Abstract
Background and Objectives
Given the increased number of treatment options for stage IA lung cancer patients, there is a growing body of literature that focuses on comparing each option’s relative impact on quality of life (QoL). The current study seeks to further understand the differences in these patients’ QoL according to surgical approach.
Methods
Screening-diagnosed first primary pathologic stage IA non-small-cell lung cancer surgical patients from the I-ELCAP cohort who answered a baseline and 1-year follow-up QoL questionnaire (SF-12) were included in the analysis. Thoracotomy patients (N = 85) were compared with VATS patients (N = 15) using paired t-tests and analysis of variance tests.
Results
Multivariate analyses indicated no differences in QoL change between the two groups from pre- to post-surgery. Physical and emotional role functioning significantly improved among VATS patients and worsened among thoracotomy patients. Among thoracotomy patients, a significant decrease in post-surgical physical QoL was observed only in those who underwent lobectomy (−3.3; 95% CI: −5.1, −1.5), not limited resection.
Conclusions
Although the sample size is small, preliminary findings underscore that changes in overall QoL are similar in VATS and thoracotomy stage IA lung cancer patients. Extension of the resection may be a more relevant factor on QoL post-surgery.
Keywords: thoracotomy, VATS, lobectomy, limited resection
INTRODUCTION
Among early stage lung cancer patients, quality of life (QoL) is of outmost concern because, for the majority, the risk of death is relatively low thanks to the advances in surgical treatment, so patients are able to focus on the physical and mental health sequelae of lung cancer [1–3]. Further, compared to other cancers, lung cancer patients experience especially high symptom burdens including pain and lung function difficulties, multiple comorbidities, and numerous mental health concerns [1–8].
Given the increased potential number of treatment options for early stage lung cancer patients, there is a growing body of literature that focuses on the comparison of each option’s impact on QoL [9–11]. Changes in QoL among lung cancer patients who undergo surgery is often dependent on the extent of surgery, with less invasive approaches generally associated with better post-surgery physical QoL scores, and faster return to pre-surgical conditions [9–10]. However, the findings of the literature are mixed. One study involving patients with stage-I lung cancer that were randomized to either sublobar resection (SR) or SR with brachytherapy, found that there was no significant differences pre-to post-surgery in the percentage of QoL score change between the two treatment groups [10]. Similarly, a recent study comparing bilobectomy and lobectomy patients, found no differences in QoL changes [11]. Ostroff et al. found that early stage lung cancer survivors who had undergone surgery 1–6 years prior had lower physical health QoL scores as compared to a screening sample, but there was no difference between the two samples on mental health QoL scores, nor was there a baseline QoL assessment on which to compare the data [12]. When compared to healthy controls, however, mental and physical health QoL was found to be significantly worse among post-lobectomy lung cancer patients only [13]. Recent research from our group indicated that, in multivariate analyses, only lobectomy, not limited resection had a negative impact on QoL from pre- to post-surgery and that impact was limited to physical health QoL [14].
Research involving the specific comparison of VATS (video-assisted thoracic surgery) and thoracotomy on early stage lung cancer patients’ QoL is limited. One study of stage I lung cancer patients, found that an improvement in physical health QoL was more common among VATS patients as compared to thoracotomy patients [15]. However, Rizk et al. found that pain and physical health QoL were similar between VATS and thoracotomy patients at follow-up, while mental health QoL was worse among VATS patients [16]. This study, however, did not disentangle the effect of surgical approach (VATS vs. thoracotomy) from the effect of type of resection (lobectomy vs. limited resection) on QoL. The purpose of the current study is to further understand the differences between VATS and thoracotomy patients on baseline QoL, post-surgical QoL, and change in QoL from baseline to post-surgery in a sample of screening-detected early stage (IA) lung cancer patients. In addition, a secondary aim is to compare limited resection and lobectomy QoL scores among the thoracotomy patients. This is one of the first studies in which specific changes in QoL subscale scores will be examined in addition to overall physical and mental health QoL. In addition, differences based on patient-specific demographic factors such as gender and smoking status will also be examined.
MATERIALS AND METHODS
This report draws from the database of newly diagnosed cases of non-small cell lung cancer (NSCLC) identified as a result of computed tomography (CT) screening in the International Early Lung Cancer Action Program (I-ELCAP) cohort. The screenings were performed under an IRB approved, HIPAA compliant common protocol at each of the participating institutions from 2001 (when the SF-12 QoL measure started to be collected) to 2014 on smokers, never smokers, and participants with occupational exposure to airborne carcinogens or exposure to secondhand smoke aged 40 years and older [17]. Consent was obtained from all participants at the time of enrollment. Participants were interviewed to obtain information on relevant demographics, occupational history, smoking habits, and co-morbidities. For purposes of this study, only participants diagnosed with their first primary pathologic stage IA non-small-cell lung cancer that underwent surgery and provided follow-up information 1 year later (7–18 months) were included in the present analysis. This time interval corresponds to the I-ELCAP screening clinical regimen for repeat CT scans. Staging classification for lung cancer was made centrally based on the American Joint Committee on Cancer Staging Manual, sixth edition, with one exception. Cases of multiple adenocarcinomas (<30 mm in diameter) without lymph node metastases were classified as synchronous primaries and considered to be stage IA. Information regarding resection method (i.e., VATS or thoracotomy) was collected for all resection patients.
QoL information was collected using a standard SF-12 form which is a shorter version of the SF-36 questionnaire [18]. The SF-12 assesses eight domains of health: physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF-12 is used to calculate two component scores, the Physical Component Summary Score (PCS) and the Mental Component Summary Score (MCS). Both MCS and PCS have scores that range from 0 to 100 (worst to best QoL). The PCS is a combination of SF-12 items that focus on participants’ perceptions of their general health, mobility, limitations due to physical problems, and limitations in work/productivity due to physical problems and pain. Similarly, the MCS focuses on participants’ experiences of symptoms of depression and anxiety, difficulties with social activity, and amount accomplished due to emotional difficulties.
Statistical Analysis
Continuous variables are presented as means and standard deviations, categorical variables as percentages. Univariate and multivariate analyses were performed to examine the difference in physical (PCS) and mental (MCS) health component scores of the SF-12 before and after surgery using paired t-test and analysis of variance tests. Univariate and multivariate analysis of variance were also performed to examine the difference in SF-12 subscales before and after surgery. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). Covariates include age, education, ethnicity, gender, pack-years of smoking, and presence of comorbid conditions (e.g., liver disease, asthma, diabetes) as these are often associated with lung cancer QoL outcomes in the literature.
RESULTS
There were 100 participants (50 women, 50 men) who matched the inclusion criteria and had an SF-12 questionnaire completed at baseline CT screening and at 1-year follow up after surgery for pathologic stage IA non-small cell lung cancer. Their mean age was 63 years (SD = 8.2); 85 patients underwent thoracotomy and 15 underwent video-assisted thoracoscopic surgery (VATS). The average time of follow up was 11 months after surgery (SD: 2.3 months; range: 7–18 months post-surgery). See Table I for a description of the patient sample.
TABLE I.
Patient Characteristics (N = 100)
| n (%) | |
|---|---|
| Age (mean ± SD) | 62.7 ± 8.2 |
| Gender | |
| Female | 50 (50%) |
| Male | 50 (50%) |
| White | 88 (88%) |
| College education | 45 (45%) |
| Smoking status | |
| Never smoker | 1 (1%) |
| Former smoker | 48 (48%) |
| Current smoker | 51 (51%) |
| Packyears of smoking among smokers (mean ± SD) | 49.6 ± 24.9 |
| Lesion size prior to resection (mm) (mean ± SD) | 14.3 ± 6.0 |
| Tumor pathology size (mm) | |
| Mean ± SD | 13.8 ± 6.0 |
| 1–10 mm | 35 (35%) |
| 11–20 mm | 52 (52%) |
| 21–30 mm | 13 (13%) |
| Lesion location | |
| LLL | 12 (12%) |
| LUL | 26 (26%) |
| RLL | 17 (17%) |
| RML | 2 (2%) |
| RUL | 43 (43%) |
| Extent of surgery | |
| Wedge resection | 12 (12%) |
| Segmentectomy | 11 (11%) |
| Lobectomy | 73 (73%) |
| Bilobectomy | 4 (4%) |
| Type of surgery | |
| Limited resection | 23 (23%) |
| Lobectomy | 77 (77%) |
| Method of resection | |
| VATS | 15 (15%) |
| Thoracotomy | 85 (85%) |
| Baseline SF-12 (mean ± SD) | |
| Mental health component | 52.2 ± 9.6 |
| Physical health component | 49.1 ± 6.9 |
| Time of follow-up (mean ± SD) | 11.4 ± 2.3 |
Changes in Quality of Life After Surgery
On baseline screening CT, average PCS and MCS scores were 49.1 (SD = 6.9) and 52.2 (SD = 9.6), respectively. Mean post-surgery PCS was 46.9 (SD = 8.7) and MCS was 53.9 (SD = 9.6). There was a statistically significant decrease in PCS from baseline to post-surgery follow-up (−2.2; 95% CI: −3.62, −0.71), while MCS showed a non-statistically significant increase at post-surgery follow-up (+1.7; 95% CI: −0.22/3.63) (Table II; Figs. 1 and 2).
TABLE II.
SF-12 Quality of Life Scores Pre- and Post-Surgery Overall and According to Gender (N = 100)
| AH (n = 100) |
Male (n = 50) |
Female (n = 50) |
Male versus female (male = 1) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusteda
|
Adjustedb
|
||||||||||||||
| Mean | SD | 95% CI for mean | Mean | SD | 95% CI for mean | Mean | SD | 95% CI for Mean | Parameter estimates | 95% CI | Parameter estimates | 95% CI | |||
| PCS | |||||||||||||||
| Baseline | 49.1 | 6.9 | 49.4 | 6.7 | 48.7 | 7.2 | 0.76 | 1.99 | 3.51 | 1.40 | 1.37 | 4.17 | |||
| Follow-up | 46.9 | 8.7 | 47.2 | 8.6 | 46.6 | 8.9 | 0.67 | 2.82 | 4.15 | 1.83 | 1.42 | 5.07 | |||
| Difference (follow-up-baseline) | −2.2* | 7.4* | (−3.62, −0.71)* | −2.2* | 7.5* | (−4.33, −0.09)* | −2.1* | 7.3* | (4.20, −0.04)* | 0.09 | 3.02 | 2.84 | 0.42 | 2.47 | 3.32 |
| MCS | |||||||||||||||
| Baseline | 52.2 | 9.6 | 53.7 | 9.5 | 50.7 | 9.6 | 2.98 | 0.81 | 6.77 | 3.17 | 0.71 | 7.05 | |||
| Follow-up | 53.9 | 9.6 | 55.4 | 9.9 | 52.4 | 9.1 | 2.94 | 0.83 | 6.70 | 3.57 | 0.27 | 7.41 | |||
| Difference (Follow-up-baseline) | 1.7 | 9.7 | (−0.22, 3.63) | 1.7 | 8.4 | (−0.70, 4.07) | 1.7 | 11 | (−1.38, 4.84) | −0.04 | 3.91 | 3.82 | 0.39 | 3.50 | 4.29 |
PCS, Physical Component Score; MCS, Mental Component Score.
Regression analysis comparing QoL score between male and female.
Multivariate regression analysis comparing QoL score between male and female after adjusting for age, pack-years of smoking, ethnicity, and education.
Significant at P > 05.
Fig. 1.
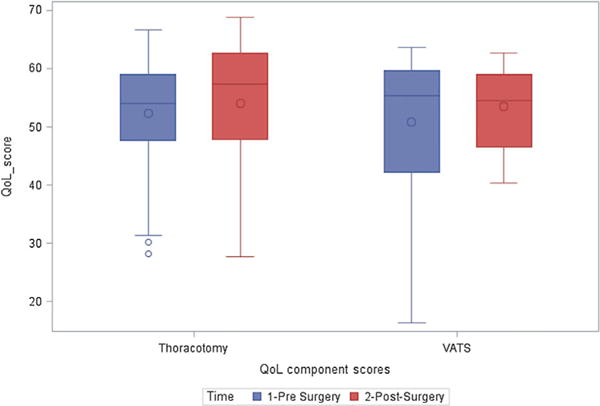
Pre- and post-surgery mental health component scores according to resection method; QoL, quality of life.
Fig. 2.
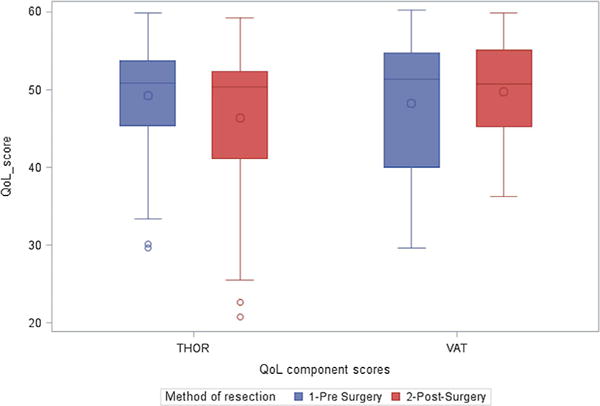
Pre- and post-surgery physical health component scores according to resection method; QoL, quality of life.
Effect of Method of Resection on PCS
A significant decrease in PCS score was observed among those who underwent resection via thoracotomy (−2.8; 95% CI: −4.38, −1.23), while there was a non-statistically significant improvement in post-surgical PCS among patients who underwent VATS resection (+1.4; 95% CI: −2.30, 5.20). The difference in PCS from baseline to follow-up between the two methods of resection was statistically significant (+4.25). At multivariate analysis, adjusting for sex, age, pack-years of smoking, ethnicity, and education, the mean PCS at baseline did not vary by method of resection (Table III; Figs. 1 and 2).
TABLE III.
SF-12 Quality of Life Scores Pre- and Post-Surgery According to Method of Resection
| VATS (n = 15) |
Thoracotomy (n = 85) |
VATS versus thoracotomy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusteda
|
Adjustedb
|
|||||||||||
| Mean | SD | 95% CI for mean | Mean | SD | 95% CI for mean | Parameter estimates | 95% CI | Parameter estimates | 95% CI | |||
| PCS | ||||||||||||
| Baseline | 48.3 | 9.2 | 49.2 | 6.5 | −0.94 | −4.80 | 2.91 | −1.49 | −5.49 | 2.50 | ||
| Follow-up | 49.7 | 6.6 | 46.4 | 9.0 | 3.31 | −1.52 | 8.14 | 2.56 | −2.11 | 7.22 | ||
| Difference (follow-up-baseline) | 1.4 | 6.8 | (−2.30, 5.20) | −2.8* | 7.3* | (−4.38, −1.23)* | 4.25* | 0.23* | 8.27* | 4.05 | −0.06 | 8.15 |
| MCS | ||||||||||||
| Baseline | 50.9 | 12.9 | 52.4 | 9.0 | −1.49 | −6.86 | 3.88 | −0.62 | −6.23 | 4.99 | ||
| Follow-up | 53.6 | 7.1 | 53.9 | 10.0 | −0.36 | −5.69 | 4.98 | −0.08 | −5.63 | 5.48 | ||
| Difference (follow-up-baseline) | 2.7 | 10.4 | (−3.06, 8.41) | 1.5* | 9.6* | (0.54, 3.62)* | 1.13 | −4.28 | 6.55 | 0.55 | −5.09 | 6.18 |
PCS, Physical Component Score; MCS, Mental Component Score.
Regression analysis comparing QoL score between VATS and thoracotomy.
Multivariate regression analysis comparing QoL score between VATS and thoracotomy after adjusting for sex, age, pack-years of smoking, ethnicity, and education.
Significant at P > 05.
Effect of Method of Resection on MCS
MCS was statistically significantly improved after thoracotomy (+1.5; 95% CI: 0.54, 3.62), but the improvement was non-statistically significant after VATS (+2.7; 95% CI: −3.06, 8.41). The difference in MCS from baseline to follow-up was not significantly different between the two methods of resection in both the univariate and multivariate regression models adjusting for sex, age, pack-years of smoking, ethnicity, and education (Table III).
SF-12 Subscales
On baseline screening CT, the mean general health score was significantly higher among patients who underwent resection via VATS compared to patients who underwent resection via thoracotomy (65.0 ± 22.8 vs. 54.4 ± 16.0). Patients who underwent resection via thoracotomy had a higher baseline score in the remaining seven subscales compared to patients who underwent resection via VATS. However, the difference in score between the two methods of resection was non-statistically significant (Table IV). (see Fig. 3).
TABLE IV.
SF-12 Subscale Scores According to Method of Resection
| Variable | VATS (n = 15) |
THOR (n = 85) |
Unadjusteda
|
Adjustedb
|
||
|---|---|---|---|---|---|---|
| Mean ± Std Dev | Mean ± Std Dev | Parameter estimates | 95% CI | Parameter estimates | 95% CI | |
| Baseline | ||||||
| Physical function | 73.3 ± 34.7 | 83.2 ± 21.6 | −9.90 | (−23.20, 3.39) | −10.27 | (−24.00, 3.46) |
| Role functioning—physical | 80.8 ± 27.5 | 86.9 ± 19.8 | −6.08 | (−17.77, 5.61) | −5.46 | (−16.92, 5.99) |
| Bodily pain | 78.3 ± 26.5 | 83.8 ± 23.1 | −5.49 | (−18.59, 7.61) | −5.34 | (−19.02, 8.33) |
| General health | 65 ± 22.8 | 54.4 ± 16 | 10.59* | (1.07, 20.10)* | 8.79 | (−1.10, 18.68) |
| Vitality | 65 ± 20.7 | 70.3 ± 23.3 | −5.29 | (−18.05, 7.46) | −4.29 | (−17.33, 8.75) |
| Social function | 86.7 ± 28.1 | 87.9 ± 21.7 | −1.27 | (−13.90, 11.35) | −2.70 | (−16.14, 10.75) |
| Role functioning—emotional | 80 ± 31.6 | 87.6 ± 19.4 | −7.65 | (−19.65, 4.36) | −7.68 | (−20.08, 4.71) |
| Mental health | 70.8 ± 29 | 73.2 ± 19.7 | −2.40 | (−14.22, 9.42) | 1.50 | (−10.51, 13.51) |
| Follow-up | ||||||
| Physical function | 71.7 ± 29.7 | 70.9 ± 34.3 | 0.78 | (−17.91,19.48) | 0.20 | (−18.57, 18.98) |
| Role functioning—physical | 91.7 ± 16.1 | 77.4 ± 28.1 | 14.31 | (−0.54, 29.17) | 11.70 | (−3.07, 26.48) |
| Bodily pain | 90 ± 18.4 | 85 ± 24.8 | 5.00 | (−8.31, 18.31) | 3.92 | (−9.16, 17.00) |
| General health | 70 ± 14 | 57.1 ± 21.3 | 12.94* | (1.57, 24.31)* | 11.36 | (−0.58, 23.29) |
| Vitality | 61.7 ± 18.6 | 68.5 ± 27.3 | −6.86 | (−21.45, 7.73) | −7.18 | (−21.52, 7.16) |
| Social function | 86.7 ± 24.8 | 87.6 ± 23 | −0.98 | (−13.92, 11.96) | −0.43 | (−13.74, 12.88) |
| Role functioning—emotional | 91.7 ± 13.1 | 85 ± 25.1 | 6.67 | (−6.55, 19.89) | 6.12 | (−7.69, 19.93) |
| Mental health | 77.5 ± 17.2 | 77.1 ± 21.6 | 0.44 | (−11.22, 12.10) | 1.03 | (−11.16, 13.22) |
| Difference (follow-up-baseline) | ||||||
| Physical function | −1.7 ± 33.4 | −12.4 ± 28.2 | 10.69 | (−5.45, 26.82) | 10.47 | (−6.50, 27.44) |
| Role functioning—physical | 10.8 ± 27.5 | −9.6 ± 23.6 | 20.39* | (6.95, 33.84)* | 17.17* | (3.32, 31.02)* |
| Bodily pain | 11.7 ± 22.9 | 1.2 ± 23.1 | 10.49 | (−2.34, 23.32) | 9.26 | (−4.28, 22.80) |
| General health | 5 ± 16.9 | 2.6 ± 17.7 | 2.35 | (−7.42, 12.12) | 2.57 | (−7.72, 12.86) |
| Vitality | −3.3 ± 16 | −1.8 ± 20.7 | −1.57 | (−12.74, 9.60) | −2.89 | (−14.61, 8.83) |
| Social function | 0 ± 16.4 | −0.3 ± 24.5 | 0.29 | (−12.0, 13.38) | 2.27 | (−11.22, 15.75) |
| Role functioning—emotional | 11.7 ± 31.9 | −2.6 ± 20 | 14.31* | (2.06, 26.57)* | 13.80* | (0.82, 26.79)* |
| Mental health | 6.7 ± 28.3 | 3.8 ± 23 | 2.84 | (−10.39, 16.08) | −0.47 | (−14.02, 13.08) |
Regression analysis comparing SF-12 subscale score between VATS and thoracotomy.
Multivariate regression analysis comparing SF-12 subscale score between VATS and thoracotomy after adjusting for sex, age, pack-years of smoking, ethnicity, and education.
Significant at P > 05.
Fig. 3.
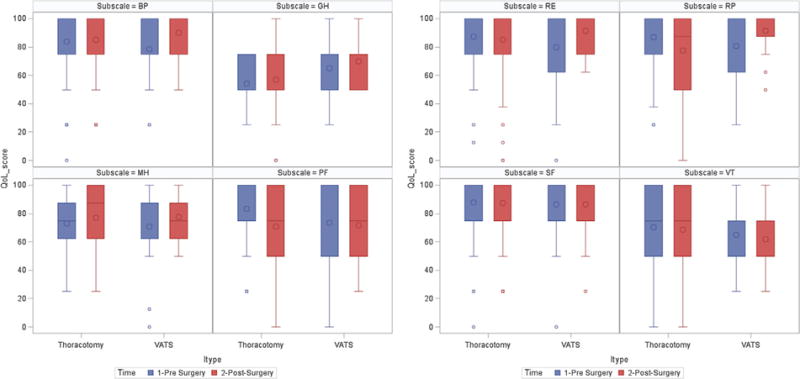
Pre- and post-surgery subscale scores according to resection method; QoL, quality of life; BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, role function-emotional; RP, role function-physical; SF, social functioning; VT, vitality.
During follow-up 7–18 months post-surgery, the mean general health score was significantly higher among patients who underwent resection via VATS compared to patients who went resection via thoracotomy (70 ± 14 vs. 57.1 ± 21.3). In the remaining seven SF-12 subscales, the average score was higher among patients who underwent resection via VATS compared to patients who underwent resection via thoracotomy, except for vitality and social function. These differences in subscale score post-surgery were not statistically significant.
The method of resection had a significant impact on both role function-physical and role function-emotional subscales from pre- to post-surgery. There was a decrease in average physical (−9.6 ± 23.6) and emotional (−2.6 ± 20.0) role function scores from pre-surgery to follow-up among patients who underwent resection via thoracotomy, while an increase in average physical (10.8 ± 27.5) and emotional (11.7 ± 31.9) role function scores was observed among patients who underwent VATS resection. After adjusting for sex, age, pack-years of smoking, ethnicity, and education, the difference in method of surgery on QoL was significant only for role function (physical and emotional) subscales in that role function was significantly higher among patients who underwent resection via VATS (Table IV).
MANCOVA results suggested that the method of resection has no overall effect on any of the SF-12 subscales after adjusting for other covariates (Wilks’ Lambda = 0.89, F(8,86) = 1.32, P = 0.25).
Effect of Sex and Smoking
There were a significant decrease in PCS in both males (−2.2; 95% CI: −4.33, −0.09) and females (−2.1; 95% CI: −4.20, −0.04) after surgery. A non-statistically significant increase in post-surgical MCS was observed in both males and females. After adjustment for age, pack-years of smoking, ethnicity, and education, the difference in baseline to post-surgical score for both PCS and MCS were non-statistically significant between males and females (Table II). A significant decrease in PCS score was observed among former smokers (−2.8; 95% CI: −4.38, −0.67). A non-statistically significant increase in post-surgical MCS was observed in both current and former smokers. No differences between current and former smokers were observed in the change of both PCS and MCS post-surgery (data not shown).
Stratified Analysis by Type of Surgery Among Patients Who Underwent Thoracotomy
A decrease in post-surgical PCS was observed in both patients who underwent lobectomy and those who underwent limited resection (−0.6; 95% CI: −4.0, 2.8); however, the decrease was only significant among those who underwent lobectomy (−3.3; 95% CI: −5.1, −1.5). When comparing the average change in PCS post-surgery, patients who underwent limited resection had a significantly higher PCS than those who underwent lobectomy, and the difference persisted at multivariate analysis. No significant differences between types of surgery were found in the change of MCS post-surgery (Table V).
TABLE V.
SF-12 Quality of Life Scores Pre- and Post-Surgery According to Type of Surgery Among Patients Who Underwent Thoracotomy
| Lobectomy (n = 70) |
Limited resection (n = 15) |
Limited resection versus lobectomy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusteda
|
Adjustedb
|
|||||||||||
| Mean | SD | 95% CI for mean | Mean | SD | 95% CI for mean | Parameter estimates | 95% CI | Parameter estimates | 95% CI | |||
| PCS | ||||||||||||
| Baseline | 49.4 | 6.6 | 48.4 | 6.0 | −0.51 | −3.78 | 2.78 | −0.36 | −3.63 | 2.91 | ||
| Follow-up | 46.1 | 9.2 | 47.7 | 8.1 | 3.18 | −0.91 | 7.27 | 3.21 | −0.57 | 6.99 | ||
| Difference (follow-up-baseline) | −3.3* | 7.5* | (−5.1, −1.5)* | −0.6 | 6.1 | (−4.0, 2.8) | 3.69* | 0.28* | 7.09* | 3.57* | 0.23* | 6.91* |
| MCS | ||||||||||||
| Baseline | 52.4 | 8.5 | 52.4 | 11.6 | −1.01 | −5.56 | 3.55 | −0.90 | −5.48 | 3.68 | ||
| Follow-up | 54.4 | 9.6 | 51.9 | 11.6 | −1.63 | −6.14 | 2.89 | −1.32 | −5.85 | 3.21 | ||
| Difference (follow-up-baseline) | 2.0 | 9.3 | (−0.2, 4.2) | −0.5 | 11.2 | (−6.7, 5.7) | −0.62 | −5.22 | 3.97 | −0.42 | −5.02 | 4.19 |
PCS, Physical Component Score; MCS, Mental Component Score.
Regression analysis comparing QoL score between limited resection and lobectomy (limited = 1).
Multivariate regression analysis comparing QoL score between limited resection and lobectomy after adjusting for sex, age, pack-years of smoking, ethnicity, and education.
Significant at P > 05.
DISCUSSION
The current study is one of the first to examine the impact on QoL of VATS as compared to thoracotomy among early stage IA lung cancer patients. Although the sample size is small, and the results should be considered preliminary and interpreted with caution, the data suggest that there is no clinically significant difference in change from baseline to post-surgery in overall physical health or mental health QoL between the two groups. This somewhat corroborates Rizk et al.’s findings that there is no difference between the two in terms of physical health QoL, although they found that mental health was consistently worse in the VATS group. However, the study by Rizk did not examine the changes from baseline to post-surgery according to type of surgery, but rather compared the baseline QoL scores between the two groups and the follow-up QoL scores between the two groups, separately [16].
Further, the current study was able to look at more subtle differences in QoL between the two groups, as QoL role functionality subscales were examined. These adjusted results indicated that the improvement in physical health role functioning from baseline to post-surgery among VATS patients significantly differed from the decrease in physical health role functioning among thoracotomy patients. Similarly, the increase in emotional health role functioning among VATS patients differed significantly from the slight decrease in emotional health role functioning among thoracotomy patients. It is possible that role functioning, which is specific to the impact of the physical or emotional correlates of the illness on the ability to accomplish tasks and participate in activities, is able to assess the more subtle differences that the two procedures have on QoL. It would be expected that VATS would have a more positive impact on QoL role functioning and garners greater patient acceptance largely due to less post-surgical pain as compared to thoracotomy [19].
Despite the small sample size, we were able to disentangle the effect of type of resection among those who underwent thoracotomy, something that was not done in previously published work [16]; we were able to assess that the extension of lung resection (lobectomy vs. limited resection) rather than type of surgical approach (thoracotomy vs. VATS) may be an important factor in determining physical QoL post-surgery. This novel, important aspect needs to be further explored in larger studies comparing both the extent of the resection and the surgical approach at the same time.
It was somewhat surprising that no differences were seen in the adjusted analyses regarding gender. Physical health QoL significantly decreased from pre- to post-surgery among both men and women, but this change was not significantly different when compared between the two. Previous lung cancer surgical studies have found women to generally fare worse than men in terms of post-surgical QoL [15,20], although the literature is somewhat mixed with some findings not supporting gender differences [14,21].
The current study findings should be interpreted with caution given the small sample size. Other limitations include the relatively wide window of follow-up time. However, analyses conducted with a narrower follow-up window (9–15 months; results not shown) revealed comparable findings. Further, the SF-12 is a general measure of QoL, not specific to cancer or lung cancer; the result is a measure that is inferior to other disease-oriented questionnaires used to assess QoL in cancer survivors, and is more susceptible to floor and ceiling effects. Also, conducting multiple comparisons using both overall and multiple subscale QoL scores as outcomes could result in chance findings. Future research that employs a prospective, longitudinal design will be useful in verifying these findings and understanding the chronicity of these effects on QoL. The study team, through the prospective design of the new Initiative for Early Lung Cancer Research on Treatment (IELCART) protocol, plans to collect prospective data from a large sample of early stage lung cancer patients in order to support or refute the current study findings with greater statistical power. This prospective study will also include a more expanded, comprehensive assessment of QoL.
Finally, given that this is an observational study in which randomization was not feasible, it is possible that multiple sources of bias existed within and between the two groups. However, the current study was able to adjust for many potential demographic differences between the two groups.
CONCLUSIONS
Although the current study findings must be considered preliminary and interpreted with caution, the findings underscore that surgeons cannot necessarily assume that VATS, a less invasive approach, will have an overall more positive impact on post-surgical QoL and that thoracotomy will have a more negative impact on post-surgical QoL. It is likely that there are some differences, given the study findings regarding role functioning, but these differences may be nuanced and more complicated than just overall improvement or worsening. The more important message of these findings is that QoL does change post-surgery, and the changes may not differ greatly by surgical approach but rather by extent of the resection. Surgeons and other lung cancer health care providers should consider having more in-depth conversations with early stage lung cancer patients regarding post-surgical QoL issues so that these patients, for whom mortality is less of an issue, feel better prepared for life post-surgery.
SYNOPSIS.
This study investigated whether there were Quality of Life (QoL) differences between thoracotomy and VATS patients among screening-diagnosed first primary pathologic stage IA non-small-cell lung cancer surgical patients from the I-ELCAP cohort. Multivariate analyses indicated that there was no difference in changes in QoL from pre-surgery to 1-year post surgery between the two groups. Physical and emotional role functioning significantly improved among VATS patients and worsened among thoracotomy patients. Among thoracotomy patients, a significant decrease in post-surgical physical QoL was observed only in those who underwent lobectomy, but not limited resection. Results must be interpreted with caution given the small sample size. Further research with larger sample sizes will seek to replicate these findings.
Acknowledgments
I-ELCAP Investigators are:
Mount Sinai School of Medicine, New York, NY: Claudia I. Henschke, Principal Investigator, David F. Yankelevitz, Rowena Yip, Dongming Xu, Mary Salvatore, Raja Flores, Andrea Wolf; Weill Cornell Medical College: Dorothy I. McCauley, Mildred Chen, Daniel M. Libby, James P. Smith, Mark Pasmantier; Nasser Altorki; Cornell University: A. P. Reeves; CBNS, City University of New York at Queens College, Queens, NY; Steven Markowitz, Albert Miller; Fundacion Instituto Valenciano de Oncologia, Valencia, Spain: Jose Cervera Deval; University of Toronto, Princess Margaret Hospital, Toronto, Canada: Heidi Roberts, Demetris Patsios; Azumi General Hospital, Nagano, Japan: Shusuke Sone, Takaomi Hanaoka; Clinica Universitaria de Navarra, Pamplona, Spain: Javier Zulueta, Juan de Torres, Maria D. Lozano; Swedish Medical Center, Seattle, WA: Ralph Aye, Kristin Manning; Christiana Care, Helen F. Graham Cancer Center, Newark, DE: Thomas Bauer; National Cancer Institute Regina Elena, Rome, Italy: Stefano Canitano, Salvatore Giunta; St. Agnes Cancer Center, Baltimore, MD: Enser Cole; LungenZentrum Hirslanden, Zurich, Switzerland: Karl Klingler; Columbia University Medical Center, New York, NY: John H.M. Austin, Gregory D. N. Pearson; Hadassah Medical Organization, Jerusalem, Israel: Dorith Shaham; Holy Cross Hospital Cancer Institute, Silver Spring, MD: Cheryl Aylesworth; Nebraska Methodist Hospital, Omaha NE: Patrick Meyers; South Nassau Communities Hospital, Long Island, NY: Shahriyour Andaz; Eisenhower Lucy Curci Cancer Center, Rancho Mirage, CA; Davood Vafai; New York University Medical Center, New York, NY: David Naidich, Georgeann McGuinness; Dorothy E. Schneider Cancer Center, Mills-Peninsula Health Services, San Mateo, CA: Barry Sheppard; State University of New York at Stony Brook, Stony Brook, NY: Matthew Rifkin; ProHealth Care Regional Cancer Center, Waukesha & Oconomowoc Memorial Hospitals, Oconomowoc, WI: M. Kristin Thorsen, Richard Hansen; Maimonides Medical Center, Brooklyn, NY: Samuel Kopel; Wellstar Health System, Marietta GA: William Mayfield; St. Joseph Health Center, St. Charles, MO: Dan Luedke; Roswell Park Cancer Institute, Buffalo, NY: Donald Klippenstein, Alan Litwin, Peter A. Loud; Upstate Medical Center, Syracuse, NY: Leslie J. Kohman, Ernest M. Scalzetti; Jackson Memorial Hospital, University of Miami, Miami, FL; Richard Thurer, Nestor Villazamar; State University of New York, North Shore-Long Island Jewish Health System, New Hyde Park, NY: Arfa Khan, Rakesh Shah; The 5th Affiliated Hospital of Sun Yat-Sen University, Zhuhai, China: Xueguo Liu; Mercy Medical Center, Rockville Center, NY: Gary Herzog; Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan: Diana Yeh; National Cancer Institute of China, Beijing, China: Ning Wu; Staten Island University Hospital, Staten Island NY: Joseph Lowry, Mary Salvatore; Central Main Medical Center: Carmine Frumiento; Mount Sinai School of Medicine, New York, NY: David S. Mendelson; Georgia Institute for Lung Cancer Research, Atlanta, GA: Michael V. Smith; The Valley Hospital Cancer Center, Paramus NJ: Robert Korst; Health Group Physimed/McGill University, Montreal, CA: Jana Taylor; Memorial Sloan-Kettering Cancer Center, New York, NY: Michelle S. Ginsberg; John Muir Cancer Institute, Concord CA: Michaela Straznicka; Atlantic Health Morristown Memorial Hospital, Morristown NJ: Mark Widmann; Alta Bates Summit Medical Center, Berkeley CA: Gary Cecchi; New York Medical College, Valhalla, NY: Terence A.S. Matalon; St. Joseph’s Hospital, Atlanta GA: Paul Scheinberg; Mount Sinai Comprehensive Cancer Center, Miami Beach, FL: Shari-Lynn Odzer; Aurora St. Luke’s Medical Center, Milwaukee WI: David Olsen; City of Hope National Medical Center, Duarte, CA: Fred Grannis, Arnold Rotter; Evanston Northwestern Healthcare Medical Group, Evanston, IL: Daniel Ray; Greenwich Hospital, Greenwich, CT: David Mullen; Our Lady of Mercy Medical Center, Bronx, NY: Peter H. Wiernik; Baylor University Medical Center, Dallas TX: Edson H. Cheung; Sequoia Hospital, Redwood City CA: Melissa Lim; Glens Falls Hospital, Glens Falls NY: Louis DeCunzo; Atlantic Medical Imaging, Atlantic City NJ: Robert Glassberg; Karmanos Cancer Institute, Detroit, MI: Harvey Pass, Carmen Endress; Rush University, Chicago IL: Mark Yoder, Palmi Shah; Building Trades, Oak Ridge TN: Laura Welch; Sharp Memorial Hospital, San Diego, CA: Michael Kalafer; Newark Beth Israel Medical Center, Newark NJ Jeremy Green; Guthrie Cancer Center, Sayre PA: James Walsh, David Bertsch; Comprehensive Cancer Centers of the Desert, Palm Springs CA: Elmer Camacho; Dickstein Cancer Treatment Center, White Plains Hospital, White Plains NY: Cynthia Chin; Presbyterian Healthcare, Charlotte NC: James O’Brien; University of Toledo, Toledo OH: James C. Willey.
Funding/sponsorship: This research was made possible by a generous gift from Sonia Lasry Gardner given in memory of her father, Moise Lasry, to support lung cancer research, outreach, and treatment.
Footnotes
DISCLOSURES
Dr. Henschke reports grants from FAMRI outside the submitted work and Dr. Henschke is a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on CT scans of the chest which are owned by Cornell Research Foundation (CRF). Since April 2009, Dr. Henschke does not accept any financial benefit from these patents including royalties and any other proceeds related to the patents or patent applications owned by CRF. In addition, Dr. Henschke is the President of the Early Diagnosis and Treatment Research Foundation; she receives no compensation for this service. Dr. Taioli is the President of the Science and Policy Institute; she receives no compensation for this service. Remaining co-authors have no conflicts of interest to disclose.
References
- 1.Buzaglo J, Gayer C, Mallick R, et al. Understanding the experience of living with non-small-cell lung cancer (NCLC): A qualitative study. J Community Support Oncol. 2014;2:6–12. doi: 10.12788/jcso.0010. [DOI] [PubMed] [Google Scholar]
- 2.Lehto RH. Identifying primary concerns in patients newly diagnosed with lung cancer. Oncol Nurs Forum. 2011;38:440–447. doi: 10.1188/11.ONF.440-447. [DOI] [PubMed] [Google Scholar]
- 3.Pozo CL, Morgan MA, Gray JE. Survivorship issues in patients with lung cancer. Cancer Control. 2014;21:40–50. doi: 10.1177/107327481402100106. [DOI] [PubMed] [Google Scholar]
- 4.Akin S, Can G, Aydiner A, et al. Quality of life, symptom experience and distress of lung cancer patients undergoing chemotherapy. Eur J Oncol Nurs. 2010;14:400–409. doi: 10.1016/j.ejon.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Le Corroller-Soriano AG, Bouhnik AD, Preau M, et al. Does cancer survivors’ health-related quality of life depend on cancer type? Findings from a large French national sample 2 years after cancer diagnosis. Eur J Cancer Care. 2011;20:132–140. doi: 10.1111/j.1365-2354.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee LJ, Chung CW, Chang YY, et al. Comparison of the quality of life between patients with non-small-cell lung cancer and healthy controls. Qual Life Res. 2011;20:415–423. doi: 10.1007/s11136-010-9761-y. [DOI] [PubMed] [Google Scholar]
- 7.Rauma V, Sintonen H, Rasanen JV, et al. Long-term lung cancer survivors have permanently decreased quality of life after surgery. Clin Lung Cancer. 2015;16:40–45. doi: 10.1016/j.cllc.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Sanders SL, Bantum EO, Owen JE, et al. Supportive care needs in patients with lung cancer. Psychooncology. 2010;19:480–489. doi: 10.1002/pon.1577. [DOI] [PubMed] [Google Scholar]
- 9.Kenny PM, King MY, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non-small-cell lung cancer. J Clin Oncol. 2008;26:233–241. doi: 10.1200/JCO.2006.07.7230. [DOI] [PubMed] [Google Scholar]
- 10.Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol. 2012;7:1148–1154. doi: 10.1097/JTO.0b013e318252cfef. [DOI] [PubMed] [Google Scholar]
- 11.Xie D, Deschamps C, Shen RK, et al. Bilobectomy versus lobectomy for non-small-cell lung cancer: A comparative study of outcomes, long-term survival, and quality of life. Ann THorac Surg. 2015;100:242–250. doi: 10.1016/j.athoracsur.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Ostroff JS, Krebs P, Coups EJ, et al. Health-related quality of life among early-stage, non-small cell, lung cancer survivors. Lung Cancer. 2011;71:103–108. doi: 10.1016/j.lungcan.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalheri V, Jenkins S, Cecins N, et al. Impairments after curative intent treatment for non-small cell lung cancer: A comparison with age and gender-matched healthy controls. Respir Med. 2015;109:1332–1339. doi: 10.1016/j.rmed.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14:37–44. doi: 10.12788/jcso.0205. [DOI] [PubMed] [Google Scholar]
- 15.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Analysis of longitudinal quality of life data in high-risk operable patients with lung cancer: Results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg. 2015;149:718–725. doi: 10.1016/j.jtcvs.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizk NP, Ghanie A, Meier H, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg. 2014;98:1160–1166. doi: 10.1016/j.athoracsur.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henschke CI. International early lung cancer action program: Enrollment and screening protocol: enrollment and screening protocol. 2014 [Google Scholar]
- 18.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scale and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: Video-assisted thoracic surgery versus thoracot-omy. Ann Thorac Surg. 1993;56:1285–1289. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 20.Chang NW, Lin KC, Lee SC, et al. The effect of gender on health-related quality of life and related factors in post-lobectomy lung-cancer patients. Eur J Oncol Nurs. 2015;19:292–300. doi: 10.1016/j.ejon.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Park S, Kang CH, Hwang Y, et al. Risk factors for postoperative anxiety and depression after surgical treatment for lung cancer. Eur J Cardio-Thorac Surg. 2016;49:e16–e21. doi: 10.1093/ejcts/ezv336. [DOI] [PubMed] [Google Scholar]


