Abstract
Background
Diet supplementation with polyphenols is a novel strategy to improve meat quality in livestock, by preventing oxidative deterioration of lipids and protein. Polyphenols have beneficial effects on both human and animal health and can be obtained from several sources, such as olive mill wastewaters (OMWW). These are severe environmental pollutants and therefore may be recycled and utilized in other sectors. The aim of this study was to evaluate growth performance, meat characteristics, fatty acid composition, antioxidant status, different forms of myoglobin and malondialdehyde formation in kids who received a diet supplemented with polyphenols obtained from OMWW. Weaned goat kids (n = 18) were divided into two homogenous groups: control (C) group (n = 9) received a fattening standard diet while the other group (n = 9) received the same diet, supplemented with 3.2 mg/day of polyphenols powder extract (PE group). Average daily gain (ADG) was calculated 10 days apart throughout the study. After 78 days, the kids were slaughtered and pH and carcass yield were evaluated. Longissimus thoracis et lumborum muscle was collected and utilized for chemical analysis, meat quality evaluation and oxidative stability.
Results
No differences were recorded in ADG, carcass weight, pH and dressing between the two groups. Furthermore a similar meat proximate composition, texture and color was observed. Dietary polyphenols supplementation significantly (P < 0.01) decreased short chains (<C12:0) (2.93 + 0.50 and 0.35 + 0.40 g/100 g of fatty acids, for C and PE Group, respectively), and saturated (49.22 ± 2.39 and 39.51 ± 1.95 g/100 g, in C and PE Group, respectively) fatty acids. Furthermore, a higher (P < 0.05) proportion of monounsaturated (34.35 ± 2.84 and 42.22 ± 2.32 g/100 g, in C and PE Group, respectively) fatty acids was recorded. Malondialdehyde formation was significantly (P < 0.05) lower in PE compared to C Group (0.25 ± 0.005 and 0.15 ± 0.005, in C and PE Group, respectively).
Conclusions
Polyphenols dietary supplementation has positive effects on kid meat, improving fatty acid profile and reducing malondialdehyde contents. Furthermore the utilization of OMWW as the source of polyphenols may represent an innovative strategy to re-utilize agri-food industry wastes.
Keywords: Polyphenols, Kids, Fatty acids, Sensory evaluation, Malondialdehyde
Background
An increased awareness of the consumers was recently observed regarding red meat’s production and consumption. A typical example was the latest report of the World Health Organization (WHO), which classified the consumption of red meat as “probably carcinogenic for man” [1], although, as outlined by other authors [2], there are still a number of gaps in the current knowledge about this topic. Furthermore, eating red meat has been correlated with higher incidence of chronic diseases [3]. For this reason there has been an increased interest for new breeding techniques which confer potential health benefits to the consumers [4].
The supplementation of ruminants diet with antioxidants is considered an effective strategy for changing and ameliorating the fatty acid composition of meat in response to consumer demands [4]. In fact, it has been observed that the inclusion of linseed and fish oil on the lamb diet may have a negative impact on the oxidative stability and on physicochemical properties of the meat [5]. In addition, the changes that occur in the muscle during the post-mortem period may create an unbalanced proportion between the antioxidant and pro-oxidant capability, increasing the risks of oxidative damage [6]. The antioxidant status of the muscle is the main factor affecting oxidative deterioration in meat [7]. Oxidative deterioration of lipids and proteins in meat could adversely affect its nutritional quality and shelf life, reducing flavor, color and quality of meat, with a negative impact on meat consumption.
Thus, it is essential to preserve the quality and the safety of the meat by attenuating oxidative deterioration. Recently, the interest of food processing industries in the use of natural antioxidants rather than synthetic counterparts was increased, for either low environmental impact and economical reasons [8]. Furthermore natural antioxidants are well accepted by the consumers, because they are considered safe and healthy [9]. For these reasons, many studies have been carried out to develop new natural antioxidants, especially from plants [8], among which polyphenols. Polyphenols are a large family of more than 8000 natural compounds derived from plants and characterized by the presence of a phenol ring in their structure [10, 11]. Epidemiological, clinical and nutritional studies strongly support the evidence that dietary phenolic compounds are effective in the prevention of common diseases, including cancer, neurodegenerative diseases and gastrointestinal disorders [12]. Furthermore, a large number of studies suggest several immunomodulatory and anti-inflammatory properties of these compounds in humans [13, 14]. Some studies, performed in livestock, reported the positive effects of diet supplementation with polyphenols on pigs and chicken health. [11, 15, 16]. In addition, it has been hypothesized that dietary supplementation with polyphenols would increase beneficial lipids and oxidative stability of myoglobin and reduce the content of malondialdehyde (MDA) [see 11 for review].
Polyphenols can be obtained from several sources. One of these is the olive oil sector, particularly important in Mediterranean countries, such as Spain (the leading producer), Italy, Greece, Turkey, Syria and Tunisia. Olive mill wastewaters (OMWW) are the main pollutant by-phase and traditional suction systems Mills. The management of OMWW is a serious environmental issue for the presence of organic compounds that turn OMWW into phytotoxic materials [17]. Nevertheless, OMWW contains valuable resources, such as polyphenols, which can represent about 10% of OMWW dry weight [18]. Some olive oil derived-compounds, such as hydroxytyrosol, have an important role in preventing cardiovascular diseases [19]. Thus OMWW could be recycled and utilized in other sectors, such as animal feeding, to reduce its environmental impact.
Therefore, the objective of this study was to investigate the effects of the supplementation with polyphenols obtained from OMWW in the diet of Saanen goat kids on growth performance and meat quality. In particular, meat proximate composition, texture and colorimetric properties, fatty acid composition, lipid oxidation and the level of the different forms of myoglobin of Longissimus thoracis et lumborum (LTL) muscle were evaluated.
Methods
Animals, experimental design and diet composition
All experimental procedures were approved by the Ethical Committee on Animal Research of the University of Naples (Protocol number: 2014/0105988 of 1st December 2014), and the study was carried out in accordance with EU Directive 2010/63/EU for animal experiments.
The trial was performed on 18 Saanen female kids maintained in the experimental farm of CREA (Research Centre for Animal Production and Aquaculture) located in Bella (PZ), in the South of Italy, at 40°75′ latitude and 15°67′ W longitude, and 802 m above sea level. The kids were allowed natural suckling until weaning, that occurred at 69 days of age. After weaning the animals were divided into 2 homogeneous groups, according to age and live body weight (LW), recorded at both birth and weaning: Control (C) group (n = 9): received a fattening standard diet (Table 1); Polyphenols extract (PE) group (n = 9): received the same diet of control group, supplemented with a powder of polyphenols extract from OMWW (3.2 mg/day, see below). The amount of polyphenols powder was defined according to antioxidant activity (see below) and some studies performed in growing lambs [5].
Table 1.
Ingredients (%) and proximate composition (% of dry matter) of the basal diet administered to the kids throughout the experimental period
| Ingredients (%) of concentrate administered | ||
|---|---|---|
| Maize meal | 60 | |
| Faba bean (Viccia faba minor) | 15 | |
| Alfalfa pellets | 5 | |
| Soybean meal | 5 | |
| Wheat bran | 10 | |
| Oat | 5 | |
| Concentrate | Dehydrated alfalfa | |
| Dry Matter (g/100 g weight) | 87.5 | 85.6 |
| Ash | 2.7 | 8.3 |
| Crude protein | 11.8 | 19.5 |
| Crude fiber | 6.19 | 25.6 |
| Neutral detergent fiber | 18.2 | 37.5 |
| Acid detergent fiber | 8.9 | 34.3 |
| Lignin | 2.1 | 9.8 |
| Ether extract | 3.8 | 1.9 |
| Non structural carbohydrates | 63.5 | 25.6 |
| Starch | 56.8 | – |
The kids of each group were individually penned in boxes (1 m2) throughout the study that lasted 78 days. All kids had free access to water and received the same ration, consisting of alfalfa hay ad libitum and a starter concentrate administered in increasing amount according to the growth following the recommendation of National Research Council [20]. Feed was administered twice a day, at 8.00 and 16.00 and polyphenols powder was mixed on daily bases with the concentrate in the PE Group. The inclusion level of polyphenols extract was variable throughout the study according the amount of administered concentrate from 1.70% at the start of the trial to 0.83% in the last week (1.16% on average). Feed intake was determined from orts (refusals) collected daily in the morning (when present) before the next feed administration. The amount and the composition of orts were utilized to calculate the dry matter (DM) intake and the composition of the ingested diet. Individual feedstuff and orts were sampled every 15 days and the analyses were carried out as per AOAC (Association of Official Analytical Chemists) procedures [21] after drying at 65 °C and mechanical reduction of the samples (granulometry 1 mm for all analyses). The chemical composition of individual feedstuff is reported in Table 1.
Each animal was individually weighed 10 days apart and average daily gain (ADG) was calculated by dividing the difference between two consecutive LW measurements (LW1 and LW2) with the number of days elapsed (LW2 − LW1/days).
Polyphenols determination
Polyphenols extract from OMWW was obtained and characterized as reported by Parrillo et al. [22]. Briefly, the polyphenolic extract was obtained by membrane separation of OMWW according to previous studies [23] and the main phenolic compounds were identified by HPLC (LC-4000 Series Integrated HPLC Systems, JASCO, Japan) according to Azaizeh et al. [18] (Table 2). Total phenols content in OMWW extract was determined by the Folin-Ciocalteu method [24]. The antioxidant activity of the OMWW extract was evaluated by using the free radical ABTS (2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid), according to the procedures described by Re et al. [25].
Table 2.
Main phenolic compounds of olive mill wastewaters (OMWW) extracts
| Main compounds | mg/kg | Percent |
|---|---|---|
| Hydroxytyrosol | 20,829 | 21.27 |
| Flavonoid | 3278 | 3.35 |
| Tyrosol | 3947 | 4.03 |
| Caffeic acid | 9991 | 10.20 |
| Verbascoside | 17,449 | 17.82 |
| Hydroxytyrosol derivatives (OHTY-glycol, OHTY-glucoside, 3,4-diidrossifenilethanol – 3,4-DHPEA Elenolic acid mono-Aldehyde, 3,4-DHPEA– AC hydroxytyrosol acetate) | 26,826 | 27.40 |
| Verbascoside derivatives (isoverbascoside, β-hydroxyverbascoside,β-hydroxyisoverbascoside) | 6498 | 6.64 |
| Other derivatives of cinnamic acid (cinnamic acid, o-, p- coumaric acid, ferulic acid) | 3372 | 3.44 |
| Caffeic acid derivatives (chlorogenic acid, neochlorogenic acid, 1-O-caffeoylquinic acid, 3,5-O-dicaffeoylquinic acid) | 4681 | 4.78 |
| Other polyphenols | 1055 | 1.08 |
| Total polyphenols (TPC) (mg/kg) | 97,926 | |
| Antioxidant activity (mmolitrolox/kg sample) | 8521 |
Slaughtering procedure and muscle sampling
At the end of the experimental period (147 days of age), all the kids were left overnight with ad libitum access to water and slaughtering procedures were carried out in accordance to the EU Regulation 2009/1099/EC on the protection of animals at the time of killing. The animals were stunned by captive bolt and the exsanguination from the jugular vein was carried out. After slaughtering, evisceration and dressing, each carcass was weighed and the pH was measured 45 min post-mortem in the LTL muscle (between 11th and 13th thoracic vertebra), using an automatic digital pH-metertest-205 (TestoInc, Sparta, NJ, USA), equipped with a penetrating electrode. The probe was calibrated with pH 4 and 7 standard buffer solutions. The dressed carcass comprises the body after removing skin, head, fore feet (at the carpal–metacarpal joint), hind feet (at the tarsal–metatarsal joint), lung, heart, liver, spleen, kidneys, kidney fat, and gastro-intestinal tract fat. Furthermore, the stomachs (rumen, reticulum, omasum, and abomasum) and the postruminal tract (intestine and caecum) were removed. Dressing percentage (DP) was calculated according to the following formula:
All carcasses were stored at 2 °C for 24 h. Ultimate pH was assessed 24 h post-mortem on LTL muscle and the carcasses were weighed again. After that, a professional butcher removed the LTL muscle, between the sixth thoracic and 5th lumbar vertebra, from the right side of each carcass. LTL samples were stored at 4 °C and used for chemical analysis, meat quality evaluation and oxidative stability. Furthermore, pH was also assessed 48 h post-mortem on LTL muscle as described above.
Chemical analyses
All analyses were carried out in the Laboratories of the Department of Veterinary Medicine and Animal Production (DMVPA), and Department of Agriculture Sciences (DIA) of University of Naples Federico II (Italy). The chemical composition of LTL muscle was determined on refrigerated (4 °C) samples according to the AOAC procedures [26] by using a Foodscan equipment (Food Scan™Lab 78,810). Each sample was analyzed in duplicate.
Texture measurements
Tenderness was evaluated on meat cooked in a thermostatically controlled water bath at 90 °C on day 1 after slaughtering. To monitor temperature achieved in the middle of the sample (70 °C), a portable digital thermometer (TEMP7 thermometer digital microprocessor for Pt100 probes, TECNAFOOD MO, IT) was used. After cooking the samples were dried with paper, in order to eliminate the moisture on the surface, and held in a cooler at 4 °C before coring. A minimum of four cores of 1.27 cm2 from each LTL muscle were obtained parallel to the longitudinal orientation of the muscle fibers. The Instron 5565 with a Warner–Bratzler shear (WBS) device and crosshead speed set at 100 mm/min and a load cell of 500 kg [27] was used. According to Girard et al. [28], the measured parameters were Shear myofibrillar force (SMF) and Warner-Bratzler Shear Force (WBSF) both expressed in kg. Indeed in a shear force curve some peaks of less importance may be observed, before and after maximum positive peak shear force (WBSF). Bouton & Harris [29] related these first small peaks to the myofibrillar component of shear force coinciding with initial yield (SMF). WBSF minus SMF estimates the connective tissue contribution.
Instrumental color
The color of the meat was determined on the surface of samples using a U3000 spectrophotometer, equipped with integrating sphere (Hitachi, Tokyo, Japan). Although illuminant D65 is largely utilized, the use of Illuminant A is recommended by AMSA [30]. For this reason color coordinates, employing the CIEL*a*b* system with two illuminants, D65 (6500 K) and A (2856 K) and 10° standard observer, were Lightness (L*); redness (a*), and yellowness (b*) were also calculated, according to AMSA [30]. The color analyses were carried out in duplicate every day, from 24 h post-mortem until day 7, on samples (diameter 2.54 cm, thick 2 cm) stored at 4 °C. The samples were placed on white trays and wrapped with oxygen-permeable film.
Color difference (∆E*) between each day of storage and the day 0 was calculated as follows:
Where AL*, ∆a*and ∆b*are the differences between L*, a* and b* values at time 0 and the individual readings each day.
Metmyoglobin (MMb), deoxymyoglobin (DMb) and oxymyoglobin (OMb) percentages were estimated according to [30] on the basis of the Reflex Attenuance (A) at the isobestic points 572, 525, 473 and 730 nm (nm). The Reflex Attenuance (A) was identified as:
where R expresses the reflectance at a specific wavelength in decimal (0.30 rather than 30%).
Therefore from the Reflex Attenuance (A), it was possible to estimate the three forms of myoglobin:
Fatty acid analysis
Fatty acid composition of the meat was assessed on fresh samples on day 1 post-slaughtering. The muscle was blended in a food processor and the lipids were extracted from 5 g samples in duplicate, using chloroform:methanol (2:1, v/v) [31]. The extracted lipids were transmethylated to their fatty acid methyl esters (FAME) according to Christie [32]. The amount of fatty acid (g/100 g of FAME) was determined by gas chromatography using a chromatograph (DANI fast GC, Italy), with a flame ionization detector and equipped with a capillary column (TR-CN 100) (60 m × 0.25 mm diameter × 20 μm). Helium was used as carrier gas at a flow rate of 1.2 mL/min. The split ratio was 50:1, the injector was set at 280 °C and the detector at 240 °C. The oven temperature was programmed and held at 80 °C for 5 min, then increased to 165 °C at 5 °C/min, held at 165 °C for 1 min, and then increased to 260 °C at 3 °C/min, and then held at 260 °C for 1 min.
Identification of each fatty acid was obtained by comparing the chromatogram with the reference standard mixture of Supelco 37 component series FAME MIX (Supelco Bellefonte, PA, USA) and a mixture of CLA isomers (Nu-Chek-Prep, Inc., Elysian, MN, USA). Retention time and area of each peak were calculated using the Clarity software (Clarity v.2.4.1.77, Data Apex Ltd., 2005). The atherogenic index (AI) and the thrombogenic index (TI) were obtained by using the following equations [33].
Evaluation of lipid oxidation
The evaluation of lipid oxidation in meat was based on the determination of malondialdehyde (MDA), a secondary lipid oxidation product (nmol/μg of meat). MDA was determined by a specific kit (Lipid Peroxidation (MDA) Assay Kit, Sigma-Aldrich, USA) according to the manual instructions. MDA was quantified by spectrophotometric analysis (Model 680 Microplate Reader, Biorad, Italy), at a wave length of 532 nm. MDA content was evaluated at 1, 3 and 7 days post-slaughtering.
Statistical analysis
The experimental data were subjected to one way analysis of variance (ANOVA) using the linear model of the SAS software [34], following confirmation of normality and homogeneity of variance. The influence of dietary treatment (polyphenols supplemented diet or control diet),storage (day 1, 3 and 7) and their interaction (dietary treatment x storage) were used as main factors to analyze color parameters, MDA levels and the different forms of myoglobin. Results are presented as mean values with standard error of mean (SEM). The mean were compared using the Student’s t-test. The differences were considered significant when P values were lower than 0.05, whereas a tendency was considered for P < 0.10.
Results
Animal performance and carcass characteristics
The effects of polyphenols supplementation on growth performance and carcass properties are shown in Table 3. A similar ADG from weaning to slaughter and a similar weight at slaughter were observed in the two Groups. Furthermore, carcass weights and yields were similar between groups at 0 and 24 h post mortem (Table 3). No significant differences were observed in the pH decline values between the groups both at 45′ minutes and 24 h post-mortem (Table 3).
Table 3.
Age and weight at weaning and at slaughter, average daily gain (ADG), feed consumption, carcass characteristics and dressing percentage in Control (C) and polyphenols extract (PE) group (mean values ± SEM)
| Groups | Significance | ||
|---|---|---|---|
| C | PE | P | |
| Age at weaning (days) | 69.8 ± 1.30 | 68.9 ± 1.50 | 0.65 |
| Age at slaughtering (days) | 146.8 ± 1.30 | 145.9 ± 1.50 | 0.65 |
| Weaning body weight (kg) | 11.0 ± 0.75 | 10.7 ± 0.86 | 0.78 |
| Final body weight (kg) | 17.9 ± 0.99 | 18.6 ± 1.28 | 0.69 |
| Weight gain (kg) | 6.94 ± 0.60 | 7.93 ± 0.61 | 0.26 |
| ADG (kg/g) | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.26 |
| Concentrate intake (kg/day) | 0.30 ± 0.00 | 0.29 ± 0.00 | 0.33 |
| Hay intake (kg/day) | 0.68 ± 0.01 | 0.67 ± 0.01 | 0.39 |
| DM intake (kg/day) | 0.84 ± 0.02 | 0.85 ± 0.03 | 0.57 |
| Carcass weight at slaughtering (kg) | 10.0 ± 0.51 | 10.0 ± 0.77 | 0.98 |
| Carcass weight at 24 h (kg) | 9.7 ± 0.50 | 9.7 ± 0.74 | 0.99 |
| pH at slaughtering | 6.8 ± 0.11 | 6.8 ± 0.12 | 0.90 |
| pH at 24 h | 5.8 ± 0.03 | 5.8 ± 0.03 | 0.64 |
| Dressing at slaughtering (%) | 55.8 ± 1.41 | 54.0 ± 0.48 | 0.15 |
| Dressing at 24 h (%) | 54.6 ± 1.39 | 52.3 ± 0.45 | 0.14 |
Meat quality
A similar meat chemical composition was recorded in C and PE group (Table 4).
Table 4.
Proximate meat composition of LTL muscle in control (C) and polyphenols extract (PE) group (mean values ± SEM)
| Groups | Significance | ||
|---|---|---|---|
| C | PE | P | |
| Moisture (%) | 66.6 ± 0.46 | 64.1 ± 0.35 | 0.11 |
| Fat (%) | 13.7 ± 0.46 | 16.5 ± 0.31 | 0.09 |
| Protein (%) | 18.3 ± 0.30 | 18.7 ± 0.40 | 0.43 |
| Collagen (%) | 2.3 ± 0.28 | 3.0 ± 0.16 | 0.14 |
| Ash (%) | 0.91 ± 0.12 | 0.84 ± 0.05 | 0.58 |
| pH* | 6.1 ± 0.01 | 6.2 ± 0.01 | 0.51 |
*pH was assessed on LTL sample 48 h after slaughtering
The texture assay highlights a double peak (SMF = 1st peak and WBSF = 2nd peak)in 9/9 animals of C samples compared to 3/9 animals of PE counterparts. SMF values obtained by deformation curves where a double peak was evident, tended to be higher (P = 0.06) in PE compared to C group, whereas similar WBSF values were recorded (Table 5).
Table 5.
Shear myofibrillar force (SMF) and Warner-Bratzler Shear Force (WBSF) recorded in the LTL muscle of control (C) and polyphenols extract (PE) group (mean values ± SEM)
| Groups | Significance | ||
|---|---|---|---|
| C | PE | P | |
| WBSF, kg | 4.5 ± 0.10 | 4.6 ± 0.10 | 0.86 |
| SMF, kg | 0.53 ± 0.07 | 1.14 ± 0.33 | 0.06 |
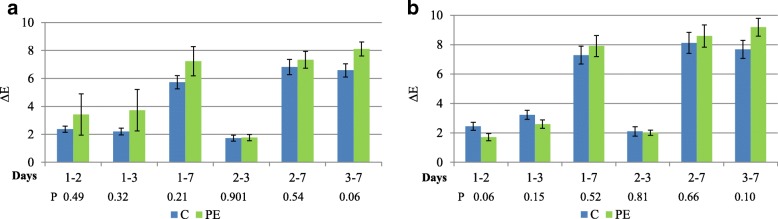
The color coordinates detected by using the two illuminants (A and D65) were similar between the two groups. In fact, although the illuminant A would give more emphasis on the proportion of red wave lengths, no significant differences were recorded in lightness (L*); redness (a*), yellowness (b*) between the two groups and throughout the storage. Furthermore, no significant interaction storage x treatment was assessed. Slight differences were observed in color differences (∆E*) during the first three days of storage in the meat of PE Group compared to the control when analyzed by illuminant A, while an opposite trend was recorded with the illuminant D65. In any case, no statistical differences were recorded (Fig. 1) and subsequently the changes showed the same trend with both illuminants.
Fig. 1.
Difference of color (∆E) by 1 to 7 days of storage with illuminant D65 (a) and illuminant A (b), in control (c) and polyphenols extract (PE) group (blue) and treated (green) group. The error bars represent standard error
Neither dietary treatment (P = 0.77 and P = 0.99, for OMb and MMb, respectively) nor storage period (P = 0.33 and P = 0.10, for OMb and MMb, respectively) and their interaction (P = 0.98 and P = 0.95, for OMb and MMb, respectively) influenced the estimated levels of myoglobin forms. As expected, during the storage period, the estimated level of MMb tended to increase in both groups, while the levels of OMb decreased until 3 days and increased after 7 days of storage (Table 6).
Table 6.
Estimated levels (mean percentage ± SEM) of metmyoglobin (MMb) and oxymyoglobin (OMb), in the LTL muscle of control (C) and treated (PE) group, throughout the storage at 4 °C
| Groups | 1 | 2 | 3 | 7 | |
|---|---|---|---|---|---|
| MMb | C | 40.73 ± 1.88 | 45.28 ± 1.18 | 53.57 ± 1.67 | 61.78 ± 3.60 |
| PE | 40.99 ± 1.94 | 47.67 ± 1.52 | 54.41 ± 1.88 | 63.37 ± 3.94 | |
| OMb | C | 68.93 ± 2.70 | 34.04 ± 3.04 | 15.07 ± 3.24 | 43.87 ± 9.42 |
| PE | 62.66 ± 7.02 | 38.13 ± 4.83 | 21.42 ± 5.69 | 51.48 ± 10.28 |
Fatty acid composition and oxidative stability
The fatty acids composition of LTL intramuscular lipids in the two groups is shown in Table 7. Dietary polyphenols supplementation determined a decrease of short chain fatty acids (<C12:0) as well as of Myristic (C14:0) Myristoleic (C14:1), Pentadecanoic (C15:0), Palmitic (C16:0) and Palmitoleic (C16:1) acids.
Table 7.
Mean fatty acid composition (g/100 g of FAME) of LTL muscle in control (C) and polyphenols extract (PE) group (mean values ± SEM)
| Groups | Significance | ||
|---|---|---|---|
| Fatty Acids | C | PE | P |
| < C12:0 | 2.93 ± 0.50 B | 0.35 ± 0.40 A | 0.001 |
| C12:0 | 0.45 ± 0.36 | 0.20 ± 0.07 | 0.06 |
| C14:0 | 4.00 ± 0.58 b | 2.28 ± 0.47 a | 0.04 |
| C14:1 | 0.46 ± 0.06 B | 0.23 ± 0.05 A | 0.01 |
| C15:0 | 0.58 ± 0.06 b | 0.41 ± 0.05 a | 0.04 |
| C16:0 | 23.81 ± 0.85B | 20.22 ± 0.70A | 0.01 |
| C16:1 | 3.05 ± 0.41 b | 1.63 ± 0.33 a | 0.02 |
| C17:0 | 1.12 ± 0.17 | 1.17 ± 0.14 | 0.79 |
| C17:1 | 1.33 ± 0.17 | 0.91 ± 0.14 | 0.08 |
| C18:0 | 15.60 ± 1.25 | 14.31 ± 1.02 | 0.43 |
| C18:1 cis | 27.16 ± 3.15 a | 36.01 ± 2.57 b | 0.05 |
| C18:1 trans | 0.08 ± 0.73 | 1.64 ± 0.60 | 0.12 |
| C18:2 cis | 12.22 ± 1.07 | 13.64 ± 0.88 | 0.32 |
| C18:2 trans n6 | 0.48 ± 0.05 | 0.37 ± 0.04 | 0.09 |
| C18:3 n6 γ-linolenic | 0.10 ± 0.03 | 0.07 ± 0.02 | 0.43 |
| C20:0 | 0.05 ± 0.04 | 0.03 ± 0.03 | 0.74 |
| C20:1 | 1.62 ± 0.15 | 1.59 ± 0.12 | 0.89 |
| C20:2 n6 | 0.10 ± 0.03 | 0.05 ± 0.02 | 0.31 |
| CLA cis 9–trans 11 | 1.22 ± 0.12 | 1.02 ± 0.10 | 0.24 |
| C20:3 n3 | 1.00 ± 0.40 | 1.22 ± 0.33 | 0.67 |
| C20:4 n6 | 0.72 ± 0.75 | 1.63 ± 0.61 | 0.36 |
| C22:0 | 0.32 ± 0.12 | 0.27 ± 0.10 | 0.76 |
| C22:6 n3 | 0.63 ± 0.40 | 0.24 ± 0.33 | 0.47 |
| C24:0 | 0.33 ± 0.20 | 0.26 ± 0.16 | 0.77 |
| C24:1 | 0.63 ± 0.38 | 0.20 ± 0.31 | 0.39 |
| PUFA n6 | 13.62 ± 0.96 | 15.77 ± 1.30 | 0.31 |
| PUFA n3 | 1.63 ± 0.75 | 1.47 ± 0.61 | 0.86 |
| n6/n3 | 8.36 ± 0.86 | 10.73 ± 0.91 | 0.41 |
| ΣSFA | 49.22 ± 2.39B | 39.51 ± 1.95A | 0.01 |
| ΣMUFA | 34.35 ± 2.84a | 42.22 ± 2.32b | 0.05 |
| ΣPUFA | 16.47 ± 2.02 | 18.26 ± 1.65 | 0.51 |
| UFA:SFA | 1.08 ± 0.13 A | 1.55 ± 0.07 B | 0.01 |
| PUFA:SFA | 0.34 ± 0.04 | 0.47 ± 0.05 | 0.09 |
| AI | 1.28 ± 0.19 b | 0.69 ± 0.16 a | 0.03 |
| TI | 2.13 ± 0.32B | 1.38 ± 0.26 A | 0.01 |
Different superscript letters within the same row indicate significant differences (a.,b.: P < 0.05; A.,B.: P < 0.01)
Note: Σ SFA = (<C12:0 + C12:0 + C14:0 + C15:0 + C16:0 + C17:0 + C18:0 + C20:0 + C22:0 + C24:0), Σ MUFA = (C14:1 + C16:1 + C17:1 + C18:1cis + C18:1trans + C20:1 + C24:1), Σ PUFA = (ΣCLA+Σn3 + Σn6),
UFA:SFA = [(ΣMUFA+ΣPUFA)/ΣSFA],
PUFA:SFA = (ΣPUFA/ΣSFA),
AI: [(4 x C14:0) + C16:0 + C18:0] / [ΣMUFA +ΣPUFA-n6 + ΣPUFA-n3],
TI: [(C14:0 + C16:0 + C18:0) / (0.5xMUFA) + (0.5xPUFA-n6) + (3xPUFA-n3) + (PUFA-n3 / PUFA-n6)]
A significant lower (P < 0.01) percentage of saturated (SFA) and higher (P < 0.05) proportion of monounsaturated (MUFA) fatty acids were recorded in PE group compared to C group. However, no differences were recorded in the total amount of polyunsaturated fatty acids (PUFA), while the unsaturated fatty acids (UFA):SFA ratio was influenced by the treatment (P < 0.01). Furthermore, the PUFA:SFA (P:S) ratio tended to be higher (P = 0.09) in PE group, compared to C group. Therefore, the meat of PE group was also characterized by a significant lower AI index (P < 0.05) and TI index (P < 0.01), compared to the control counterparts.
In relation to lipid oxidation, a significant effect of dietary treatment was observed. In particular, lower (P < 0.05) MDA values were recorded in PE group, compared to C group (0.25 ± 0.005 and 0.15 ± 0.005 in Group C and PE, respectively), as showed in Fig. 2, whereas neither the storage (P = 0.96) nor the interaction storage x dietary treatment (P = 0.97) influence the results.
Fig. 2.
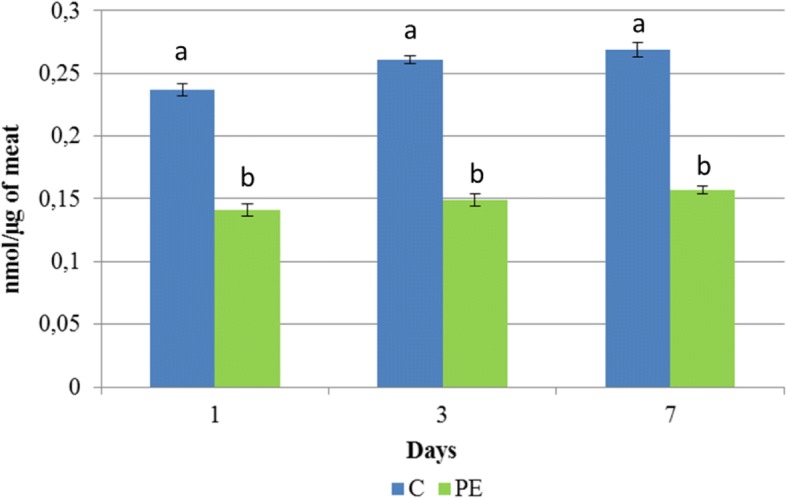
Lipid peroxidation in meat during the days of storage (expressed in MDA nmol/μg of meat), the error bars represent standard error
Discussion
This study aimed to ascertain the influence of dietary polyphenols administration on growth performance and meat quality in kids. Contrasting results have been reported on the effects of plant extracts administration on live weight gain, carcass weight and dressing percentage either in ruminants [35, 36], and not ruminants [11, 37]. According to some authors [37] no adverse effect on growth performance or protein and aminoacids digestibility were recorded in broilers after 2.5 g/kg of grape seed extracts administration, while a delayed growth rate was observed after 5 g/kg of supplementation. On the contrary, other authors [38] report an increase of final body weight and DWG in broilers fed dietary polyphenol-rich grape seed. Few studies have been performed in ruminants till now. Similar growth performance were observed in growing lambs fed a diet supplemented with grape pomace, vitamin E or grape seeds extract [39]. Similarly, the supplementation of the diet with pomegranate seed pulp [40] or with vitamin E, Turmeric powder or Andrographis paniculata powder [35] did not affect the growth of kids.
Also in our study, growth performance was not influenced by dietary polyphenols supplementation, but some aspects need to be considered. First of all about 8000 phenolic compounds have been identified in different plant species [11] and in different amounts, creating serious difficulties in making a comparison among the studies. Furthermore, fewer information is present on bioavailability of phenolic compounds in ruminants and their effects on bacterial rumen population, fermentation and absorption [10] compared to non-ruminants [11]. It can not be ruled out that different compounds and/or different amounts of polyphenols may lead to different results.
Also the proximate composition of the meat in our trial was not influenced by dietary polyphenols supplementation. This result is in agreement with previous studies carried out in goat, in which dietary herbal antioxidants [35] or vitamin E [36] were administered. Similarly, vitamin E supplementation did not affect carcass traits and dressing percentage in lambs [41]. Regarding the texture, the meat of control kids showed the occurrence in all samples of an initial yield putatively related to the myofibrillar component of shear force and classified as shear myofibrillar force [29]. On the contrary, a single peak was recorded in 6/9 animals of the PE group. For this reason, SMF values tended to be higher in PE group compared to C group. However, no differences were recorded for WBSF. This last result may indicate a similar collagen content of the meat recorded in the two groups. The evaluation of the contribution of either myofibrillar and connective tissue to meat texture after the cooking process can not be obtained without performing additional measurements [28]. Some authors [42, 43] suggested that the connective tissue contribution may be recorded by subtracting the initial yield from the shear force peak: although the shear force does not accurately represent the contribution of connective tissue to muscle tenderness [27, 44, 45], it may be hypothesized a larger contribution of the connective component to the final hardness in the control group, compared to the PE counterparts. To our knowledge, few studies have been performed to evaluate texture parameters in small ruminants after polyphenols diet supplementation. Carnosic acid supplementation seems to be an useful tool to improve meat sensory characteristics in fattening lambs, similarly to Vitamin E [46]. On the contrary, quercetin dietary supplementation seemed to worsen texture in fattening lambs [47].
No differences were observed in our study on color stability between the two groups, in accordance with previous reports carried out in lambs fed naringin supplementation [48]. The color is an important meat quality attribute because it is the first aspect which attracts consumers when choosing fresh meat. Color stability is mainly due to the increase in myoglobin oxidation and consequent metmyoglobin formation and accumulation. Kid’s meat is generally characterized by lower a* values compared to beef and lamb meat, outlining the difference in myoglobin concentration that reduces the discoloration. The effects of antioxidants on color stability in small ruminants have been analyzed in several studies in both kids [36, 40, 49] and lambs [39, 50] with contrasting results. According to some authors [40] no differences were recorded in kids fed pomegranate seed pulp on L*, while an increase of a* together with a decrease of b* was assessed. Similarly, no influence of vitamin E supplementation on color parameters was recorded in kids reared both in pen groups or maintained on pastures, although the rearing system significantly influenced meat color [36]. Dietary supplementation with antioxidants (vitamin E and flavonoids in particular) influences color parameters in some [39, 51], but not all [50] studies performed in growing lambs. Other authors reported a significantly lighter meat after polyphenols administration in lambs, probably because of their action in chelating iron and consequent low haemoglobin concentration [52]. Definitely, the results obtained in this study suggest that the concentration of MMb and OMb in meat, and consequently meat color, was not affected by dietary polyphenols supplementation. Only a slight lower, although not significant, ∆E* was observed in PE group, when detected by illuminant A. The latter determined higher value placing more emphasis on the proportion of red wave lengths. This aspect is important when the differences between treatments have to be highlighted, as outlined by AMSA [30]. Similar results were obtained by other authors [7] by using a dietary supplementation with a blend of 80% canola oil and 20% palm oil, and were justified by physical changes that occur during the storage, and not directly related to the oxidative process [49], since no significant effects on color and oxidative stability of myoglobin was recorded. However, it can not be ruled out that the amount of dietary polyphenols was not able to highlight significant effects, thus further studies are needed to evaluate different dosages.
In our study polyphenols extract supplementation affected the fatty acid profile of the meat. In particular, a significantly higher concentration of MUFA, together with a reduction of SFA was observed in PE Group compared to control, but no effects were recorded on PUFA. The acidic composition of meat in ruminants is generally different from that of non-ruminants. The PUFA/SFA ratio is lower because of the hydrogenation of UFA in the rumen, while this process does not occur in monogastrics that absorb UFA without any transformation in the gastrointestinal tract. Therefore, several UFA that are normally present in the diet of ruminants, are saturated and can not be present in derived food [53]. Thus, the diet plays the main role in the modification of fatty acid composition and rumen microbial population, as demonstrated by the evidence that rumen population activity on dietary UFA hydrogenation depends by the administered diet [54]. Several studies carried out in vitro [55–57] demonstrated that some phenolic compounds can modulate rumen fermentation, reducing the rate of biohydrogenation of fatty acids. In particular, some plants with high polyphenols content would be able to lower the biohydrogenation of PUFA in the rumen, increasing their by-pass and, consequently, the formation of CLA [56]. Similarly, an increase of C18 PUFA was recorded after 24 h in vitro incubation of an experimental diet with leaf fractions of papaya, extracted with hexane or chloroform [57]. However, only an increase of C18:1 (oleic acid) was recorded in our study, together with a similar n6 and n3 PUFA and a similar concentration of CLA, although a slight increase of C18:1 trans was observed. It is known that the presence of CLA in the tissues depends from the desaturation of C18:1 trans-11, catalyzed through Δ9 desaturase [58]. However, the amount of C18:1 trans-11 in ruminant meat and milk is influenced by its hydrogenation to stearic acid (C18:0) or other C18:1 isomers at the rumen level by the microbial population [59]. It can not be ruled out that the amount of polyphenols extract utilized in our study might need to be increased to further improve the fatty acid profile of the meat. In a recent study [36], a lower amount of SFA, together with an increase of MUFA and lower AI and TI indexes were observed in kids after 450 mg/kg of vitamin E diet supplementation compared to control counterparts. Adeyemi et al. [4], reported a lower concentration of C16:0 and C16:1 n7 in goats fed 4 and 8% oil blend compared with control, justified by displacement or dilution effects of other fatty acids and by the decrease in the activity of lipogenic enzymes responsible for the synthesis of medium chain FA or the preferential incorporation of long chain FA from diet and/or adipose tissues. In a recent trial [5] dietary supplementation with red wine extract significantly increased the levels of C20:5 n3 in lambs, but no influence on oxidative stability was recorded. In any case, the P:S ratio, recognized as a healthy index, tended to be higher in PE group compared to control. Because of the hydrogenation of PUFA by the microbial population, the meat of ruminants is usually characterized by a low P:S ratio [60], leading to an increased risk of cardiovascular diseases. Therefore, the modification of fatty acid profile obtained in the present study suggests that dietary polyphenols administration may result in kid meat with beneficial effects on human health.
A further positive effect of polyphenols administration was recorded on oxidative stability of the meat, as demonstrated by the reduction of MDA, an aldehyde commonly utilized as marker of secondary lipid oxidation in meat and characterized by mutagenic and carcinogenic properties. In fact, MDA changes the link between lipoproteins and scavenger receptors on the surface of macrophages causing cholesterol ester buildup and foam cell formation and may also react with deoxyadenosine and deoxyguanosine, nitrogenous DNA bases, potentially causing mutations [61].
Although the values recorded in our study may be considered a limiting point from where rancid flavor overpowers flavor in beef [62] and MDA concentration is probably overestimated by spectrophotometric method [63], a significant decrease was observed in PE group compared to C group.
The use of antioxidant substances, such as high polyphenols content in the diet, reduces lipid peroxidation and the formation of DNA additives, by inhibiting the formation of MDA. A possible explanation of the antioxidant activity of polyphenols can be sought in the potential of chelating metal ions [64]: they are able to bind iron, forming insoluble compounds at the gastrointestinal lumen level and making it unavailable for absorption. Iron has been recognized as the most likely catalyst to promote lipid peroxidation by the formation of free radicals formed following the Fenton reaction [65]. This is supported by several studies carried out in chicken poultry [37], rats [66] and pigs [67], in which the inclusion in the diet of polyphenols obtained by different sources significantly reduces plasma iron concentration. Therefore, the reduction of iron absorption may explain the lower MDA content recorded in PE group compared to C group. However, it can not be ruled out that this phenomenon may be due to an enhancement of muscle oxidative stability, via an increased expression of Δ9 desaturase enzyme, as demonstrated in lambs fed tannins supplementation [68], or to the increase of endogenous antioxidant defense [69]. In any case, our results are in agreement with other studies in which a significant reduction in MDA formation following polyphenols administration was recorded in goat [40], chicken [70] and turkey meat [71].
Conclusions
In conclusion, although no effects were observed on meat proximate composition, texture and colorimetric properties, the results of this study demonstrated that the supplementation of the diet with polyphenols extracts by OMWW is able to improve the fatty acid profile of kid meat, together with a reduction of MDA content. The utilization of this source of polyphenols may represent a novel strategy to re-utilize agri-food industry wastes as additives for livestock, meeting either meat industry and consumers requests of natural additives in animal farming. In any case, further studies are needed to test different doses of polyphenols and to better understand the role that the different categories of these compounds may have on meat quality.
Acknowledgements
The authors gratefully acknowledge Mr. Roberto Di Matteo for his technical support during meat quality analyses.
The authors gratefully acknowledge Dr. Emiliana Capurro, PhD, Dr. Trevor Cullen (Dublin, Ireland) and Dr. Claire Teter for language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ∆E*
Color difference
- A
Reflex attenuance
- a*
Redness
- ADG
Average daily gain
- AI
Atherogenic index
- ANOVA
analysis of variance
- AOAC
Association of official analytical chemists
- b*
Yellowness
- C group
Control group
- CREA
Research centre for animal production and aquaculture
- DIA
Department of agriculture sciences
- DM
Dry matter
- DMb
Deoxymyoglobin
- DMVPA
Department of veterinary medicine and animal production
- DP
Dressing percentage
- FAME
Fatty acid methyl esters
- L*
Lightness
- LTL
Longissimus thoracis et lumborum
- LW
Live body weight
- MDA
Malondialdehyde
- MMb
Metmyoglobin
- MUFA
Monounsaturated fatty acid
- OMb
Oxymyoglobin
- OMWW
Olive mill wastewaters
- PE group
Polyphenols extract group
- PUFA
Polyunsaturated fatty acid
- SEM
Standard error of mean
- SFA
Saturated fatty acid
- SMF
Shear myofibrillar force
- TI
Thrombogenic index
- TPC
Total polyphenols
- UFA
unsaturated fatty acid
- WBS
Warner–bratzler shear
- WBSF
Warner-bratzler Shear Force
Authors’ contributions
RC, GC and GN designed the experiment. EV, SC and MC prepared the polyphenols and determined their antioxidant activity. DR, SC, CMAB, MA and RC carried out the experiments. CMAB, RC and PDP performed the statistical analysis of the results. RC, GN, PDP and MC prepared the draft of the manuscript. All authors contributed to the analysis of the data, discussion of results and implications and commented on the manuscript at all stages. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental procedures were approved by the Ethical Committee on Animal Research of the University of Naples (Protocol number: 2014/0105988 of 1st December 2014), and the study was carried out in accordance with EU Directive 2010/63/EU for animal experiments.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roberta Cimmino, Phone: +39 0823 356743, Email: r.cimmino@anasb.it.
Carmela M. A. Barone, Email: cmbarone@unina.it
Salvatore Claps, Email: salvatore.claps@crea.gov.it.
Ettore Varricchio, Email: varricchio@unisannio.it.
Domenico Rufrano, Email: drufrano@tiscali.it.
Mariangela Caroprese, Email: mariangela.caroprese@unifg.it.
Marzia Albenzio, Email: marzia.albenzio@unifg.it.
Pasquale De Palo, Email: p.depalo@veterinaria.uniba.it.
Giuseppe Campanile, Email: giucampa@unina.it.
Gianluca Neglia, Email: neglia@unina.it.
References
- 1.International Agency for Research on Cancer . Carcinogenicity of consumption of red and processed meat. Lyon France: Report of the International Agency for Research on cancer. Press release no 240; 2015. [Google Scholar]
- 2.Domingo JL, Nadal M. Carcinogenicity of consumption of red meat and processed meat: a review of scientific news since the IARC decision. Food Chem Toxicol. 2017;105:256–261. doi: 10.1016/j.fct.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Wolk A. Potential health hazards of eating red meat. J Intern Med. 2017;281:106–122. doi: 10.1111/joim.12543. [DOI] [PubMed] [Google Scholar]
- 4.Adeyemi KD, Shittu RM, Sabow AB, Ebrahimi M, Sazili AQ. Influence of diet and postmortem ageing on oxidative stability of lipids, myoglobin and myofibrillar proteins and quality attributes of gluteus medius muscle in goats. PLoS One. 2016;11:e0154603. doi: 10.1371/journal.pone.0154603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muíño I, Apeleo E, de la Fuente J, Pérez-Santaescolástica C, Rivas-Cañedo A, Pérez C, et al. Effect of dietary supplementation with red wine extract or vitamin E, in combination with linseed and fish oil, on lamb meat quality. Meat Sci. 2014;98:116–123. doi: 10.1016/j.meatsci.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Sabow AB, Sazili AQ, Aghwan AA, Zulkifli I, Goh YM, Ab-Kadir MZA, et al. Changes of microbial spoilage lipid-protein oxidation and physicochemical properties during postmortem refrigerated storage of goat meat. Anim Sci J. 2016;87:816–826. doi: 10.1111/asj.12496. [DOI] [PubMed] [Google Scholar]
- 7.Adeyemi KD, Sabow AB, Shittu RM, Karim R, Karsani SA, Sazili AQ. Impact of chill storage on antioxidant status, lipid and protein oxidation, color, drip loss and fatty acids of semimembranosus muscle in goats. CyTA-J Food. 2015; 10.1080/19476337.2015.1114974.
- 8.Jiang J, Xiong Y. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: a review. Meat Sci. 2016;120:107–117. doi: 10.1016/j.meatsci.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Naveena BM, Sen AR, Vaithiyanathan S, Babji Y, Kondaiah N. Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 2008;80:1304–1308. doi: 10.1016/j.meatsci.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Gessner DK, Ringseis R, Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol An Nutr (Berl) 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- 11.Lipiński K, Mazur M, Antoszkiewicz Z, Purwin C. Polyphenols in monogastric nutrition: a review. Ann Anim Sci. 2017;17:41–58. [Google Scholar]
- 12.Zhang PY. Polyphenols in health and disease. Cell Biochem Biophys. 2016;73:649–664. doi: 10.1007/s12013-015-0558-z. [DOI] [PubMed] [Google Scholar]
- 13.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 15.Brenes A, Viveros A, Chamorro S, Arija I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review Anim Feed Sci Tech. 2016;211:1–17. doi: 10.1016/j.anifeedsci.2015.09.016. [DOI] [Google Scholar]
- 16.Laudadio V, Ceci E, Lastella NM, Tufarelli V. Dietary high-polyphenols extra-virgin olive oil is effective in reducing cholesterol content in eggs. Lipids Health Dis. 2015;14:5. doi: 10.1186/s12944-015-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federici F, Fava F, Kalogerakis N, Mantzavinos C. Valorisation of agro-industrial by-products effluents and waste: concept opportunities and the case of olive mill wastewaters. J Chem Technol Biot. 2009;84:895–900. doi: 10.1002/jctb.2165. [DOI] [Google Scholar]
- 18.Azaizeh H, Halahlih F, Najami N, Brunner D, Faulstich M, Tafesh A. Antioxidant activity of phenolic fractions in olive mill wastewater. Food Chem. 2012;134:2226–2234. doi: 10.1016/j.foodchem.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Bolaños JG, López O, López-García MA, Marset A. Biological properties of hydroxytyrosol and its derivatives. In: Boskou D, editor. Olive oil - constituents. Quality. Health properties and bioconversions. Croatia: InTech open; 2012. pp. 375–396. [Google Scholar]
- 20.NRC (National Research Council). Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. Washington, DC: The National Academies Press; 2007. 10.17226/11654
- 21.AOAC. Association of official analytical chemists: official methods of analysis. 18thed. 2004, Arlington, VA, USA: Association of Official Analytical Chemists.
- 22.Parrillo L, Coccia E, Volpe MG, Siano F, Pagliarulo C, Scioscia E, Varricchio E, Safari O, Eroldogan T, Paolucci M. Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish. Astacusleptodactylus Aquaculture. 2017;473:161–8.
- 23.Cassano A, Conidi C, Giorno L, Drioli E. Fractionation of olive mill wastewaters by membrane separation techniques. J Hazard Mat. 2013;248-249:185–193. doi: 10.1016/j.jhazmat.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 25.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying and improved ABTS radical action decolorization assay. Free Radical Bio Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 26.AOAC . Determination of Fat, Moisture, and Protein in Meat and Meat Products: official Methods of Analysis. 18th ed. Gaithersburg: Method; 2007. [Google Scholar]
- 27.American Meat Science Association (AMSA) Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of meat. 2nd Edition Version 1.02. Chicago: Illinois: American Meat Science Association; 2016. [Google Scholar]
- 28.Girard I, Bruce HL, Basarab JA, Larsen IL, Aalhus JL. Contribution of myofibrillar and connective tissue components to the Warner–Bratzler shear force of cooked beef. I Meat Sci. 2012;92:775–782. doi: 10.1016/j.meatsci.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Bouton PE, Harris PV. Factors affecting tensile and Warner–Bratzler shear values of raw and cooked meat. J Texture Stud. 1978;9:395–413. doi: 10.1111/j.1745-4603.1978.tb01215.x. [DOI] [Google Scholar]
- 30.AMSA. Meat color measurements guidelines. Chicago: IL, USA: American Meat Science Association; 2012.
- 31.Folch J, Lees M. Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 32.Christie WW. Preparation of methyl esters-part 2. Lipid Technol. 1990;2:79–80. [Google Scholar]
- 33.Ulbricht TLV, Southgate DAT. Coronary hearth disease: seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-M. [DOI] [PubMed] [Google Scholar]
- 34.SAS Institute . SAS procedures guide version 6. 3. SAS Institute Inc: Cary, NC; 1990. [Google Scholar]
- 35.Karami M, Alimon AR, Goh YM, Sazili AQ, Ivan M. Effects of dietary herbal antioxidants supplemented on feedlot growth performance and carcass composition of male goats. Am J Anim Vet Sci. 2010;5:33–39. doi: 10.3844/ajavsp.2010.33.39. [DOI] [Google Scholar]
- 36.Yakan A, Ates CT, Alasahana S, Odabasioglua F, Unal N, Ozturkc OH, Gungor OF, Ozbeyaz C. Damascus kids’ slaughter, carcass and meat quality traits in different production systems using antioxidant supplementation. Small Ruminant Res. 2016;136:43–53. doi: 10.1016/j.smallrumres.2016.01.002. [DOI] [Google Scholar]
- 37.Chamorro S, Viveros A, Centeno C, Romero C, Arija I, Brenes A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal. 2013;7:555–561. doi: 10.1017/S1751731112001851. [DOI] [PubMed] [Google Scholar]
- 38.Abu Hafsa SH, Ibrahim SA. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J Anim Physiol An Nutr (Berl). 2017; 10.1111/jpn.12688. [DOI] [PubMed]
- 39.Guerra-Rivas C, Vieira C, Rubio B, Martínez B, Gallardo B, Mantecón AR, Lavín P, Manso T. Effects of grape pomace in growing lamb diets compared with vitamin E and grape seed extract on meat shelf life. Meat Sci. 2016;116:221–229. doi: 10.1016/j.meatsci.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Emami A, FathiNasri MH, Ganjkhanlou M, Zali A, Rashidi L. Effects of dietary pomegranate seed pulp on oxidative stability of kid meat. Meat Sci. 2015;104:14–19. doi: 10.1016/j.meatsci.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Macit M, Aksakal V, Emsen E, Esenbu˘ga N, Aksu MI. Effect of vitamin E supplementation on fattening performance: non carcass components and retail cut percentages and meat quality traits of Awassi lambs. Meat Sci. 2003;64:1–6. doi: 10.1016/S0309-1740(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 42.Bouton PE, Harris PV, Shorthose WR. Changes in shear parameters of meat associated with structural changes produced by aging, cooking and myofibrillar contraction. J Food Sci. 1975;40:1122–1126. doi: 10.1111/j.1365-2621.1975.tb01032.x. [DOI] [Google Scholar]
- 43.Lawrie RA, Ledward DA. Lawrie’s meat science. 7th edition. Cambridge England: Woodhead Publishing limited p. 2006;442
- 44.Bouton PE, Ford AL, Harris PV, Shorthose WR, Ratcliff D, Morgan JHL. Influence of animal age on the tenderness of beef: muscle differences. Meat Sci. 1978;2:301–311. doi: 10.1016/0309-1740(78)90031-1. [DOI] [PubMed] [Google Scholar]
- 45.Harris PV, Shorthose WR. Meat texture. In: Lawrie RA, editor. Developments in meat science-4. London, England: Elsevier Applied Science Publishers; 1988. pp. 245–286. [Google Scholar]
- 46.Morán L, Andrés S, Bodas R, Prieto N, Giráldez FJ. Meat texture and antioxidant status are improved when carnosic acid is included in the diet of fattening lambs. Meat Sci. 2012;91:430–434. doi: 10.1016/j.meatsci.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 47.Andrés S, Huerga L, Mateo J, Tejido ML, Bodas R, Morán L, Prieto N, Rotolo L, Giráldez FJ. The effect of quercetin dietary supplementation on meat oxidation processes and texture of fattening lambs. Meat Sci. 2014;96:806–811. doi: 10.1016/j.meatsci.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Bodas R, Prieto N, Jordán MJ, López-Campos O, Giráldez FJ, Morán L, et al. The liver antioxidant status of fattening lambs is improved by naringin dietary supplementation at 0.15% rates but not meat quality. Animal. 2012;6:863–870. doi: 10.1017/S175173111100214X. [DOI] [PubMed] [Google Scholar]
- 49.Morales-delaNuez A, Moreno-Indias I, Falcón A, Argüello A, Sánchez-Macias D, Capote J, Castro N. Effects of various packaging systems on the quality characteristic of goat meat. Asian-Austral J Anim. 2009;22:428–432. doi: 10.5713/ajas.2009.80488. [DOI] [Google Scholar]
- 50.Muela E, Alonso V, Campo MM, Sañudo C, Beltrán JA. Antioxidant diet supplementation and lamb quality throughout preservation time. Meat Sci. 2014;98:289–295. doi: 10.1016/j.meatsci.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 51.Inserra L, Priolo A, Lanza M, Bognanno M, Gravador R, Luciano G. Dietary citrus pulp reduces lipid oxidation in lamb meat. Meat Sci. 2014;96:1489–1493. doi: 10.1016/j.meatsci.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Samman S, Sandström B, Toft MB, Bukhave K, Jensen M, Sørensen SS, Hansen M. Green tea or rosemary extract added to foods reduces non heme-iron absorption. Am J Clin Nutr. 2001;73:607–612. doi: 10.1093/ajcn/73.3.607. [DOI] [PubMed] [Google Scholar]
- 53.Chilliard Y, Glasser F, Ferlay A, Bernard L, Rouel J, Doreau M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur J Lipid Sci Technol. 2007;109:828–855. doi: 10.1002/ejlt.200700080. [DOI] [Google Scholar]
- 54.Bessa RJ, Alves SP, Jeronimo E, Alfaia CM, Prates JA, Santos-Silva J. Effect of lipid supplements on ruminal biohydrogenation intermediates and muscle fatty acids in lambs. Eur J Lipid Sci Technol. 2007;109:868–878. doi: 10.1002/ejlt.200600311. [DOI] [Google Scholar]
- 55.Cabiddu A, Salis L, Tweed JK, Molle G, Decandiaa M, Lee M. The influence of plant polyphenols on lipolysis and biohydrogenation in dried forages at different phenological stages: in vitro study. J Sci Food Agric. 2010;90:829–835. doi: 10.1002/jsfa.3892. [DOI] [PubMed] [Google Scholar]
- 56.Jayanegara A, Kreuzer M, Wina E, Leiber E. Significance of phenolic compounds in tropical forages for the ruminal bypass of polyunsaturated fatty acids and the appearance of biohydrogenation intermediates as examined in vitro. Anim Prod Sci. 2011;51:1127–1136. doi: 10.1071/AN11059. [DOI] [Google Scholar]
- 57.Jafari S, Meng GY, Rajion MA, Jahromi MF, Ebrahimi M. Manipulation of rumen microbial fermentation by polyphenol rich solvent fractions from papaya leaf to reduce green-house gas methane and biohydrogenation of C18 PUFA. J Agric Food Chem. 2016;64:4522–30. [DOI] [PubMed]
- 58.Palmquist DL, St-Pierre N, McClure KE. Tissue fatty acid profiles can be used to quantify endogenous rumenic acid synthesis in lambs. J Nutr. 2004;134:2407–2414. doi: 10.1093/jn/134.9.2407. [DOI] [PubMed] [Google Scholar]
- 59.Griinari JM, Bauman DE. Biosynthesis of conjugated linoleic acid and its incorporation into meat and milk in ruminants. In: Yurawecz MP, Mossoba MM, JKG K, Pariza MW, Nelson GJ, editors. Advances in conjugated linoleic acid research. Champaign: AOCS press; 1999. pp. 180–200. [Google Scholar]
- 60.Nieto G, Ros G. Modification of fatty acid composition in meat through diet: effect on lipid peroxidation and relationship to nutritional quality – a review. Lipid peroxidation, Dr. angel Catala: In Tech; 2012. 10.5772/51114. Available from: https://www.intechopen.com/books/lipid-peroxidation/modification-of-fatty-acid-composition-in-meat-through-diet-effect-on-lipid-peroxidation-and-relatio
- 61.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Campo MM, Nute GR, Hughes SI, Enser M, Wood JD, Richardson RI. Flavour perception of oxidation in beef. Meat Sci. 2006;72:303–311. doi: 10.1016/j.meatsci.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 63.Reitznerová A, Šuleková M, Nagy J, Marcinˇcák S, Semjon B, Cˇertík M, Klempová T. Lipid peroxidation process in meat and meat products: a comparison study of malondialdehyde determination between modified 2-Thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules. 2017;22 [DOI] [PMC free article] [PubMed]
- 64.Shahidi F, Janitha PK, Wanasundara PD. Phenolic antioxidants. Critical Reviews in Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 65.Liochev SI, Hausladen A, Beyer WF, Jr, Fridovich I. NADPH: ferredoxin oxidoreductase acts as a paraquatdiaphorase and is a member of the soxrs regulon. P Natl AcadSci USA. 1994;91:1328–1331. doi: 10.1073/pnas.91.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marouani N, Chahed A, Hedhili A, Hamdaoui MH. Both aluminium and polyphenols in green tea decoction (Camelia sinensis) affect iron status and haematological parameters in rats. Eur J Nutr. 2007;46:453–459. doi: 10.1007/s00394-007-0685-4. [DOI] [PubMed] [Google Scholar]
- 67.Lee SH, Shinde PL, Choi JY, Kwon IK, Lee JK, Pak SI, Cho WT, Chae BJ. Effects of tannic acid supplementation on growth performance, blood haematology, iron status and faecal microflora in weanling pigs. Livestock Sci. 2010;131:281–286. doi: 10.1016/j.livsci.2010.04.013. [DOI] [Google Scholar]
- 68.Vasta V, Priolo A, Scerra M, Hallet KG, Wood JD, Doran O. Δ(9) desaturase protein expression and fatty acid composition of longissimus dorsi muscle in lambs fed green herbage or concentrate with or without added tannins. Meat Sci. 2009;82:357–364. doi: 10.1016/j.meatsci.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 69.López-Andrés P, Luciano G, Vasta V, Gibson TM, Biondi L, Priolo A, Mueller-Harvey I. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br J Nutr. 2013;110:1–8. doi: 10.1017/S0007114512005703. [DOI] [PubMed] [Google Scholar]
- 70.Goňi I, Brenes A, Centeno C, Viveros A, Saura-Calixto F, Rebole´ A, Arija I, Esteve R. Effect of dietary grape pomace and vitamin E on growth performance, nutrient digestibility and susceptibility to meat lipid oxidation in chickens. Poultry Sci. 2007;86:508–516. doi: 10.1093/ps/86.3.508. [DOI] [PubMed] [Google Scholar]
- 71.Juskiewicz J, Jankowski J, Zielinski H, Zdunczyk Z, Mikulski D, Antoszkiewicz Z, Kosmala M, Zdunczyk P. The fatty acid profile and oxidative stability of meat from turkeys fed diets enriched with n-3 polyunsaturated fatty acids and dried fruit pomaces as a source of polyphenols. PLoS One. 2017;12:e0170074. doi: 10.1371/journal.pone.0170074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during the current study are available from the corresponding author on reasonable request.