Abstract
Recent clinical trials investigating cardiovascular (CV) safety of newer antidiabetic agents have been rapidly and largely changing the landscape of diabetes care and providing highly important clinical information on decision-making regarding the choice of antidiabetic agents. Similar to the sodium-glucose cotransporter 2 (SGLT2) inhibitors, some glucagon-like peptide-1 receptor agonists (GLP-1RAs) have also demonstrated a marked risk reduction in major adverse CV events (MACE) in patients with type 2 diabetes at high risk of CV events. However, the two classes of agents differ largely in their pharmacological modes of action on glucose-lowering and CV parameters. Furthermore, CV benefits on individual components of MACE and other outcomes, including heart failure (HF), appear to differ partly between the two classes. Specifically, improvement of overall CV outcomes was likely driven by reduction in HF-related events in trials investigating SGLT2 inhibitors, and by reduction in atherosclerotic events in those investigating GLP-1RAs. This difference in CV benefit observed in the trials has important clinical implications regarding how to use the two classes of agents and how to identify suitable patients to obtain the best benefit from each class during routine diabetes care, possibly leading to a treatment plan tailored to an individual patient’s CV risk and clinical condition. At this stage, however, we cardiologists may overlook such differences and may be unfamiliar with GLP-1RAs specifically. Herein, we highlight the potential benefits of GLP-1RAs on CV parameters observed in recent CV outcomes trials and further discuss clinical application of GLP-1RAs in CV medicine.
Keywords: Type 2 diabetes, Cardiovascular outcomes trial, Glucagon-like peptide-1 receptor agonist, Sodium-glucose cotransporter 2 inhibitor, Major adverse cardiovascular event
Health authority regulations in the US and Europe require pharmaceutical companies to conduct a pre-approval trial showing at least non-inferiority of cardiovascular (CV) safety compared with placebo treatment, for any new therapy to treat type 2 diabetes (T2D), prior to granting market approval. Following the momentous CV outcomes trial with sodium-glucose cotransporter 2 (SGLT2) inhibitor (empagliflozin) [1], two CV outcomes trials with glucagon-like peptide-1 receptor agonists (GLP-1RAs) have also demonstrated superior CV benefits compared with placebo [2, 3]. Furthermore, CV benefits have since been demonstrated for another SGLT2 inhibitor in the more recent CANVAS program [4]. These trials highlight the favorable effects of each new class of T2D therapy on CV parameters, seemingly resulting in a large paradigm shift in diabetes care and impact on decision-making in the selection of antidiabetic agents to improve prognosis [5, 6].
The two drug classes (SGLT2 inhibitors and GLP-1RAs) differ largely in their pharmacological actions. Compared with orally administered SGLT2 inhibitors, GLP-1RAs need to be subcutaneously injected, which appears to be a somewhat hurdle for patients to accept the therapy. However, given the superior CV benefits of GLP-1RAs, which differ somewhat from those attributed to SGLT2 inhibitors, we cardiologists also need to consider the CV benefits of GLP-1RAs and become more comfortable prescribing the agents for appropriate patients who may benefit the most from them. In this commentary, we summarize the potential effects of GLP-1RAs on CV parameters based on results from recent CV outcomes trials, and further discuss the clinical application of the agents in CV medicine.
Detailed biological characteristics of GLP-1 and proposed modes of action of GLP-1RAs on the metabolic and CV systems have been investigated in preclinical and clinical studies and summarized elsewhere [7–12]. In the LEADER (liraglutide) and SUSTAIN-6 (semaglutide) trials [2, 3], the GLP-1RAs rapidly and greatly decreased HbA1c levels compared to placebo, which may have partly contributed to the increased incidence of diabetic retinopathy complications observed in SUSTAIN-6 (HR: 1.76 [95% CI 1.11, 2.78]). This suggests that appropriate management of diabetic retinopathy should be further required, especially when using semaglutide. On the other hand, the rates of occurrence of hypoglycemia in the active drug groups were similar (or even lower) to those of the placebo groups. Similar to the SGLT2 inhibitors [1, 4, 13], GLP-1RAs also mildly reduced systolic and diastolic blood pressure in the trials. Moreover, GLP-1RAs also modestly increased heart rate, although the exact mechanisms are still unclear. Similarly, body weight loss associated with GLP-1RAs was evident in the trials. The mechanism underlying body weight loss associated with SGLT2 inhibitors was speculated to be mainly due to plasma volume contraction and body fat mass reduction [14, 15], while body weight loss associated with GLP-1RAs appears to be at least in part due to a central effect. Interestingly, GLP-1RAs are known to reduce appetite and food intake partly via appetite-inhibitory neurotransmission signaling within the arcuate nucleus [16, 17], possibly resulting in subsequent improvements in insulin resistance and serum lipid profiles (e.g., reduction in triglyceride levels). Furthermore, decreases in body weight associated with GLP-1RAs differed between the CV outcomes trials, with mean body weight decreases from baseline of 2.3 kg at 36 months in the LEADER trial (median dose of liraglutide: 1.78 mg once-daily) and 3.6 and 4.9 kg at 104 weeks in the SUSTAIN-6 trial (for 0.5 and 1.0 mg once-weekly, respectively). This may indicate that the effect of semaglutide on body weight reduction is relatively rapid-acting and dose-dependent, possibly resulting from the higher albumin affinity of semaglutide compared with liraglutide [18]. Taken together, the metabolic modifications associated with GLP-1RA treatment appear to have a favorable impact on CV systems, such as vascular function [19, 20], subsequently leading to anti-atherosclerotic effects in T2D patients. In an experimental study, liraglutide treatment could also reduce vascular inflammation and suppress neointimal hyperplasia via improvement of nitric oxide bioavailability [21].
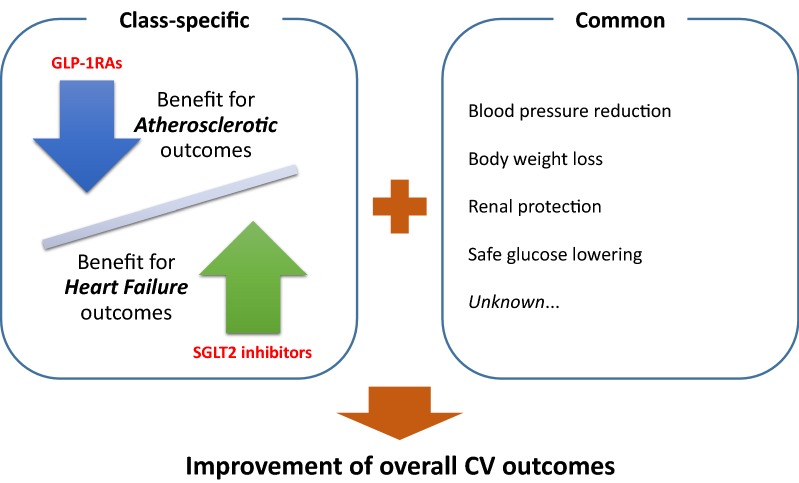
Although CV outcomes trials with GLP-1RAs (LEADER and SUSTAIN-6) and SGLT2 inhibitors (EMPA-REG OUTCOME and CANVAS) showed significantly reduced occurrences of the pre-specified primary composite endpoint comprised of CV death, non-fatal myocardial infarction, and non-fatal stroke in T2D patients at high risk of CV events, the treatment effects on the individual components of the primary composite endpoint differed between drug classes, and even between different members of the same class [22, 23]. While a consistent reduction in hospitalization for heart failure (HF) was confirmed in the CV outcomes trials with SGLT2 inhibitors, the effect of GLP-1RAs on hospitalization for HF was neutral [24]. This inconsistency between the drug classes may, in part, be caused by the presence or absence of diuretic action and increase in heart rate, both of which generally have a large impact on HF management. Furthermore, a previous randomized trial with liraglutide demonstrated that use of liraglutide in T2D patients with advanced HF and reduced left ventricular ejection fraction was associated with a numerically increased risk of death or rehospitalization for HF [25]. Atherosclerotic CV events (myocardial infarction and stroke) were totally and numerically prevented in LEADER and SUSTAIN-6. In contrast, both GLP-1RAs and SGLT2 inhibitors are known to have protective effects on renal function and improved renal outcomes were observed in CV outcomes trials [1, 3, 4, 26, 27]. Taken together, in addition to some common benefits for CV systems, it is likely that GLP-1RAs improved overall CV outcomes mainly by reduction in atherosclerotic events, whereas SGLT2 inhibitors improved CV outcomes mainly via prevention of HF-related outcomes (Fig. 1).
Fig. 1.
Potential effects of GLP-1RAs and SGLT2 inhibitors in CV outcomes trials. Based on the results from recent CV outcomes trials with GLP-1RAs and SGLT2 inhibitors, in addition to some common favorable CV effects associated with both classes, different class-specific effects (GLP-1RAs: benefit for risk of atherosclerotic outcomes, SGLT2 inhibitors: benefit for risk of HF outcomes) largely contributed to the overall improvement of CV outcomes. CV cardiovascular, GLP-1RA glucagon-like peptide-1 receptor agonist, SGLT2 sodium-glucose cotransporter 2
We also considered differences in trial designs and results among the CV outcomes trials with GLP-1RAs (Table 1). To date, four trials have been completed, and two trials are ongoing. Among the four completed trials, two (LEADER and SUSTAIN-6) demonstrated significant risk reduction in MACE [2, 3], and one (EXSCEL) demonstrated a numerically reduced risk of MACE [28]. In SUSTAIN-6, semaglutide treatment reduced the risk of MACE by 26% in a short follow-up duration (median 2.1 years). In contrast, in the ELIXA trial (once-daily lixisenatide) [29], neither an increase nor a decrease in MACE and the individual component CV events was observed during the follow-up duration (median 2.1 years). This may suggest the possibility that the short-acting nature of GLP-1 activation enhancement was insufficient to reduce the occurrence of CV events, beyond improved glycemic metabolism. Compared to the other trials investigating GLP-1RAs, the ELIXA trial included T2D patients with recent acute coronary syndrome (ACS), suggesting that perhaps their CV risk was too severe to demonstrate a simple decline in CV events via short-term pharmacological intervention. Furthermore, the patients in the trial had received higher rates of statin treatment (> 90%), and their levels of HbA1c were lower (< 8.0%) than those of patients in the other trials. Such differences in trial designs and medical history of the patient populations may be a major determinant of the discrepancy in the results, although the exact reasons are currently unclear. In the more recent EXSCEL trial (once-weekly exenatide), all-cause mortality was statistically decreased by 14%, and CV death was numerically decreased by 12% [28]. Such outcomes were also significantly reduced by liraglutide treatment in the LEADER trial, but were not significantly reduced in the SUSTAIN-6 trial [2]. Focusing on the differences between the two trials, the LEADER trial included a higher rate (> 80%) of patients receiving secondary prevention of CV events, while the rate was 60% in the SUSTAIN-6 trial. Interestingly, among the CV outcomes trials with SGLT2 inhibitors, the EMPA REG OUTCOME trial, in which almost all patients had a history of CV events at baseline, showed marked reduction in all-cause mortality and CV death [1]. On the other hand, the CANVAS program, in which one-third of patients had no prior history of CV events, demonstrated no significant risk reduction in such outcomes [4]. We therefore speculate that the reduction in risk of all-cause mortality and CV death following pharmacological intervention depends, at least in part, on history of CV events at baseline. In the EXSCEL trial, > 70% of patients had a previous history of CV events, which was higher than in the SUSTAIN-6 and CANVAS trials. Moreover, a subgroup analysis revealed that exenatide treatment was numerically better at preventing MACE in patients with a previous history of CV events relative to those without, although there was no statistically significant interaction of the treatment effect across the subgroups [28]. A similar trend was also demonstrated in a subgroup analysis of the CANVAS trial [30]. Finally, the treatment effect of GLP-1RAs on the hospitalization for HF was consistently neutral across the trials [2, 3, 28, 29]. Thus, these results suggest that GLP-1RAs exhibit superior preventive effects on atherosclerotic CV events and can improve CV prognosis in T2D patients with a previous history of CV events (except ACS), and that such treatment effects could become apparent by, for example, comparing the results of CV outcomes trials with another class of incretin-based agents, namely dipeptidyl peptidase-4 (DPP-4) inhibitors [31–33].
Table 1.
Comparion of recent CV outcomes trials completed with GLP-1RAs and SGLT2 inhibitors
| GLP-1RA | SGLT2 inhibitor | |||||
|---|---|---|---|---|---|---|
| Once-daily | Once-weekly | |||||
| ELIXA (lixisenatide) | LEADER (liraglutide) | SUSTAIN-6 (semaglutide) | EXSCEL (exenatide) | EMPA-REG OUTCOME (empagliflozin) | CANVASa (canagliflozin) | |
| Patient number | 6058 | 9340 | 3297 | 14,752 | 7020 | 10,142 |
| Median follow-up duration (years) | 2.1 | 3.8 | 2.1 | 3.2 | 3.1 | 2.4 |
| Key eligibility | T2D with recent ACS | T2D with high CV risk | T2D with high CV risk | T2D with high CV risk | T2D with previous CV disease | T2D with high CV risk |
| Prior CV disease (%) | 100 | 81 | 60 | 73 | 99 | 66 |
| Mean baseline HbA1c (%) | 7.7 | 8.7 | 8.7 | 8.0 | 8.1 | 8.2 |
| Metformin use (%) | 66 | 76 | 73 | 77 | 74 | 77 |
| Statin use (%) | 93 | 72 | 73 | 74 | 77 | 75 |
| RAAS inhibitor use (%) | 85 | 83 | 84 | 80 | 81 | 80 |
| Outcomes (HR [95% CI]) | ||||||
| MACEb | 1.02 [0.89–1.17] | 0.87 [0.78–0.97] | 0.74 [0.58–0.95] | 0.91 [0.83–1.00] | 0.86 [0.74–0.99] | 0.86 [0.75 – 0.97] |
| CV death | 0.98 [0.78–1.22] | 0.78 [0.66–0.93] | 0.98 [0.65–1.48] | 0.88 [0.76–1.02] | 0.62 [0.49 – 0.77] | 0.87 [0.72–1.06] |
| Myocardial infarction | 1.03 [0.87–1.22] | 0.86 [0.73–1.00] | 0.74 [0.51–1.08] | 0.97 [0.85–1.10] | 0.87 [0.70–1.09] | 0.85 [0.69–1.05] |
| Stroke | 1.12 [0.79–1.58] | 0.86 [0.71–1.06] | 0.61 [0.38–0.99] | 0.85 [0.70–1.03] | 1.18 [0.89–1.56] | 0.90 [0.71–1.15] |
| All-cause death | 0.94 [0.78–1.13] | 0.85 [0.74–0.97] | 1.05 [0.74–1.50] | 0.86 [0.77–0.97] | 0.68 [0.57 – 0.82] | 0.87 [0.74–1.01] |
| Hospitalization for heart failure | 0.96 [075–1.23] | 0.87 [0.73–1.05] | 1.11 [0.77–1.61] | 0.94 [0.78–1.13] | 0.65 [0.50 – 0.85] | 0.67 [0.52 – 0.87] |
| Composite renal outcomes | – | 0.78 [0.67–0.92] | 0.64 [0.46–0.88] | – | 0.61 [0.53 – 0.70] | 0.60 [0.47–0.77] |
Italic values indicate significance of p value (p < 0.05)
ACS acute coronary syndrome, CI confidence interval, CV cardiovascular, GLP-1RA glucagon-like peptide-1 receptor agonist, MACE major adverse cardiovascular events, HR hazard ratio, RAAS renin–angiotensin–aldosterone system, SGLT2 sodium-glucose cotransporter 2, T2D type 2 diabetes
aPooled data from CANVAS and CANVAS-R
b4-point MACE in the ELIXA trial and 3-point MACE in the other trials
Assessment of the reasons for the mismatch between the results of CV outcomes trials with GLP-1RAs and DPP-4 inhibitors remains a challenge. Both are incretin-based glucose-lowering agents that act via enhancement of GLP-1 activity; however, CV outcomes trials with these agents showed apparently different treatment effects on CV outcomes. In contrast to the CV outcomes trials with GLP-1RAs, those with DPP-4 inhibitors demonstrated consistent non-inferiority in both the primary composite outcome and individual components compared to placebo, and even showed some unexpected increases in risk of hospitalization for HF, acute pancreatitis, and hypoglycemia [31–34]. Although the exact mechanisms by which both classes of agents exert their inconsistent effects on CV safety remain unknown, there are apparently distinct modes of action resulting in different enhancement of GLP-1 levels and non-glycemic effects [35]. Dr. Packer recently proposed an intriguing hypothesis that DPP-4 inhibitors potentiate some endogenous peptides, such as stromal cell-derived factor-1, in addition to activation of GLP-1, and that unfavorable effects of those peptides on CV parameters (e.g., inflammation, fibrosis, plaque growth, and sympathetic activation) could prevail against the CV protective effects of GLP-1, possibly leading to worsened CV complications and HF [36, 37]. Thus, based on the results of CV outcomes trials with incretin-based agents, clinical priority of GLP-1RAs may be accentuated when choosing antidiabetic agents, at least for T2D patients at higher risk of CV events. Furthermore, interestingly a recent report from Japan demonstrated that dulaglutide 0.75 mg once weekly was a cost-effective therapy, compared to insulin glargine [38].
In summary, recent CV outcomes trials with GLP-1RAs have shown promising benefits in CV care of T2D patients, and GLP-1RAs have been accordingly listed as a second line therapy in T2D patients with established atherosclerotic CV disease, alongside SGLT2 inhibitors [5, 6]. As the pharmacological action and impact on CV parameters interpreted from the CV outcomes trials seemingly differed between both classes of glucose-lowering agents, they should be used accordingly, that is, after taking into consideration a patient’s needs and drug tolerability. Further studies are also needed to assess mechanistic insights into GLP-1RAs on CV parameters and clinical safety of GLP-1RAs especially in elderly populations.
Authors’ contributions
AT wrote the draft of the article, which was critically supervised by KN. Both authors read and approved the final manuscript.
Acknowledgements
Authors thank Ms. Aya Yamada for her excellent secretarial assistance.
Competing interests
AT has no financial interests to disclose related to this manuscript. KN has received honoraria from Boehringer Ingelheim, Daiichi Sankyo, Mitsubishi Tanabe, Astellas, Merck, Takeda, and Ono; research funding from Teijin, Mitsubishi Tanabe, Boehringer Ingelheim, Astellas, and Bayer; and scholarships from Astellas, Daiichi Sankyo, Takeda, Mitsubishi Tanabe, Boehringer Ingelheim, Bristol-Myers Squibb, and Teijin.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
None.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACS
acute coronary syndrome
- CV
cardiovascular
- GLP-1RA
glucagon-like peptide-1 receptor agonist
- DPP-4
dipeptidyl peptidase-4
- HF
heart failure
- MACE
major adverse CV event
- SGLT2
sodium-glucose cotransporter 2
- T2D
type 2 diabetes
Contributor Information
Atsushi Tanaka, Phone: +81-952-34-2364, Email: tanakaa2@cc.saga-u.ac.jp.
Koichi Node, Email: node@cc.saga-u.ac.jp.
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes care. 2018;41(Suppl 1):S73–S85. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association 9. Cardiovascular disease and risk management: standards of medical care in diabetes-2018. Diabetes care. 2018;41(Suppl 1):S86–S104. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 7.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 8.Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297(1–2):127–136. doi: 10.1016/j.mce.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Davidson MH. Cardiovascular effects of glucagonlike peptide-1 agonists. Am J Cardiol. 2011;108(3 Suppl):33b–41b. doi: 10.1016/j.amjcard.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Nauck MA, Meier JJ, Cavender MA, Abd El MA, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136(9):849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 11.Lim S, Kim KM, Nauck MA. Glucagon-like peptide-1 receptor agonists and cardiovascular events: class effects versus individual patterns. Trends Endocrinol Metab TEM. 2018;29(4):238–248. doi: 10.1016/j.tem.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19(4):524–536. doi: 10.1111/dom.12849. [DOI] [PubMed] [Google Scholar]
- 13.Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6(6):e004007. doi: 10.1161/JAHA.116.004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, Sugg J, Parikh S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16(2):159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 15.Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum-Morschel L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16(11):1087–1095. doi: 10.1111/dom.12322. [DOI] [PubMed] [Google Scholar]
- 16.Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Investig. 2014;124(10):4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen LB, Secher A, Hecksher-Sorensen J, Pyke C. Long-acting glucagon-like peptide-1 receptor agonists have direct access to and effects on pro-opiomelanocortin/cocaine- and amphetamine-stimulated transcript neurons in the mouse hypothalamus. J Diabetes Invest. 2016;7(Suppl 1):56–63. doi: 10.1111/jdi.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau J, Bloch P, Schaffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58(18):7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 19.Koska J, Sands M, Burciu C, D’Souza KM, Raravikar K, Liu J, Truran S, Franco DA, Schwartz EA, Schwenke DC, et al. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes. 2015;64(7):2624–2635. doi: 10.2337/db14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambadiari V, Pavlidis G, Kousathana F, Varoudi M, Vlastos D, Maratou E, Georgiou D, Andreadou I, Parissis J, Triantafyllidi H, et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):8. doi: 10.1186/s12933-017-0646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushima H, Mori Y, Koshibu M, Hiromura M, Kohashi K, Terasaki M, Fukui T, Hirano T. The role of endothelial nitric oxide in the anti-restenotic effects of liraglutide in a mouse model of restenosis. Cardiovasc Diabetol. 2017;16(1):122. doi: 10.1186/s12933-017-0603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaul S. Mitigating cardiovascular risk in type 2 diabetes with antidiabetes drugs: a review of principal cardiovascular outcome results of EMPA-REG OUTCOME, LEADER, and SUSTAIN-6 trials. Diabetes Care. 2017;40(7):821–831. doi: 10.2337/dc17-0291. [DOI] [PubMed] [Google Scholar]
- 23.Sattar N, Petrie MC, Zinman B, Januzzi JL., Jr Novel diabetes drugs and the cardiovascular specialist. J Am Coll Cardiol. 2017;69(21):2646–2656. doi: 10.1016/j.jacc.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316(5):500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 27.Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornoe K, Zinman B, Buse JB. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 28.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, Ping L, Wei X, Lewis EF, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 30.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (canagliflozin cardiovascular assessment study) Circulation. 2018;137(4):323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 32.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 33.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Chen X, Lu P, Zhang J, Xu Y, He W, Li M, Zhang S, Jia J, Shao S, et al. Incretin-based agents in type 2 diabetic patients at cardiovascular risk: compare the effect of GLP-1 agonists and DPP-4 inhibitors on cardiovascular and pancreatic outcomes. Cardiovasc Diabetol. 2017;16(1):31. doi: 10.1186/s12933-017-0512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203–216. doi: 10.1111/dom.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Packer M. Have dipeptidyl peptidase-4 inhibitors ameliorated the vascular complications of type 2 diabetes in large-scale trials? The potential confounding effect of stem-cell chemokines. Cardiovasc Diabetol. 2018;17(1):9. doi: 10.1186/s12933-017-0648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packer M. Do DPP-4 inhibitors cause heart failure events by promoting adrenergically mediated cardiotoxicity? clues from laboratory models and clinical trials. Circ Res. 2018;122(7):928–932. doi: 10.1161/CIRCRESAHA.118.312673. [DOI] [PubMed] [Google Scholar]
- 38.Ishii H, Madin-Warburton M, Strizek A, Thornton-Jones L, Suzuki S. The cost-effectiveness of dulaglutide versus insulin glargine for the treatment of type 2 diabetes mellitus in Japan. J Med Econ. 2018;21(5):488–496. doi: 10.1080/13696998.2018.1431918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.