Abstract
Advanced gastric cancer (AGC) has high morbidity and mortality in East Asia, and it is urgent to explore new treatments to improve patient prognosis. Programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors have exhibited remarkable activity in clinical trials and were approved by the FDA for clinical therapy in several types of tumors. Here, we evaluated PD-L1 expression and T cell infiltration in AGC. Positive tumor PD-L1 expression was detected in 171 AGCs (33.60%) out of 509 AGCs. PD-L1 expression was positively correlated with CD8+ T cell infiltration. Then, PD-L1 and CD8A mRNA expression was analyzed using gastric cancer data from the TCGA database, confirming a positive correlation. Patient survival was assessed according to PD-L1 status and the T cell infiltration density. PD-L1 expression and a high density of CD8+ T cells in AGCs were associated with improved prognosis, whereas no significant difference was noted between PD-1 and CD3 expression. In contrast, a high density of FOXP3+ T cells in AGCs indicated a poor prognosis. Multivariate Cox regression analysis revealed that CD8+ T cell density acts as an independent predictor of overall survival (OS) in AGC patients. Taken together, this study further highlights targets for immune checkpoint-based therapy in AGC.
1. Introduction
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer-related death worldwide [1]. The recent CONCORD-3 study was published in Lancet this year. The worldwide surveillance of cancer survival reported in this study demonstrated that GC ranked second in cancer incidence in China with lung cancer ranking first (GC, 15.6%; lung cancer, 22.6%) [2]. Currently, surgical resection and perioperative chemotherapy are routine treatments for AGC. The prognosis of this cancer is dismal, and the need for new strategies to treat AGC is pressing.
Immunotherapy, especially immune checkpoint blockade, has emerged as a promising cancer treatment [3]. Immune checkpoint inhibitors, such as anti-PD-1 (nivolumab and pembrolizumab), anti-PD-L1 (atezolizumab), and anti-CTLA-4 (ipilimumab) drugs, were approved by the Food and Drug Administration (FDA) to treat various types of cancer [4, 5]. The above inhibitors were recommended by the FDA for the treatment of melanoma, non-small-cell lung cancer, and other cancers. Regarding GC therapy with immune checkpoint inhibitors, although no drug has been recommended by the FDA, several clinical trials revealed survival benefits after anti-PD-1 treatment [6, 7].
PD-L1 expression in tumor cells and the immune checkpoint blockade therapeutic response have a close relationship. In a previous study involving 17 patients with PD-L1-negative tumors, none of the patients exhibited an objective response, while for PD-L1-positive tumors, 9 of 25 patients (36%) exhibited an objective response [8]. In another study involving patients with recurrent or metastatic PD-L1-positive gastric cancer, the anti-PD-1 drug pembrolizumab exhibited a promising antitumor effect [7]. Mismatch repair deficiency can also indicate the response of immune checkpoint blockage, and a large proportion of mutant neoantigens in mismatch repair-deficient cancers make them sensitive to anti-PD-1 therapy [9]. PD-L1 expression was significantly associated with mismatch repair deficiency in a large number of patients representing several tumor types [10, 11]. Based on the above data, we concluded that tumor PD-L1 expression status in cancer played an important role in the immune microenvironment. However, studies on tumor PD-L1 expression in GC with large patient numbers are needed to dissect the detailed mechanism.
We investigated PD-L1 expression and T cell infiltration in a tumor microarray (TMA) representing 509 AGC patients. The correlation between PD-L1 expression and T cell infiltration was examined in our study and using GC data from the TCGA database. Finally, the relationships between PD-L1 status and T cell infiltration with patient overall survival (OS) were analyzed.
2. Materials and Methods
2.1. Patients and TMA Construction
This is a retrospective analysis of 509 patients with primary gastric cancer who underwent gastrectomy at the Department of Gastrointestinal Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, from January 2006 to December 2011. The final follow-up date was December 31, 2017, for all cases examined. OS time was defined as the interval between the gastrectomy and patient death or survival. A total of 242 mortalities occurred, which were all due to cancer-associated causes. All patients received the standard treatments such as D2 radical resection and first-line adjuvant chemotherapy according to the NCCN guide. Only 25 patients did not finish the standard chemotherapy for their personal reasons or inability to tolerate side effects. There was no difference in the number of not finishing standard chemotherapy between PD-L1-positive and -negative groups. We excluded the following types of patients: (1) patients with recurrent gastric cancer after the radical operation, (2) patients receiving neoadjuvant chemotherapy or previous radiotherapy, (3) patients suffering from other malignant tumors, and (4) patients with autoimmune or immunodeficiency diseases.
We collected formalin-fixed paraffin-embedded (FFPE) tissue blocks from the pathology department of Renji hospital. Tumor TNM stage was assigned based on pathological tumor, node, and metastasis staging per the American Joint Committee on Cancer (AJCC 8th edition) staging system. For each case, the diagnosis was confirmed by two senior pathologists through a review of H&E-stained slides. Representative FFPE blocks were chosen to punch onto glass slides to construct the TMA. Every patient's tumor tissue on the TMA was consecutive, and the TMA was constructed using a tissue arrayer with 5 μm thickness.
This study was approved by the ethics committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine, for the use of samples. Informed consent was obtained from all enrolled patients before study inclusion.
2.2. Immunohistochemistry
Immunohistochemistry (IHC) was performed on the TMA using antibodies specific to PD-L1 (1 : 100, Abcam, UK, ab205921), PD-1 (1 : 100, CST, USA, 43248), CD3 (1 : 200, Wuhan Goodbio Technology Co., Ltd., China), CD8 (1 : 100, Wuhan Goodbio Technology Co., Ltd., China), and FOXP3 (1 : 200, CST, USA, 98377). Briefly, after tissue sections were deparaffinized, rehydrated with graded ethanol, incubated with 0.3% hydrogen peroxide for 30 minutes, and blocked with 10% BSA (Sangon, Shanghai, China), slides were first incubated using the antibody at 4°C overnight and then labeled with the HRP second antibody (Thermo Scientific, US) at room temperature for 1 h. Positive staining was visualized with DAB substrate liquid (Gene Tech, Shanghai) and counterstained with hematoxylin [12]. All the sections were observed and photographed with a microscope (Carl Zeiss, Germany). In the following analysis, we excluded immune cells in vessels, lymph nodes and lymphatics, necrotic tissue, or necrosis-adjacent areas.
2.3. IHC Evaluation
Tumor PD-L1 expression in the cytoplasm and membrane of tumor cells was evaluated based on immunostaining. The PD-L1-positive group was defined based on greater than 5% of stained cells regardless of cytoplasmic or membrane staining. The remaining cases comprised the negative group. The criterion for classification in the PD-1 high expression group was greater than 5 cells stained per high-power field (HPF), whereas the remaining cases comprised the low expression group [13]. We chose four random areas (amplification 200x, 0.34 mm2) on the TMA for each case and counted the average CD3+, CD8+, and FOXP3+ cell density. According to the median number of stained cells (CD3, 80/0.34 mm2; CD8, 35/0.34 mm2), patients were dichotomized into the high and low density group. To evaluate Foxp3+ T cells, given that few cells were stained, we defined the high infiltration group as greater than 5 stained cells/HPF, whereas the remaining cases comprised the low infiltration group. Digital image analysis and Nikon DR-Si2 cell count software were used for the staining evaluation described above, and the results were verified by two senior pathologists who were blinded to the clinicopathological data.
2.4. TCGA Database Analysis
From 450 GC samples from the TCGA database, we selected 415 tumor tissue samples except 35 normal tissue samples. We analyzed the correlation between PD-L1 and CD8A expression at the mRNA level.
2.5. Statistical Analysis
Standard statistical tests were used to analyze clinical data. Associations between PD-L1 expression and clinicopathological factors were tested using a χ 2 test or Fisher's exact test. The correlation between PD-L1 and CD8A mRNA expression was calculated using the Spearman correlation test. Survival analysis was performed using the Kaplan-Meier method and the long-rank test. Univariate and multivariate analysis were conducted using the Cox proportional hazards model to analyze prognostic factors. All statistical tests were 2-sided, and P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 statistical package software (SPSS, Chicago, IL, USA) or GraphPad Prism (GraphPad Software Inc., San Diego, CA).
3. Results
3.1. Clinicopathological Findings
A retrospective cohort study of 509 AGC patients, including 49 TNM stage I cases, 172 TNM stage II cases, and 288 TNM stage III cases, was conducted. The median age of the AGC patients was 62 (22–89) years, and the median OS time was 48 (2–117) months. A total of 242 (47.54%) patients died during the follow-up period. Among the 509 cases, male patients (347/509) and a low position of the lesion (360/509) represented a large proportion of the cohort. In total, 96 and 80 cases exhibited blood vessel and perineuronal invasion, respectively, among the 509 AGC patients. The detailed clinicopathological characteristics of the patients are presented in Table 1.
Table 1.
Correlation between tumor PD-L1 expression and clinicopathological parameters in GC patients.
| Clinicopathological features | Cases | PD-L1 expression | P value (χ 2 test) | |
|---|---|---|---|---|
| Positive | Negative | |||
| 171 | 338 | |||
| Gender | ||||
| Male | 347 | 116 | 231 | 0.9077 |
| Female | 162 | 55 | 107 | |
| Age(years) | ||||
| ≤60 | 224 | 65 | 159 | 0.0526 |
| >60 | 285 | 106 | 179 | |
| Tumor location | ||||
| Up | 73 | 22 | 51 | 0.8970 |
| Middle | 105 | 35 | 70 | |
| Low | 260 | 89 | 171 | |
| Total | 66 | 24 | 42 | |
| Remnant | 5 | 1 | 4 | |
| Length-diameter | ||||
| <5 cm | 232 | 93 | 139 | 0.0045∗∗ |
| ≥5 cm | 277 | 78 | 199 | |
| Lauren type | ||||
| Intestinal | 163 | 62 | 101 | 0.3268 |
| Diffuse | 326 | 102 | 224 | |
| Mix | 20 | 7 | 13 | |
| Blood vessel invasion | ||||
| Absent | 413 | 139 | 274 | 0.9519 |
| Present | 96 | 32 | 64 | |
| Perineuronal invasion | ||||
| Absent | 429 | 151 | 278 | 0.0762 |
| Present | 80 | 20 | 60 | |
| pT stage | ||||
| T2 | 85 | 33 | 52 | 0.5263 |
| T3 | 151 | 50 | 101 | |
| T4 | 273 | 88 | 185 | |
| pN stage | ||||
| N0 | 162 | 59 | 103 | 0.4947 |
| N1 | 96 | 35 | 61 | |
| N2 | 112 | 37 | 75 | |
| N3 | 139 | 40 | 99 | |
| TNM stage | ||||
| I | 49 | 17 | 32 | 0.2255 |
| II | 172 | 66 | 106 | |
| III | 288 | 88 | 200 | |
∗∗ P < 0.01.
3.2. PD-L1 Expression in AGC and Its Association with Clinicopathological Parameters
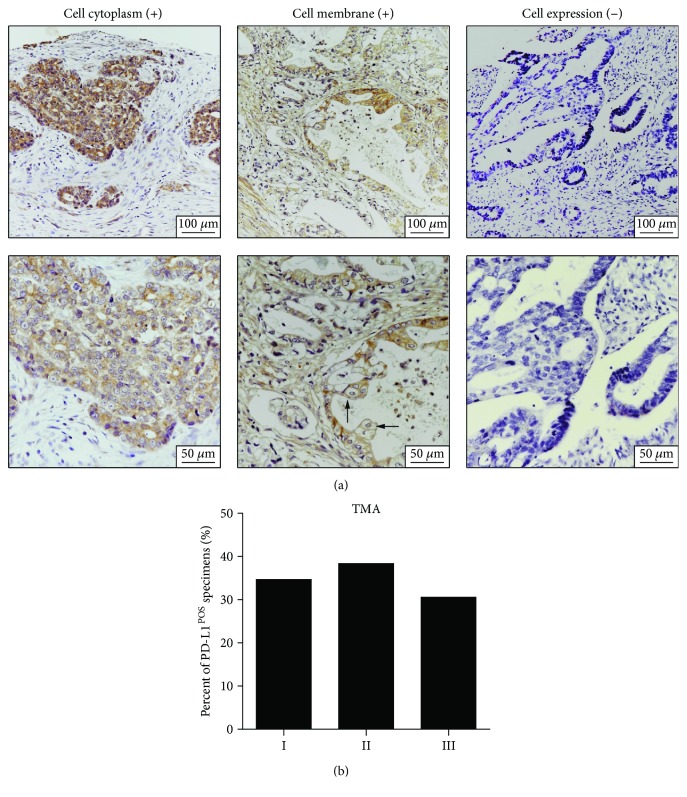
In tumor cells, PD-L1 is expressed in the cytoplasm and on membranes (Figure 1(a)). Tumor PD-L1 expression was detected in 171 (33.60%) cases among 509 AGC patients. Regarding TNM stage I, II, and III patients, the percent of tumor PD-L1-positive patients among the three stages did not differ significantly (P = 0.2255) (Figure 1(b)).
Figure 1.
PD-L1 expression in AGCs. (a) Representative samples of IHC staining of PD-L1, including tumor cell cytoplasm staining, tumor cell membrane staining, and negative control, are presented. The lower panel (400x original magnification) is the zoom-in image of the upper panel (200x original magnification). The arrows indicate the membrane PD-L1 expression in tumor cells. (b) The percent of tumor PD-L1-positive specimens in TNM stage I, II, and III GC patients.
The relationship between tumor PD-L1 expression and the clinical characteristics of AGC patients is presented in Table 1. PD-L1 expression was positively associated with tumor length-diameter (P = 0.0045). No significant relationship was found between tumor PD-L1-positive status and other clinicopathological features.
3.3. T Cell Infiltration in Tumor Tissues and Its Association with Clinicopathological Parameters
PD-1 expression was present in infiltrating immune cells, and CD3+, CD8+, and FOXP3+ T cell infiltration in tumor tissues was evident (Figure 2(a)). Regarding the assessments of PD-1 expression and clinicopathological characteristics, we found that PD-1 expression exhibited a close relationship with perineuronal invasion (P = 0.0241). No close relationship between PD-1 expression and other clinicopathological features was found (Supplementary Table S1). Regarding the T cell infiltration and patient clinicopathological characteristics analysis, high CD3+ T cell infiltration in tumor tissues was positively associated with the patient's Lauren type (P = 0.0243). CD8+ T cell infiltration in tumor tissues exhibited a close relationship with lymph node metastasis (P = 0.0242) (Supplementary Table S2 and S3). No significant relationship was noted between FOXP3+ T cell infiltration and any clinicopathological feature (Supplementary Table S4).
Figure 2.
PD-1+ immune cell and T cell infiltration in AGCs. (a) Representative samples of IHC staining of PD-1, CD3, CD8, and FOXP3, including high and low infiltration for each marker, are presented. (b) The percent of tumor PD-L1-positive specimens in the high and low expression groups of PD-1, CD3, CD8, and FOXP3. ∗∗ P < 0.01; ns: no statistical significance.
3.4. Association of PD-L1 Expression with T Cell Infiltration
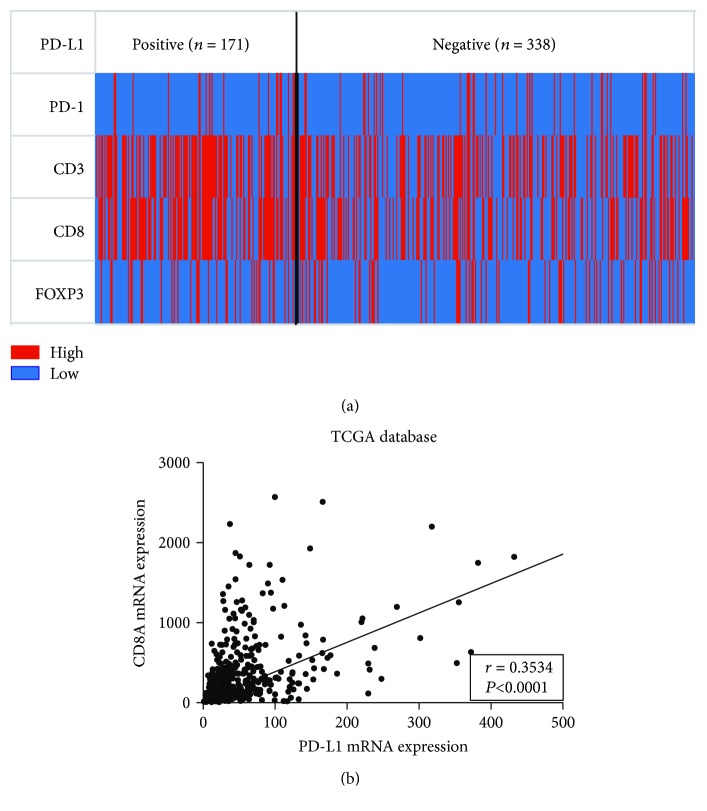
No significant difference in the number of PD-L1-positive patients was noted between the PD-1 high and low expression groups (P = 0.8860). Significant differences in the number of PD-L1-positive patients were noted between the CD3+ and CD8+ T cell high and low infiltration groups (P = 0.0018 and P = 0.0001, resp.). Regarding FOXP3, no significant difference in PD-L1-positive patients was noted between the high and low expression groups (P = 0.9215) (Figure 2(b)). We constructed a heat map to analyze PD-L1 expression based on T cell tumor infiltration and found that the percentages of CD3+ and CD8+ T cell high infiltration patients in the PD-L1-positive group were increased compared with the PD-L1-negative group (Figure 3(a)). Next, the association between PD-L1 and CD8A expression at the mRNA level was analyzed using GC data from TCGA database, and a positive correlation was noted (r = 0.3534, P < 0.0001) (Figure 3(b)).
Figure 3.
The relationship between PD-L1 expression and T cell infiltration in 509 AGC patients and TCGA database. (a) The heat map of PD-1, CD3, CD8, and FOXP3 high and low infiltration in tumors of PD-L1-positive and -negative AGC patients. (b) The correlation between PD-L1 and CD8A mRNA expression levels in GC patients in the TCGA database.
3.5. PD-L1 Expression and T Cell Infiltration Is Associated with Patient OS
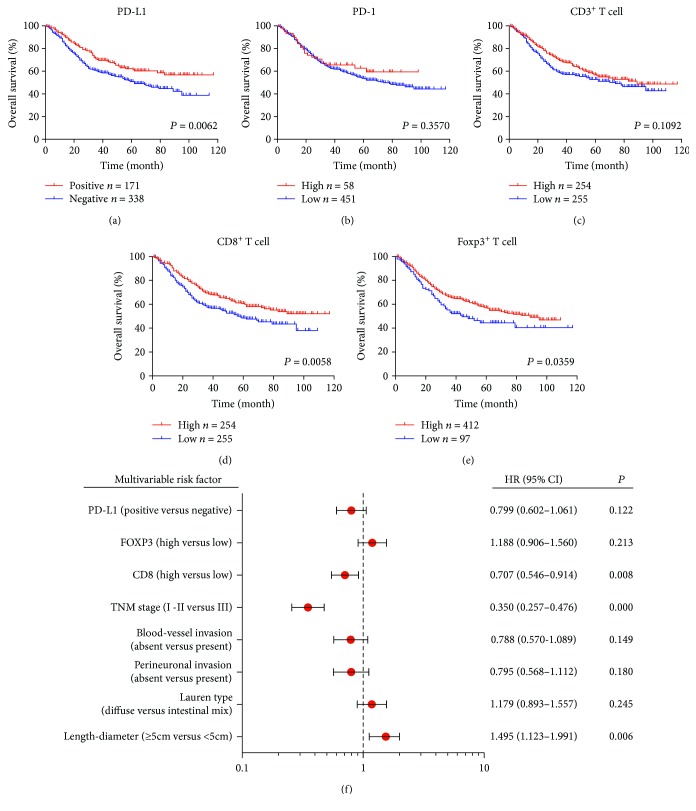
Kaplan-Meier analysis was performed to evaluate OS according to PD-L1, PD-1, CD3, CD8, and FOXP3 expression in AGC tumors (Figures 4(a)–4(e)). Positive tumor PD-L1 expression and high CD8+ T cell infiltration were associated with improved OS compared with negative expression or the low infiltration group (P = 0.0062 and P = 0.0058, resp.) (Figures 4(a) and 4(d)). Conversely, high FOXP3+ T cell infiltration was associated with worse OS than low infiltration (P = 0.0359) (Figure 4). No significant differences were noted between the high and low PD-1 and CD3 expression groups (P = 0.3570 and P = 0.1092) (Figures 4(b) and 4(c)).
Figure 4.
Prognostic value of tumor PD-L1 expression and T cell infiltration in AGC patients. (a) Kaplan-Meier survival curves for OS based on PD-L1, PD-1, CD3, CD8, and FOXP3 status. (b) After univariate analysis, we selected statistically significant risk factors, including PD-L1, FOXP3, CD8, and other clinicopathological parameters, for multivariable analysis.
3.6. Univariate and Multivariable Analysis of Prognostic Parameters for Survival in AGC Patients
Characteristics, including PD-L1 status, T cell infiltration density, and clinicopathological features, were analyzed using Cox proportional hazards regression models to assess the prognostic values (Table 2 and Figure 4(f)). In the univariate analysis of AGC patients, tumor PD-L1-positive status (HR = 0.668, 95% CI: 0.505–0.885, P = 0.005), high CD8+ T cell infiltration in tumor (HR = 0.691, 95% CI: 0.536–0.891, P = 0.004), and high FOXP3+ T cell infiltration in tumor (HR = 1.434, 95% CI: 1.061–1.938, P = 0.019) were revealed as protective or risk factors for OS in AGC patients. Next, we selected potential prognostic factors based on univariate results (P < 0.05) to conduct multivariable analysis. High CD8+ T cell infiltration in tumor (HR = 0.707, 95% CI: 0.546–0.914, P = 0.008), TNM stage (HR = 0.350, 95% CI: 0.257–0.476, P ≤ 0.001), and length-diameter (HR = 1.495, 95% CI: 1.123–1.991, P = 0.006) could act independent predictors of OS for AGC patients. However, tumor PD-L1-positive status (HR = 0.799, 95% CI: 0.602–1.061, P = 0.122) and high FOXP3+ T cell infiltration in tumor (HR = 1.188, 95% CI: 0.906–1.560, P = 0.213) were not independent predictors for AGC prognosis. Other clinicopathological parameters, including blood vessel invasion (HR = 0.788, 95% CI: 0.570–1.089, P = 0.149), perineuronal invasion (HR = 0.795, 95% CI: 0.568–1.112, P = 0.180), and Lauren type (HR = 1.179, 95% CI: 0.893–1.557, P = 0.245) exhibited no significant differences in the multivariable analysis (Figure 4(f)).
Table 2.
Univariate analysis of prognostic parameters for survival in GC patients.
| Prognostic parameter | HR | 95% CI | P value |
|---|---|---|---|
| PD-L1 (positive versus negative) | 0.668 | 0.505–0.885 | 0.005 |
| PD-1 (high versus low) | 0.767 | 0.495–1.189 | 0.236 |
| CD3 (high versus low) | 0.782 | 0.608–1.008 | 0.057 |
| CD8 (high versus low) | 0.691 | 0.536–0.891 | 0.004 |
| FOXP3 (high versus low) | 1.434 | 1.061–1.938 | 0.019 |
| Age (>60 versus ≤60) | 1.057 | 0.820–1.363 | 0.668 |
| Gender (male versus female) | 0.850 | 0.652–1.109 | 0.230 |
| Tumor location (low versus others) | 0.828 | 0.644–1.066 | 0.144 |
| Length-diameter (≥5 cm versus <5 cm) | 2.012 | 1.542–2.624 | 0.000 |
| Lauren type (diffuse versus intestinal mix) | 1.340 | 1.023–1.753 | 0.033 |
| Perineuronal invasion (absent versus present) | 0.578 | 0.419–0.796 | 0.001 |
| Blood vessel invasion (absent versus present) | 0.500 | 0.372–0.671 | 0.000 |
| TNM stage (I–II versus III) | 0.291 | 0.216–0.390 | 0.000 |
HR: hazard ratio; CI: confidence interval.
4. Discussion
Gastric cancer, especially at an advanced stage, has limited therapeutic options. The majority of patients are diagnosed at an advanced stage in China as gastroscopy is not as commonly applied in China as in other developed countries. Current conventional treatments for gastric cancer include surgery and perioperative chemotherapy [14]. Immunotherapy, especially immune checkpoint inhibitors, may provide a new opportunity for the treatment of gastric cancer in the future as they have succeeded in the treatment of other solid tumors. In this context, we conducted this study to characterize the roles of PD-L1 and the immune microenvironment in GC patients.
PD-L1, which is also named CD274 or B7H1, is one ligand of PD-1 that is expressed on various types of tumor cells [15]. PD-L1-positive tumors may indicate immune-active tumors that can respond to anti-PD-1 and/or PD-L1 therapies [16]. PD-L1 interaction with its receptor, PD-1, impairs T cell activation and cytokine production. During infection or inflammation in normal tissue, this interaction plays an important role in preventing autoimmunity during the immune response by maintaining homeostasis. In the tumor microenvironment, PD-L1 and PD-1 interaction imparts tumor immunity evasion by inactivating cytotoxic T lymphocytes (CTLs). Previous studies have reported that PD-L1 expression in tumor cells can act as a prognostic factor in various human malignancies, but the conclusion was not consistent even among the same type of tumors. This controversy underscores the importance of our study assessing PD-L1 status in the prognosis of GC patients.
By analyzing the proportion of PD-L1-positive patients among GC patients at different TNM stages, we observed no significant differences. A previous study on PD-L1 expression in non-small-cell lung cancer (NSCLC) demonstrated that high PD-L1 expression was associated with younger patient age and high tumor grade. No associations with sex, tumor size, stage, nodal status, EGFR, or KRAS mutation status were noted. PD-L1 expression appeared to be a favorable prognostic factor in early-stage disease, and the results differed for advanced-stage patients [17]. In our study, we analyzed tumor length-diameter and PD-L1-positive status. We found that the small tumor size (<5 cm) group contained a larger percent of PD-L1-positive GC patients. Other clinicopathological parameters showed no differences in the PD-L1-positive and PD-L1-negative groups.
High PD-L1 expression was associated with high CD8+ T cell infiltration in a pancancer analysis study. The results demonstrated that PD-L1 expression exhibited a positive correlation with CD8A expression. Patients exhibiting high or low expression of both proteins were increased compared with patients with high or low expression of only one protein [18]. In our study analysis, we also found that PD-L1-positive status exhibited a tight relationship with CD3+ or CD8+ T cell infiltration given that the PD-L1 high expression group contained a larger percent of CD3+ or CD8+ T high infiltration patients than did the low expression group. GC analysis of the PD-L1 and CD8A mRNA expression levels reported in TCGA database revealed a positive correlation between PD-L1 and CD8A mRNA. Another study that classified melanoma tumors based on T cell infiltration and PD-L1 expression demonstrated that 38% were type I cancers (PD-L1+TIL+), 41% were type II cancers (PD-L1−TIL−), 1% were type III cancers (PD-L1+TIL−), and 20% were type IV cancers (PD-L1− TIL+) [19]. PD-L1 expression exhibited a positive correlation with CD8+ T cell infiltration. This result was consistent with that of our study and another study of synovial sarcoma [20].
Next, we analyzed PD-L1 expression based on GC patient survival. Patients with high PD-L1 expression exhibited prolonged OS times compared with the low expression group. In fact, high PD-L1 expression was associated with a better prognosis than low PD-L1 expression in several cancer types, including gastric cancer [21], colorectal cancer [22, 23], breast cancer [24, 25], metastatic melanoma [26], Merkel cell carcinoma [27], glioblastoma [28], and other cancer [29–31]. Other studies reported that PD-L1 expression status is associated with poor prognosis. PD-L1 expression is upregulated in multiple human cancers and attenuates the antitumor immune response [32–35].
Two mechanisms of the upregulation of PD-L1, including the innate immune response and adaptive immune response, have been proposed. The innate immune response leads to PD-L1 upregulation due to dysregulated oncogenic signaling pathways and chromosomal alterations and amplifications in the tumor. In the adaptive immune response, tumor-infiltrating cytotoxic T lymphocytes (CTLs) secrete IFN-γ when they encounter tumor antigens, causing an adaptive response to IFN-γ and leading to the upregulation of PD-L1 in tumor cells. The induced expression of PD-L1 in the tumor microenvironment creates a “shield” to avoid attack from activated effector T cells. Consequently, under these circumstances, PD-L1 expression is considered a marker of an active host antitumor immune response [16]. It is not contradictory that high PD-L1 expression levels are associated with better clinical outcomes for patients with an activated immune status.
Based on the above analysis, PD-L1 expression associated with tumor-infiltrating immune cells was a positive prognostic feature. In a study to determine whether tumor-infiltrating lymphocytes (TILs) can predict the clinical prognosis in gastric cancer, the densities of CD3+ and CD8+ TILs remained independent prognostic factors in multivariate survival analysis [36]. In our study, a high density of CD8+ T cell tumor infiltrate indicated an improved prognosis compared with the low density group, and CD8+ T cell infiltration was an independent prognostic factor in the multivariate survival analysis. In CD3+ T cell infiltration analysis, no significant difference was noted between GC patients with high and low infiltration. It is possible that CD3+ T cells contained various T cell types with different functions in the immune response. CD8+ T cells act as a type of immune cell that directly kills or eliminate tumor cells in the tumor microenvironment. Conversely, FOXP3+ T cells can suppress antitumor immunity. Next, FOXP3+ T cell high and low tumor infiltration were analyzed based on OS. We found that a higher density of FOXP3+ T cell tumor infiltration was associated with a worse survival. This result was consistent with a previous study in other tumors [37].
5. Conclusions
In our study, we explored the relationship between PD-L1 expression and T cell tumor infiltration among 509 AGC patients. We found that PD-L1-positive status was correlated with high CD3+ and CD8+ T cell infiltration. PD-L1 and CD8A mRNA expression levels were positively correlated among GC patients in the TCGA database. Positive PD-L1 tumor expression and a high density of CD8+ T cells in AGCs were both associated with increased OS time, whereas no significant differences were noted in the PD-1 and CD3 high and low groups. In contrast, a high density of FOXP3+ T cell infiltration was associated with poor prognosis. Multivariate Cox regression analysis revealed that CD8+ T cell density could act as an independent predictor of OS in AGC patients. Taken together, positive tumor PD-L1 expression and high CD8+ T cell infiltration might have implications for targeting the PD-L1/PD-1 axis and the treatment of GCs. The prognostic value and immune pattern might be useful for guiding treatment in the future.
Acknowledgments
The authors thank all members of the Department of Pathology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, for their assistance. This work was supported by the National Natural Science Foundation of China (no. 81272743).
Contributor Information
Gang Zhao, Email: zhaogang@renji.com.
Hui Cao, Email: caohui@renji.com.
Data Availability
The TCGA database analyzed during the current study is available in The Cancer Genome Atlas website (https://cancergenome.nih.gov/). Other data used to support the findings of this study can be requested from the corresponding author by email (zhaogang@renji.com).
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Yangyang Wang, Chunchao Zhu, and Wei Song share co-first authorship.
Supplementary Materials
Supplementary Table S1: correlation between PD-1 expression and clinicopathological characteristics in AGC patients. Supplementary Table S2: correlation between CD3 expression and clinicopathological characteristics in AGC patients. Supplementary Table S3: correlation between CD8 expression and clinicopathological characteristics in AGC patients. Supplementary Table S4: correlation between FOXP3 expression and clinicopathological characteristics in AGC patients.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C., Matsuda T., di Carlo V., et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian S. L., Drake C. G., Pardoll D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll D. M. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanella V., Festino L., Strudel M., Simeone E., Grimaldi A. M., Ascierto P. A. PD-L1 inhibitors in the pipeline: promise and progress. OncoImmunology. 2017;7(1, article e1365209) doi: 10.1080/2162402X.2017.1365209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang Y.-K., Boku N., Satoh T., et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 7.Muro K., Chung H. C., Shankaran V., et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. The Lancet Oncology. 2016;17(6):717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 8.Topalian S. L., Hodi F. S., Brahmer J. R., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le D. T., Durham J. N., Smith K. N., et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S. T., Klempner S. J., Park S. H., et al. Correlating programmed death ligand 1 (PD-L1) expression, mismatch repair deficiency, and outcomes across tumor types: implications for immunotherapy. Oncotarget. 2017;8(44):77415–77423. doi: 10.18632/oncotarget.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawazoe A., Kuwata T., Kuboki Y., et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20(3):407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S. H., Li J., Dong F. Y., et al. Increased serotonin signaling contributes to the Warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice. Gastroenterology. 2017;153(1):277–291.e19. doi: 10.1053/j.gastro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Masugi Y., Nishihara R., Yang J., et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66(8):1463–1473. doi: 10.1136/gutjnl-2016-311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20(1):1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Liu F., Liu L. Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Medicine. 2017;96(18, article e6369) doi: 10.1097/MD.0000000000006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian S. L., Taube J. M., Anders R. A., Pardoll D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature Reviews Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper W. A., Tran T., Vilain R. E., et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89(2):181–188. doi: 10.1016/j.lungcan.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Ock C. Y., Keam B., Kim S., et al. Pan-cancer immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration. Clinical Cancer Research. 2016;22(9):2261–2270. doi: 10.1158/1078-0432.CCR-15-2834. [DOI] [PubMed] [Google Scholar]
- 19.Teng M. W. L., Ngiow S. F., Ribas A., Smyth M. J. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Research. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowicki T. S., Akiyama R., Huang R. R., et al. Infiltration of CD8 T cells and expression of PD-1 and PD-L1 in synovial sarcoma. Cancer Immunology Research. 2017;5(2):118–126. doi: 10.1158/2326-6066.CIR-16-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J. W., Nam K. H., Ahn S. H., et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19(1):42–52. doi: 10.1007/s10120-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 22.Dunne P. D., McArt D. G., O'Reilly P. G., et al. Immune-derived PD-L1 gene expression defines a subgroup of stage II/III colorectal cancer patients with favorable prognosis who may be harmed by adjuvant chemotherapy. Cancer Immunology Research. 2016;4(7):582–591. doi: 10.1158/2326-6066.CIR-15-0302. [DOI] [PubMed] [Google Scholar]
- 23.Droeser R. A., Hirt C., Viehl C. T., et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. European Journal of Cancer. 2013;49(9):2233–2242. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Cimino-Mathews A., Thompson E., Taube J. M., et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Human Pathology. 2016;47(1):52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schalper K. A., Velcheti V., Carvajal D., et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clinical Cancer Research. 2014;20(10):2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 26.Taube J. M., Anders R. A., Young G. D., et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science Translational Medicine. 2012;4(127, article 127ra37) doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipson E. J., Vincent J. G., Loyo M., et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunology Research. 2013;1(1):54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Carlsson R., Ambjorn M., et al. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. The Journal of Neuroscience. 2013;33(35):14231–14245. doi: 10.1523/JNEUROSCI.5812-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt L. H., Kummel A., Gorlich D., et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One. 2015;10(8, article e0136023) doi: 10.1371/journal.pone.0136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velcheti V., Schalper K. A., Carvajal D. E., et al. Programmed death ligand-1 expression in non-small cell lung cancer. Laboratory Investigation. 2014;94(1):107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim W. Y., Jung H. Y., Nam S. J., et al. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Archiv. 2016;469(5):581–590. doi: 10.1007/s00428-016-2011-0. [DOI] [PubMed] [Google Scholar]
- 32.Leite K. R. M., Reis S. T., Junior J. P., et al. PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagnostic Pathology. 2015;10(1):p. 189. doi: 10.1186/s13000-015-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin T., Zeng Y. D., Qin G., et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6(32):33972–33981. doi: 10.18632/oncotarget.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao Y., Li W., Chen K., et al. B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget. 2015;6(5):3452–3461. doi: 10.18632/oncotarget.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanishi J., Wada Y., Matsumoto K., Azuma M., Kikuchi K., Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunology, Immunotherapy. 2007;56(8):1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H. E., Chae S. W., Lee Y. J., et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. British Journal of Cancer. 2008;99(10):1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Reuver P. R., Mehta S., Gill P., et al. Immunoregulatory Forkhead box protein p3-positive lymphocytes are associated with overall survival in patients with pancreatic neuroendocrine tumors. Journal of the American College of Surgeons. 2016;222(3):281–287. doi: 10.1016/j.jamcollsurg.2015.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: correlation between PD-1 expression and clinicopathological characteristics in AGC patients. Supplementary Table S2: correlation between CD3 expression and clinicopathological characteristics in AGC patients. Supplementary Table S3: correlation between CD8 expression and clinicopathological characteristics in AGC patients. Supplementary Table S4: correlation between FOXP3 expression and clinicopathological characteristics in AGC patients.
Data Availability Statement
The TCGA database analyzed during the current study is available in The Cancer Genome Atlas website (https://cancergenome.nih.gov/). Other data used to support the findings of this study can be requested from the corresponding author by email (zhaogang@renji.com).