Abstract
Exposure to nerve agents (NAs) and other organophosphates (OPs) can initiate seizures that rapidly progress to status epilepticus (SE). While the electrographic and neuropathological sequelae of SE evoked by NAs and OPs have been characterized in adult rodents, they have not been adequately investigated in immature animals. In this study postnatal day (PND) 14, 21 and 28 rat pups, along with PND70 animals as adult controls, were exposed to NAs (sarin, VX) or another OP (diisopropylfluorophosphate, DFP). We then evaluated behavioral and electrographic (EEG) correlates of seizure activity, and performed neuropathology using Fluoro-Jade B. Although all immature rats exhibited behaviors that are often characterized as seizures, the incidence, duration, and severity of the electrographic seizure activity were age-dependent. No (sarin and VX) or brief (DFP) EEG seizure activity was evoked in PND14 rats, while SE progressively increased in severity as a function of age in PND21, 28 and 70 animals. Fluoro-Jade B staining was observed in multiple brain regions of animals that exhibited prolonged seizure activity. Neuronal injury in PND14 animals treated with DFP was lower than in older animals and absent in rats exposed to sarin or VX. In conclusion, we found that NAs and an OP provoked robust SE and neuronal injury similar to adults in PND21 and PND28, but not in PND14, rat pups. Convulsive behaviors were often present independent of EEG seizures and were unaccompanied by neuronal damage. These differential responses should be considered when investigating medical countermeasures for NA and OP exposure in pediatric populations.
Keywords: Nerve agents, Diisopropylfluorophosphate, Seizure, Status epilepticus, Pediatric, Rats
1. Introduction
Exposure to nerve agents (NAs) and other organophosphates (OPs) can produce prolonged repetitive seizures and status epilepticus (SE), a medical condition that causes high morbidity and mortality. NAs and other OPs irreversibly bind to the active site of acetylcholinesterase (AChE) and thus inhibit the degradation of the neurotransmitter acetylcholine, which then results in excess cholinergic activation (Aas, 2003). Centrally, excessive activation at muscarinic receptors by acetylcholine can initiate extended seizures (Hamilton et al., 1997), and these extended seizures can rapidly progress to SE which in turn produces marked neuronal death and mortality in animals (Apland et al., 2010; Crawford et al., 2004; Li et al., 2011; McDonough and Shih, 1997; Tuovinen, 2004).
The human pediatric population is thought to be more susceptible than adults to seizure development in general (Schaffer and Sirven, 2013; Volpe, 2008), and very young children (<2 yr old) are more likely to experience SE than older patients (Shinnar et al., 1997). Children who were acutely poisoned with carbamates or OPs displayed characteristic peripheral and central symptoms of anticholinesterase intoxication, with convulsive seizures appearing in 8–30% of cases (Lifshitz et al., 1999; Verhulst et al., 2002; Zwiener and Ginsburg, 1988). Similarly, in animal models, results from studies in which the chemoconvulsants pilocarpine or kainic acid were administered to immature and adult rats suggested that seizure susceptibility and mortality are greater in younger animals than they are in adults (Albala et al., 1984; Cavalheiro et al., 1987; Priel et al., 1996; Tremblay et al., 1984; Yang et al., 1998). Conversely, seizure-associated neuronal injuries in response to these agents increase as the age of the animal increases (Cavalheiro et al., 1987; Haut et al., 2004; Scantlebury et al., 2007). These data suggest that there are age-specific effects of NAs and other OPs. However, while age-related responses to other chemoconvulsant agents have been investigated (Cavalheiro et al., 1987; Haut et al., 2004; Scantlebury et al., 2007), and there are also electrographic and neuropathological characterizations of acute NA and OP poisoning in adult rats (Crawford et al., 2004; Deshpande et al., 2010; McDonough et al., 1995, 1998; Todorovic et al., 2012), characterizing the effects of these agents in immature animals has only just begun (Fawcett et al., 2009; Miller et al., 2015; Shih et al., 1990; Wright et al., 2016). As there are evident differences in the seizures of patients based on their age, therapies administered to adults many not be optimal for children (Baker, 2007; Rotenberg and Newmark, 2003). Therefore, an animal model of OP and NA exposure is required to evaluate the therapeutic efficacy of potential treatments in young individuals.
The current paper describes and validates age-appropriate models to evaluate OP- and NA-induced seizures in immature rats. Immature rat pups at postnatal day (PND) 14, 21, and 28, as well as adult rat controls (PND70), were administered the OP DFP or the NAs sarin or VX and then were examined for behavioral convulsions, electroencephalographic (EEG) evidence of seizure activity, and neuropathology. The results demonstrate that there is a strong relationship between age and both seizure susceptibility and neuronal damage, and that behavioral convulsive activity is not always predictive of EEG recorded seizures in immature animals.
2. Materials and methods
2.1. Animals
Pregnant Sprague-Dawley rats (at 13–15 days of pregnancy) were received from Charles River (Raleigh, NC); pups were delivered in the animal facility approximately 1 week following arrival of the pregnant female. Litters were culled to 8 or 10 pups to maintain consistent weights. Animals were kept on a 12-hr light-dark cycle and had access to food ad libitum. DFP administration was performed at the University of Utah, and NA administration procedures were performed at the U.S. Army Medical Research Institute of Chemical Defense (USAMRICD). All surgical and experimental procedures were performed under protocols approved by the respective Institutional Animal Care and Use Committees at the University of Utah and USAMRICD, and were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and the Animal Welfare Act of 1966 (P.L. 89–544), as amended.
2.2. Implantation of EEG electrodes
University of Utah
Pups at PND12, 19, and 26, and adult rats at PND68 were anesthetized with 2.5–3% isoflurane and placed into a stereotaxic frame. An incision was made along the midline of the scalp to expose the skull. Burr holes were made using a high-speed dental drill. Electrode wires and the reference wire of the telemetric implant (Epitel, Inc., Salt Lake City, UT) were placed in the burr holes so that the wires were touching the dura, and were secured using cyanoacrylate gel compound with accelerator (Zayachkivsky et al., 2013). The incision was sutured shut using dissolvable suture. Post-surgery, the animals received 0.5–3 ml lactated Ringer’s solution and 0.1 ml marcaine (MWI Veterinary Supplies, Boise, ID) or 0.015 mg/kg (PND12) or 0.03 mg/kg (PND19, 26 and 68) buprenorphine (MWI Veterinary Supplies, Boise, ID). All surgical procedures were performed under sterile conditions. The pups were allowed to recover in a heated recovery chamber until conscious before being returned to their dam (PND 12) or to a new cage (PNDs 19, 26, and 68).
USAMRICD
In animals that were administered NA, EEG recordings were made with a tethered system using implanted three-channel electrodes (Plastics One, Roanoke, VA) for the PND14, 21, and 28 groups or three cortical stainless-steel screw electrodes secured to a connector plug implant for the PND70 group. Pups at PND12 (PND14 group), 19 (PND21 group), and 25 or 26 (PND28 group), and adult rats PND63 or 64 (PND70 group) were anesthetized with isoflurane (5% induction; 0.5–3.0% maintenance, with oxygen) and placed into a stereotaxic frame. An incision was made along the midline of the scalp to expose the skull. For the PND14, 21, and 28 groups, the electrode wires were scraped bare of insulation, coiled and positioned in a triangular fashion flush with the skull, and the headpiece was secured using glass ionomer cement (Instech Laboratories, Plymouth Meeting, PA) or methyl methacrylate fast curing acrylic resin (Lang Dental, Wheeling, IL). In addition, two miniature anchoring screws were implanted in the skull to secure the headpiece in PND21 and 28 animals. For the PND70 group animals, burr holes were drilled over each hemisphere midway between bregma and lambda and 3 mm lateral to the midline, and an additional hole was drilled over the cerebellum. Stainless steel screw electrodes were placed in the holes and connected to a miniature plug via wires previously soldered to the screws; the whole assembly was then secured using glass ionomer or methyl methacrylate. Incisions were closed with non-absorbable monofilament suture material. Immediately after surgery all animals received warmed Ringer’s solution (0.5–2 ml, s.c.) and PND14 pups received 0.015 mg/kg buprenorphine (s. c.), while the PND21, PND28 and PND70 animals received 0.03 mg/ kg buprenorphine (s.c.), for analgesia. Animals of all age groups were placed in a heated recovery chamber until normal ambulation returned, and then were returned to their dam (PND14 group) or moved to a new cage (PND21, 28, and 70 groups).
2.3. NA and OP exposures and subsequent EEG recordings
University of Utah
Rat pups that received DFP (Sigma-Aldrich, St. Louis, MO) were pretreated with 0.026 mg/kg pyridostigmine bromide (Sigma-Aldrich, St. Louis, MO) i.p. 30 min before DFP treatment to reduce the peripheral effects of the OP. DFP was administered s.c. in ice-cold PBS in doses as follows: PND14, 4.0–4.5 mg/kg; PND21, 4.5–6.0 mg/kg; PND28, 4.0–6.5 mg/kg; and PND70, 4.0–6.25 mg/kg. At least three different batches of DFP were used for these experiments. Their potency in the animals was varied, even when fresh. Furthermore, aliquots of DFP stored at −80 °C would lose potency over several months. This resulted in a range of DFP doses that were employed to produce the greatest probability of animals exhibiting electrographic seizures without causing excess mortality. An admixture of 0.1 mg/kg atropine sulfate (Sigma-Aldrich, St. Louis, MO) and 25 mg/kg 2-PAM (Sigma-Aldrich, St. Louis, MO) was given i.p.1 min after DFP administration to reduce respiratory distress. Behavioral responses were continuously observed for a minimum of 1 h following DFP administration in all age groups. EEG data were acquired using the Epoch™ wireless EEG system (Epitel, Inc., Salt Lake City, UT), and recording occurred for >30 min before and 3 h following DFP treatment for PND14 rat pups, and 24 h following DFP treatment for the PND21, PND28 and PND70 animals. EEG signals were amplified in the transmitter and converted to a frequency-modulated signal that subsequently activated the receiver antenna located in the base, which was placed underneath each animal’s cage (Zayachkivsky et al., 2013). The EEG data were recorded and stored on a computer (Acqknowledge software, BIOPAC Systems, Inc, Goleta, CA) for subsequent analysis. Simultaneous video monitoring was performed using EZWatch pro, Version 3.1HD (Louisville, KY), a video surveillance system with infrared cameras.
USAMRICD
Rat pups of different ages were administered either sarin or VX s.c. The doses of sarin and VX were based on initial data (Wright, personal communication) from the Wright et al. (2016) study who reported that PND14 and PND21 rats were more than 2.7 fold more sensitive to the lethal effects of sarin than older (PND28, PND70) animals, while the lethality of VX was far less varied across age groups. Within an age group the doses were then ranged up and down over successive animals in an attempt to elicit a high percentage of seizure responses yet minimize mortality with these highly toxic compounds. For sarin the following doses were used: PND14, 41.5–68.1 μg/kg; PND21, 88.9–142.5 μg/kg; PND28, 110.0–262.5 μg/kg; and PND70, 125.0–187.5 μg/kg. For VX, the following doses were used: PND14, 22.0–33.0 μg/kg; PND21, 19.9–34.5 μg/kg; PND28, 19.9–34.5 μg/kg; and PND70, 16.0–24.0 μg/kg. Animals then received 0.1 mg/kg atropine methyl nitrate (Wedgewood Pharmacy, Swedesboro, NJ) s.c. 1 min later. Animals received 25 mg/kg of 2-PAM (Sigma-Aldrich, St. Louis, MO) s.c. at the onset of behavioral signs of toxicity, typically 2–13 min after sarin administration and 7–23 min after VX administration. At the onset of electrographic seizure activity (3–22 min after sarin administration, 7–28 min after VX administration), animals received 0.1–0.45 mg/kg atropine sulfate (Wedgewood Pharmacy, Swedesboro, NJ) s.c. Behavioral observations were made continuously for 1 h following NA administration. EEG activity was recorded for a minimum of 30 min before NA administration as a baseline, for the 4 h following agent administration, and for 30 min at 24 h after agent administration if the animals survived. All EEG signals were recorded using CED 1902 amplifiers to display and record the EEG signals on a computer with Spike2 software (Cambridge Electronic Design, Ltd., Cambridge, UK).
2.4. Classification of electrographic seizure activity
Animals were classified into one of three groups for electro-graphic seizure activity. The first group (SE) consisted of animals exhibiting continuous rhythmic high-amplitude spikes or sharp-wave epileptiform activity for ≥ 5 min (as in Shih et al., 2011). The second group (epileptiform activity, EA) was comprised of animals that showed continuous epileptiform activity of ≥ 10 s but <5 min, and the third group consisted of animals that did not exhibit any change in electrographic activity compared to their baseline signals. For both the SE and EA groups, seizure onset was operationally defined as the appearance of continuous rhythmic, high-amplitude spikes or sharp-wave activity for ≥ 10 s. Both of these groups were included in the analysis of latency to abnormal electrographic activity (Figs. 1 and 2). However, only the SE group was included in further analyses because the intention of this paper was to focus on OP- or NA-induced SE in an immature population. “Electrographic/EEG seizure activity” refers to both SE and periodic EA.
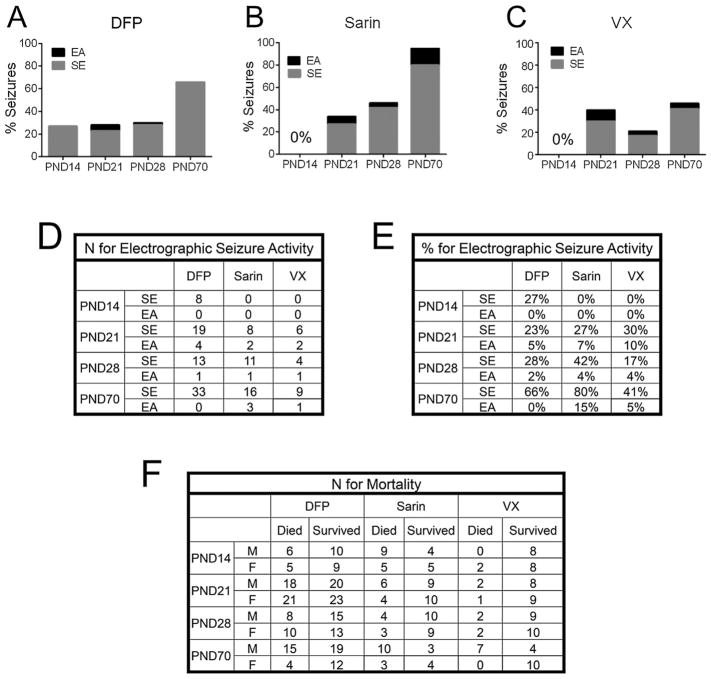
Fig.1.
A–C, Percentage of PND14, PND21, PND28 and PND70 animals that displayed electrographic seizure activity following the administration of DFP (A), sarin (B) or VX (C). EA is defined as continuous epileptiform activity of ≥10 s but <5 min, and SE is defined as continuous rhythmic high-amplitude or sharp-wave activity for ≥ 5 min. D, n for electrographic seizure activity ((A–C)). E, percentage of animals with electrographic seizure activity ((A–C)). F. numbers of male and female animals in each age group that died or survived for 24 h following exposure to each agent.
Fig. 2.
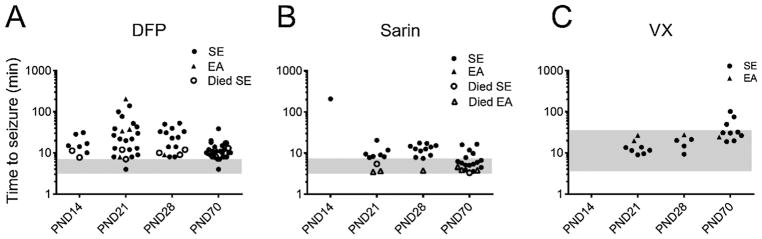
The latency to abnormal electrographic activity was dissociated from the latency to behavioral signs following the administration of DFP (A), sarin (B) or VX (C). Although toxic signs that included convulsive-like behaviors began within minutes of NA or DFP administration, the first electrographic signs of seizure activity sometimes did not occur until an hour or more following the administration of one of the OPs. The shaded area on each graph represents the time within which toxic signs appeared. Rats that survived for more than 15 min had either SE (●) or epileptiform activity (▲, EA). Some animals died within 15 min after DFP administration, but exhibited abnormal electrographic activity before death (○, SE; △; EA).
2.5. Fluoro-Jade B staining
University of Utah
After DFP exposure and the 24 h of EEG and video recording, rats were deeply anesthetized with isoflurane and perfused with 10% neutral-buffered formalin (Sigma-Aldrich, St. Louis, MO). Following several days incubation in 10% formalin, brains were transferred to 30% w/v sucrose prior to subsequent freezing. Consecutive 40 μm coronal sections were made using a cryostat and mounted onto slides. Sections were then stained with Fluoro-Jade B, a marker for injured neurons (Schmued et al., 1997). Sections were incubated with 0.06% KMnO4 (Sigma-Aldrich, St. Louis, MO) followed by 0.001% Fluoro-Jade B (Millipore, Temecula, CA). Following air drying, slides were cleared with xylene, and coverslips were mounted with DPX (VWR, Radnor, PA). Every third section was visualized on a Hamamatsu Nanozoomer 2.0 HT (Olympus, Center Valley, PA).
USAMRICD
Following the 30-min EEG recording period performed 24 h after sarin or VX administration, rats were deeply anesthetized with sodium pentobarbital (75.0 mg/kg, i.p.) and perfused with saline, followed by 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). Brains were post-fixed in 4% paraformaldehyde for at least 24 h, then cut in the coronal plane into 3–4 mm blocks, paraffin-processed, and sectioned on a microtome at 5 μm. Mounted sections were then incubated in 0.06% KMnO4 followed by 0.001% Fluoro-Jade B (Millipore, Temecula, CA). Sections were air dried, cleared with xylene and coverslipped with DPX (Sigma-Aldrich, St. Louis, MO). For both DFP- and NA-exposed animals, presence or absence of Fluoro-Jade B staining in 3 consecutive visualized slides was the criterion for whether a particular animal was considered to have damage (Table 1).
Table 1.
Incidence of neural damage in animals that displayed SE >1 h.
| HILUS | LATERODORSAL THALAMUS | BASOLATERAL AMYGDALA | PIRIFORM CORTEX | PARIETAL CORTEX | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| DFP | SARIN | VX | DFP | SARIN | VX | DFP | SARIN | VX | DFP | SARIN | VX | DFP | SARIN | VX | |
| PND14 | N/A | 0/1 | N/A | N/A | 0/1 | N/A | N/A | 0/1 | N/A | N/A | 0/1 | N/A | N/A | 0/1 | N/A |
| PND21 | 6/10 | 3/3 | 2/3 | 5/10 | 2/3 | 2/3 | 10/10 | 3/3 | 2/3 | 10/10 | 2/3 | 2/3 | 4/10 | 2/3 | 3/3 |
| PND28 | 9/9 | 0/1 | N/A | 9/9 | 1/1 | N/A | 9/9 | 1/1 | N/A | 9/9 | 1/1 | N/A | 7/9 | 0/1 | N/A |
| PND70 | 9/9 | 4/4 | 1/1 | 9/9 | 4/4 | 1/1 | 9/9 | 4/4 | 1/1 | 9/9 | 4/4 | 1/1 | 9/9 | 4/4 | 1/1 |
2.6. Statistical analyses
Differences between the PND14-PND70 age groups for mortality and the presence or absence of seizures were examined using a Chi-squared test of independence. Differences between male and female animals within each age group were compared using Fisher’s exact tests. Significant differences between ages for latency to the first electrographic seizure were calculated using a one-way ANOVA with further comparisons using Tukey’s test. Comparisons between male and female animals for latency to the first electrographic seizure in the PND21 and 28 groups were performed using Student’s t-tests; for the PND70 group the comparison was performed with a Mann-Whitney test due to significant differences in variance. Statistical tests were performed using GraphPad Prism 6 (San Diego, CA). For all tests, p < 0.05 was considered significant.
3. Results
3.1. Behavior in rat pups and young adult controls in response to OP or NA exposure
Animals exposed to DFP, sarin or VX displayed some similar behaviors across all age groups. Rat pups and PND70 adults showed toxic signs characteristic of excessive cholinergic stimulation, which included gasping, gagging, lacrimation, urination, hypersalivation, muscle twitching and/or fasciculations. Scratching, limb extension, Straub tail, chewing, ataxia, and head bobbing were also observed; full body tremors or what might be considered behavioral convulsions were seen and sometimes occurred later than the initial cholinergic toxic signs, as was the case after exposure to VX. Not every animal had the full complement of these behaviors, and the severity of the behaviors varied between animals, even within the same age group; the different doses of agents used within an age group certainly contributed to this variability in responses. In addition, the acute stereotyped clonic seizures that are typically associated with the initiation of electrographic seizure activity following chemoconvulsant administration or kindling in adult (Racine, 1972) or immature (Scholl et al., 2013; Tremblay et al., 1984) animals were not evident in any age group of these immature (PND14, 21 or 28) rats. Rearing accompanied by forelimb clonus was rarely observed in the hours after the initiation of electrographic seizure activity following DFP or NA administration in these younger age groups. Furthermore, we did not observe any discrete behaviors or an obvious increase in severity of the behaviors associated with the initiation of abnormal EEG activity in animals exhibiting electrographic seizures. It was quite common for animals to display forelimb extension, chewing, Straub tail, and robust full body tremors with no accompanying EEG indication of seizure, particularly in the youngest age groups. In some cases these behaviors foreshadowed the development of EEG seizure activity, but just as often they could persist for >1 h and then slowly fade away without the animal ever developing EEG evidence of seizure activity. In pups that did not exhibit electrographic seizure activity or that developed only brief EA activity, DFP-associated behaviors began to diminish 2–3 hr following administration in PND21 and 28 pups, and approximately 1 h after DFP administration in PND14 pups. Similarly, in rats that did not develop electrographic seizures in response to sarin or VX exposure or developed just brief EA activity, toxic signs began to decrease 1–2 h following administration. In animals that did display SE seizures, the OP-induced behaviors continued longer, although with diminishing intensity over time. Recovery from OP-induced behaviors was faster in younger animals (PND14-28) than in PND70 adult animals. Adult rats that developed SE seizure activity following sarin or VX exposure displayed all the behavioral signs and EEG changes described in rats that developed seizures following intoxication with the nerve agent soman (McDonough and Shih, 1993), although the behavioral activity and EEG changes were slower in animals exposed to VX compared to those exposed to sarin. As the seizures persisted, these animals often became prone. Adult animals that did not develop EEG seizure activity or only developed brief EA often still exhibited chewing, head bobbing, and whole-body tremors with occasional Straub tail, but never developed the initial rhythmic clonic movements of the facial muscles and ears or clonic forepaw movements seen in the animals at the onset of SE seizures. The chewing, head bobbing, and whole-body tremors and Straub tail could persist for as long as 1–2 hr after administration and then slowly diminished. In animals that developed SE following NA exposure, the rhythmic head clonus continued with diminishing intensity as the recording session progressed. This head clonus was most apparent in the PND70 adults and less so in the younger (PND14-28) animals.
3.2. Susceptibility to electrographic seizure activity
DFP
Following DFP administration, 23–28% of PND14, 21, and 28 animals and 66% of PND70 animals developed electrographic seizure activity (Fig. 1A and E). Of those animals that developed electrographic seizure activity, the majority in all age groups, 8/8 PND14, 19/23 PND21, 13/14 PND28, and 33/33 PND70, developed SE and the rest displayed EA (Fig.1D). It is important to note that all animals displayed the behavioral signs detailed above. Using a Chi-square test for trend, we found a strong relationship between increased age and seizure susceptibility (p < 0.0001). However, Fisher’s exact tests showed no significant differences between male and female rats within each age group in the development of EEG seizures (data not shown).
Sarin
Of the 25 PND14 animals included in the analysis (see note below) that were exposed to varying doses of sarin, none exhibited either SE or EA. Relative to DFP, sarin treatment elicited more electrographic seizure activity across the other age groups; the rate in PND21 animals was 34%, and in PND28 the rate was 46%, while PND70 adults had the highest rate of electrographic seizure activity, with 95% of animals entering SE or EA (Fig. 1B and E). Similar to DFP, there was a significant association between age and seizure susceptibility (p < 0.0001), as is evident in Fig.1A. As in the DFP-treated animals, most sarin-treated animals that exhibited EEG seizure activity developed SE (8/10 PND21s,11/12 PND28s, and 16/19 PND70s) with the remainder displaying EA as defined above (Fig. 1D). Also, note that one PND14 did develop spike and wave electrographic seizure activity that began 208 min after sarin exposure (Fig. 2B) that was >5.5 times longer than the next longest latency to abnormal electrographic activity after sarin administration in any of the other age groups. This was the only PND14 pup to show any seizure or abnormal electrographic activity of the 26 animals in this age group exposed to sarin. Because this single animal was an apparent outlier, its latency and neuropathology data were omitted from the analysis.
VX
As with sarin, of the 22 PND14 pups exposed to VX, none displayed abnormal electrographic activity (Fig. 1C and D). In PND21 pups treated with VX, 40% exhibited electrographic seizures, while only 21% of the PND28 animals developed seizures (Fig. 1E). Only 46% of the PND70 animals displayed abnormal electrographic activity, as compared to 66% with DFP and 95% with sarin (Fig. 1E). Although there was a significant correlation between seizure susceptibility and age (p < 0.01), the relationship was not as clear as for DFP or sarin. Similar to DFP and sarin, if electrographic seizures were generated following VX administration, those animals most often experienced SE (6/8 PND21 pups, 4/5 PND28 pups, and 9/10 PND70 adults; see Fig. 1D).
Note that the PND21, 28, and 70 OP- and NA-exposed animals in the EA category showed high-amplitude continuous seizures consistent with SE, but died before exhibiting these electrographic characteristics for the required 5 min and therefore were categorized as having EA. Finally, there was no apparent relationship between the severity of toxic signs and the subsequent development of electrographic seizure activity. This divergence was most pronounced in the younger age groups (PND14, 21 and 28) with DFP and VX administration.
3.3. Latencies to toxic signs and first electrographic seizure activity
DFP
While toxic signs and convulsive behavior (defined as whole-body tremors) began within minutes of DFP administration for all ages, the latency to the first electrographic seizure (for animals that showed EEG seizure activity) was variable across the age groups, and many animals did not display electrographic seizures until well over 1 h after initial DFP treatment (Fig. 2A). Following DFP exposure, PND21 and PND28 animals displayed a wide range of latencies to the initiation of electrographic seizure activity (44.0 ± 48.9 min and 29.4 ± 18.0 min, respectively; mean ± SD). PND14 (17.6 ± 8.9 min) and PND70 (11.6 ± 6.5 min) animals showed a more discrete clustering of latencies to SE or EA. There were no significant differences in the latency to first EEG seizure between male and female rats at PND21, 28 or 70 (two-tailed Student’s t tests with post-hoc Mann-Whitney tests; data not shown).
Sarin
Administration of sarin usually elicited toxic signs and behavioral convulsions within minutes, and these were followed—most often within 10 min or less and never more than 21 min after administration—by electrographic seizure activity (Fig. 2B). Sarin initiated electrographic seizure activity in a more narrow time window than did either DFP or VX. This latency was 8.8 ± 4.9 min (mean + SD) for PND21 animals, 12.0 ± 4.3 min for PND28 animals, and 6.8 ± 4.0 min for PND70 animals. Although most sarin-exposed animals showed toxic signs, not all showed behavioral convulsions (tremors and fasciculations), which was most likely due to rapid mortality.
VX
In animals exposed to VX, toxic signs occurred later in relation to administration than with either DFP or sarin, usually 5–20 min post-exposure (Fig. 2C). Behavioral convulsions were also much later relative to exposure with VX and were usually disassociated in time from initial toxic signs, such as chewing, often by many minutes. Electrographic seizure activity in response to VX generally occurred later in PND70 animals than in PND21 and 28 pups, with electrographic seizure activity initiating 40.9 ± 27.0 min (mean + SD) after VX administration in PND70 animals and 14.5 ± 6.1 and 18.7 ± 7.0 min post-VX in PND21 and 28 animals, respectively. It is important to note that it was common for VX-exposed animals to show an array of toxic signs, sometimes up to and including behavioral convulsions, without ever showing abnormal electrographic seizure activity. While there was divergence between behavioral signs and electrographic activity in response to all compounds, it occurred more often in response to VX and DFP, and occurred not only in pups but even in some adult animals exposed to these compounds.
3.4. Electrographic activity
DFP
Eight of 30 PND14 rat pups treated with DFP showed brief periods (~30 min) of rhythmic, polyspike SE activity that spontaneously ended with no further epileptiform activity (Fig. 3A). In contrast, PND21, 28 and 70 animals experienced robust SE that lasted 4–6 hr and then tapered off into interictal spiking and/or spontaneous bursts of seizure activity (Figs. 4–6). In the PND21 animals, 19 of 82 had electrographic SE, as did 13 of 46 PND28 animals and 33 of 50 PND70 adults; 4 PND21 and 1 PND28 had EA (Fig. 2A). While the EEGs of PND14 pups returned to baseline following cessation of SE (Fig. 3A), the EEGs in older animals that developed SE rarely returned to pre-DFP levels during the time of recording (Fig. 5). At 23–24 hr following DFP administration, 7 of 9 PND21 SE rats had either spontaneous bursts of seizure activity (n = 4) or interictal spiking (n = 3), while 7 of 12 PND28 SE rats had interictal spiking, and 1 of 12 showed both spiking and spontaneous bursts of seizure activity (Fig. 7A and B). Of the 9 PND70 SE animals, 2 had spiking, 2 had spontaneous bursts of seizure activity, 4 had both types of abnormal electrographic behavior, and 1 animal continued in SE during the 23–24 hr period after DFP administration.
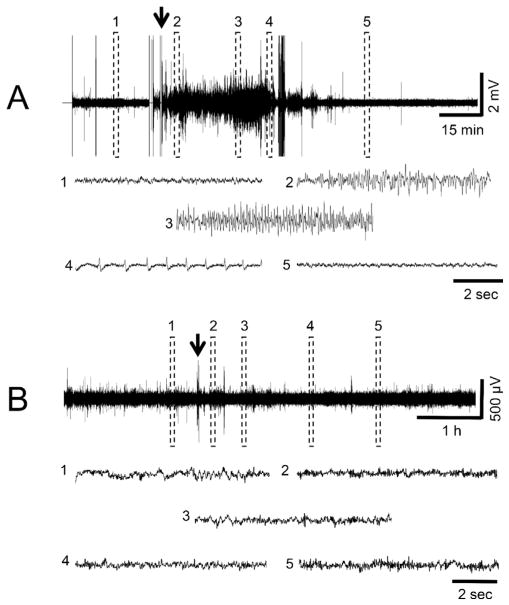
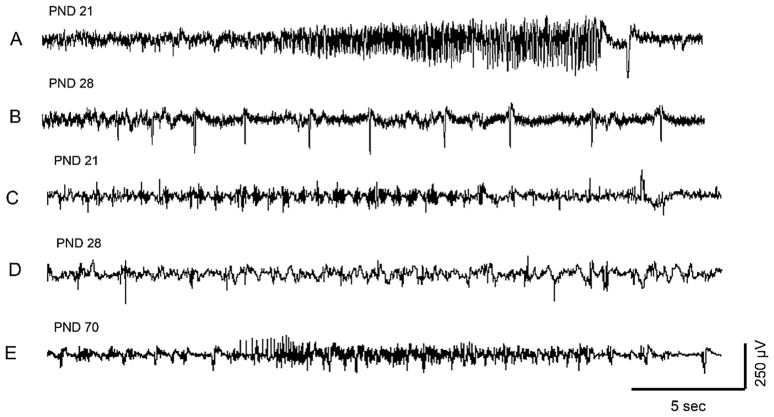
Fig. 3.
EEG recordings of representative PND14 pups following DFP (A) or VX (B) administration. For A, the traces show the different stages of DFP-induced SE; (1) shows baseline, (2) indicates initiation of seizures, (3) shows SE, (4) indicates SE attenuation, (5) shows the return to baseline. The arrow indicates the time of DFP administration. PND14 pups treated with DFP had seizure activity that lasted <1 h. In B, 5 regions throughout the EEG record were expanded and illustrate that EEG activity did not change over time compared to baseline in response to VX administration (arrow). With the exception of one animal, PND14 pups exposed to sarin showed the same lack of change from baseline activity as those exposed to VX.
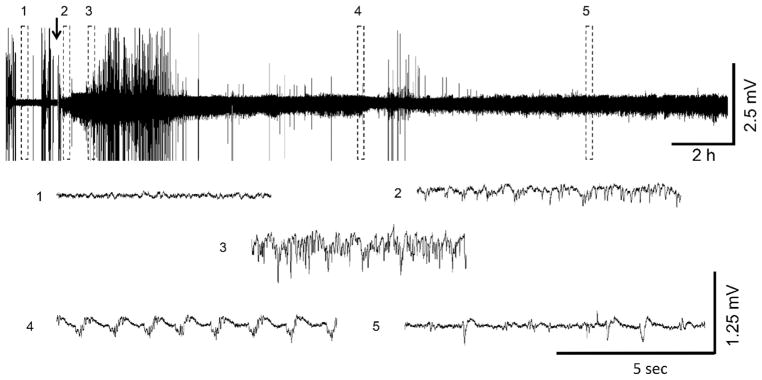
Fig. 4.
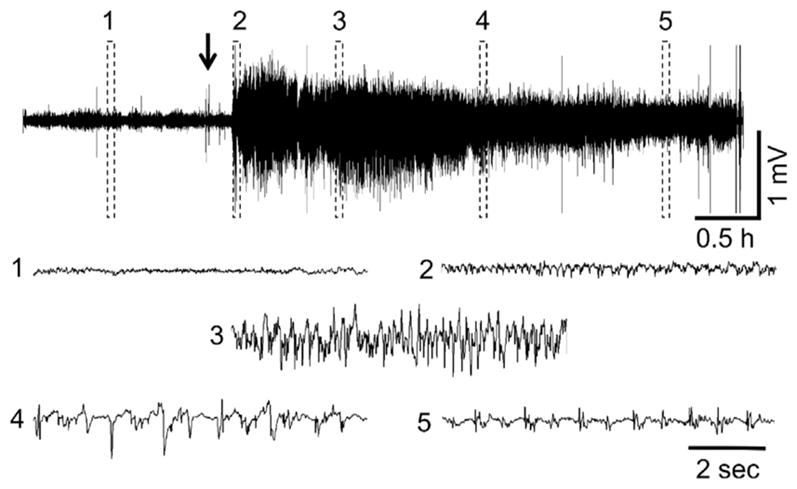
Representative PND21 EEG recording following VX administration. (1) shows baseline, (2) initiation of seizures, (3) SE, (4) SE attenuation, (5) continued epileptiform spiking. The arrow indicates VX administration. Exposure to DFP and sarin at this age resulted in similar EEG profiles when animals developed SE.
Fig. 6.
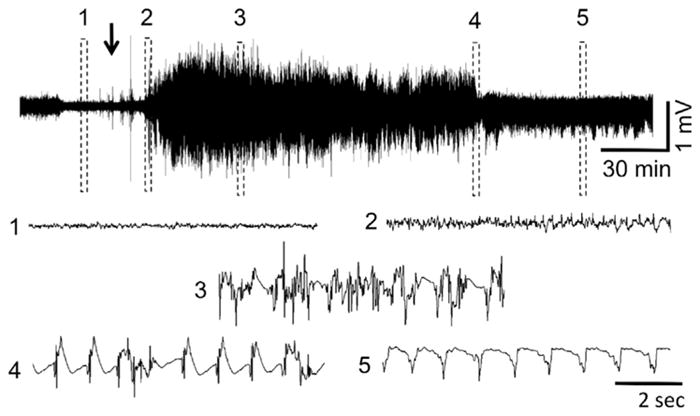
Representative EEG recording at PND70 after sarin administration. (1) baseline, (2) initiation of seizures, (3) SE, (4) SE attenuation, (5) continued epileptiform spiking. The arrow indicates sarin administration. Exposure to DFP and VX at this age resulted in similar EEG profiles when animals developed SE.
Fig. 5.
Representative EEG record at PND28 following DFP administration. (1) baseline, (2) initiation of seizures, (3) SE, (4) SE attenuation, (5) continued epileptiform spiking. The arrow indicates DFP delivery. Exposure to sarin and VX at this age resulted in similar EEG profiles when animals developed SE.
Fig. 7.
Electrographic seizure or epileptiform activity is still present in many of the animals that developed SE at 24 h following OP or NA administration. A. Spontaneous seizure in PND21 rat pup (DFP); B. Interictal spiking in PND28 rat pup (DFP); C. Repetitive high frequency bursts interspersed with high-amplitude spikes in PND21 rat pup (sarin); D. Interictal spiking in PND28 rat pup (VX); E. Spiking and brief seizure in PND70 adult (VX).
Sarin
With the exception of the one pup noted earlier, none of the 23 PND14 pups that were exposed to sarin showed any abnormal electrographic seizure activity (Fig. 3B). For the PND21 pups exposed to sarin,10 of 29 exhibited either EA or SE; 2 of the 10 animals exhibited EA and spontaneously ceased seizure activity after 5–8 min, while the other 8 developed SE (Fig. 2B). In the PND28 group, 12 of 26 animals exhibited EA or SE; the one animal that developed EA spontaneously stopped seizing after 17 min, while the remaining 11 animals developed SE (Fig. 2B). PND70 animals showed a much more robust and consistent response to sarin than did younger animals (Fig. 6), with 19 of 20 PND70 animals exhibiting EA or SE; 3 animals developed EA seizures that stopped within 5–15 min, while the remaining 16 animals developed SE (Figs. 2B and Fig. 6). At 24 h following administration of sarin, of the animals that exhibited SE and survived to that point, 1 of 3 PND21 animals, 1 of 1 PND 28 animal, and 4 of 4 PND70 animals still exhibited electrographic seizure activity that consisted of interictal spiking and/or spontaneous bursts of seizure activity (Fig. 7C).
VX
As with sarin administration, none of the PND14 animals exposed to VX (0 of 18) exhibited any abnormal electrographic seizure activity during the recording periods (Figs. 2C and Fig. 3B). In PND21 pups, 8 of 20 animals displayed EA or SE; the 3 animals that developed EA spontaneously stopped 3–10 min after seizure onset, while the other 5 animals developed SE (Figs. 2C and Fig. 4). In the PND28 pups, 5 of 23 displayed EA or SE in response to VX administration, and, as in the PND21 pups, the one animal that showed EA spontaneously stopped seizing 2 min after seizure onset, while the other 4 animals developed SE (Fig. 2C). VX elicited more consistent electrographic seizure activity in PND70 animals than in younger animals with 10 of 21 PND70 animals displaying seizure activity,1 having brief EA and the others SE (Fig. 2C). At 24 h post-exposure, of the animals that experienced EEG seizure activity and survived to that point, 2 of 3 PND21 pups and 1 of 1 PND70 animal still exhibited interictal spiking usually in combination with spontaneous bursts of seizure activity (Fig. 7D and E).
3.5. 24 h Mortality
DFP
Mortality resulting from DFP occurred in all age groups: PND14 pups 37%; PND21 pups 48%; PND28 pups 39%; and adult PND70 rats 38%. There were no significant differences in mortality between age groups (Chi-square test; data not shown).
Sarin
Mortality due to sarin also occurred in all age groups: PND14 pups 61%; PND21 pups 34%; PND28 pups 27%; and adult PND70 rats 65%. This U-shaped curve displayed a significant relation between age and mortality as shown by a Chi-square test for trend (p < 0.01), which was no doubt due to the different challenge doses of sarin chosen for each age group.
VX
VX also caused mortality at all ages, but the proportion of mortality was notably lower than with administration of DFP or sarin: PND14 pups 11%; PND21 pups 15%; PND28 pups 17%; and adult PND70 rats 33%. There were no significant differences in mortality between age groups (Chi-square test).
Across all agents and age groups, there were no statistical differences in mortality between male and female rats (Fisher’s exact tests with two-tail P values). Fig. 1F shows the numbers of male and female animals in each age group that died or survived for 24 h following exposure to each agent. For DFP and sarin, much of the mortality occurred within the first 15 min after administration, presumably from acute respiratory effects. Once animals entered SE, for animals exposed to DFP, the mortality rate dropped: only 2 of 8 PND14, 2 of 24 PND21, 3 of 15 PND28, and 5 of 30 PND70 rats died after the onset of SE. With sarin and VX, animals were more likely to die during SE. Of the animals that entered SE following sarin exposure, 4 of 7 PND21, 5 of 6 PND28, and 14 of 18 PND70 rats died. Similarly for VX, 2 of 5 PND21, 2 of 5 PND28, and 7 of 8 PND70 rats died during SE.
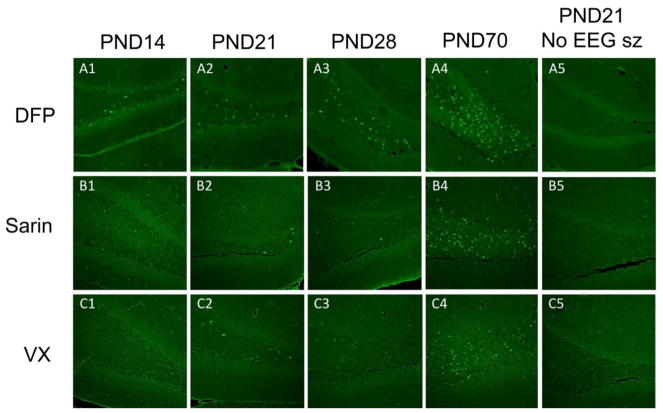
3.6. Fluoro-Jade B staining
Neural damage was apparent in several regions of the brains of animals in all age groups that experienced SE for greater than 1 h in response to DFP or the NAs (Table 2), and this damage was age-specific. Fluoro-Jade staining was evident in at least two of the five structures that were evaluated, and the frequency and extent of the damage was greatest in the adult PND70 animals (Fig. 8, Table 1). Following DFP exposure, there was an increase in the number of animals sustaining damage in the hilus, laterodorsal thalamus, and parietal cortex that correlated with increasing age of the animal (Fig. 8A1–A4). PND14 rat pups and a PND28 rat that exhibited SE for <1 h after administration of DFP showed some neuronal damage (Fig. 8A1 and A3; Table 2), but not to the extent that older animals did. All the animals (PND21, 28, and 70) exposed to either sarin or VX that showed seizure activity for >1 h showed damage to at least two of the five structures that were evaluated, and the frequency and extent of the damage was again greatest in the adult PND70 animals (Table 1; Fig. 8B1–4 and C1–4). Also, animals in all age groups that did not develop SE following DFP, sarin, or VX administration did not have neuronal damage in the regions listed, nor in any other region in the brain, even if they displayed prolonged behavioral convulsions (Fig. 8A5, B5 and C5). In addition, in sarin- and VX-treated animals in which seizure activity occurred for <1 h following onset, there was no neuronal damage in the brain regions that were analyzed quantitatively or observed informally in any other regions.
Table 2.
Incidence of neural damage in animals that displayed SE <1 h.
| HILUS | LATERODORSAL THALAMUS | BASOLATERAL AMYGDALA | PIRIFORM CORTEX | PARIETAL CORTEX | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| DFP | SARIN | VX | DFP | SARIN | VX | DFP | SARIN | VX | DFP | SARIN | VX | DFP | SARIN | VX | |
| PND14 | 3/8 | N/A | N/A | 0/8 | N/A | N/A | 0/8 | N/A | N/A | 8/8 | N/A | N/A | 0/8 | N/A | N/A |
| PND21 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 1/2 | 0/2 | 0/2 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| PND28 | N/A | 0/3 | 0/3 | N/A | 0/3 | 0/3 | N/A | 0/3 | 0/3 | N/A | 0/3 | 0/3 | N/A | 0/3 | 0/3 |
| PND70 | N/A | N/A | 0/1 | N/A | 0/1 | N/A | N/A | N/A | 0/1 | N/A | N/A | 0/1 | N/A | N/A | 0/1 |
Fig. 8.
Increasing neuronal damage in the hilus correlated with increasing age of the rat pup. Brain sections stained with Fluoro Jade B from representative animals are shown here. Note that no Fluoro Jade B staining was observed in the brains of rat pups that only displayed convulsive behaviors and did not develop sustained SE (last column). Bregma −2.16 to −3.00. Cal bar is 250 μm.
4. Discussion
This study characterized the relations between toxic behavioral responses, electrographically recorded seizure activity and neurological damage in immature and young adult rats using the OP compound DFP and the nerve agents sarin and VX. While exposure to each of these three agents elicited some distinct characteristics in all of the different age groups, in general the animals displayed similar responses to all of the agents and did so in an age-dependent manner. It should be noted that comparisons between age groups need be made with caution since different doses of the three toxic agents were used both within and across the different age groups; however, at least for DFP, the differences in doses across the three immature age groups (even PND14) were relatively small and seemed unlikely to be the basis for the apparent age-related differences in seizure probability and intensity. Furthermore, while different doses of the agents were used, they were adjusted for each agent to be age-appropriate and approximately equipotent for eliciting seizure activity.
4.1. Age-dependence of seizure activity and SE after OP or NA exposure
Across all three agents, the adult PND70 animals were more susceptible to developing consistent seizures and SE than the immature animals. PND14 animals almost never developed seizures or SE in response to sarin or VX. With DFP, periods of seizure-like activity did develop in a subset of PND14 animals, but this abnormal activity was not sustained for >1 h and spontaneously stopped in all cases. With all three agents, seizure activity developed in greater proportions as older age groups were tested, and the proportion of animals in which this seizure activity developed and then spontaneously stopped declined in a similar age-dependent fashion. Admittedly, different dose ranges were used depending upon the agent and age group, so direct comparisons must be made with this caution. Yet when agent-and age-appropriate doses were used, the ability of these three toxic compounds to elicit sustained seizure activity that developed into SE appeared to be age-dependent and similar across compounds.
4.2. Behavioral effects of OP or NA administration
The behavioral correlates of OP- or NA-intoxication in the immature animals most often did not predict electrographic seizure activity. Animals in each age group displayed similar behavioral effects after exposure to DFP, sarin or VX whether seizures developed or not. Many toxic signs and behaviors, such as chewing, head bobbing, whole-body tremor, convulsive-like movements, and Straub tail, were common to all ages across all exposure agents as might be expected for compounds that share a similar toxic mechanism of action. Behaviors that might be expected to occur during the initiation of electrographic seizures, (i.e., forelimb clonus, rearing and falling), and commonly seen in kindling (Racine, 1972) were surprisingly absent, especially in the younger animals (PND14, 21 and 28).
4.3. Relative value of electrographic activity versus behavior in assessment of seizures and subsequent brain damage
Another finding common to all agents and all age groups was that neural damage was only observed in animals that developed sustained SE, and in the case of the two NAs, this activity had to persist for >1 h. Because younger animals (i.e., PND14, 21 and 28) were less susceptible to developing SE with these agents, and when SE did develop it tended to resolve earlier than in the PND70 adults, the younger age groups showed damage in fewer brain areas than the adults. All PND70 animals that developed SE that was sustained for >1 h, regardless of whether the SE was elicited by DFP, sarin or VX, had damage in all the brain areas that were studied. The finding that animals that developed toxic/convulsive signs alone, but did not exhibit prolonged SE did not show neurological damage indicates that caution must be used in interpreting convulsive behavior as indicative of seizure activity in the absence of EEG monitoring. In a recent paper, Miller et al. (2015) stated that PND21 rats exposed to the NA soman developed convulsive behaviors which they interpreted as SE, but these animals did not display any evidence of neuropathology as determined by absence of Fluoro-Jade staining in the amygdala, hippocampus or other brain areas at either 24 h or 7 days after exposure. They also reported a reduction in amygdala and hippocampal volumes in PND21 animals exposed to soman when measured 30 or 90 days after exposure along with impairment in fear conditioning and an increase in anxiety-like behaviors at 30 days, but not 90 days, following exposure; these brain volumetric changes and behavioral deficits could be blocked if animals received a GluK1 receptor antagonist 60 min after exposure. In contrast, the present results show that frank neural damage, as indicated by robust Fluoro-Jade staining 24 h after exposure, can be elicited by the NAs sarin and VX when sustained SE is confirmed with EEG. Many research groups use the Racine scale (Racine, 1972) to grade the development of convulsive behaviors that presumably indicate the concurrent electrographic seizure activity. The Racine scale of behavioral concomitants of seizures was originally based on observations in kindled animals, not in animals treated with chemoconvulsants, especially OP or NA compounds. Several toxic signs of excess cholinergic stimulation, such as chewing, tremors, head bobbing and fasciculations, are defining features of early stage Racine scale seizures, but can also be elicited by a purely peripherally acting compound (Abou-Donia et al., 1996). Straub tail has also been used as a behavioral indication of seizure in developing rats (Veliskova, 2006), although this phenomenon is simply a reflex action that originates in the spinal cord and manifests at the neuromuscular junction (Leimdorfer, 1948; Bilbey et al., 1960). OPs and NAs often render the animal unable to stand, such that rearing or rearing with falling accompanied by forelimb clonus is only rarely seen in the development of SE with NAs (Carpentier et al., 1990; McDonough and Shih, 1993). Therefore, although use of behavioral measures alone to assess the effects of convulsive compounds is known to have limitations in adult animals, this approach is even more problematic in immature rodents.
4.4. Decreased – not increased – susceptibility of immature animals to the seizure-inducing effects of OPs and NAs
The current thinking that suggests that very young animals are more susceptible to OP- or NA-induced seizures is not supported by these results. The data with DFP, sarin and VX support the contrary notion that while the compounds are highly lethal, it is more difficult to induce electrographic seizure activity in immature animals using age-appropriate and approximately equipotent toxic doses. The most striking example of this was in PND14 animals, where more than 45 rats were exposed to sarin or VX, and although all of the animals displayed at least some convulsive behavior (as described), none of them demonstrated electrographic seizure activity. There could be several explanations for these observations. An immature cholinergic system could contribute to the low percentage of EEG seizures in immature animals. Consistent with this proposal, genetic knockout studies in mice have shown that seizures are elicited by cholinomimetics, such as pilocarpine, through stimulation of the M1 muscarinic receptor (Hamilton et al.,1997). In rats, the M1 muscarinic receptor is only at 70% of adult levels at PND14 and at 90% at PND21 (Lee et al., 1990). However, recent work from Torolira et al. (2016) showed that rat pups as young as PND7 can develop SE and widespread neuronal injury in response to lithium-pilocarpine treatment, which suggests that the M1 receptor system is mature enough at that age to support cholinergically induced SE under the appropriate experimental conditions. The biggest difference between the lithium-pilocarpine SE models and the OPs used here is that pilocarpine is a direct agonist at M1 receptors. In contrast, OPs act as indirect agonists by inhibiting AChE, and the resultant high levels of acetylcholine act at all central and peripheral muscarinic and nicotinic receptors. It is quite possible that the indirect cholinergic agonists – like the OPs – cannot mimic the high levels of M1 stimulation produced by pilocarpine in these younger animals. Several studies have shown that brain AChE levels in subcortical regions of rodents progressively increase postnatally to adult levels, usually by 20–60 days (Thal et al., 1992; Berdel et al., 1996; Lassiter et al., 1998). The expression pattern appears to be more complex in the neocortex, depending upon the cortical area studied (Geula et al., 1995). Postmortem studies in humans show an increasing level of choline acetyltransferase, which commonly parallels AChE levels, in hippocampus and entorhinal cortex throughout the first several decades of life (Perry et al., 1993). As stated above, M1 receptors in rats show a progressive increase from 30% in PND7, 70% in PND14, 90% in PND21, to adult levels in PND28 animals that parallels the increase in AChE levels (Lee et al., 1990). Because of these differences between enzyme levels and receptor levels, it might be speculated that this could account for the increased lethal effects of these agents at lower doses in younger animals in the absence of seizures, while post-weanling animals require higher doses and are susceptible to the seizurogenic potential of these agents.
4.5. OP- or NA-induced neuronal damage was highly dependent on the duration of seizure activity
Most investigators agree that neuronal damage after administration of chemoconvulsant compounds correlates with the age of the animal at the time of exposure. Similar to the current results, other workers have also observed increases in neuronal damage, with the increasing age of the animal at the time of the injury, with SE-provoking chemoconvulsants such as kainic acid and pilocarpine (Cavalheiro et al., 1987; Sperber et al., 1991; Priel et al., 1996; Sankar et al.,1998; Yang et al.,1998; Druga et al., 2005; Mares et al., 2005). Specifically, 24 h after treatment with pilocarpine, older but still immature animals had greater neuronal damage in the parietal cortex (Mares et al., 2005) and hilus (Sankar et al., 1998) than did younger immature animals.
In our study, neuronal damage in response to DFP-, sarin- or VX-induced SE increased not only with age, but also with the length of electrographic SE. For example, PND21 animals that were treated with DFP and displayed electrographic SE for >1 h had a lower percentage of damage in the hilus, LD thalamus and parietal cortex than did PND28 and PND70 rats. Furthermore, following DFP treatment, fewer PND28 rats showed damage in the parietal cortex than PND70 animals with damage in this region. PND14 animals showed the least amount of damage, although this could also be due to the short duration of SE after DFP treatment (Table 2). A time-dependence for neuronal injury and death has been described in kainate-treated (Covolan and Mello, 2000), pilocarpine-treated (Lemos and Cavalheiro,1996) and NA-treated animals (Lallement et al., 1994; McDonough et al.,1995). These trends were also observed in our DFP- and NA-treated animals, animals that only exhibited short SE or EA showed little or no damage, whereas animals with longer sustained SE experienced greater neuronal damage.
4.6. Dose dependence of OP and NA effects on seizures and neuronal injury
For all agents and ages, the dose window between evoking EEG seizures versus causing death was very narrow, which has long been a methodological problem for studies with chemoconvulsive compounds, particularly with OPs and NAs. If no pretreatments or therapies are used with OPs and NAs, and the challenge dose of DFP or NA is high enough to elicit seizures, test animals fall into one of three categories: (1) The animals develop toxic signs but do not develop EEG evidence of sustained seizures. In these animals the toxic signs usually subside slowly over many hours, and most of these animals will survive for 24 h and will display no brain pathology. This first category represents ~40% of a tested group. (2) The animals die shortly (5–10 min) after agent exposure or seizure onset from either fulminating pulmonary edema or apnea. In the case of NAs, some of the remaining animals that do not die immediately continue to have seizures but die overnight. This second category represents ~40% of a test group. (3) Animals develop sustained SE with obvious robust seizures and survive for 24 h. Most of these animals still display epileptiform activity, continued SE, bursts of EEG spikes and/or isolated spike discharges or some combination of the above. This abnormal activity typically resolves over several days. These survivors at this point are also in a fragile physical condition having lost substantial body weight and require special care (e.g., s.c. fluids, mash) over several days to encourage feeding and minimize further mortality. These animals will invariably display widespread neuropathology, as has been described in rodent models of DFP-, NA-, or pilocarpine-induced SE (Turski et al., 1983; McDonough et al., 1995, 1998; Crawford et al., 2004; Todorovic et al., 2012; Scholl et al., 2013; Pouliot et al., 2016). This third category typically represents ~20% of a test group. The use of specific pretreatments (e.g., pyridostigmine, HI-6) along with low doses of centrally or peripherally acting anticholinergics (e.g., atropine sulfate, atropine methyl nitrate) allows the use of higher challenge doses of either DFP or the NAs (Shih et al., 1990; Shih and McDonough et al., 1999; Deshpande et al., 2010; Pessah et al., 2016; Pouliot et al., 2016). The use of peripherally acting anticholinergics is also common in the pilocarpine and lithium-pilocarpine models of SE to minimize mortality (Turski et al., 1983; Cavalheiro et al., 1987; Scholl et al., 2013). This increases the percentage of animals that develop sustained seizures and minimizes, but does not eliminate, the early or late mortality. This conclusion about the percentage of animals that display seizures and consequent brain damage is subject to the problems of batch-to-batch variability of DFP potency (Heiss et al., 2016), which is also seen with pilocarpine and kainate. These factors are the main challenges in achieving substantial numbers of brain damaged long-term survivors when conducting these types of SE experiments.
5. Conclusions
Few substantial differences were found between the effects of DFP and those of the two NAs as a function of age in immature rats. This lends weight to the use of DFP as a surrogate for NA effects in both immature and adult animals (Deshpande et al., 2010; Pessah et al. 2016; Pouliot et al., 2016). Likewise, no notable differences were found between male and female animals at any age in response to DFP or the NAs in development of SE or neuronal injury. Older immature rat pups (PND21 and PND28) were more similar to adult animals (PND70) than to PND14 pups in terms of EEG seizure behavior and neuronal damage. Since the youngest animals, (i.e., the PND14 pups) were unable to sustain electrographic seizures for very long periods (DFP), or even at all (sarin, VX), the older groups of immature animals (i.e., PND21 and 28 pups) may be more suited as models to evaluate the seizurogenic effects of exposure to OP pesticides or NAs in the pediatric population. As in adult animals, only those immature animals that developed sustained electrographic seizure activity had neuropathological damage. Because no reliable behavioral indicators correlated with electrographic seizures in immature rats, this study reinforces the conclusion that behavioral indices alone are insufficient to establish the presence or absence of electrographic seizures. Therefore, it is crucial to instrument animals when investigating the effects of chemoconvulsants such as OPs and NAs on electrographic seizure activity and neuronal damage.
Acknowledgments
The views expressed in this paper are those of the authors and do not reflect the official policy of the Countermeasures Against Chemical Threats (CounterACT) program, National Institutes of Health (NIH), Department of Health and Human Services, Department of Army, Department of Defense (DoD), or the U.S. Government. This research was supported by the NIH Office of the Director through an Inter-Agency Agreement (Y1-O6-9613-01) between the National Institute of Allergy and Infectious Diseases (NIAID) and DoD USAMRICD (A120-B.P2009-2) and under subcontract W81XWH-14-C-0119 to the University of Utah. Support was also provided by an appointment to the Research Participation Program for the U.S. Army Medical Research Institute of Chemical Defense administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and U.S. Army Medical Research and Materiel Command.
References
- Aas P. The threat of mid-spectrum chemical warfare agents. Prehosp Disaster Med. 2003;18:306–312. doi: 10.1017/s1049023x00001254. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Wilmarth KR, Jensen KF, Oehme FW, Kurt TL. Neurotoxicity resulting from coexposure to pyridostigmine bromide, deet, and permethrin: implications of Gulf War chemical exposures. J Toxicol Environ Health. 1996;48:35–56. doi: 10.1080/009841096161456. doi: http://dx.doi.org/10.1080/009841096161456. [DOI] [PubMed] [Google Scholar]
- Albala BJ, Moshe SL, Okada R. Kainic-acid-induced seizures: A developmental study. Dev Brain Res. 1984;13:139–148. doi: 10.1016/0165-3806(84)90085-3. doi: http://dx.doi.org/10.1016/0165-3806(84)90085-3. [DOI] [PubMed] [Google Scholar]
- Apland JP, Figueiredo TH, Qashu F, Aroniadou-Anderjaska V, Souza AP, Braga MF. Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology. 2010;31:485–492. doi: 10.1016/j.neuro.2010.05.014. doi: http://dx.doi.org/10.1016/j.neuro.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD. Antidotes for nerve agent poisoning: should we differentiate children from adults? Curr Opin Pediatr. 2007;19:211–215. doi: 10.1097/MOP.0b013e328012cba2. doi: http://dx.doi.org/10.1097/MOP.0b013e328012cba2. [DOI] [PubMed] [Google Scholar]
- Berdel B, Moryś J, Maciejewska B, Narkiewicz O. Acetylcholinesterase activity as a marker of maturation of the basolateral complex of the amygdaloid body in the rat. Int J Dev Neurosci. 1996;14:543–549. [PubMed] [Google Scholar]
- Bilbey DLJ, Salem H, Grossman MH. The anatomical basis of the straub phenomenon. Brit J Pharmacol. 1960;15:540–543. doi: 10.1111/j.1476-5381.1960.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier P, Iroudayanadin S, Delamanche S, Le Bert M, Blanchet G, Bouchaud S. Seizure-related opening of the blood-brain barrier induced by soman: possible correlation with the acute neuropathology. Neuro Toxicol. 1990;11:493–508. [PubMed] [Google Scholar]
- Cavalheiro EA, Silva DF, Turski WA, Calderazzo-Filho LS, Bortolotto ZA, Turski L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Brain Res. 1987;37:43–58. doi: 10.1016/0165-3806(87)90227-6. [DOI] [PubMed] [Google Scholar]
- Covolan L, Mello LE. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000;3:133–152. doi: 10.1016/s0920-1211(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Crawford SM, Compton JR, Tetz LM, Ratcliffe RH, Steele KH, Gordon RK, Nambiar MP, Avenue RG, Spring S. Development of a rat diisopropylfluorophosphate-induced seizure/status epilepticus model for screening of neuroprotectants following exposure to chemical warfare agents. 2004 Scientific Conference on Chemical and Biological Defense Research.2004. [Google Scholar]
- Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ. Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate. Toxicol Sci. 2010;116:623–631. doi: 10.1093/toxsci/kfq157. doi: http://dx.doi.org/10.1093/toxsci/kfq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druga R, Mareš P, Otáhal J, Kubová H. Degenerative neuronal changes in the rat thalamus induced by status epilepticus at different developmental stages. Epilepsy Res. 2005;63:43–65. doi: 10.1016/j.eplepsyres.2004.11.001. doi: http://dx.doi.org/10.1016/j.eplepsyres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Fawcett WP, Aracava Y, Adler M, Pereira EF, Albuqueque EX. Acute toxicity of organophosphorus compounds in guinea pigs is sex- and age-dependent and cannot be solely accounted for by acetylcholinesterase inhibition. J Pharmacol Exp Ther. 2009;328:516–524. doi: 10.1124/jpet.108.146639. doi: http://dx.doi.org/10.1124/jpet.108.146639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Mesulam MM, Kuo CC, Tokuno H. Postnatal development of cortical acetylcholinesterase-rich neurons in the rat brain: permanent and transient patterns. Exp Neurol. 1995;134:157–178. doi: 10.1006/exnr.1995.1046. doi: http://dx.doi.org/10.1006/exnr.1995.1046. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci U S A. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. doi: http://dx.doi.org/10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut SR, Veliskova J, Moshe SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3:608–617. doi: 10.1016/S1474-4422(04)00881-6. doi: http://dx.doi.org/10.1016/S1474-4422(04)00881-6. [DOI] [PubMed] [Google Scholar]
- Heiss DR, Zehnder DW, Jett DA, Platoff GE, Jr, Yeung ST, Brewer BN. Synthesis and storage stability of diisopropylfluorophosphate. J Chem. 2016;(2016):3190891. doi: 10.1155/2016/3190891. doi: http://dx.doi.org/10.1155/2016/3190891. [DOI] [PMC free article] [PubMed]
- Lallement G, Pernot-Marino I, Baubichon D, Burckhart MF, Carpentier P, Blanchet G. Modulation of soman-induced neuropathology with an anticonvulsant regimen. Neuroreport. 1994;5:2265–2268. doi: 10.1097/00001756-199411000-00015. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Barone S, Jr, Padilla S. Ontogenetic differences in the regional and cellular acetylcholinesterase and butyrylcholinesterase activity in the rat brain. Brain Res Dev Brain Res. 1998;105:109–123. [PubMed] [Google Scholar]
- Lee W, Nicklaus KJ, Manning DR, Wolfe BB. Ontogeny of cortical muscarinic receptor subtypes and muscarinic receptor-mediated responses in rat. J Pharmacol Exp Ther. 1990;252:482–490. [PubMed] [Google Scholar]
- Leimdorfer A. An electroencephalographic analysis of the action of amidone, morphine and strychnine on the central nervous system. Arch Int Pharmacodyn Ther. 1948;76:153–162. [PubMed] [Google Scholar]
- Lemos T, Cavalheiro EA. Status epilepticus and the late development of spontaneous seizures in the pilocarpine model of epilepsy. Epilepsy Res. 1996;(Suppl 12):137–144. [PubMed] [Google Scholar]
- Li Y, Lein PJ, Liu C, Bruun DA, Tewolde T, Ford G, Ford BD. Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication. Toxicol Appl Pharmacol. 2011;253:261–269. doi: 10.1016/j.taap.2011.03.026. doi: http://dx.doi.org/10.1016/j.taap.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshitz M, Shahak E, Sofer S. Carbamate and organophosphate poisoning in young children. Pediatr Emerg Care. 1999;15:102–103. doi: 10.1097/00006565-199904000-00006. [DOI] [PubMed] [Google Scholar]
- Mares P, Tsenov G, Aleksakhina K, Druga R, Kubova H. Changes of cortical interhemispheric responses after status epilepticus in immature rats. Epilepsia. 2005;46(Suppl 5):31–37. doi: 10.1111/j.1528-1167.2005.01004.x. doi: http://dx.doi.org/10.1111/j.1528-1167.2005.01004. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Shih TM. Pharmacological modulation of soman-induced seizures. Neurosci Biobehav Rev. 1993;17:203–215. doi: 10.1016/s0149-7634(05)80151-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Dochterman LW, Smith CD, Shih TM. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;16:123–132. [PubMed] [Google Scholar]
- McDonough JH, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Clark TR, Slone TW, Zoeffel D, Brown K, Kim S, Smith CD. Neural lesions in the rat and their relationship to EEG delta activity following seizures induced by the nerve agent soman. Neurotoxicology. 1998;19:381–392. [PubMed] [Google Scholar]
- Miller SL, Aroniadou-Anderjaska V, Figueiredo TH, Prager EM, Almeida-Suhett CP, Apland JP, Braga MFM. A rat model of nerve agent exposure applicable to the pediatric population: the anticonvulsant efficacies of atropine and GluK1 antagonists. Toxicol Appl Pharmacol. 2015;284:204–216. doi: 10.1016/j.taap.2015.02.008. doi: http://dx.doi.org/10.1016/j.taap.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Piggott MA, Court JA, Johnson M, Perry RH. Transmitters in the developing and senescent human brain. Ann N Y Acad Sci. 1993;1695:69–72. doi: 10.1111/j.1749-6632.1993.tb23030.x. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Rogawski MA, Tancredi DJ, Wulff H, Zolkowski D, Bruun DA, Hammock BD, Lein PJ. Models to identify treatments for the acute and persistent effects of seizure-inducing chemical threat agents. Ann NY Acad Sci. 2016;1378:124–136. doi: 10.1111/nyas.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot W, Bealer SL, Roach B, Dudek FE. A rodent model of human organophosphate exposure producing status epilepticus and neuropathology. Neurotoxicology. 2016;56:196–203. doi: 10.1016/j.neuro.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel MR, dos Santos NF, Cavalheiro EA. Developmental aspects of the pilocarpine model of epilepsy. Epilepsy Res. 1996;26:115–121. doi: 10.1016/s0920-1211(96)00047-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure Electroencephalogr. Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rotenberg JS, Newmark J. Nerve agent attacks on children: diagnosis and management. Pediatrics. 2003;112:648–658. doi: 10.1542/peds.112.3.648. doi: http://dx.doi.org/10.1542/peds.112.3.648. [DOI] [PubMed] [Google Scholar]
- Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantlebury MH, Heida JG, Hasson HJ, Veliskova J, Velisek L, Galanopoulou AS, Moshe SL. Age-dependent consequences of status epilepticus: animal models. Epilepsia. 2007;48:75–82. doi: 10.1111/j.1528-1167.2007.01069.x. doi: http://dx.doi.org/10.1111/j.1528-1167.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- Schaffer PO, Sirven JI. Epilepsy Statistics. Epilepsy Foundation; Oct, 2013. Epilepsy.com/Learn/Epilepsy-Statistics. Web. 14 Feb. 2017. [Google Scholar]
- Schmued LC, Albertson C, Slikker W. Fluoro-jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Scholl EA, Dudek FE, Ekstrand JJ. Neuronal degeneration is observed in multiple regions outside the hippocampus after lithium pilocarpine-induced status epilepticus in the immature rat. Neuroscience. 2013;252:45–59. doi: 10.1016/j.neuroscience.2013.07.045. doi: http://dx.doi.org/10.1016/j.neuroscience.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Penetar DM, McDonough JH, Romano JA, King JM. Age-related differences in soman toxicity and in blood and brain regional cholinesterase activity. Brain Res Bull. 1990;24:429–436. doi: 10.1016/0361-9230(90)90097-j. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JM., Jr Organophosphorus nerve agents-induced seizures and efficacy of atropine sulfate as anticonvulsant treatment. Pharmacol Biochem Behav. 1999;64:147–153. doi: 10.1016/s0091-3057(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Shih TM, Guarisco JA, Myers TM, Kan RK, McDonough JH. The oxime pro-2-PAM provides minimal protection against the CNS effects of the nerve agents sarin, cyclosarin, and VX in guinea pigs. Toxicol Mech Methods. 2011;21:53–62. doi: 10.3109/15376516.2010.529190. doi: http://dx.doi.org/10.3109/15376516.2010.529190. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Pellock JM, Moshe SL, Maytal J, O’Dell C, Driscoll SM, Alemany M, Newstein D, DeLorenzo RJ. In whom does status epilepticus occur: age-related differences in children. Epilepsia. 1997;38:907–914. doi: 10.1111/j.1528-1157.1997.tb01256.x. doi: http://dx.doi.org/10.1111/j.1528-1157.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Haas KZ, Stanton PK, Moshé SL. Resistance of the immature hippocampus to seizure-induced synaptic reorganization. Dev Brain Res. 1991;60:88–93. doi: 10.1016/0165-3806(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Thal L, Gilbertson E, Armstrong DM, Gage FH. Development of the basal forebrain cholinergic system: phenotype expression prior to target innervation. Neurobiol Aging. 1992;13:67–72. doi: 10.1016/0197-4580(92)90011-l. [DOI] [PubMed] [Google Scholar]
- Todorovic MS, Cowan ML, Balint CA, Sun C, Kapur J. Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res. 2012;101:268–276. doi: 10.1016/j.eplepsyres.2012.04.014. doi: http://dx.doi.org/10.1016/j.eplepsyres.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torolira D, Suchomelova L, Wasterlain CG, Niquet J. Widespread neuronal injury in a model of cholinergic status epilepticus in postnatal day 7 rat pups. Epilepsy Res. 2016;120:47–54. doi: 10.1016/j.eplepsyres.2015.11.005. doi: http://dx.doi.org/10.1016/j.eplepsyres.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay E, Nitecka L, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. I clinical, electrographic and metabolic observations. Neuroscience. 1984;13:1051–1072. doi: 10.1016/0306-4522(84)90288-4. [DOI] [PubMed] [Google Scholar]
- Tuovinen K. Organophosphate-induced convulsions and prevention of neuropathological damages. Toxicology. 2004;196:31–39. doi: 10.1016/j.tox.2003.10.013. doi: http://dx.doi.org/10.1016/j.tox.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Brain Behav Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Veliskova J. Behavioral characterization of seizures in rats. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier Inc; 2006. pp. 601–611. [Google Scholar]
- Verhulst L, Waggie Z, Hatherill M, Reynolds L, Argent A. Presentation and outcome of severe anticholinesterase insecticide poisoning. Arch Dis Child. 2002;86:352–355. doi: 10.1136/adc.86.5.352. doi: http://dx.doi.org/10.1136/adc.86.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the Newborn. Saunders; Philadelphia, PA: 2008. pp. 178–214. [Google Scholar]
- Wright LKM, Lee RB, Vincelli NM, Whalley CE, Lumley LA. Comparison of the lethal effects of chemical warfare nerve agents across multiple ages. Toxicol Lett. 2016;241:167–174. doi: 10.1016/j.toxlet.2015.11.023. doi: http://dx.doi.org/10.1016/j.toxlet.2015.11.023. [DOI] [PubMed] [Google Scholar]
- Yang Y, Tandon P, Liu Z, Sarkisian MR, Stafstrom CE, Holmes GL. Synaptic reorganization following kainic acid-induced seizures during development. Brain Res Dev Brain Res. 1998;107:169–177. doi: 10.1016/s0165-3806(97)00211-3. [DOI] [PubMed] [Google Scholar]
- Zayachkivsky A, Lehmkuhle MJ, Fisher JH, Ekstrand JJ, Dudek FE. Recording EEG in immature rats with a novel miniature telemetry system. J Neurophysiology. 2013;114:900–911. doi: 10.1152/jn.00593.2012. doi: http://dx.doi.org/10.1152/jn.00593.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiener RJ, Ginsburg CM. Organophosphate and carbamate poisoning in infants and children. Pediatrics. 1988;81:121–126. [PubMed] [Google Scholar]