Abstract
Both gestational cortisol exposure (GCE) and variability in postnatal environments can shape the later-life behavioral and endocrine outcomes of the hypothalamic-pituitary-adrenal (HPA) axis. We examined the influence of GCE and social play on HPA functioning in developing marmosets. Maternal urinary cortisol samples were collected across pregnancy to determine GCE for 28 marmoset offspring (19 litters). We administered a social separation stressor to offspring at 6, 12, and 18 months of age, during which we collected urinary cortisol samples and behavioral observations. Increased GCE was associated with increased basal cortisol levels and cortisol reactivity, but the strength of this relationship decreased across age. Increased social play was associated with decreased basal cortisol levels and a marginally greater reduction in cortisol reactivity as offspring aged, regardless of offspring GCE. Thus, GCE is associated with HPA functioning, but socially enriching postnatal environments can alter the effects associated with increased fetal exposure to glucocorticoids.
Keywords: fetal programming, social play, HPA axis, prenatal cortisol, cortisol reactivity, development, non-human primate, marmoset
INTRODUCTION
There is a robust link between glucocorticoid exposure during pregnancy and later-life outcomes in offspring including alterations in hypothalamic-pituitary-adrenal (HPA) axis activity, propensities for particular behavioral phenotypes, and vulnerabilities for adult diseases (Barker, 1990, 1998; Davis et al., 2007; Weinstock, 2008). An emerging model proposes that prenatal exposure to glucocorticoids (cortisol in primates; corticosterone in rodents) programs the fetus to adapt to the subsequent environmental demands, which may be signaled through changes in maternal physiology and behavior including the mother’s own activation of glucocorticoids in response to stressful stimuli (Harris & Seckl, 2011; Glover, O’Connor, & O’Donnell, 2010). The prenatal period is a time where offspring undergo massive ontogenetic changes, particularly brain development, and, consequently, offspring phenotypes are exceptionally vulnerable to changes in the maternal uterine environment (Charil, Laplante, Vaillancourt, & King, 2010). Cortisol concentrations naturally rise during pregnancy in both nonhuman primates and humans (Mustoe, Birnie, Korgan, Santo, & French, 2012; Sandman et al., 2006), and alterations in metabolism, efficacy of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), or increases in maternal stress, anxiety, or depression can affect the fetus’ exposure to glucocorticoids and later-life outcomes (Field, Diego, & Hernandez-Reif, 2006; Holmes et al., 2006). Given that glucocorticoids vary considerably among mothers during gestation, it appears that the effects of prenatal stress and other alterations in glucocorticoid exposure during pregnancy may alert fetuses to demands in their imminent environment (Glover, 2011). Importantly, the relationship between glucocorticoid exposure and later-life outcomes in offspring are evident when measuring maternal glucocorticoid levels directly via maternal plasma or amniotic fluid (Davis, Glynn, Waffarn, & Sandman, 2011; O’Connor, Bergman, Sarkar, & Sandman, 2012), or when using maternal stress or anxiety as proxy measures of glucocorticoids (Davis et al., 2007; O’Connor et al., 2005). Furthermore, early postnatal experiences, such as parental care and early-life adversity, can also play a major role in shaping developmental outcomes including HPA responsiveness (Birnie, Taylor, Cavanaugh, & French, 2013; Francis & Meaney, 1999; Laurent et al., 2013; Liu et al., 1997). Thus, the interaction between intra-uterine environments such as variation in glucocorticoid exposure and diverse postnatal experiences can mold the developmental trajectory of many endocrine and behavioral later-life outcomes.
Exposure to glucocorticoids can exert a long-lasting influence on normal HPA activity and the HPA response to stress. Adult male guinea pigs exposed to greater prenatal stress had a reduced basal and stress-induced plasma glucocorticoid concentrations, while females exhibited increased basal and stress-induced plasma glucocorticoid concentrations (Lingas & Matthews, 2001). In general, rats exposed to increased prenatal glucocorticoid exposure show HPA hyperactivity, that is, increase in circulating glucocorticoids, in response to stress (Barbazanges, Piazza, Le Moal, & Maccari, 1996; Weinstock, Poltyrev, Schorer-Apelbaum, Men, & McCarty, 1998); however, there are exceptions (Cannizzaro et al., 2006; Van den Hove et al., 2005). The HPA response to stress in rats appears to be sex specific, with females being more susceptible to the effects of prenatal programming on later-life HPA activity (Bhatnagar, Lee, & Vining, 2005; McCormick, Smyth, Sharma, & Meaney, 1995; Weinstock, 2007). Moreover, the effect of prenatal programming of later-life HPA activity is strongly linked to the timing of gestational stress, where the extent of perturbation of later-life HPA activity is often greatest when maternal stressors are imposed during the period of maximal fetal brain growth (Kapoor & Matthews, 2005). In primates, prenatal stress is associated with increased levels of ACTH and cortisol to both basal and stressed conditions (Clarke, Wittwer, Abbott, & Schneider, 1994) and increased basal cortisol levels following a dexamethasone (DEX) suppression in juvenile rhesus monkeys (Coe et al., 2003). Conversely, marmosets do not show an increase in basal cortisol following prenatal exposure to DEX (Hauser et al., 2008). Studies on humans have shown a similar programming-like effect of prenatal cortisol exposure on HPA activity. Increased maternal cortisol levels or maternal reports of high stress or anxiety during pregnancy were positively associated with levels of cortisol across the day in children at 4–10 years of age (Gutteling, de Weerth, & Buitelaar, 2005; O’Connor et al., 2005) and this association between maternal anxiety and children’s cortisol persists into adolescence (Van den Bergh, Van Calster, Smits, Van Huffel, & Lagae, 2008). Unlike with fetal programming in rodents, the effect of prenatal cortisol exposure on HPA activity in primates does not appear to be as strongly linked to the timing of stressful stimuli during gestation (or the data are mixed). However, in cases where fetal programming is linked to gestational timing in primates, early- or mid-gestation exposure appears to be the more sensitive period (Mustoe et al., 2012; Schneider, Roughton, Koehler, & Lubach, 1999).
The programming effects of glucocorticoids are not limited to HPA activity; behavioral and cognitive programming appears to occur as well. Offspring exposed to higher levels of gestational glucocorticoids generally show more reactive temperaments and slower cognitive and motor development. For example, juvenile rhesus monkeys exposed to higher gestational cortisol show decreased levels of exploratory behavior and attention, while exhibiting increases in emotionality, inactivity, and immature motor reflexes and motor impairments (Clarke & Schneider, 1993; Clarke, Soto, Bergholz, & Schneider, 1996; Coe et al., 2003; Schneider, 1992; Schneider et al., 1999). Marmosets show similar responses to artificially-elevated gestational cortisol exposure (DEX treatment), in that offspring born to DEX-treated mothers show more inactivity and a decrease in initiating social play with siblings and parents compared to controls (Hauser et al., 2008). Human infants and toddlers exposed to higher prenatal cortisol and/or maternal anxiety showed greater negative reactivity (Davis et al., 2007; de Weerth, van Hees, & Buitelaar, 2003), more behavioral agitation such as crying and deficits in attention (Gutteling, de Weerth, Willemsen-Swinkels, et al. 2005; Huizink, Robles de Medina, Mulder, Visser, & Buitelaar, 2002; O’Connor, Heron, Goldin, & Glover, 2003), and slower rates of behavioral recovery in response to stressful stimuli (Davis et al., 2011). Taken as a whole, the programming of prenatal glucocorticoid exposure can enduringly influence both later-life normative HPA functioning and individual HPA and behavioral responses to stressful stimuli.
The exact nature of the relationship between prenatal programming effects linked to gestational glucocorticoid exposure and later-life outcomes is unclear given the moderating influences of varying postnatal environments on these same outcome measures. Like prenatal stress and glucocorticoid exposure, poor or insecure mother-infant attachments, parental maltreatment, or early social deprivation influence the HPA response to stress (Capitanio, Mendoza, Mason, & Maninger, 2005; Dettling, Parker, Lane, Sebanc, & Gunnar, 2000; Gunnar & Donzella, 2002; Koch, McCormack, Sanchez, & Maestripieri, 2012; Tarullo & Gunnar, 2006), alter behavioral trajectories (Corcoran et al., 2012; Dettling, Feldon, & Pryce, 2002a; Dettling, Feldon, & Pryce 2002b; Suomi, 1997), and lead to vulnerabilities to mood and anxiety disorders in both human and non-human primates (reviewed in Heim & Nemeroff, 2001; Pryce et al., 2005). Importantly, in contrast to early social deprivation, some evidence shows that socially and physically enriching environments can offset or reverse the effects of prenatal glucocorticoid exposure or stress on later-life reactivity and behavior. In particular, peripubertal social and physical environmental enrichment reversed the effect of maternal separation on HPA and behavioral responses to stress in rats (Francis, Diorio, Plotsky, & Meaney, 2002; Morley-Fletcher, Rea, Maccari, & Laviola, 2003). Thus, an infant’s early social and physical environment has the potential to moderate the effects of fetal programming through gestational glucocorticoids. The extent to which early socially enriching environments may offset gestational programming of later-life neuroendocrine and behavioral reactivity to stressful stimuli is not as thoroughly explored, especially in non-human primates.
Marmosets are a highly social primate species that show sensitivity to early social environments and reach maturation quickly (Birnie et al., 2013; Dettling et al., 2002a,b; Pryce, Dettling, Spengler, Spaete, & Feldon, 2004). The development of HPA responses and stress reactivity to a social separation paradigm in marmosets are characterized by high inter-individual variability, but individual patterns of stress reactivity are highly stable within individuals across broad developmental stages, suggesting a “trait” like response to stressful stimuli (French et al., 2012). Consequently, marmosets serve as an exemplary nonhuman primate model for examining the effects of prenatal glucocorticoid exposure and varying social conditions during development on early adult outcomes in stress reactivity and behavior, and linking these early-life experiences with trait-like responses to stressful stimuli. One of the primary gaps in the literature is the investigation of natural variation in glucocorticoid exposure across gestation and sequelae of glucocorticoid exposure longitudinally in order to determine the extent and longevity of the fetal programming on both neuroendocrine and behavioral outcomes. Furthermore, it is unclear if enriching postnatal experiences moderate the influence of gestational cortisol exposure on later-life HPA and behavioral responses to stressful stimuli. Therefore, the thrust of this paper is to explore the relationship between variation in cortisol exposure across gestation and subsequent HPA response characteristics, and to assess whether variation in early social experiences (in this case, social play) can moderate the effects of glucocorticoid exposure. If gestational exposure to glucocorticoids alters early programming of the HPA axis, then we would expect elevated cortisol and greater behavioral agitation in response to a standardized stressor in marmoset offspring exposed to higher levels of gestational glucocorticoids. Moreover, if enhanced socially rich environments, by increased juvenile social play behavior, offset or attenuate the outcomes associated with increased prenatal cortisol exposure, then we expect individual marmosets who initiate and receive more social play will have an attenuated HPA and behavioral response to social stress beyond the potentially resultant early-life programming from the gestational glucocorticoid exposure. Finally, we seek to explore if the nature of any of these effects depends on the gestational timing of cortisol exposure, and the age and sex of the offspring.
METHODS
Subjects
The study included longitudinal data collected from 28 white-faced marmosets (Callithrix geoffroyi) born into the colony at the Callitrichid Research Center at the University of Nebraska at Omaha. Mothers (maternal age range: 2.22–9.13 years) were housed in large breeding enclosures with an unrelated adult male, and offspring were housed with family units including parents and siblings. Colony rooms were maintained at 19.7–22.1°C and on a 12:12 light-dark cycle. Wire-mesh enclosures varied in size depending on the number of offspring in the breeding group, with a minimum of 0.8 m3 per animal. Enclosures included branches, rope vines, nest boxes, and various enrichment items. All animals were fed twice each day, first at 0800–0900 hr and then later in the afternoon at 1300–1500 hr. The Institutional Animal Care and Use Committee at the University of Nebraska at Omaha approved all animal use procedures (IACUC 07-033-05-FC) and these procedures followed all ethical guidelines and principles endorsed by the International Society for Developmental Psychobiology. Further details of animal housing and husbandry have been previously reported (Schaffner, Shepard, Santos, & French, 1995).
Homecage Urine Collection From Pregnant Mothers and Offspring First-Void Samples
First-void urine samples from breeding females and offspring were collected using noninvasive collection techniques (French et al., 1996). One to three times a week, we collected samples between 0700 and 0830 hr by holding aluminum pans under a stream of urine in exchange for a valued food reward. We ensured only one individual’s urine was present in each pan, and the timing of urine collection coincided with the waking of marmosets. Urine samples were transferred into individual microcentrifuge tubes, and the samples were centrifuged at 5,000 rpm for approximately 2 min to separate the urine from any sediment contamination. The urine was transferred into a clean test tube and stored at −20°C until assayed. Urine sampling reflects total circulating hormones levels since the last time the bladder was emptied, and first-void urine samples are an accurate and readily available representation for the composite daily average of circulating hormones (French et al., 1996).
Homecage Play Behavior Observations
We collected play behavior data on offspring between the ages of 5–10 months. This time period coincides with the juvenile period in marmosets in which a well-developed and highly variable repertoire of play behaviors is exhibited (Yamamoto, 1993). We collected two to five 20-min observations per week for each offspring. A trained observer sat approximately one meter from the home cage with a laptop computer and recorded behaviors with Observer software (Noldus Technologies, Houston, TX). All-occurrences of behaviors for play-bout frequencies were scored among all family members. For each play bout, we scored three components of rough-and-tumble play: initiate play, receive play, and overall play (composite of initiate and receive). Successful play initiations were combined to give a composite “initiate play” score, and the animal that engaged in rough- and-tumble play with the play initiator was given a “receive play” score. Overall, social play was the combined initiated and received play scores. A new play bout was scored only if 5 s elapsed without a play bout between interactants. See Birnie, Hendricks, Smith, Milam, and French (2012) for a full ethogram of play behaviors.
Social Separation Stressors
Marmosets underwent social separation stress challenges at 6, 12, and 18 months of age. Data for the current study were obtained from the same population of animals described in previous reports on stress reactivity from our lab (Birnie et al., 2013; French et al., 2012). First-void urine samples were collected on the day prior to, and immediately before separation on the day of the stressor manipulation. At 0900 hr, the marmoset was removed from its home cage and placed in a small wire mesh separation cage measuring 0.5 m3. Separation cages were placed in a separate, quiet room some distance away from the home cage. These cages were furnished with a food dish and a water bottle filled with diluted apple juice to facilitate urination. Animals remained in the separation cage until 1700 hr (total of 8 hr of separation). This type of social separation has been shown to induce a measurable and significant increase in urinary glucocorticoid excretion compared to normal circadian variation (Johnson et al., 1996; Rukstalis & French, 2005; Smith & French, 1997). A final first-void urine sample was collected between 0700 and 0830 hr on the day after the social separation paradigm.
During the social separation, we attempted to collect urine from the isolated marmoset every hour from 1000 to 1700 hr. Clean polyethylene sheets were placed underneath the separation cage, and sheets were replaced after each hourly collection. Not all marmosets provided samples at each hourly interval, but samples were collected on at least every 2-hr interval for all marmosets.
During the first 60 min of the social separation challenge, animals were observed by trained technicians to evaluate behavioral responses to the initial separation challenge. Behavioral measures considered in this study included locomotion (the frequency the individual moved across the six sides of the isolate cage), and cage manipulations (the frequency the individual grabbed, bit, or manipulated in any way parts of the cage, or items connected or near to the cage, such as the plastic lining, food trays, or water bottles). Increases in these behaviors are associated with increases in stress or anxiety associated with the social separation challenge (French et al., 2007).
Pregnanediol-3-Glucuronide (PdG) Enzyme Immunoassay (EIA)
Determination of conception and gestational timing was evaluated by monitoring excreted levels of the progesterone metabolite, PdG, in breeding females. Urinary PdG levels were monitored by EIA following the protocol established for marmosets (for details, see French et al., 1996; Mustoe, Jensen, & French, 2012). Inter-assay coefficients of variation (CV), determined from high and low concentration pool samples ran on each plate, were 22.0% and 6.1%, respectively. Intra-assay CVs for the high and low pools 28.0% and 6.8%, respectively. Variation in fluid intake and output was indexed by measuring concentrations of urinary creatinine (Cr). The creatinine assay utilized a modified Jaffe end-point assay, previously described and validated for use in marmosets (French et al., 1996). Pregnancy was defined as PdG concentrations >10 µg/mg Cr for a minimum of 30 days, and the estimated day of conception was defined as the first sample proceeding the beginning of the 30-day consecutive samples of PdG concentrations >10 µg/mg Cr (Mustoe et al., 2012).
Cortisol EIA
We measured urinary cortisol concentrations in samples collected from pregnant mothers in their homecage and from samples collected from offspring during social separation challenges via an EIA previously developed and validated for marmoset urine (Smith & French, 1997). Microtiter plates were coated with rabbit anticortisol antibody and 50 µl of standard and urine sample (diluted 1:6,400 in distilled, deionized water) was added. Standards were serially diluted in distilled and deionized water, with a lower-bound sensitivity of 7.8 pg/well. Labeled cortisol (horseradish peroxidase, HRP) was added to each well, and the plates were incubated for 2 hr. We separated free from bound hormone in each well by washing the plate four times with saline-buffered detergent solution (Tween 20; Sigma Chemicals, St. Louis, MO) and then added 100 µl of substrate (ABTS and H2O2) and allowed the plate to develop until the B0 wells reached an optical density of approximately 1.0. The sample concentration was determined by interpolating optical densities with a four-parameter sigmoidal fit function. Inter-assay coefficients of variation (CV), determined from high and low concentration pool samples ran on each plate, were 12.28% and 14.90%, respectively. Intra-assay CVs for the high and low pools 8.94% and 9.78%, respectively. To minimize procedural variability, all samples for a single individual were assayed at the same time whenever possible. As with PdG, urinary cortisol concentrations in each sample were adjusted for variable fluid intake and output by correcting for urinary creatinine concentration.
Gestational Cortisol Exposure (GCE)
First-void urine samples were collected two to five times per week and first and third trimester durations in pregnant females were determined by individual pregnancy gestation length divided into thirds where pregnancy and conception was determined using the PdG EIA procedure described above. Cortisol concentrations for first-void urine samples were determined using the Cortisol EIA procedure discussed above. Thus, GCE for the first and third trimesters were estimated by the average cortisol concentrations across the complete duration of the first and third trimester for each pregnancy.
Data Analysis
Overview of Model
Multi-level modeling (MLM) was used to assess the relationship of GCE, juvenile social play, and offspring sex on HPA reactivity across developing marmoset offspring. MLM functions similarly to traditional hierarchical regression, but one of the primary advantages of MLM over traditional hierarchical regression is the ability to account for non-independence of longitudinal data (Raudenbush and Bryk, 2002). Non-independence of data in this study results from the same individuals measured repeatedly across time and age. Specifically, cortisol data (the outcome or dependent variable) is measured across time during the social separation challenge (three first void urine samples across three consecutive days, and 8 hr of urine samples across the separation challenge on Day 2), and across age (social separation challenge occurred at 6, 12, and 18 months of age). We were interested in whether basal cortisol levels and levels of cortisol reactivity (nsamples = 865) were influenced by time (ntimepoints = 924), age (nmarmosets × age points = 84), and, most importantly, of individual offspring (nmarmosets = 28). Factors for individual marmosets included offspring sex, offspring GCE, and the amount of juvenile social play between 5 and 10 months of age. Consequently, these data are represented in three levels: Cortisol (the dependent variable) is accounted for by both the linear and curvilinear (time2) change in time during the social separation challenge (level 1); maturation or change in age from 6, 12, and 18 months postnatal (level 2); and offspring sex, GCE, and levels of juvenile social play (level 3). Information about higher levels, such as individual differences in levels of exposure to cortisol during gestation and levels of early juvenile social play were used to predict the intercept (individual levels of basal cortisol) and slope (individual levels of cortisol reactivity), that is, change in cortisol reactivity during the stressor and change in cortisol levels across age. More specifically, we evaluated whether GCE or juvenile social play predicted HPA activity, that is, basal cortisol intercept and cortisol reactivity slope, and the maturation (change across age) of these factors across the developing marmoset. It is worth noting that multiple offspring from the same litters were analyzed at the individual level rather than the litter level to account for greater individual postnatal variation in juvenile social play and HPA characteristics. Twin offspring from the same litter were treated as having identical GCE. All variables in the MLM models other than time are grand centered. Finally, correlations between GCE and individual behaviors during the social separation challenge such as locomotion and cage manipulations and juvenile social play were tested. Only first and third trimester analyses were included to emphasize the notion of early or later gestational effects on postnatal outcomes. All multi-level analyses were conducted using HLM version 6.08, and bivariate correlations were calculated using SPSS 19.0.
Justification of Model
The unconditional model, where no variables were regressed on urinary cortisol concentrations following the social separation challenge, resulted in an intra-class correlation wherein 67.2%, 15.2%, and 17.6% of the variance in cortisol concentrations was attributable to variability over time (level 1), variability across age (level 2), and variability between individual factors including prenatal cortisol exposure, juvenile social play, and offspring sex (level 3), respectively. This highlights the sources of variation in cortisol, and the final estimation of variation in cortisol concentrations following the social separation challenge resulted in a significant amount of variability left to account for in differences in maturation of the marmoset across age ( , p < .001) and differences in basal cortisol concentrations and cortisol reactivity explained by GCE, juvenile social play, and offspring sex ( , p < .001). This indicates that variations in GCE, juvenile social play, and offspring sex are related to basal cortisol and cortisol reactivity above and beyond variance accounted for by the change in cortisol over the duration of the stressor (time) and marmoset age alone (time of the stressor: 6, 12, or 18 months). Cortisol concentrations significantly increased in response to the social separation stressor (β’s > .95, all p’s < .001) indicating the social separation paradigm elicits a robust glucocorticoid stress response. Basal concentrations of cortisol and cortisol reactivity decreased across age (β’s > .29, all p’s < .003). The change from pre- to post-stressor basal concentrations of cortisol were positively associated with cortisol reactivity (β = 0.08, p < .001). However this change was not significantly accounted for by variance in GCE, juvenile social play, and offspring sex ( , p > .50), so pre- and post-basal concentrations were treated as an overall basal cortisol concentration. A summary of the normative cortisol response to the social separation challenge and the development of stress reactivity across age are described in French et al. (2012).
RESULTS
Association Between GCE and HPA Activity
First Trimester GCE
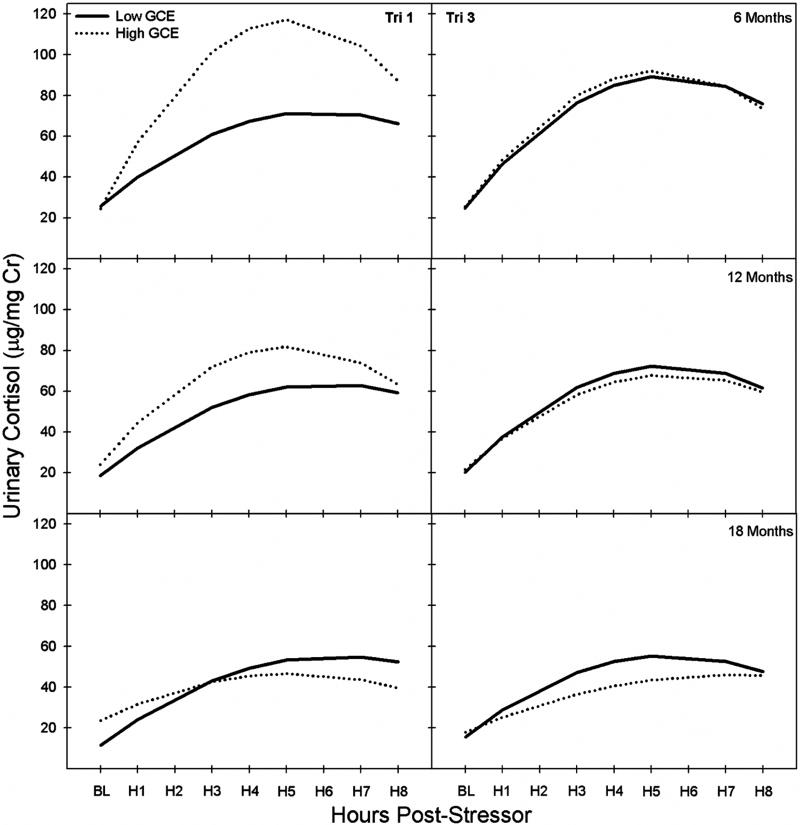
Variation in marmoset GCE affected multiple components of HPA function in prepubertal, peripubertal, and postpubertal marmosets. Overall, first trimester GCE predicted differences in cortisol concentrations above and beyond the effects of time, age, and sex ( , p = .001). Higher GCE during the first trimester was associated with elevated offspring basal cortisol concentrations (β = .23, p < .001), and increased offspring cortisol reactivity, that is, change in cortisol over the duration of the stressor (linear: β = .24, p = .02; curvilinear: β = −.18, p = .02; see Fig. 1). The strength of the association between GCE and cortisol reactivity during the stressor decreased significantly across age (linear: β = −.20, p = .01; curvilinear: β = .14, p = .05; see Fig. 1), and these data suggest that the relationship between gestational cortisol and later-life HPA activity weakens throughout development. Overall, increased first trimester GCE is associated with increased basal cortisol concentrations, increased cortisol reactivity to stressful stimuli, and greater reduction in cortisol reactivity across developing offspring.
FIGURE 1.
Modeled graph of the association between 1st and 3rd trimester (Tri) gestational cortisol exposure (GCE) on marmoset offspring’s pre- and post-stressor basal (BL) and cortisol (µg/mg Cr) reactivity, that is, change in urinary cortisol over time in H (hours), at 6, 12, and 18 M (months) of age after controlling for offspring sex and individual differences in HPA activity across age and time. High and low GCE reflect modeled cortisol reactivity change over time for upper and lower quartiles of GCE.
Third Trimester GCE
Third trimester GCE did not predict differences in cortisol concentrations above and beyond the effects of time, age, and sex ( , p > .50), suggesting that third trimester GCE was not a good predictor of HPA functioning. Third trimester GCE was not associated with offspring basal concentrations of cortisol in marmosets or with changes in cortisol reactivity during the social separation (β’s < .11, all p > .32). As with first trimester GCE, there was a decrease in the association between third trimester GCE and offspring cortisol reactivity across age (β = −.10, p = .05), but this effect size was modest, and the overall association between late gestational cortisol and cortisol reactivity was not significant. It appears that the association between GCE and later-life basal cortisol concentrations and cortisol reactivity is stronger during first trimester GCE than third trimester GCE (Fig. 1 and Tab. 1).
Table 1.
Association Between Offspring GCE and Normative and Stress-Induced HPA Activity Across Development
| Predictor | 1st Trimester | 3rd Trimester | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| β | b | SE | t-Ratio | β | b | SE | t-Ratio | |
| Basal CORT | ||||||||
| Sex | 0.07 | 3.32 | 2.34 | 1.42 | −0.01 | 0.48 | 2.65 | 0.18 |
| GCE | 0.24 | 0.54 | 0.11 | 4.70** | −0.06 | −0.002 | 0.04 | −0.04 |
| Basal CORT across age | ||||||||
| Sex | −0.06 | −0.73 | 0.40 | −1.84ψ | −0.04 | −0.77 | 0.41 | −1.89ψ |
| GCE | −0.10 | 0.02 | 0.02 | 0.76 | −0.03 | 0.002 | 0.005 | 0.44 |
| CORT reactivity | ||||||||
| Linear | ||||||||
| Sex | 0.02 | 0.48 | 3.31 | 0.15 | −0.06 | −1.69 | 3.45 | −0.48 |
| GCE | 0.24 | 0.40 | 0.15 | 2.61* | −0.11 | −0.04 | 0.04 | −1.01 |
| Curvilinear | ||||||||
| Sex | 0.01 | 0.02 | 0.37 | 0.04 | 0.06 | 0.22 | 0.38 | 0.59 |
| GCE | −0.18 | −0.04 | 0.02 | −2.45** | 0.08 | 0.004 | 0.004 | 0.90 |
| CORT reactivity across age | ||||||||
| Linear | ||||||||
| Sex | −0.01 | −0.05 | 0.39 | −0.17 | 0.04 | 0.22 | 0.43 | 0.52 |
| GCE | −0.20 | −0.07 | 0.02 | −2.72** | −0.10 | −0.01 | 0.003 | −2.08* |
| Curvilinear | ||||||||
| Sex | −0.01 | −0.001 | 0.04 | −0.06 | −0.04 | −0.03 | 0.05 | −0.53 |
| GCE | 0.14 | 0.006 | 0.003 | 2.09* | 0.10 | 0.001 | 0.001 | 1.69 |
Note: t-Ratios are of unstandardized coefficients. SE, standard error.
p < .10,
p < .05,
p < .01 and are two-tailed.
GCE refers to gestational cortisol exposure, and CORT refers to cortisol. Model examined the relationship between GCE and offspring sex, on different HPA parameters.
Offspring Sex Differences
There were no major sex differences in the relationship between GCE and HPA activity. Offspring sex does not predict differences in cortisol concentrations above and beyond the effects of time and age ( , p > .50). There was a trend for females to show a stronger reduction in basal cortisol across development than males (β = −.03, p = .06). However, this relationship is not significantly strengthened when controlling for both first and third trimester GCE (first: β = −.06, p = .08; third: β = −.04, p = .07). Overall, when controlling for the effect of GCE, there was no association between sex and marmosets’ cortisol reactivity in response to the stressor across development.
Association Between GCE and Behavioral Phenotypes
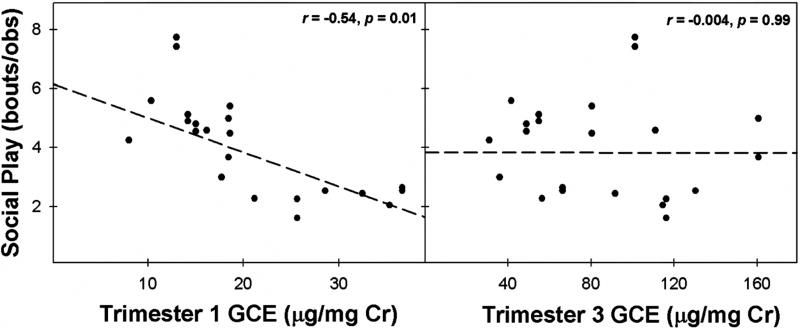
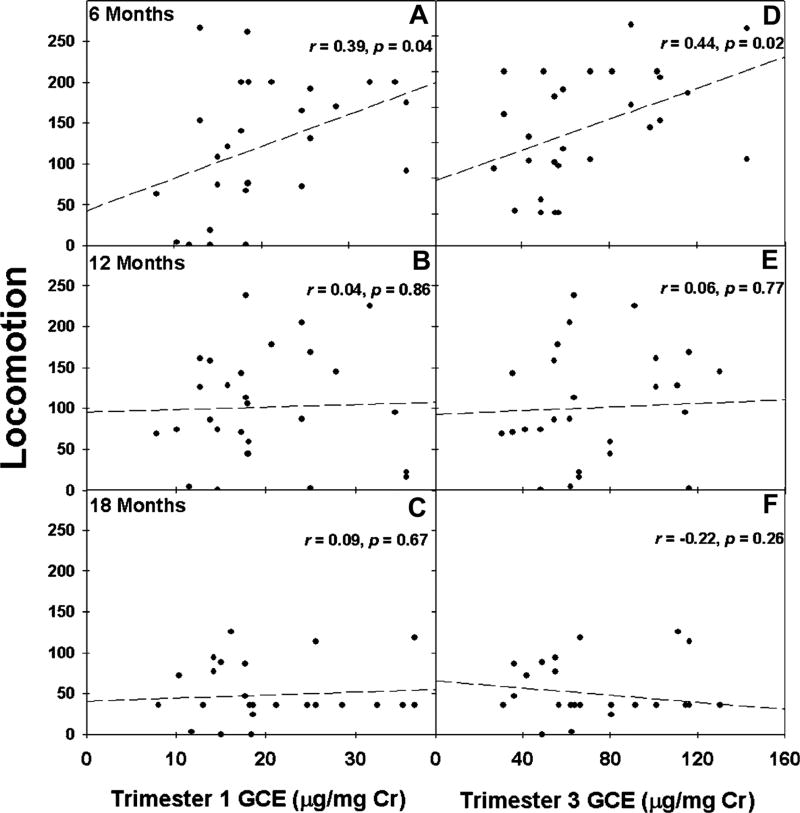
First trimester GCE was negatively correlated with offspring levels of juvenile social play, while third trimester GCE was not significantly correlated with offspring levels juvenile social play (Fig. 2). This relationship, like the relationship between gestational cortisol and HPA activity, shows that first trimester GCE is more strongly associated with juvenile social play in marmosets than third trimester GCE. First trimester GCE is positively correlated with locomotion behaviors at 6 months of age but not at 12 or 18 months of age (Fig. 3a–c). Likewise, third trimester GCE was positively associated with locomotion at 6 months of age but not 12, or 18 months of age (Fig. 3d–f). Conversely, cage manipulations during the social separation stressor at any age were not significantly associated with both first and third trimester GCE (all r’s < .21, p’s > .28). The relationship between GCE and behavioral responses to the social separation challenge appear early in marmoset maturation (6 months) and only for locomotive behaviors. These data are aligned with the endocrine data showing that first trimester GCE is associated with cortisol activity, especially for prepubertal marmosets. However, unlike for patterns of cortisol activity, the patterns of behavioral responses during the social separation stressor are not specifically associated with the timing of GCE.
FIGURE 2.
Association between first and third trimester gestational cortisol exposure (µg/mg Cr) (GCE) and levels of social play (number of play bouts/observation) in individual juvenile marmosets.
FIGURE 3.
Association between first and third trimester gestational cortisol exposure (µg/mg Cr) (GCE) with individual locomotion behavioral responses (frequency of locomotion) during the social separation stressor at 6, 12, and 18 months of age. A–C reflect first trimester GCE and D–F reflect third trimester GCE across 6, 12, and 18 months of age.
Association Between Juvenile Social Play and HPA Activity
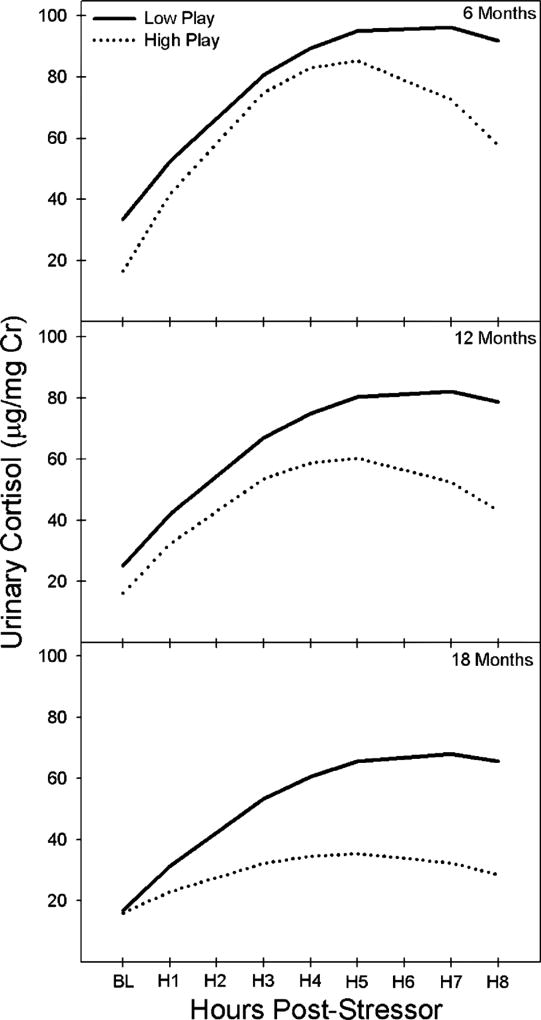
Juvenile social play significantly affected normative HPA activity and HPA responses to stress throughout development. Variation in juvenile social play accounted for differences in HPA functioning above and beyond the effects associated with GCE ( , p = .04), indicating that social play is an important predictor of HPA parameters. Specifically, marmosets who engaged in higher social play showed decreased basal concentrations of cortisol (β = −.18, p = .02), and showed a marginally greater reduction in cortisol reactivity as they aged (β = −.19, p = .08; Fig. 4 and Tab. 2). In light of the finding that the relationship between GCE and cortisol reactivity decreases significantly across development, these data suggest a potential marginal attenuation in stress-induced HPA activity later in development at 12 and 18 months of age for marmosets who show high juvenile social play. For individual marmosets in the highest and lowest quartiles, the relationship between GCE and cortisol reactivity is marginally reduced across age, where the difference in cortisol reactivity is greatest in 6-month-old marmosets. Conversely, the relationship between juvenile social play and cortisol reactivity is augmented across age after controlling for GCE, where the difference in cortisol reactivity is greatest in 18-month-old marmosets. These data suggest that early social play is an important variable in the shaping of adolescent HPA development, especially when accounting for GCE as social play significantly improves the overall model prediction above and beyond the effects of GCE.
FIGURE 4.
Modeled graph of the association between levels of juvenile social play (number of play bouts/observation) on marmoset offspring’s pre- and post-stressor basal (BL) and cortisol (µg/mg Cr) reactivity, that is, change in cortisol over time in H (hours), at 6, 12, and 18 M (months) of age after controlling for, GCE, offspring sex, and individual differences in HPA activity across age and time. High and low play reflect modeled cortisol reactivity change over time for upper and lower quartiles of levels of juvenile social play.
Table 2.
Association Between Juvenile Social Play and GCE on Normative and Stress-Induced HPA Activity Across Development
| Predictor | Final Model | |||
|---|---|---|---|---|
|
| ||||
| β | b | SE | t-Ratio | |
| Basal CORT | ||||
| Sex | −0.02 | −0.09 | 2.83 | −0.03 |
| GCE | 0.13 | 0.27 | 0.16 | 1.72ψ |
| Play | −0.18 | −1.61 | 0.63 | −2.57* |
| Basal CORT across age | ||||
| Sex | −0.07 | −0.23 | 0.41 | −0.55 |
| GCE | −0.10 | 0.06 | 0.03 | 2.00ψ |
| Play | −0.02 | 0.24 | 0.12 | 2.05ψ |
| CORT reactivity | ||||
| Linear | ||||
| Sex | 0.01 | 0.32 | 4.95 | 0.06 |
| GCE | 0.23 | 0.39 | 0.28 | 1.41 |
| Play | −0.01 | −0.07 | 1.19 | −0.06 |
| Curvilinear | ||||
| Sex | −0.04 | −0.12 | 0.53 | −0.24 |
| GCE | −0.23 | −0.05 | 0.03 | −1.61 |
| Play | −0.08 | −0.07 | 0.14 | −0.48 |
| CORT reactivity across age | ||||
| Linear | ||||
| Sex | −0.10 | −0.55 | 0.37 | −1.50 |
| GCE | −0.31 | −0.10 | 0.04 | −2.95** |
| Play | −0.19 | −0.23 | 0.13 | −1.79ψ |
| Curvilinear | ||||
| Sex | 0.07 | 0.05 | 0.05 | 0.97 |
| GCE | 0.22 | 0.01 | 0.004 | 2.46* |
| Play | 0.15 | 0.03 | 0.02 | 1.51 |
Note: t-Ratios are of unstandardized coefficients. SE, standard error.
p < .10,
p < .05,
p < .01 and are two-tailed.
GCE refers to gestational cortisol exposure, and CORT refers to cortisol. Model examined juvenile social play, GCE, and offspring sex on different HPA parameters.
DISCUSSION
The findings presented here document how naturally occurring variation in exposure of marmoset fetuses to cortisol of maternal origin across gestation is associated with multiple features of endocrine and behavioral development. Specifically, we found that marmosets with increased first trimester GCE exhibited increased basal cortisol concentrations and higher cortisol reactivity in response to a social separation stressor, and this relationship between first trimester GCE and cortisol reactivity weakened across development. Moreover, this study is the first to indicate that this gestational cortisol programming of HPA activity in marmosets is potentially attenuated by increased participation in juvenile social play after controlling for the effects of time, age, sex, and GCE. Marmosets that engaged in increased levels of social play between 5 and 10 months of age show decreased basal concentrations in cortisol and a marginally greater reduction of the cortisol response to the social separation stressor as the marmosets develop. Lastly, our findings highlight that the relationship between GCE and later-life HPA outcomes appear more strongly linked to variation in maternal cortisol in early gestation rather than late gestation.
Gestational Cortisol Exposure and Later-Life HPA Activity
It is expected that basal and reactive functioning of the HPA axis would be sensitive to variation in cortisol during pregnancy. The “fetal programming hypothesis” posits that differences in behavioral and health outcomes can be attributed to differences in fetal environments (Barker, 1990, 1998). It is likely that intrauterine influences on the developing fetus would serve as an adaptive preparation for the fetus’ subsequent environment and development. Since cortisol is an end-product of the HPA response to stress and arousal, mothers who exhibit increased cortisol during pregnancy are more likely to be mothers in environments where increased arousal, anxiety, and/or stress are more frequently experienced. Consequently, the more arousing or stressful the environment is during the pregnancy, the more likely a reactive HPA system may be beneficial for offspring. The effects associated with increased cortisol exposure or stress during pregnancy, such as increased anxiety, attentional distractions, impulsivity, and altered HPA functioning could serve adaptive functions in highly stressful or arousing environments, such as increased vigilance, alertness, or sensitivity to dangerous or stressful signals (reviewed in Glover, 2011; Matthews, 2002). Here, we reveal that marmoset mothers that showed greater increases in cortisol during early gestation were more likely to have offspring that showed greater basal cortisol levels and greater reactive cortisol responses to stressful stimuli. Furthermore, we would additionally expect alterations in behavioral phenotypes to match the reactive endocrine responses. First, we found a relationship between increased first trimester GCE and decreased social play. Second, we found a relationship between increased overall GCE and increased stressor-induced locomotion. Together, these data suggest that marmosets’ GCE are associated with behavioral phenotypes typical of a more anxious and reactive manner, at least early in life.
We found that endocrine responses to stress were only associated with variation during first trimester, while the behavioral response to stressors (locomotion) was associated across the entire gestation but only in young marmosets and not adolescent marmosets. Previous research has linked early GCE to human infant behavioral responses and later GCE to infant cortisol responses (Davis et al., 2011; Davis and Sandman, 2010). Our findings suggest that later-life marmoset basal cortisol concentrations and cortisol reactivity are more sensitive to variation in first trimester GCE where sensitivity to alterations in brain development and organization is higher. Conversely, behavioral phenotypes like social play and locomotion responses to the stressor were also correlated with early GCE, but, also, later GCE (for locomotive behaviors in young marmosets). However, this effect is less permanent in that this relationship does not persist into adolescence. Given the varying levels of cortisol exposure, the nature of the gestational stressors, species differences in gestational physiology, length of gestation, and timing of key developmental milestones, the effect of specific timing on GCE on postnatal outcomes can range considerably, but in many cases, these effects are malleable based on the quality of postnatal social interactions.
Postnatal Reprogramming of HPA Activity
Though marmoset’s normative and stress-induced HPA function was associated with increased GCE, it remains foremost to consider that postnatal developmental plasticity may play a pivotal role in the overall development trajectory of offspring. First, fetal programming may not be a completely satisfactory conclusion without evidence that the effects on offspring HPA functioning persist into adolescence and adulthood. Given that the normative HPA functioning undergoes considerable maturation postnatally (French et al., 2012; Gunnar, Brodersen, Krueger, & Rigatuso, 1996; Pryce, Palme, & Feldon, 2002), firm conclusions of postnatal effects attributed to GCE potentially underestimate the influences associated with early social and physical environments, especially for adolescent and early adulthood behavioral and endocrine phenotypes. Our data support this conclusion, as the association between GCE and HPA reactivity significantly declines from 6-month-old marmosets (juvenile) to 18-month-old marmosets (young adults). This suggests that either the first trimester GCE programming of HPA activity does not persist into adolescence, and is therefore not permanent, or environmental factors, such as social interactions, may reprogram the HPA activity independently of GCE.
Juvenile social play in marmosets appears to play an important role in adolescent HPA activity regardless of individual GCE. Marmosets’ patterns of basal HPA activity are significantly associated with their early social environment (social play) and their change in HPA responses to stressors across development are marginally associated with their early social environment (social play). This is congruent with other findings that early social environments may also program offspring HPA and behavioral responses to stressful stimuli (Green, Barnes, & McCormick, 2012; Liu et al., 1997). Specifically, adolescent rats that were prenatally stressed and received physical environmental enrichment showed increased play and a reduced glucocorticoid response to stressful stimuli, while rats that were not prenatally stressed did not show a reduction in glucocorticoid reactivity, though, interestingly, they still showed an increase in social play (Morley-Fletcher et al., 2003). Moreover, Francis et al. (2002) showed that only rat infants who were separated from their mothers for periods postnatally were positively impacted by peripubertal environmental enrichment, while rats that did not receive maternal deprivation were relatively unaffected. This suggests that enriching environments are effective at alleviating heightened HPA reactivity, such as instances commonly observed following high GCE or deprived early social environments. Thus, in both rats and non-human primates, environmental enrichment such as toys and social play may operate as important moderating variables in either attenuating or even reversing the fetal programming associated with increased gestational glucocorticoids, and these, themselves, can have potentially long-lasting changes on HPA and behavioral outcomes.
The response to stressors in rats and primates is intimately tied to social play. This relationship appears to be somewhat paradoxical though. In some cases, immediately following an acute stressor play levels in adolescent rats are completely suppressed, but in cases following repeated restraint stressors, the negative impact of stress on play is only short-term following termination of the stressor (Klein, Padow, & Romeo, 2010). Conversely, play can aid in the response to stressors. Specifically, marmoset scratching, a behavioral indicator of stress or agitation, was reduced following increased play, while levels of play remained unchanged with or without scratching (Norscia & Palagi, 2011). This finding suggests that play reduces behavioral indicators of stress more than do the onset of stressors influence long-term play. Moreover, when play is deprived during adolescence, rats show decreased social activity as adults and their corticosterone levels remained elevated longer following social defeat (van den Berg et al., 1999). The juvenile to adult transition appears to be an especially sensitive period for the development of stress reactivity (Romeo, 2010). Our findings support this notion, since, in relation to social play, it is the marmosets during and following puberty that show the greatest differences in HPA activity. Because play seems to play an important role in countering the stress response, increased social play during this sensitive period may condition the HPA to be less reactive to typical or mild stressors.
It is important to consider whether gestational and early postnatal programming affect offspring HPA activity and behavior similarly or differentially. In this study, we found that increased GCE was associated with increases in both stress-induced HPA activity and locomotion in young marmosets, and at this early stage, there appears to be a co-activation of GCE on HPA and behavioral activity. Later in development, however, increased GCE was still associated with heightened HPA activity, but no longer had an effect on stressor-induced behaviors. While overall activation of behavioral and endocrine responses to the social separation challenge are to some extent independent, (Taylor, Mustoe, & French, 2013), we documented fetal programming on both stressor-induced locomotive behavior and endocrine responses for 6-month-old marmosets. However, the effect of GCE on behavioral responses to stressors is much weaker than its effect on endocrine response to stressors, and weakens further across development. This difference educes the nature of the relationship between environmental influences such as the social environment or endocrine influences such as puberty and their alteration on the trajectory of HPA development. While GCE and the development of the HPA stressor response system most likely use similar mechanisms, behavioral adaptations are more sensitive to environmental and experiential influences (Coe, Glass, Wiener, & Levine, 1983; Levine, Wiener, & Coe, 1993). Furthermore, locomotive behaviors in response to a stressor do not stabilize until puberty in marmosets (Taylor et al., 2013), and this suggests that other behavioral adaptations, like engaging in play with others, could play an important role in modifying responses to stressors. While more work is essential to uncover the specific relationship between postnatal social environments and behavioral responses to stressors, factors like gestational programming, postnatal influences, and changing behavioral repertoires may potentially reflect three different adaptation strategies. First, gestational programming provides a biological/organizational adaptation to the environment; second, positive social interactions modify stress reactivity to regulate social and physical interactions with the environment; and third, behavioral adaptation provides a response strategy via learning which may be tailored to specific situations and experiences.
CONCLUSIONS
These findings are among the first to document the relationship between natural variation of cortisol exposure across the entire pregnancy with behavioral and endocrine outcomes both before and during adolescence in non-human primates. Having multiple time points of both GCE and longitudinal developmental outcomes is a primary strength of this study. In addition, the use of a non-human primate model of a highly social species such as the marmoset can provide valuable insight as to the degree and nature of the moderation of early-life social experiences on both endocrine and behavioral later-life outcomes. However, some limitations are worth mentioning. The measures of GCE are indirect, and the degree to which the cortisol influenced fetal and placental physiology was not tested. Additionally, other postnatal sources of cortisol delivery to the offspring, such as lactation have been shown to influence behavioral outcomes (Hinde, 2013). Finally, the interdependence of the offspring’s relationship with their mothers, fathers, siblings, and other social partners, which may influence the response to arousing stimuli (Birnie et al., 2013; Hennessy, Kaiser, & Sachser, 2009; Parker & Maestripieri, 2011), or many other sources of positive social interactions, were not accounted for directly in this analysis. The nature of these limitations warrants further investigation. Here, we suggest that the adaptability of HPA functioning can be viewed as resulting in both positive and negative outcomes, but, importantly, the adaptability should be viewed in the context of the offspring’s current environmental demands, and HPA functioning, to some extent, exhibits a degree of malleability across development. Finally, these findings suggest two important conclusions. First, increased early GCE exposure is related to more reactive behavioral and endocrine phenotypes. Second, increased levels of juvenile social play are associated with decreased basal levels of cortisol in adolescence and a marginal decrease in cortisol reactivity across development regardless of the offspring’s GCE exposure. Thus, socially enriching postnatal environments can shape the development of later-life HPA functioning either in spite of or apart from the prenatal effects associated with increased fetal exposure to glucocorticoids.
Acknowledgments
The authors thank Heather Jensen for her excellent care of the marmosets and Jonathan Santo for statistical assistance. The research presented here is supported by the National Institutes of Health (HD042882) awarded to J.A.F. We also wish to acknowledge the foresight and inspiration of Jeffrey E. Fite in the initiation of the long-term research program on the developmental socioendocrinology of marmosets and all members of the Callitrichid Research Center for the diligent effort in the care of the marmosets during the research procedures. We wish to thank the anonymous reviewers for their insightful comments and suggestions for improvement of the manuscript.
Footnotes
The authors have no conflict of interest to declare.
References
- Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. The Journal of Neuroscience. 1996;16(12):3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. British Medical Journal. 1990;301(6761):1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clinical Science. 1998;95(2):115–1128. [PubMed] [Google Scholar]
- Bhatnagar S, Lee TM, Vining C. Prenatal stress differentially affects habituation of corticosterone responses to repeated stress in adult male and female rats. Hormones and Behavior. 2005;47(4):430–438. doi: 10.1016/j.yhbeh.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Birnie AK, Hendricks SE, Smith AS, Milam R, French JA. Maternal gestational androgens are associated with decreased juvenile play in white-faced marmosets Callithrix geoffroyi. Hormones and Behavior. 2012;62(2):136–145. doi: 10.1016/j.yhbeh.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie AK, Taylor JH, Cavanaugh J, French JA. Quality of maternal and paternal care predicts later stress reactivity in the cooperatively-breeding marmoset (Callithrix geoffroyi) Psychoneuroendocrinology. 2013;38(12):3003–3014. doi: 10.1016/j.psyneuen.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro C, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, Cannizzaro E. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: Interaction with a brief, daily maternal separation. Behavioural Brain Research. 2006;169(1):128–136. doi: 10.1016/j.bbr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Developmental Psychobiology. 2005;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Research Reviews. 2010;65(1):56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Schneider ML. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Developmental Psychobiology. 1993;26(5):293–304. doi: 10.1002/dev.420260506. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Soto A, Bergholz T, Schneider ML. Maternal gestational stress alters adaptive and social behavior in adolescent rhesus monkey offspring. Infant Behavior and Development. 1996;19(4):451–461. [Google Scholar]
- Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Developmental Psychobiology. 1994;27(5):257–269. doi: 10.1002/dev.420270502. [DOI] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8(4):401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biological Psychiatry. 2003;54(10):1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Corcoran CA, Pierre PJ, Haddad T, Bice C, Suomi SJ, Grant KA, Bennett AJ. Long-term effects of differential early rearing in rhesus macaques: Behavioral reactivity in adulthood. Developmental Psychobiology. 2012;54(5):546–555. doi: 10.1002/dev.20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2011;52(2):119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81(1):131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development. 2003;74(2):139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacology Biochemistry and Behavior. 2002a;73(1):259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus primates) and analysis of its effects on early development. Biological Psychiatry. 2002b;52(11):1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Parker SW, Lane S, Sebanc A, Gunnar MR. Quality of care and temperament determine changes in cortisol concentrations over the day for young children in childcare. Psychoneuroendocrinology. 2000;25(8):819–836. doi: 10.1016/s0306-4530(00)00028-7. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: A review. Infant Behavior and Development. 2006;29(3):445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. The Journal of Neuroscience. 2002;22(18):7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9(1):128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower-Merritt D, Smith TE, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied’s black tufted-ear marmosets (Callithrix kuhli) American Journal of Primatology. 1996;40(3):231–245. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- French JA, Fite JE, Jensen H, Oparowski K, Rukstalis MR, Fix H, Power ML. Treatment with CRH-1 antagonist antalarmin reduces behavioral and endocrine responses to social stressors in marmosets (Callithrix kuhlii) American Journal of Primatology. 2007;69(8):877–889. doi: 10.1002/ajp.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Smith AS, Gleason AM, Birnie AK, Mustoe A, Korgan A. Stress reactivity in young marmosets (Callithrix geoffroyi): Ontogeny, stability, and lack of concordance among co-twins. Hormones and Behavior. 2012;61(2):196–203. doi: 10.1016/j.yhbeh.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V. Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. Journal of Child Psychology and Psychiatry. 2011;52(4):356–367. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, O’Donnell K. Prenatal stress and the programming of the HPA axis. Neuroscience & Biobehavioral Reviews. 2010;35(1):17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Developmental Psychobiology. 2012;55(8):849–859. doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development. 1996;67(3):877–889. [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30(6):541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. European Child & Adolescent Psychiatry. 2005;14(1):41–51. doi: 10.1007/s00787-005-0435-1. [DOI] [PubMed] [Google Scholar]
- Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Hormones and Behavior. 2011;59(3):279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Hauser J, Knapman A, Zürcher NR, Pilloud S, Maier C, Diaz-Heijtz R, Pryce CR. Effects of prenatal dexamethasone treatment on physical growth, pituitary-adrenal hormones, and performance of motor, motivational, and cognitive tasks in juvenile and adolescent common marmoset monkeys. Endocrinology. 2008;149(12):6343–6355. doi: 10.1210/en.2008-0615. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hinde K. In building babies. New York, NY: Springer; 2013. Lactational programming of infant behavioral phenotype; pp. 187–207. [Google Scholar]
- Holmes MC, Sangra M, French KL, Whittle IR, Paterson J, Mullins JJ, Seckl JR. 11β-Hydroxysteroid dehydrogenase type 2 protects the neonatal cerebellum from deleterious effects of glucocorticoids. Neuroscience. 2006;137(3):865–873. doi: 10.1016/j.neuroscience.2005.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(9):1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, Chrousos GP. The biobehavioral consequences of psychogenic stress in a small, social primate (Callithrix jacchus) Biological Psychiatry. 1996;40(5):317–337. doi: 10.1016/0006-3223(95)00397-5. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. The Journal of Physiology. 2005;566(3):967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ZA, Padow VA, Romeo RD. The effects of stress on play and home cage behaviors in adolescent male rats. Developmental Psychobiology. 2010;52(1):62–70. doi: 10.1002/dev.20413. [DOI] [PubMed] [Google Scholar]
- Koch H, McCormack K, Sanchez MM, Maestripieri D. The development of the hypothalamic-pituitary-adrenal axis in rhesus monkeys: Effects of age, sex, and early experience. Developmental Psychobiology. 2012;56(1):86–95. doi: 10.1002/dev.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D, Leve LD. Stress system development from age 4.5 to 6: Family environment predictors and adjustment implications of HPA activity stability versus change. Developmental Psychobiology. 2013 doi: 10.1002/dev.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Wiener SG, Coe CL. Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psychoneuroendocrinology. 1993;18(4):297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- Lingas RI, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 2001;73(5):302–311. doi: 10.1159/000054647. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends in Endocrinology & Metabolism. 2002;13(9):373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Developmental Brain Research. 1995;84(1):55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. European Journal of Neuroscience. 2003;18(12):3367–3374. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- Mustoe AC, Birnie AK, Korgan AC, Santo JB, French JA. Natural variation in gestational cortisol is associated with patterns of growth in marmoset monkeys (Callithrix geoffroyi) General and Comparative Endocrinology. 2012;175(3):519–526. doi: 10.1016/j.ygcen.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe AC, Jensen HA, French JA. Describing ovarian cycles, pregnancy characteristics, and the use of contraception in female white-faced marmosets, Callithrix geoffroyi. American Journal of Primatology. 2012;74(11):1044–1053. doi: 10.1002/ajp.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norscia I, Palagi E. When play is a family business: Adult play, hierarchy, and possible stress reduction in common marmosets. Primates. 2011;52(2):101–104. doi: 10.1007/s10329-010-0228-0. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological Psychiatry. 2005;58(3):211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Developmental Psychobiology. 2012;55(20):145–155. doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: A test of a programming hypothesis. Journal of Child Psychology and Psychiatry. 2003;44(7):1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neuroscience & Biobehavioral Reviews. 2011;35(7):1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Dettling A, Spengler M, Spaete C, Feldon J. Evidence for altered monoamine activity and emotional and cognitive disturbance in marmoset monkeys exposed to early life stress. Annals of the New York Academy of Sciences. 2004;1032(1):245–249. doi: 10.1196/annals.1314.030. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Palme R, Feldon J. Development of pituitary-adrenal endocrine function in the marmoset monkey: Infant hypercortisolism is the norm. Journal of Clinical Endocrinology & Metabolism. 2002;87(2):691–699. doi: 10.1210/jcem.87.2.8244. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience & Biobehavioral Reviews. 2005;29(4):649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Advanced quantitative techniques in the social sciences. Thousand Oaks, CA: Sage; 2002. Hierarchical linear models: applications and data analysis methods. [Google Scholar]
- Romeo RD. Adolescence: A central event in shaping stress reactivity. Developmental Psychobiology. 2010;52(3):244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: Exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Hormones and Behavior. 2005;47(1):1–7. doi: 10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27(6):1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in wied’s black tufted-ear marmosets (Callithrix kuhli) American Journal of Primatology. 1995;36(3):185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Schneider ML. Prenatal stress exposure alters postnatal behavioral expression under conditions of novelty challenge in rhesus monkey infants. Developmental Psychobiology. 1992;25(7):529–540. doi: 10.1002/dev.420250706. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: An examination of ontogenetic vulnerability. Child Development. 1999;70(2):263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiology & Behavior. 1997;62(2):225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. British Medical Bulletin. 1997;53(1):170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Taylor JH, Mustoe AC, French JA. Behavioral responses to social separation stressor change across development and are dynamically related to HPA activity in marmosets. American Journal of Primatology. 2013 doi: 10.1002/ajp.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology. 1999;34(2):129–138. [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Pinna Puissant S, Van Huffel S. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Hormones and Behavior. 2008;54(2):253–257. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Van den Hove DLA, Blanco CE, Aendekerk B, Desbonnet L, Bruschettini M, Steinbusch HP, Steinbusch HWM. Prenatal restraint stress and long-term affective consequences. Developmental Neuroscience. 2005;27(5):313–320. doi: 10.1159/000086711. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochemical Research. 2007;32(10):1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience & Biobehavioral Reviews. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R. Effect of prenatal stress on plasma corticosterone and catecholamines in response to foot-shock in rats. Physiology & Behavior. 1998;64(4):439–444. doi: 10.1016/s0031-9384(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME. From dependence to sexual maturity: The behavioral ontogeny of Callitrichidae. In: Rylands AB, editor. Marmosets and Tamarins: Systematics, behaviour, and ecology. New York: Oxford University Press; 1993. pp. 235–254. [Google Scholar]