajr.173.2.10430155. [DOI] [PubMed] [Google Scholar]
- 12.Henschke C, Yankelevitz D, Mirtcheva R, McGuinness G, McCauley D, Miettinen O. CT screening for Lung Cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002;178(5):1053–1057. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 13.Sone S, Nakayama T, Honda T, Tsushima K, Li F, Haniuda M, et al. Long-term follow-up study of a population-based 1996-1998 mass screening programme for Lung Cancer using mobile low-dose spiral computed tomography. Lung Cancer. 2007;58(3):329–341. doi: 10.1016/j.lungcan.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa M, Sone S, Takashima S, Li F, Yang ZG, Maruyama Y, et al. Growth rate of small Lung Cancers detected on mass CT screening. Br J Radiol. 2000;73(876):1252–1259. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 15.Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304–317. doi: 10.1148/radiol.12120628. [DOI] [PubMed] [Google Scholar]
- 16.MacMahon H, Naidich DP, Goo JM, Lee KS, Leung AN, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology. 2017:161659. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 17.Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70(Suppl 2):ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen OS, Henschke CI, Smith JP, Yankelevitz DF. Is ground glass descriptive of a type of pulmonary nodule? Radiology. 2014;270(1):311–312. doi: 10.1148/radiol.13131665. [DOI] [PubMed] [Google Scholar]
- 19.Sugi K, Kobayashi S, Sudou M, Sakano H, Matsuda E, Okabe K. Long-term prognosis of video-assisted limited surgery for early Lung Cancer. Eur J Cardiothorac Surg. 2010;37(2):456–460. doi: 10.1016/j.ejcts.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Matsuguma H, Mori K, Nakahara R, Suzuki H, Kasai T, Kamiyama Y, et al. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest. 2013;143(2):436–443. doi: 10.1378/chest.11-3306. [DOI] [PubMed] [Google Scholar]
- 21.Kim EA, Johkoh T, Lee KS, Han J, Fujimoto K, Sadohara J, et al. Quantification of ground-glass opacity on high-resolution CT of small peripheral adenocarcinoma of the lung: pathologic and prognostic implications. AJR Am J Roentgenol. 2001;177(6):1417–1422. doi: 10.2214/ajr.177.6.1771417. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura H, Saji H, Ogata A, Saijo T, Okada S, Kato H. Lung Cancer patients showing pure ground-glass opacity on computed tomography are good candidates for wedge resection. Lung Cancer. 2004;44(1):61–68. doi: 10.1016/j.lungcan.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Tateishi U, Muller NL, Johkoh T, Maeshima A, Asamura H, Satake M, et al. Mucin-producing adenocarcinoma of the lung: thin-section computed tomography findings in 48 patients and their effect on prognosis. J Comput Assist Tomogr. 2005;29(3):361–368. doi: 10.1097/01.rct.0000162820.08909.e1. [DOI] [PubMed] [Google Scholar]
- 24.Fukui T, Katayama T, Ito S, Abe T, Hatooka S, Mitsudomi T. Clinicopathological features of small-sized non-small cell Lung Cancer with mediastinal lymph node metastasis. Lung Cancer. 2009;66(3):309–313. doi: 10.1016/j.lungcan.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Vazquez M, Carter D, Brambilla E, Gazdar A, Noguchi M, Travis WD, et al. Solitary and multiple resected adenocarcinomas after CT screening for Lung Cancer: histopathologic features and their prognostic implications. Lung Cancer. 2009;64(2):148–154. doi: 10.1016/j.lungcan.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg. 2012;143(3):607–612. doi: 10.1016/j.jtcvs.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S, Fukui T, Taniguchi T, Usami N, Kawaguchi K, Ishiguro F, et al. Prognostic impact of tumor size eliminating the ground glass opacity component: modified clinical T descriptors of the tumor, node, metastasis classification of Lung Cancer. J Thorac Oncol. 2013;8(12):1551–1557. doi: 10.1097/JTO.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 28.Gierada DS, Pinsky P, Nath H, Chiles C, Duan F, Aberle DR. Projected outcomes using different nodule sizes to define a positive CT Lung Cancer screening examination. J Natl Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata H, Shirahashi K, Mizuno Y, Yamamoto H, Takemura H. Feasibility of segmental resection in non-small-cell Lung Cancer with ground-glass opacity. Eur J Cardiothorac Surg. 2014;46(3):375–379. doi: 10.1093/ejcts/ezu021. discussion 9. [DOI] [PubMed] [Google Scholar]
- 30.Hattori A, Suzuki K, Takamochi K, Oh S. Clinical features of multiple Lung Cancers based on thin-section computed tomography: what are the appropriate surgical strategies for second Lung Cancers? Surg Today. 2015;45(2):189–196. doi: 10.1007/s00595-014-0921-5. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi Y, Sakao Y, Deshpande GA, Fukui T, Mizuno T, Kuroda H, et al. The association between baseline clinical-radiological characteristics and growth of pulmonary nodules with ground-glass opacity. Lung Cancer. 2014;83(1):61–66. doi: 10.1016/j.lungcan.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Kodama H, Yamakado K, Hasegawa T, Takao M, Taguchi O, Fukai I, et al. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J Vasc Interv Radiol. 2014;25(3):333–339. doi: 10.1016/j.jvir.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 33.Nakata M, Sawada S, Saeki H, Takashima S, Mogami H, Teramoto N, et al. Prospective study of thoracoscopic limited resection for ground-glass opacity selected by computed tomography. Ann Thorac Surg. 2003;75(5):1601–1605. doi: 10.1016/s0003-4975(02)04815-4. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 34.Henschke CI, Shaham D, Yankelevitz DF, Kramer A, Kostis WJ, Reeves AP, et al. CT screening for Lung Cancer: significance of diagnoses in its baseline cycle. Clin Imaging. 2006;30(1):11–15. doi: 10.1016/j.clinimag.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Henschke CI, Yankelevitz DF, Miettinen OS. International Early Lung Cancer Action Program I. Computed tomographic screening for Lung Cancer: the relationship of disease stage to tumor size. Arch Intern Med. 2006;166(3):321–325. doi: 10.1001/archinte.166.3.321. [DOI] [PubMed] [Google Scholar]
- 36.Kodama K, Higashiyama M, Takami K, Oda K, Okami J, Maeda J, et al. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg. 2008;34(5):1068–1074. doi: 10.1016/j.ejcts.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 37.Carretta A, Ciriaco P, Melloni G, Bandiera A, Libretti L, Puglisi A, et al. Surgical treatment of multiple primary adenocarcinomas of the lung. Thorac Cardiovasc Surg. 2009;57(1):30–34. doi: 10.1055/s-2008-1038989. [DOI] [PubMed] [Google Scholar]
- 38.Kohno T, Fujimori S, Kishi K, Fujii T. Safe and effective minimally invasive approaches for small ground glass opacity. Ann Thorac Surg. 2010;89(6):S2114–S2117. doi: 10.1016/j.athoracsur.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Q, Suzuki K, Anami Y, Oh S, Takamochi K. Clinicopathologic features in resected subcentimeter Lung Cancer − status of lymph node metastases. Interact Cardiovasc Thorac Surg. 2010;10(1):53–57. doi: 10.1510/icvts.2009.216119. [DOI] [PubMed] [Google Scholar]
- 40.Matsunaga T, Suzuki K, Hattori A, Fukui M, Kitamura Y, Miyasaka Y, et al. Lung Cancer with scattered consolidation: detection of new independent radiological category of peripheral Lung Cancer on thin-section computed tomography. Interact Cardiovasc Thorac Surg. 2013;16(4):445–449. doi: 10.1093/icvts/ivs520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ambrosini-Spaltro A, Ruiu A, Seebacher C, Vattemi E, Gentile L, Feil B, et al. Impact of the IASLC/ATS/ERS classification in pN0 pulmonary adenocarcinomas: a study with radiological-pathological comparisons and survival analyses. Pathol Res Pract. 2014;210(1):40–46. doi: 10.1016/j.prp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Ye B, Cheng M, Ge XX, Geng JF, Li W, Feng J, et al. Factors that predict lymph node status in clinical stage T1aN0M0 lung adenocarcinomas. World J Surg Oncol. 2014;12:42. doi: 10.1186/1477-7819-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye B, Cheng M, Li W, Ge XX, Geng JF, Feng J, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg. 2014;98(1):217–223. doi: 10.1016/j.athoracsur.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Kudo Y, Matsubayashi J, Saji H, Akata S, Shimada Y, Kato Y, et al. Association between high-resolution computed tomography findings and the IASLC/ATS/ERS classification of small lung adenocarcinomas in Japanese patients. Lung Cancer. 2015;90(1):47–54. doi: 10.1016/j.lungcan.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Yoshioka M, Ichiguchi O. Selection of sublobar resection for c-stage IA non-small cell Lung Cancer based on a combination of structural imaging by CTand functional imaging by FDG PET. Ann Thorac Cardiovasc Surg. 2009;15(2):82–88. [PubMed] [Google Scholar]
- 46.Matsuguma H, Yokoi K, Anraku M, Kondo T, Kamiyama Y, Mori K, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J Thorac Cardiovasc Surg. 2002;124(2):278–284. doi: 10.1067/mtc.2002.122298. [DOI] [PubMed] [Google Scholar]
- 47.Matsuguma H, Nakahara R, Anraku M, Kondo T, Tsuura Y, Kamiyama Y, et al. Objective definition and measurement method of ground-glass opacity for planning limited resection in patients with clinical stage IA adenocarcinoma of the lung. Eur J Cardiothorac Surg. 2004;25(6):1102–1106. doi: 10.1016/j.ejcts.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Sagawa M, Higashi K, Usuda K, Aikawa H, Machida Y, Tanaka M, et al. Curative wedge resection for non-invasive bronchiolo-alveolar carcinoma. Tohoku J Exp Med. 2009;217(2):133–137. doi: 10.1620/tjem.217.133. [DOI] [PubMed] [Google Scholar]
- 49.Kim TJ, Park CM, Goo JM, Lee KW. Is there a role for FDG PET in the management of Lung Cancer manifesting predominantly as ground-glass opacity? AJR Am J Roentgenol. 2012;198(1):83–88. doi: 10.2214/AJR.11.6862. [DOI] [PubMed] [Google Scholar]
- 50.Seok Y, Cho S, Kim K, Jheon S. Partly solid pulmonary nodules: waiting for change or surgery outright? Interact Cardiovasc Thorac Surg. 2014;19(4):556–560. doi: 10.1093/icvts/ivu205. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida J, Ishii G, Hishida T, Aokage K, Tsuboi M, Ito H, et al. Limited resection trial for pulmonary ground-glass opacity nodules: case selection based on high-resolution computed tomography-interim results. Jpn J Clin Oncol. 2015;45(7):677–681. doi: 10.1093/jjco/hyv057. [DOI] [PubMed] [Google Scholar]
- 52.Ohta Y, Shimizu Y, Kobayashi T, Matsui O, Minato H, Matsumoto I, et al. Pathologic and biological assessment of lung tumors showing ground-glass opacity. Ann Thorac Surg. 2006;81(4):1194–1197. doi: 10.1016/j.athoracsur.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki K, Kusumoto M, Watanabe S, Tsuchiya R, Asamura H. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg. 2006;81(2):413–419. doi: 10.1016/j.athoracsur.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 54.Asamura H, Suzuki K, Watanabe S, Matsuno Y, Maeshima A, Tsuchiya R. A clinicopathological study of resected subcentimeter Lung Cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg. 2003;76(4):1016–1022. doi: 10.1016/s0003-4975(03)00835-x. [DOI] [PubMed] [Google Scholar]
- 55.Ohde Y, Nagai K, Yoshida J, Nishimura M, Takahashi K, Suzuki K, et al. The proportion of consolidation to ground-glass opacity on high resolution CT is a good predictor for distinguishing the population of non-invasive peripheral adenocarcinoma. Lung Cancer. 2003;42(3):303–310. doi: 10.1016/j.lungcan.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Aokage K, Yoshida J, Ishii G, Matsumura Y, Haruki T, Hishida T, et al. Identification of early t1b lung adenocarcinoma based on thin-section computed tomography findings. J Thorac Oncol. 2013;8(10):1289–1294. doi: 10.1097/JTO.0b013e31829f6d3b. [DOI] [PubMed] [Google Scholar]
- 57.Duann CW, Hung JJ, Hsu PK, Huang CS, Hsieh CC, Hsu HS, et al. Surgical outcomes in Lung Cancer presenting as ground-glass opacities of 3 cm or less: a review of 5 years’ experience. J Chin Med Assoc. 2013;76(12):693–697. doi: 10.1016/j.jcma.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Segmentectomy for clinical stage IA lung adenocarcinoma showing solid dominance on Radiology. Eur J Cardiothorac Surg. 2014;46(4):637–642. doi: 10.1093/ejcts/ezt645. [DOI] [PubMed] [Google Scholar]
- 59.Yanagawa M, Tanaka Y, Leung AN, Morii E, Kusumoto M, Watanabe S, et al. Prognostic importance of volumetric measurements in stage I lung adenocarcinoma. Radiology. 2014;272(2):557–567. doi: 10.1148/radiol.14131903. [DOI] [PubMed] [Google Scholar]
- 60.Cho JH, Choi YS, Kim J, Kim HK, Zo JI, Shim YM. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg. 2015;99(1):218–222. doi: 10.1016/j.athoracsur.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 61.Hattori A, Suzuki K, Matsunaga T, Miyasaka Y, Takamochi K, Oh S. What is the appropriate operative strategy for radiologically solid tumours in subcentimetre Lung Cancer patients? dagger. Eur J Cardiothorac Surg. 2015;47(2):244–249. doi: 10.1093/ejcts/ezu250. [DOI] [PubMed] [Google Scholar]
- 62.Li Z, Ye B, Bao M, Xu B, Chen Q, Liu S, et al. Radiologic predictors for clinical stage IA lung adenocarcinoma with ground glass components: a multi-center study of long-term outcomes. PLoS One. 2015;10(9):e0136616. doi: 10.1371/journal.pone.0136616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura S, Fukui T, Kawaguchi K, Fukumoto K, Hirakawa A, Yokoi K. Does ground glass opacity-dominant feature have a prognostic significance even in clinical T2aN0M0 lung adenocar-cinoma? Lung Cancer. 2015;89(1):38–42. doi: 10.1016/j.lungcan.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Sakurai H, Nakagawa K, Watanabe S, Asamura H. Clinicopathologic features of resected subcentimeter Lung Cancer. Ann Thorac Surg. 2015;99(5):1731–1738. doi: 10.1016/j.athoracsur.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 65.Yano M, Yoshida J, Koike T, Kameyama K, Shimamoto A, Nishio W, et al. Survival of 1737 lobectomy-tolerable patients who underwent limited resection for cStage IA non-small-cell Lung Cancer. Eur J Cardiothorac Surg. 2015;47(1):135–142. doi: 10.1093/ejcts/ezu138. [DOI] [PubMed] [Google Scholar]
- 66.Kodama K, Higashiyama M, Yokouchi H, Takami K, Kuriyama K, Mano M, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer. 2001;33(1):17–25. doi: 10.1016/s0169-5002(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 67.Aoki T, Tomoda Y, Watanabe H, Nakata H, Kasai T, Hashimoto H, et al. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology. 2001;220(3):803–809. doi: 10.1148/radiol.2203001701. [DOI] [PubMed] [Google Scholar]
- 68.Takashima S, Maruyama Y, Hasegawa M, Saito A, Haniuda M, Kadoya M. High-resolution CT features: prognostic significance in peripheral lung adenocarcinoma with bronchioloalveolar carcinoma components. Respiration. 2003;70(1):36–42. doi: 10.1159/000068410. [DOI] [PubMed] [Google Scholar]
- 69.Ikeda N, Maeda J, Yashima K, Tsuboi M, Kato H, Akada S, et al. A clinicopathological study of resected adenocarcinoma 2 cm or less in diameter. Ann Thorac Surg. 2004;78(3):1011–1016. doi: 10.1016/j.athoracsur.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 70.Sakao Y, Nakazono T, Sakuragi T, Natsuaki M, Itoh T. Predictive factors for survival in surgically resected clinical IA peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2004;77(4):1157–1161. doi: 10.1016/j.athoracsur.2003.09.055. discussion 61-2. [DOI] [PubMed] [Google Scholar]
- 71.Seki N, Sawada S, Nakata M, Inoue T, Nishimura R, Segawa Y, et al. Lung Cancer with localized ground-glass attenuation represents early-stage adenocarcinoma in nonsmokers. J Thorac Oncol. 2008;3(5):483–490. doi: 10.1097/JTO.0b013e31816a4994. [DOI] [PubMed] [Google Scholar]
- 72.Higashi K, Sakuma T, Ito K, Niho S, Ueda Y, Kobayashi T, et al. Combined evaluation of preoperative FDG uptake on PET, ground-glass opacity area on CT, and serum CEA level: identification of both low and high risk of recurrence in patients with resected T1 lung adenocarcinoma. Eur J Nucl Med Mol Imaging. 2009;36(3):373–381. doi: 10.1007/s00259-008-0961-4. [DOI] [PubMed] [Google Scholar]
- 73.Inoue M, Minami M, Sawabata N, Utsumi T, Kadota Y, Shigemura N, et al. Clinical outcome of resected solid-type small-sized c-stage IA non-small cell Lung Cancer. Eur J Cardiothorac Surg. 2010;37(6):1445–1449. doi: 10.1016/j.ejcts.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 74.Shi CL, Zhang XY, Han BH, He WZ, Shen J, Chu TQ. A clinicopathological study of resected non-small cell Lung Cancers 2 cm or less in diameter: a prognostic assessment. Med Oncol. 2011;28(4):1441–1446. doi: 10.1007/s12032-010-9632-y. [DOI] [PubMed] [Google Scholar]
- 75.Lederlin M, Puderbach M, Muley T, Schnabel PA, Stenzinger A, Kauczor HU, et al. Correlation of radio- and histomorphological pattern of pulmonary adenocarcinoma. Eur Respir J. 2013;41(4):943–951. doi: 10.1183/09031936.00056612. [DOI] [PubMed] [Google Scholar]
- 76.Matsuguma H, Oki I, Nakahara R, Suzuki H, Kasai T, Kamiyama Y, et al. Comparison of three measurements on computed tomography for the prediction of less invasiveness in patients with clinical stage I non-small cell Lung Cancer. Ann Thorac Surg. 2013;95(6):1878–1884. doi: 10.1016/j.athoracsur.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 77.Shiono S, Yanagawa N, Abiko M, Sato T. Detection of non-aggressive stage IA Lung Cancer using chest computed tomography and positron emission tomography/computed tomography. Interact Cardiovasc Thorac Surg. 2014;19(4):637–643. doi: 10.1093/icvts/ivu188. [DOI] [PubMed] [Google Scholar]
- 78.Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest. 2014;145(1):66–71. doi: 10.1378/chest.13-1094. [DOI] [PubMed] [Google Scholar]
- 79.Hwang EJ, Park CM, Ryu Y, Lee SM, Kim YT, Kim YW, et al. Pulmonary adenocarcinomas appearing as part-solid ground-glass nodules: is measuring solid component size a better prognostic indicator? Eur Radiol. 2015;25(2):558–567. doi: 10.1007/s00330-014-3441-1. [DOI] [PubMed] [Google Scholar]
- 80.Kakinuma R, Kodama K, Yamada K, Yokoyama A, Adachi S, Mori K, et al. Performance evaluation of 4 measuring methods of ground-glass opacities for predicting the 5-year relapse-free survival of patients with peripheral nonsmall cell Lung Cancer: a multicenter study. J Comput Assist Tomogr. 2008;32(5):792–798. doi: 10.1097/RCT.0b013e31815688ae. [DOI] [PubMed] [Google Scholar]
- 81.Okada M, Nakayama H, Okumura S, Daisaki H, Adachi S, Yoshimura M, et al. Multicenter analysis of high-resolution computed tomography and positron emission tomography/computed tomography findings to choose therapeutic strategies for clinical stage IA lung adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141(6):1384–1391. doi: 10.1016/j.jtcvs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Dong B, Sato M, Sagawa M, Endo C, Usuda K, Sakurada A, et al. Computed tomographic image comparison between mediastinal and lung windows provides possible prognostic information in patients with small peripheral lung adenocarcinoma. J Thorac Cardiovasc Surg. 2002;124(5):1014–1020. doi: 10.1067/mtc.2002.125647. [DOI] [PubMed] [Google Scholar]
- 83.Murakawa T, Konoeda C, Ito T, Inoue Y, Sano A, Nagayama K, et al. The ground glass opacity component can be eliminated from the T-factor assessment of lung adenocarcinoma. Eur J Cardiothorac Surg. 2013;43(5):925–932. doi: 10.1093/ejcts/ezs467. [DOI] [PubMed] [Google Scholar]
- 84.Hashizume T, Yamada K, Okamoto N, Saito H, Oshita F, Kato Y, et al. Prognostic significance of thin-section CT scan findings in small-sized lung adenocarcinoma. Chest. 2008;133(2):441–447. doi: 10.1378/chest.07-1533. [DOI] [PubMed] [Google Scholar]
- 85.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I Lung Cancer detected on CT screening. N Engl J Med. 2006:1763–1771. doi: 10.1056/NEJMoa060476. United States: 2006 Massachusetts Medical Society. [DOI] [PubMed] [Google Scholar]
- 86.Henschke CI, Yankelevitz DF, Yip R, Reeves AP, Farooqi A, Xu D, et al. Lung Cancers diagnosed at annual CT screening: volume doubling times. Radiology. 2012;263(2):578–583. doi: 10.1148/radiol.12102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Network NCC. Clinical Practice Guidelines in Oncology (NCCN Guidelines). Version 1.2014 Lung Cancer Screening. http://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. Accessed March 27, 2015.
- 88.Higashiyama M, Kodama K, Yokouchi H, Takami K, Mano M, Kido S, et al. Prognostic value of bronchiolo-alveolar carcinoma component of small lung adenocarcinoma. Ann Thorac Surg. 1999;68(6):2069–2073. doi: 10.1016/s0003-4975(99)01064-4. [DOI] [PubMed] [Google Scholar]
- 89.International Early Lung Cancer Action Program Protocol. http://www.ielcap.org/sites/default/files/I-ELCAP%20protocol-v21-3-1-14.pdf. Accessed May 30, 2017.
- 90.Henschke C, Yankelevitz D, Naidich D, McCauley D, McGuinness G, Libby D, et al. CT screening for Lung Cancer: suspiciousness of nodules according to size on baseline scans. Radiology. 2004;231(1):164–168. doi: 10.1148/radiol.2311030634. [DOI] [PubMed] [Google Scholar]
- 91.Henschke C, Yip R, Yankelevitz D, Smith J. Definition of a positive test result in computed tomography screening for Lung Cancer: a cohort study. Ann Intern Med. 2013;158(4):246–252. doi: 10.7326/0003-4819-158-4-201302190-00004. [DOI] [PubMed] [Google Scholar]
- 92.Yip R, Henschke C, Yankelevitz D, Boffetta P, Smith J, The International Early Lung Cancer Investigators The impact of the regimen of screening on Lung Cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev. 2015;24(3):201–208. doi: 10.1097/CEJ.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 93.Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw. 2016;14(3):255–264. doi: 10.6004/jnccn.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485–491. doi: 10.7326/M14-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kazerooni E, Austin J, Black W, Dyer D, Hazelton T, Leung A, et al. ACR-STR practice parameter for the performance and reporting of Lung Cancer screening thoracic computed tomography. J Thorac Imaging. 2014;29(5):310–316. doi: 10.1097/RTI.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 96.American College of Radiology. http://www.acr.org/Quality-Safety/Resources/LungRADS. Accessed May 30, 2017.


