Abstract
Bryophyllum pinnatum belongs to the family Crassulaceae and it is commonly used in the ethnomedical practices. This study investigated the antidiarrheal and antioxidant properties of methanol extract of Bryophyllum pinnatum leaf harvested from South-Eastern Nigeria in mice. Cold maceration method in 80% methanol was adopted in the extract preparation. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays were used to evaluate the antioxidant property while castor oil-induced diarrhea, small intestinal transit, and enteropooling models were used for the antidiarrheal investigation. The effects of the extract (50, 100, and 200 mg/kg) were compared to distilled water (10 ml/kg) and loperamide (5 mg/kg). The extract produced concentration dependent increase in antioxidant effect in both DPPH and FRAP assay. The extract caused a significant (p < 0.05) reduction in mean stool output, percentage of wet stools, small intestinal transit, and intestinal fluid accumulation in the treated mice when compared to the distilled water treated mice. The study validates the use of Bryophyllum pinnatum in the ethnomedical management of diarrhea.
1. Introduction
Diarrhea is a common cause of hospitalization among children and even elderly persons in most developing countries and accounts for about 5 million deaths annually [1]. It is characterized by increased frequency, fluidity, and bowel movement of three or more times per day as well as inflammatory response and oxidative stress [2, 3]. It occurs due imbalanced in the secretory and absorptive physiologies of water and electrolyte in the gut which might be caused by microorganism (bacteria, virus, protozoa, and fungi), helminths, toxins, diet, and allergy [4]. Diarrhea is often treated with antispasmodic, antisecretory, and inhibitors of prostaglandin secretions such as loperamide, diaretyl, and atropine [3, 5]. These drug are associated with side effects such constipation, drowsiness, and colorectal cancer which limit their usage [5]. In the tropics several natural occurring compounds are employed in the treatment of diarrhea and other gastrointestinal disorders [1]. In South-Eastern Nigeria, Bryophyllum pinnatum is among the medicinal plants included in herbal preparations meant for diarrhea control.
Bryophyllum pinnatum belongs to the family Crassulaceae and the common names include life plant, love plant, miracle leaf, and Canterbury bells. It is widely distributed in tropical Africa, America, Hawaii, India, China, Australia, and Madagascar [6]. The leaf is extensively used in folk medicine as an astringent, emollient, hemostatic, carminative, disinfectant, and tonic. It is also used in conditions such as hematemesis, hemorrhoids, menstrual pain, wounds, boils, sloughing ulcers, ophthalmia, burns, scalds, diarrhea, and dysentery [7]. Some biological active compounds, including alkaloids, triterpenes, lipids, flavonoids, glycosides, kaempferol rhamnoside, bufadienolides, phenols, and organic acids have been isolated from the plant [6–8]. The leaves of B. pinnatum have been reported to possess hepatoprotective and antineoplastic [6, 9], anti-asthmatic and antitussives [9, 10], antidiabetic [10], antihypertensive [11], antimicrobial [12], anti-inflammatory and analgesic [13, 14], and antiulcer [15] activities. The antidiarrheal activity of B. Pinnatum harvested from Southwest ecological zone has been reported [16]. The ecological factors such as rainfall, soil type, light intensity, and humidity affect the phytochemical composition of medicinal plants as well as the pharmacological activities [17]. There is paucity of data on the antidiarrheal effects of B. pinnatum leaf grown in South-Eastern Nigeria ecological zone. The study investigated the antidiarrheal effects of methanol extract of B. Pinnatum leaf harvested from South-Eastern Nigeria in mice.
2. Materials and Method
2.1. Plant Collection and Identification
Fresh leaves of Bryophyllum pinnatum were collected from Ugba Junction in Isiala Ngwa South Local Government Area of Abia State, South-Eastern Nigeria in June 2016 (rainy season) and were confirmed Bryophyllum pinnatum by a plant taxonomist, Dr. M. C. Dike of College of Natural Resources and Environmental Management, Michael Okpara University of Agriculture, Umudike. A voucher specimen with catalogue number MOUAU/CVM/VPP/2016/44 was deposited in the Department of Veterinary Physiology and Pharmacology herbarium for reference.
2.2. Chemicals and Drugs
Ascorbic acid (Hopkin and Williams, England), loperamide hydrochloride (Xian-Janssen Pharmaceutical Ltd., China), charcoal, castor oil, gum acacia (Merck, Germany), 2,2-diphenyl-2-picrylhydrazyl (DPPH), methanol, sodium acetate trihydrate, glacial acetic acid, 2,4,6-tripyridyl-s-triazine (TPTZ), and hydrochloric acid purchased from Sigma-Aldrich, Germany were used for the study.
2.3. Extraction of Plant Material
The leaves of Bryophyllum pinnatum were dried under room temperature on a laboratory bench and were ground into a coarse powder using manual grinder (Corona, China). The powdered material was weighed using an electronic balance (PI 303 model, China). The plant material (150 g) was macerated with 80% methanol for 48 hours with intermittent shaking every 3 hours. The resulting mixture was filtered with Whatman No. 1 filter papers and was dried in a hot air oven at 40°C. The extract was stored in a refrigerator at 4°C until used. The percentage yield (w/w) of the Bryophyllum pinnatum extracts (BPE) was calculated using the formula below:
| (1) |
2.4. Animals
Ninety (90) mice (28–34 g), aged 8–10 weeks, sourced from the laboratory animal unit of the Department of Veterinary Physiology and Pharmacology, Michael Okpara University of Agriculture Umudike, Abia State, were used for the study. The animals were housed in aluminum cages at room temperature and under natural light/darkness cycles. The mice were supplied with clean drinking water and fed with standard commercial pelleted grower feed (Vital feed® Nigeria) ad libitum. The mice were acclimatized for two weeks prior to the study. They were maintained in accordance with the recommendations of the Guide for the care and use of laboratory animals [18] and the experimental protocol was approved by the institution's ethical committee.
2.5. Antidiarrheal Study
2.5.1. Castor Oil-Induced Diarrhea
We followed the methods of Onoja and Udeh [19]. Briefly, thirty (30) mice were divided into 5 groups (A–E) of 6 mice per group. They were fasted for about 10 h with free access to water, but water was removed 2 h prior to the test. Group A received 10 mL/kg of distilled water and Group B received loperamide (5 mg/kg) while Groups C, D, and E received 50, 100, and 200 mg/kg of BPE, respectively. One hour after administration of drugs, diarrhea was induced by administering 0.3 ml of castor oil orally. The animals were placed individually in cages lined with white paper. The numbers of both wet and dry stool droppings were counted at 1 h interval over a period of 4 h. The mean percentage of wet stool passed by the treated groups was compared with that of the control group [20].
| (2) |
2.5.2. Small Intestinal Transit
The modified method of Chime [21] as described in Onoja and Udeh [19] was followed in this experiment. Briefly, thirty mice were randomly assigned to five groups (A–E) of six mice each and were fasted for 10 h before the experiment. Group A received 10 mL/kg of distilled water and Group B received loperamide (5 mg/kg) while Groups C, D, and E received 50, 100, and 200 mg/kg of BPE, respectively. The standard charcoal meal (5% of activated charcoal suspended in 5% of gum acacia) was administered to all the animals 1 h after treatment. The animals were sacrificed 30 min after administration of charcoal meal by cervical dislocation and the intestines were immediately isolated and ligated at the pyloric sphincter and ileocecal junction. The small intestinal transit was expressed as percentage of distance travelled by the charcoal meal relative to the total length of the small intestine from the pyloric sphincter to the ileocecal junction.
| (3) |
2.5.3. Enteropooling
The method of Hassan et al. [22] as described in Onoja and Udeh. [19] was followed in this study. Briefly, thirty mice were randomly divided into five groups of six mice each and were fasted for 10 h before the experiment. Group A received 10 mL/kg of distilled water and Group B received loperamide (5 mg/kg) while Groups C, D, and E received 50, 100, and 200 mg/kg of BPE, respectively. One h after the treatment, the animals were sacrificed by cervical dislocation and laparotomized and the intestines were immediately isolated and ligated at the pyloric sphincter and ileocecal junction. The small intestines were weighed, the content of each intestine was milked out, and the empty intestines were reweighed. The difference in weight between the full and empty intestines was recorded as the weight of the intestinal content.
| (4) |
where wt = weight.
2.6. Antioxidant Study
2.6.1. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Photometric Assay
The DPPH radical scavenging activity of the extract was analyzed as reported by Onoja et al., [23] using spectrophotometer. The extract at various concentrations (25, 50, 100, 200, and 400 μg/ml) was assayed in triplicate and ascorbic acid was also assayed as a reference standard.
2.6.2. Ferric Reducing Antioxidant Power
The ferric reducing antioxidant power of BPE was evaluated as described by Onoja et al. [24]. The concentrations of 25, 50, 100, 200, and 400 μg/ml of BPE in triplicate were used in the study. It was compared with ascorbic acid at 125 μg/ml concentration.
2.7. Data Analysis
The obtained data were statistically evaluated using one-way ANOVA, followed by least significant difference test with SPSS software. The mean values were considered significant at p < 0.05.
3. Results
3.1. Effect of BPE and Loperamide on Castor Oil-Induced Diarrhea on Mice
The extract caused a significant (p < 0.05) dose-dependent decrease in the percentage of wet stools (Table 1) in the treated groups throughout the period of observation when compared with distilled water treated groups. At 4 h after diarrhea induction, the percentages of wet stool of the distilled water, loperamide, and BPE 50, 100, and 200 mg/kg were 84.62, 90.00, 83.61, 72.14, and 62.50%, respectively. The optimum antidiarrheal effect of BPE was produced at 200 mg/kg dose.
Table 1.
Effect of BPE and loperamide on castor oil induced diarrhea on mice.
| Percentage (%) wet stool | ||||
|---|---|---|---|---|
| Treatment | 1 h | 2 h | 3 h | 4 h |
| Distilled water 10 ml/kg | 76.66 ± 2.35 | 81.38 ± 2.00 | 82.57 ± 2.27 | 84.62 ± 2.23 |
| Loperamide 5 mg/kg | 0.00 ± 0.00∗ | 40.00 ± 16.32∗ | 88.75 ± 3.39 | 90.00 ± 2.98 |
| BPE 50 mg/kg | 48.33 ± 9.06∗ | 63.54 ± 11.01 | 83.06 ± 2.01 | 83.61 ± 2.18 |
| BPE 100 mg/kg | 28.33 ± 9.77∗ | 50.00 ± 11.57 | 61.66 ± 8.48∗ | 72.14 ± 5.13∗ |
| BPE 200 mg/kg | 20.83 ± 10.75∗ | 61.11 ± 2.02 | 63.49 ± 2.89∗ | 62.50 ± 4.56∗ |
∗ p < 0.05 when compared with distilled water treated group. BPE = Bryophyllum pinnatum Extract.
3.2. Effect of BPE and Loperamide on Small Intestinal Transit in Mice
The loperamide and BPE (50, 100, and 200 mg/kg) significantly (p < 0.05) reduced the small intestinal transit of charcoal meal in the treated mice (Table 2) when compared with distilled water treated mice. The extract did not produce dose-dependent effect. The maximal effect of the extract was observed at 50 mg/kg.
Table 2.
Effects of BPE and loperamide on small intestinal transit in mice.
| Treatment | % Distance travelled | % Inhibition |
|---|---|---|
| Distilled water 10 ml/kg | 60.65 ± 0.46 | 0 |
| Loperamide 5 mg/kg | 45.10 ± 2.20∗ | 25.63 |
| BPE 50 mg/kg | 43.78 ± 2.96∗ | 27.81 |
| BPE 100 mg/kg | 58.93 ± 2.76 | 2.83 |
| BPE 200 mg/kg | 54.60 ± 0.49 | 9.97 |
∗ p < 0.05 when compared with distilled water treated group. BPE = Bryophyllum pinnatum extract.
3.3. Effect of BPE and Loperamide on Enteropooling in Mice
The loperamide and BPE (50, 100, and 200 mg/kg) caused a significant (p < 0.05) reduction in the weight of the intestinal content in the treated mice when compared to the distilled water treated mice (Table 3). The BPE did not produce a dose-dependent effect. The weights of the intestinal content of the distilled water, loperamide, and BPE 50, 100, and 200 mg/kg were 0.91, 0.45, 0.60, 0.67, and 0.66 g, respectively.
Table 3.
Effects of BPE and loperamide on the enteropooling in mice.
| Treatment | WT of intestinal content (g) | % inhibition |
|---|---|---|
| Distilled water 10 ml/kg | 0.91 ± 0.01 | - |
| Loperamide 5 mg/kg | 0.45 ± 0.07∗ | 50.55 |
| BPE 50 mg/kg | 0.60 ± 0.01∗ | 34.07 |
| BPE 100 mg/kg | 0.67 ± 0.01∗ | 26.37 |
| BPE 200 mg/kg | 0.66 ± 0.01∗ | 27.47 |
∗ p < 0.05 when compared with distilled water treated group. BPE = Bryophyllum pinnatum extract, WT = weight.
3.4. DPPH Radical Scavenging Activities of BPE
The extract produced a concentration dependent increase in percentage antioxidant activity. The optimum antioxidant activity of BPE was produced at 400 μg/ml concentration (Figure 1).
Figure 1.
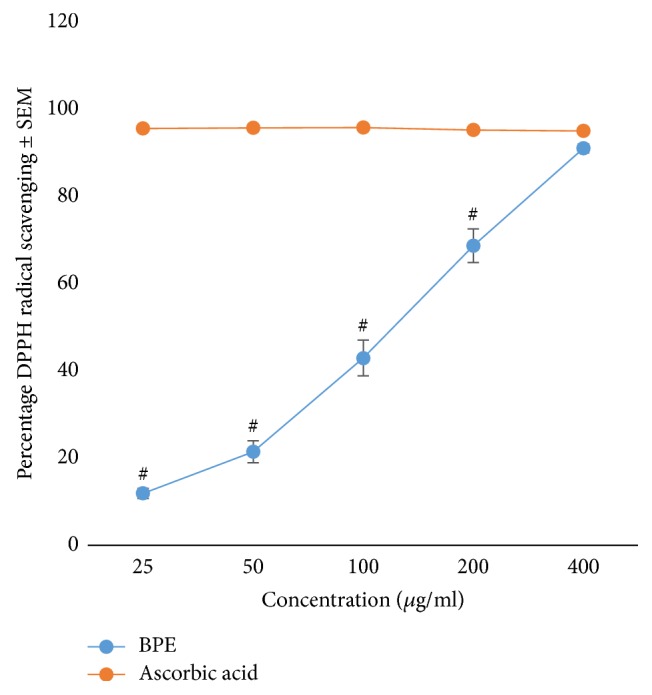
DPPH radical scavenging activities of BPE. #p < 0.05 when compared with ascorbic acid, BPE = Bryophyllum pinnatum extract.
3.5. Ferric Reducing Antioxidant Power of BPE
The extract produced a concentration dependent increase in antioxidant power. At 400 μg/ml the assay gave 2.27 (μM), showing that BPE has a high antioxidant power (Figure 2).
Figure 2.
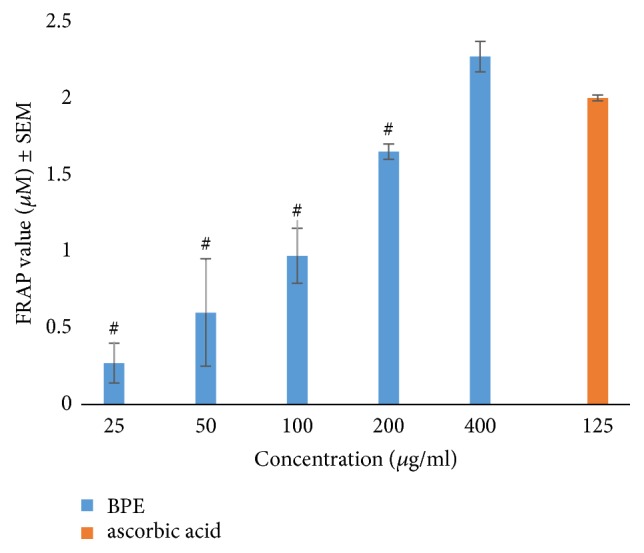
Ferric reducing antioxidant power (FRAP) of BPE. #p < 0.05 when compared with ascorbic acid, BPE = Bryophyllum pinnatum extract.
4. Discussion
The antidiarrheal property of methanol extract of Bryophyllum pinnatum was evaluated using castor oil-induced diarrhea, intestinal transit, and enteropooling models in mice. The extract significantly (p < 0.05) inhibited castor oil-induced diarrhea, reduced intestinal transit, reduced intestinal fluid accumulation, and elicited potent antioxidant activities. These pharmacological activities could be mediated by the phytochemical constituents of Bryophyllum pinnatum [25]. The presence of phytoconstituents like alkaloids, terpenes, glycosides, and flavonoids has been reported on B. pinnatum and the antidiarrheal and antioxidant activities of these phytoconstituents have been reported as well [26].
The inhibition of castor oil-induced diarrhea might be linked to protection against gastric irritation and inflammation as well as reduction in prostaglandin release [27, 28]. Ricinoleic acid, the active metabolite of castor oil, causes irritation and inflammation of the intestinal mucosa which leads to increased prostaglandin release, enhanced peristalsis, and reduced reabsorption of sodium ion, chloride ion, and water from the gut which give rise to diarrhea [29]. The inhibition of prostaglandin synthesis has been incriminated in the anti-inflammatory and analgesic activities of B. pinnatum [13, 14].
The reduction in intestinal transit may be due to reduced peristalsis which can be attributed to relaxation of the intestinal smooth muscle [30, 31]. Drugs that relax the intestinal smooth muscles are often used as antidiarrheal agent because they inhibit intestinal hypermotility (increase peristalsis) which usually accompanies diarrhea [26]. Antidiarrheal activity of loperamide is linked to inhibition of peristalsis [32, 33]. The reduction in intestinal fluid accumulation is linked to antisecretory activity. Tannins and flavonoids present in the plant extract are reported to inhibit release of prostaglandins, thereby inhibiting motility and secretion induced by castor oil. The antidiarrheal activity of the extract may also be due to denature of proteins by tannates that make intestinal mucosa more resistant and reduce secretion [25]. The reported antimicrobial activities of B. pinnatum suggest that the extract could be effective in the management of susceptible microbial induced diarrhea [34, 35]. The findings of this study corroborate the antidiarrheal activities of Combretum dolichopetalum [19] as well as the report of Adeyemi et al. [16] on the antidiarrheal effects of B. pinnatum harvested from South-Western Nigerian.
The antidiarrheal effects of B. Pinnatum may also be associated with its potent antioxidant potential as observed in this study. Umukoro and Ashorobi reported that ascorbic acid and α-tocopherol reduce prostaglandin level through the inhibition of perioxidation of phospholipids and thus ameliorate castor oil-induced diarrhea [27]. The antidiarrheal and antioxidant activities of Bryophyllum pinnatum could be mediated by the phytochemical composition. Phytochemical investigations have shown the presence of alkaloids, triterpenes, lipids, flavonoids, glycosides, kaempferol rhamnoside, bufadienolides, phenols, and organic acids [6–8].
5. Conclusion
The findings of this study demonstrated the pharmacological basis for ethnomedical use of Bryophyllum pinnatum in diarrhea treatment. However further studies are desired toward the isolation and characterization of the active compound.
Acknowledgments
The authors are grateful to Dr. M. C. Dike for the identification of the plant material.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclosure
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
References
- 1.Sebai H., Jabri M.-A., Souli A., et al. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. Journal of Ethnopharmacology. 2014;152(2):327–332. doi: 10.1016/j.jep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Suleiman M. M., Dzenda T., Sani C. A. Antidiarrhoeal activity of the methanol stem-bark extract of Annona senegalensis Pers. (Annonaceae) Journal of Ethnopharmacology. 2008;116(1):125–130. doi: 10.1016/j.jep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Agbor G. A., Longo F., Makong E. A., Tarkang P. A. Evaluation of the antidiarrheal and antioxidant properties of Justicia hypocrateriformis. Pharmaceutical Biology. 2014;52(9):1128–1133. doi: 10.3109/13880209.2013.879189. [DOI] [PubMed] [Google Scholar]
- 4.Whyte L. A., Jenkins H. R. Pathophysiology of diarrhoea. Paediatrics and Child Health. 2012;22(10):443–447. doi: 10.1016/j.paed.2012.05.006. [DOI] [Google Scholar]
- 5.Rang H. P., Dale M. M., Ritter J. M., Moore R. K. Rang & Dale's Pharmacology. 6th. Edinburgh, Scotland: Churchill Livingstone; 2007. [Google Scholar]
- 6.Afzal M., Kazmi I., Anwar F. Antineoplastic potential of Bryophyllum pinnatum lam. on chemically induced hepatocarcinogenesis in rats. Pharmacognosy Research. 2013;5(4):247–253. doi: 10.4103/0974-8490.118811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamboj A., Saluja A. K. Bryophyllum pinnatum (Lam.) Kurz.: Phytochemical and pharmacological profile: A review. Pharmacognosy Reviews. 2009;3(6):364–374. [Google Scholar]
- 8.Tatsimo S. J. N., Tamokou J. D. D., Havyarimana L., et al. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Research Notes. 2012;5, article 158 doi: 10.1186/1756-0500-5-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozolua R. I., Eboka C. J., Duru C. N., Uwaya D. O. Effects of aqueous leaf extract of Bryophyllum pinnatum on guinea pig tracheal ring contractility. Nigerian Journal of Physiological Sciences. 2010;25(2):149–157. [PubMed] [Google Scholar]
- 10.Aransiola E. F., Daramola M. O., Iwalewa E. O., Seluwa A. M., Olufowobi O. O. Antidiabetic effect of Bryophyllum pinnatum leaves. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering. 2014;8:89–93. [Google Scholar]
- 11.Ghasi S., Egwuibe C., Achukwu P. U., Onyeanusi J. C. Assessment of the medical benefit in the folkloric use of Bryophyllum pinnatum leaf among the igbos of Nigeria for the treatment of hypertension. African Journal of Pharmacy and Pharmacology. 2011;5(1):83–92. [Google Scholar]
- 12.Mudi S., Ibrahim H. Activity of Bryophyllum pinnatum Kurz extracts on respiratory tract pathogenic bacteria. Bayero Journal of Pure and Applied Sciences. 2008;1:43–48. [Google Scholar]
- 13.Chibli L. A., Rodrigues K. C. M., Gasparetto C. M., et al. Anti-inflammatory effects of Bryophyllum pinnatum (Lam.) Oken ethanol extract in acute and chronic cutaneous inflammation. Journal of Ethnopharmacology. 2014;154(2):330–338. doi: 10.1016/j.jep.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Afzal M., Gupta G., Kazmi I., et al. Anti-inflammatory and analgesic potential of a novel steroidal derivative from Bryophyllum pinnatum. Fitoterapia. 2012;83(5):853–858. doi: 10.1016/j.fitote.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Pal S., Chaudhuri A. K. N. Studies on the anti-ulcer activity of a Bryophyllum pinnatum leaf extract in experimental animals. Journal of Ethnopharmacology. 1991;33(1-2):97–102. doi: 10.1016/0378-8741(91)90168-D. [DOI] [PubMed] [Google Scholar]
- 16.Adeyemi O. O., Ishola I. O., Okoro U. Antidiarrhoeal Activity of Hydroethanolic Leaf Extract of Bryophyllum pinnatum Lam. Kurtz (Crassulaceae) Nigerian Quarterly Journal of Hospital Medicine. 2013;23(4):323–329. [PubMed] [Google Scholar]
- 17.Evans W. C. Trease and Evans Pharmacognosy. 16th. Edinburgh, Scotland: W. B. saunders Elsevier; 2009. [Google Scholar]
- 18.Bjelakovic G., Nikolova D., Gluud L. L., Simonetti R. G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. The Journal of the American Medical Association. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 19.Onoja S. O., Udeh N. E. Antidiarrheal effects of hydromethanolic extract of Combretum dolichopetalum leaves in mice. Journal of Coastal Life Medicine. 2015;3(11):910–913. [Google Scholar]
- 20.Ezeigbo I. I., Ezeja M. I., Madubuike K. G., et al. Antidiarrhoeal activity of leaf methanolic extract of Rauwolfia serpentina. Asian Pacific Journal of Tropical Biomedicine. 2012;2(6):430–432. doi: 10.1016/S2221-1691(12)60070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chime H. R. Studies on anti-diarrheal activity of Calotropis gigantean R. BR. In experimental animals. Journal of Pharmacy and Pharmaceutical Sciences. 2004;7:70–75. [PubMed] [Google Scholar]
- 22.Hassan K. A., Brenda A. T., Patrick V., Patrick O. E. In vivo antidiarrheal activity of the ethanolic leaf extract of Catharanthus roseus linn. (Apocyanaceae) in wistar rats. African Journal of Pharmacy and Pharmacology. 2011;5(15):1797–1800. doi: 10.5897/AJPP11.505. [DOI] [Google Scholar]
- 23.Onoja S. O., Ezeja M. I., Omeh Y. N., Onwukwe B. C. Antioxidant, anti-inflammatory and antinociceptive activities of methanolic extract of Justicia secunda Vahl leaf. Alexandria Journal of Medicine. 2017;53(3):207–213. doi: 10.1016/j.ajme.2016.06.001. [DOI] [Google Scholar]
- 24.Onoja S. O., Madubuike G. K., Ezeja M. I. Hepatoprotective and antioxidant activity of hydromethanolic extract of Daniella oliveri leaves in carbon tetrachloride-induced hepatotoxicity in rats. Journal of Basic and Clinical Physiology and Pharmacology. 2015;26(5):465–470. doi: 10.1515/jbcpp-2014-0087. [DOI] [PubMed] [Google Scholar]
- 25.Dey S. K., Hira A., Howlader M. S. I., Ahmed A., Hossain H., Jahan I. A. Antioxidant and antidiarrheal activities of ethanol extract of Ardisia elliptica fruits. Pharmaceutical Biology. 2014;52(2):213–220. doi: 10.3109/13880209.2013.826245. [DOI] [PubMed] [Google Scholar]
- 26.Han X., Pang Y., Liu S., et al. Antidiarrhea and antioxidant activities of honokiol extract from Magnoliae officinalis cortex in mice. Tropical Journal of Pharmaceutical Research. 2014;13(10):1643–1651. doi: 10.4314/tjpr.v13i10.11. [DOI] [Google Scholar]
- 27.Umukoro S., Ashorobi R. B. Effect of Aframomum melegueta seed extract on castor oil-induced diarrhea. Pharmaceutical Biology. 2005;43(4):330–333. doi: 10.1080/13880200590951748. [DOI] [PubMed] [Google Scholar]
- 28.Ramasamy A., Das S., Mani V., Sengottuvelu S., Vinoth Prabhu V. Evaluation of Anti-diarrheal Potential of Hydro-alcoholic Extracts of Leaves of Murraya koenigii in Experimental Animals. Journal of Dietary Supplements. 2016;13(4):393–401. doi: 10.3109/19390211.2015.1101636. [DOI] [PubMed] [Google Scholar]
- 29.Pérez Gutiérrez S., Zavala Mendoza D., Soto Peredo C., Sánchez Sánchez O., Zavala Sánchez M. A. Evaluation of the anti-diarrheal activity of Salvia connivens. Pharmaceutical Biology. 2014;52(11):1467–1470. doi: 10.3109/13880209.2014.898076. [DOI] [PubMed] [Google Scholar]
- 30.Simões-Wüst A. P., Grãos M., Duarte C. B., et al. Juice of Bryophyllum pinnatum (Lam.) inhibits oxytocin-induced increase of the intracellular calcium concentration in human myometrial cells. Phytomedicine. 2010;17(12):980–986. doi: 10.1016/j.phymed.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Yemitan O. K., Salahdeen H. M. Neurosedative and muscle relaxant activities of aqueous extract of Bryophyllum pinnatum. Fitoterapia. 2005;76(2):187–193. doi: 10.1016/j.fitote.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Fujita W., Gomes I., Dove L. S., Prohaska D., McIntyre G., Devi L. A. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochemical Pharmacology. 2014;92(3):448–456. doi: 10.1016/j.bcp.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarpellini E., Laterza L., Ianiro G., Tack J., Abenavoli L., Gasbarrini A. Eluxadoline for the treatment of diarrhoea-predominant irritable bowel syndrome. Expert Opinion on Pharmacotherapy. 2016;17(10):1395–1402. doi: 10.1080/14656566.2016.1182982. [DOI] [PubMed] [Google Scholar]
- 34.Akinpelu D. A. Antimicrobial activity of Bryophyllum pinnatum leaves. Fitoterapia. 2000;71(2):193–194. doi: 10.1016/S0367-326X(99)00135-5. [DOI] [PubMed] [Google Scholar]
- 35.Akinsulire O. R., Aibinu I. E., Adenipekun T., Adelowotan T., Odugbemi T. In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. African Journal of Traditional, Complementary and Alternative Medicines. 2007;4(3):338–344. [PMC free article] [PubMed] [Google Scholar]


