Abstract
Background:
Elevated blood pressure is still one of the major risk factors for diseases and disabilities and also a public health challenge worldwide. In the present longitudinal study, we aimed to evaluate the association between risk of hypertension and dietary advanced glycation end products (AGEs) as a recently discussed potential risk factor.
Materials and Methods:
Dietary assessment of 1775 participants in the third phase of Tehran lipid and glucose study to obtain dietary intake of AGEs was performed using a validated semi-quantitative food frequency questionnaire, and they were followed up for a mean duration of approximately 6 years. To determine the incidence of hypertension across quartiles of AGEs intake, logistic regression models with adjustment for potential confounding variables were used. All statistical analyses were conducted using SPSS, and P < 0.05 was considered statistically significant.
Results:
Higher hypertension occurrence risk was generally attributed to higher AGEs intake quartiles after adjusting for age in men (odds ratio [OR] = 1.48, 95% confidence interval [CI] = 1.11–1.52, P = 0.038) and additional adjustment for smoking, drugs, and physical activity in women (OR = 1.38%–95% CI = 1.09–1.42, P = 0.042). Moreover, across the increasing trend of dietary AGEs intake, the percentage of fat intake increased and that of carbohydrate significantly decreased (P < 0.0001).
Conclusion:
In conclusion, it is highly recommended to limit dietary AGEs consumption to prevent and manage hypertension and its complications.
Keywords: Advanced glycation end products, hypertension, risk, Tehran lipid and glucose study
INTRODUCTION
Elevated blood pressure is estimated as a leading risk factor for global mortality, and it has been considered as the reason of 9.4 million deaths and 7% of global disability-adjusted life years in 2010.[1] Likewise, the prevalence of hypertension in Iranian adults was reported 26.6% in the third national surveillance of risk factors of noncommunicable diseases (SuRFNCD-2007)[2] and also hypertension prevalence in Birjand, Center of South Khorasan, and East of Iran has previously been investigated as 20.1%.[3] Not only uncontrolled hypertension is a matter of causing human suffering and imposing severe financial and service burdens on health systems but also it eventually increases the likelihood of cardiovascular complications, the same as stroke, myocardial infarction, cardiac failure and also dementia, renal failure, and blindness.[4] Some of the major contributors to hypertension are detrimental dietary patterns, obesity, harmful alcohol use, physical inactivity, aging, genetic factors, psychological stress, socioeconomic determinants, inadequate access to health care,[4] and also a recently discussed determinant that is dietary advanced glycation end products (AGEs) intake.[2,5] The results of Hasandokht et al.[6] study suggest that the improvements in blood pressure level in Iranian women is associated with lifestyle modification strategies.
AGEs, also called glycotoxins, are dietary oxidants produced through nonenzymatic reactions between reducing sugars and free amino groups in proteins, lipids, or nucleic acids during cooking and thermal food processing.[2,7,8] In particular, AGE formation rises and accelerates mostly through grilling, broiling, roasting, searing, and frying of animal-derived foods.[8] Carboxymethyl lysine is recognized as the main type of dietary AGEs and also generally used as dietary AGEs marker.[9] Currently, this is well established that AGEs can be absorbed and significantly added to body's AGE pool, consequently lead to higher AGE plasma level.[2,8,10,11] AGEs affect different tissues by binding their receptors presented in a wide range of differentiated human cells including epithelial, immune, and endothelial cells.[2] AGEs soluble receptors (sRAGE) are a group of these receptors have been defined as a determinant index for metabolic syndrome-associated complications.[12] Since AGEs are integrated with oxidative stress and inflammation, they are responsible for different chronic disease conditions including; atherosclerosis, cardiovascular diseases, liver and kidney dysfunction, neurodegenerative disease, cancers, obesity, insulin resistance, and other metabolic syndrome components.[2,8,10,13,14] AGEs are also considered to play a leading role in developing hypertension and its related complications.[15,16] Furthermore, therapeutic methods that lead to lower tissue or plasma AGE levels are discussed as thriving treatments for hypertension and atherosclerotic vascular changes.[15,17]
In this longitudinal study, we aimed to evaluate the association between dietary AGEs consumption and risk of hypertension in a representative sample of Iranian adults.
MATERIALS AND METHODS
Study population
This study was conducted within the framework of the Tehran lipid and glucose study (TLGS). In brief, TLGS is a community-based prospective study conducted to investigate noncommunicable diseases, in a representative sample of residents, aged ≥3 years, from district 13 of Tehran, the capital city of Iran. The first phase of the TLGS began in March 1999 and data collection, at 3-year intervals, is ongoing.[18]
Baseline examination of our study included 9602 adults (4020 men and 5582 women), aged 19–70 years who participated in the third phase of TLGS (2006–2008), and had complete data, including demographics, anthropometrics, biochemical, and dietary assessments.[19] Participants were excluded from the final analysis if they had not information about dietary intake at baseline (n = 6393) or they reported implausible energy intake (<800 kcal/d or ≥4200 kcal/d) (n = 166) or they lacked information on anthropometric measurements at baseline (n = 235), had no follow-up information on anthropometrics and biochemical measurements at the second examination (2011–2014) (n = 629). Among these people, the one who had the history of hypertension by self-report (n = 258) or had hypertension; according to JNC-VI legislations (diastolic pressure ≥90 mmHg or systolic pressure ≥140 mmHg [n = 153]) or used antihypertension medication (n = 81) were excluded.
Finally, 1775 participants were included in the analysis. The mean duration of the follow-up was approximately 6 years.
Informed written consent was obtained from all participants and the study protocol was approved by the research council of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences. The study was conducted according to the Declaration of Helsinki and was approved by the Ethical Committee of the RIES, Shahid Beheshti University of Medical Sciences.
Dietary assessment
Dietary data were collected using a validated semi-quantitative food frequency questionnaire (FFQ) with 168 food items, developed for the TLGS. Trained dietitians with at least 5 years of experience in the TLGS survey interviewed participants, face-to-face, and asked them about their consumption frequency for each food item consumed during the past year on a daily, weekly, or monthly basis. The validity of the FFQ was previously evaluated by comparing food groups and nutrient values determined from the questionnaire with values estimated from the average of twelve 24-h dietary recall surveys.[20] To calculation of total dietary AGEs intake, we used the published food AGEs database[8,9] for 549 commonly consumed food items for the Northeastern American multiethnic urban population which was measured using a validated immunoassay method because the Iranian Food Composition Table has no data on AGEs content of foods. We determine the AGEs values kilo-Unit (kU) in 100 g solid food or 100 ml liquid for 168 food items evaluated in the FFQ of third phase of TLGS. The FFQ included questions on the frequency of consumption and usual portion size of food item to estimate. We calculated the total AGEs consumption per day for each participant, and for data analysis, portion sizes of consumed food items reported in household measures were converted to grams, and their intakes were categorized using quartile cutoffs. The amount of AGEs consumption was adjusted for daily energy intake. The AGEs values for some Iranian specific food items, for example, some kinds of bread and cookies were estimated from similar food items for which the AGEs value was available, AGEs value was not available for all fruits or vegetables, so we used the mean of values of fruits or vegetables available to substitute for the others.[21] Finally, we obtained the AGEs values for 132 out of 168 food items in the FFQ of third phase of TLGS and 36 items which had no similar items in the main list were set as missing.
Anthropometrics and lifestyle measurements
Trained interviewers collected the participants' characteristics using a questionnaire. Information on age (years), educational level (illiterate, primary education, and academic education), smoking behavior (yes/no), and physical activity level (MET h/week) were also assessed. Participants who smoked daily or occasionally were considered current smokers, and those who had never smoked or those who stopped smoking were considered nonsmokers. Physical activity level was assessed using the Persian-translated Modifiable Activity Questionnaire (MAQ),[22] based on the frequency and time spent on light, moderate, high, and very high-intensity activities according to the list of common activities of daily life over the past year. Physical activity levels were expressed as metabolic equivalent hours/week (METs h/week). High reliability and relatively moderate validity were reported for the Persian-translated MAQ in Tehranian adults.[23]
Weight was measured to the nearest 100 g using digital scales, while the participants were minimally clothed, without shoes. Height was measured to the nearest 0.5 cm, in a standing position without shoes, using a tape meter. Body mass index (BMI) was calculated as weight (kg) divided by square of the height (m2). Blood pressure was taken on the right arm, twice in a sitting position by a qualified physician after 15 min rest, using a standardized mercury sphygmomanometer; the mean of two measurements was considered as participants blood pressure.
Statistical analysis
Dietary AGEs were divided into quartiles. The mean (±standard deviation) values and the proportions of baseline characteristics of the participants with and without hypertension, in each sex, were compared using the independent sample t-test or Chi-square test, respectively. Dietary intakes of participants were compared across quartiles of AGEs intakes, using analysis of covariance with adjustment for energy intake (kcal/d). Systolic blood pressure and diastolic blood pressure at baseline and follow-up examinations were compared across dietary AGEs intake using analysis of variance and Bonferroni pair-wise comparison test.
A univariate analysis was performed for each potential confounder including age, using of medications, smoking, menopause status, and BMI; variables with PE < 0.2 in the univariate analysis were selected for the multivariable models; PE (P value for entry) determines which variables should be included in the final multivariable model.[24,25,26,27] To determine the incidence of hypertension (as dependent variable) across quartiles of dietary AGEs intake (as independent variable), logistic regression models with adjustment for potential confounding variables were used. To assess the overall trends of ODs across quartiles of dietary AGEs intake, the median of each quartile was used as a continuous variable in logistic regression models. All statistical analyses were conducted using SPSS (Version 20; IL, Chicago), and P < 0.05 was considered statistically significant.
RESULTS
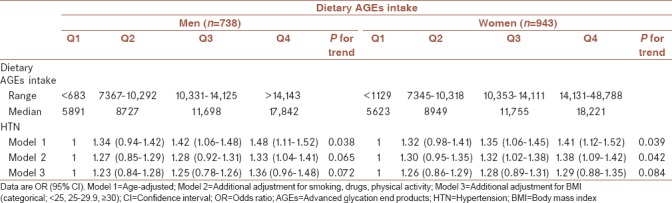
Mean age of participants was 45.3 ± 12.5 years at baseline and 43.9% were male. The cumulative incidence of hypertension was 8.7% after a median follow-up of 6.3 years. The mean of dietary AGEs intake at baseline in hypertensive men and women were 11597 kU and 10673 kU, respectively. BMI at baseline had statistically significant difference in hypertensive and normotensive participants in both sex. Women identified as hypertensive were more likely to be older. Hypertensive men had the highest dietary AGEs intake. Baseline characteristics of the study population are shown in Table 1. Dietary intakes of the study participants across quartiles of dietary AGEs intake are shown in Table 2. There was a significant difference in all dietary intakes, including energy intakes, macronutrients, and dietary fiber across dietary AGEs intake quartiles. Energy intakes, total fat, and total fiber intake increased significantly across quartiles of dietary AGEs intake (P < 0.0001). Systolic and diastolic blood pressure at baseline and follow-up examinations across quartiles of dietary AGEs intake are shown in Table 3. A higher systolic and diastolic blood pressure were observed in the highest compared to the lowest quartiles of dietary AGEs intake in men at baseline; a similar trend was also observed in follow-up examination after 3 and 6 years except in women at third and fourth quartiles of dietary AGEs intake. Men in the third quartiles of dietary AGEs intake and men in the fourth quartiles of dietary AGEs intake had also higher systolic and diastolic blood pressure after 6-year follow-up, respectively. Table 4 shows the occurrence of hypertension across quartiles of dietary AGEs intake. There was no significant association between dietary AGEs intake and the occurrence of hypertension in men in model 2 and 3 and also in women in model 3. The risk of hypertension in men, in the highest compared to the lowest quartiles of dietary AGEs intake, increased by 48% (OR = 1.48%–95% confidence interval [CI] = 1.11–1.52, P = 0.038), in age-adjusted model. A similar association was also observed in age-adjusted model (OR = 1.41%–95% CI = 1.12–1.52, P = 0.039) and after additional adjustment for using smoking, drugs, and physical activity (OR = 1.38%–95% CI = 1.09–1.42, P = 0.042) in women.
Table 1.
Baseline characteristics of the participants
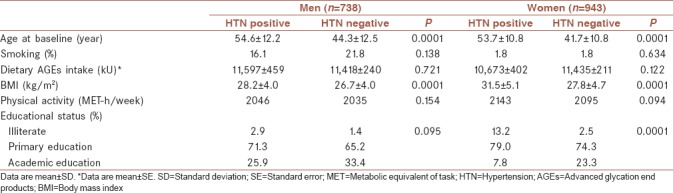
Table 2.
Baseline dietary intakes of the participants across quartiles of dietary advanced glycation end products intake
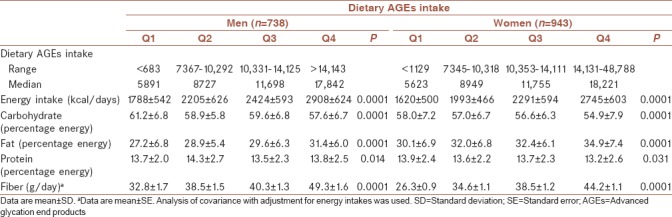
Table 3.
Systolic and diastolic blood pressure at baseline and follow-up examinations across quartiles of dietary advanced glycation end products intake
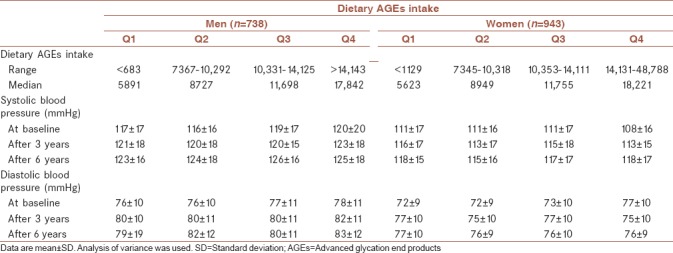
Table 4.
The occurrence of hypertension across quartiles of dietary advanced glycation end products intake
DISCUSSION
Several reports are very likely to support the association between dietary AGEs intake and risk of a vast array of metabolic complications.[14,28] In the present longitudinal study, we also observed that on the whole, higher systolic and diastolic blood pressure were reported across the ascending dietary AGEs intake quartiles mostly in men. However, higher hypertension occurrence risk was disappeared after adjustment for smoking, drugs, physical activity, and BMI in men and only after adjustment for BMI in women.
As mentioned earlier in the results, higher percentage of fat intake from total dietary energy was associated with higher dietary AGEs intake. However, it may be the outcome of increasing energy intake across quartiles of dietary AGEs intake that is associated with higher amounts of food intake and therefore higher dietary AGEs intake. Inversely, there was a decrease in the percentage of carbohydrate intake from total dietary energy in the highest compared to the lowest quartiles of dietary AGEs intake. It has been earlier noted that fat-rich foods contain higher amounts of dietary AGEs; however, carbohydrates due to higher water content and agents that prevent new AGE formation such as vitamins and antioxidants, have lower amounts of these products.[8] We certainly know little about the amount of AGE intakes of different populations with diverse dietary patterns. However, by comparing the average of dietary AGEs intake in this study with similar values from a cohort of healthy adults from New York City as a sample of Western society, notably represents lower amount of AGEs consumption in the present study.[29] This is actually a matter of differences between Iranian and Western dietary patterns since Iranian dietary pattern is rich in complex carbohydrates and contains less fat and meat and consequently has lower AGEs content in comparison with Western diet.[30,31]
We observed no association between the risk of hypertension across quartiles of dietary AGEs intake after adjusting the results for BMI in women and additional adjustments for smoking, drugs, and physical activity in men. Results also showed that among the three confounder variables in model 2 for men, only smoking was considered as a real confounding factor, and in other words, smoking in men is a factor that influences the effect of dietary AGEs intake on the occurrence of hypertension.
Hence, the increasing trend of blood pressure across dietary AGEs intake quartiles may be due to increasing BMI that is probably caused by increased energy intake toward the quartiles. Hence, it seems that the effect of confounding factors, especially BMI must be regarded in the future studies. On the other hand, in the present study, the method of cooking that is a remarkable effective factor on the AGE content of foods was skipped, and we just determined the AGE content of food items.[8,32] As a result, estimating levels of dietary AGEs intake and the following complications may be defined improperly, since the amounts of AGEs may not be representative of the total AGEs intake.
Whereas, animal studies have been verified the association between higher dietary AGEs intake and higher plasma AGE levels and the resulting metabolic complications,[33] it is still controversial based on the human surveys.[10,11] Of note, food-derived AGEs have been recently introduced as a risk factor for a wide range of metabolic disorders. Uribarri et al. emphasis the potential role of dietary AGEs as a contributor to the body's AGE pool and leading cause of oxidative stress and inflammation that eventually results in the most chronic disease.[10] Thus, AGEs are responsible for vascular disorders through inflammation and oxidative stress caused by increased glycation of low-density and high-density lipoproteins, increased production of cytokines, activation of the pro-inflammatory inducible nitric oxide synthase (iNOS), and inhibition of NO availability.[5] AGEs induce their pathologic role through two main pathways; directly crosslinking proteins and altering their functions or triggering intracellular signals through several receptor- and nonreceptor-mediated mechanisms that finally bring about the production of reactive oxygen species and inflammatory cytokines.[10] In addition, Vasdev et al. proposed that not only AGEs cause inflammation and oxidative stress but also they are responsible for endothelial dysfunction. These compounds directly or through receptors change the function of many intra- and extracellular proteins such as antioxidants and metabolic enzymes, calcium channels, lipoproteins, and transcriptional and structural proteins. AGEs can also lead to vascular stiffening by means of forming protein crosslinks and accumulating in long-lived proteins such as collagen and elastin. Hence, it is the reason that why higher plasma AGE levels are correlated with higher vascular stiffness in untreated essential hypertensives. These compounds also interfere in the renin-angiotensin system that finally results in higher blood pressure levels.[15] On the other hand, the hypothesis has been consolidated by studies advocating the effectiveness of lowering AGEs therapies in decreasing oxidative stress, lowering blood pressure, and attenuating atherosclerotic vascular changes.[15,34] It is worth mentioning that the interaction between RAGE results in the expression of factors mediated in oxidative stress.[2] The RAGE is a member of the immunoglobulin superfamily and a multi-ligand receptor and also involved in the pathogenesis of atherosclerosis.[35] For instance, the interaction between AGEs and their receptors exacerbates aortic stiffness and increases intima-media thickness.[16] sRAGE is one of secreted isoforms of RAGE that prevents the interaction between RAGE and its ligands.[36] Investigations revealed that the levels of sRAGE are inversely associated with metabolic syndrome components and especially level of blood pressure.[12,15]
Considering the detrimental effects of dietary AGEs intake, it is strongly recommended to obey some nutritional tips. Some of these advices are as follows: Increasing consumption of low AGE foods (including fruits and vegetables, seafood, whole grains, bread, and low-fat milk), in contrast lowering the intake of foods with higher AGE content (such as sugary items like candy, cookies, and beverages; highly processed foods, such as packaged meats, cheese, and snack-type foods; and fats, including butter, full-fat cheeses, and fried foods), modifying cooking methods (preferring cooking methods with low temperatures and high moisture – boiling, steaming, poaching, and stewing – for a relatively long duration), and improving lifestyle habits (the same as exercising, maintaining normal body weight, and avoiding tobacco consumption).[13]
The strength of this survey lies in longitudinal nature of the study, so the causality could be drawn between the desired exposure and outcome. Large sample size of the study and approximately 6 years' duration of follow-up were also assessed as the reasons of power. On the other hand, we encountered some limitations in this study. As we do not have a published food AGE database for Iranian food items, we had to apply the American published food-AGE database alternatively. Moreover, we had no accurate data on the methods of cooking which is strongly effective on the AGE contents of foods. In the present study, FFQ was used to calculate AGEs consumption over the previous year, but the likelihood of recall bias could not be ignored, which may result in misclassification of exposure.
CONCLUSION
In summary, higher dietary AGEs intake was associated with increased risk of hypertension in adults. However, the correlation was not independent of participants' BMI. Moreover, the percentage of fat intake from total dietary energy had been raised across the ascending quartiles of dietary AGEs intake. Taking into account the pathological side effects of consuming AGEs, recommendations on limiting AGEs intake are crucially important for preventing hypertension and other related metabolic discomforts. Further researches are necessary to identify AGE content of different foods based on different cooking methods and food culture.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the personnel of the Research Institute of Endocrine Sciences and TLGS participants. We would also wish to acknowledge Ms. N. Shiva for critical editing of English grammar and syntax of this manuscript.
REFERENCES
- 1.Rahimi K, Emdin CA, MacMahon S. The epidemiology of blood pressure and its worldwide management. Circ Res. 2015;116:925–36. doi: 10.1161/CIRCRESAHA.116.304723. [DOI] [PubMed] [Google Scholar]
- 2.Angoorani P, Ejtahed HS, Mirmiran P, Mirzaei S, Azizi F. Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int J Food Sci Nutr. 2016;67:170–6. doi: 10.3109/09637486.2015.1137889. [DOI] [PubMed] [Google Scholar]
- 3.Kazemi T, Hajihosseini M, Mashreghimoghadam H, Azdaki N, Ziaee M. Prevalence and determinants of hypertension among Iranian adults, Birjand, Iran. Int J Prev Med. 2017;8:36. doi: 10.4103/ijpvm.IJPVM_103_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Status Report on Noncommunicable Diseases 2014. Geneva: World Health Organization (WHO); 2014. [Google Scholar]
- 5.Stirban A, Tschöpe D. Vascular effects of dietary advanced glycation end products. Int J Endocrinol 2015. 2015:836498. doi: 10.1155/2015/836498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasandokht T, Farajzadegan Z, Siadat ZD, Paknahad Z, Rajati F. Lifestyle interventions for hypertension treatment among iranian women in primary health-care settings: Results of a randomized controlled trial. J Res Med Sci. 2015;20:54–61. [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Uribarri J. Dietary advanced glycation end products and their potential role in cardiometabolic disease in children. Horm Res Paediatr. 2016;85:291–300. doi: 10.1159/000444053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–16e12. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–91. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 10.Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, et al. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. 2015;6:461–73. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis KE, Prasad C, Vijayagopal P, Juma S, Adams-Huet B, Imrhan V, et al. Contribution of dietary advanced glycation end products (AGE) to circulating AGE: Role of dietary fat. Br J Nutr. 2015;114:1797–806. doi: 10.1017/S0007114515003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson BI, Dong C, Gardener H, Elkind MS, Wright CB, Goldberg R, et al. Serum levels of soluble receptor for advanced glycation end-products and metabolic syndrome: The northern manhattan study. Metabolism. 2014;63:1125–30. doi: 10.1016/j.metabol.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palimeri S, Palioura E, Diamanti-Kandarakis E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: Recommendations for dietary management. Diabetes Metab Syndr Obes. 2015;8:415–26. doi: 10.2147/DMSO.S63089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT. Dietary advanced glycation end products and risk factors for chronic disease: A Systematic review of randomised controlled trials. Nutrients. 2016;8:125. doi: 10.3390/nu8030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasdev S, Gill V, Singal P. Role of advanced glycation end products in hypertension and atherosclerosis: Therapeutic implications. Cell Biochem Biophys. 2007;49:48–63. doi: 10.1007/s12013-007-0039-0. [DOI] [PubMed] [Google Scholar]
- 16.Baumann M. Role of advanced glycation end products in hypertension and cardiovascular risk: Human studies. J Am Soc Hypertens. 2012;6:427–35. doi: 10.1016/j.jash.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Hartog JW, van de Wal RM, Schalkwijk CG, Miyata T, Jaarsma W, Plokker HW, et al. Advanced glycation end-products, anti-hypertensive treatment and diastolic function in patients with hypertension and diastolic dysfunction. Eur J Heart Fail. 2010;12:397–403. doi: 10.1093/eurjhf/hfq001. [DOI] [PubMed] [Google Scholar]
- 18.Azizi F, Rahmani M, Emami H, Mirmiran P, Hajipour R, Madjid M, et al. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1) Soz Praventivmed. 2002;47:408–26. doi: 10.1007/s000380200008. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini-Esfahani F, Jessri M, Mirmiran P, Bastan S, Azizi F. Adherence to dietary recommendations and risk of metabolic syndrome: Tehran lipid and glucose study. Metabolism. 2010;59:1833–42. doi: 10.1016/j.metabol.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the tehran lipid and glucose study. J Epidemiol. 2010;20:150–8. doi: 10.2188/jea.JE20090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao L, Kramer JR, Chen L, Rugge M, Parente P, Verstovsek G, et al. Dietary consumption of meat, fat, animal products and advanced glycation end-products and the risk of barrett's oesophagus. Aliment Pharmacol Ther. 2013;38:817–24. doi: 10.1111/apt.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in pima indians. Diabetes Care. 1990;13:401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 23.Momenan AA, Delshad M, Sarbazi N, Rezaei Ghaleh N, Ghanbarian A, Azizi F, et al. Reliability and validity of the modifiable activity questionnaire (MAQ) in an iranian urban adult population. Arch Iran Med. 2012;15:279–82. [PubMed] [Google Scholar]
- 24.Kritz-Silverstein D, Laughlin GA, McEvoy LK, Barrett-Connor E. Sex and age differences in the association of blood pressure and hypertension with cognitive function in the elderly: The rancho bernardo study. J Prev Alzheimers Dis. 2017;4:165–73. doi: 10.14283/jpad.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng S, Shen T, Liu J, Tomlinson B, Sun H, Chen X, et al. Uncontrolled hypertension increases with age in an older community-dwelling chinese population in shanghai. Aging Dis. 2017;8:558–69. doi: 10.14336/AD.2016.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Someya Y, Tamura Y, Kohmura Y, Aoki K, Kawai S, Daida H, et al. Slightly increased BMI at young age is a risk factor for future hypertension in japanese men. PLoS One. 2018;13:e0191170. doi: 10.1371/journal.pone.0191170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand A, Sk MIK. The risk of hypertension and other chronic diseases: Comparing smokeless tobacco with smoking. Front Public Health. 2017;5:255. doi: 10.3389/fpubh.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagishi S, Matsui T. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. Nutrition. 2016;32:157–65. doi: 10.1016/j.nut.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, et al. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–33. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghassemi H, Harrison G, Mohammad K. An accelerated nutrition transition in Iran. Public Health Nutr. 2002;5:149–55. doi: 10.1079/PHN2001287. [DOI] [PubMed] [Google Scholar]
- 31.Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr Rev. 2013;71:511–27. doi: 10.1111/nure.12035. [DOI] [PubMed] [Google Scholar]
- 32.Chen G, Smith JS. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 2015;168:190–5. doi: 10.1016/j.foodchem.2014.06.081. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann SM, Dong HJ, Li Z, Cai W, Altomonte J, Thung SN, et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51:2082–9. doi: 10.2337/diabetes.51.7.2082. [DOI] [PubMed] [Google Scholar]
- 34.Luévano-Contreras C, Garay-Sevilla ME, Wrobel K, Malacara JM, Wrobel K. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J Clin Biochem Nutr. 2013;52:22–6. doi: 10.3164/jcbn.12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: Potential therapeutic targets for cardiovascular diseases. Mol Med. 2007;13:625–35. doi: 10.2119/2007-00087.Koyama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson BI, Harja E, Moser B, Schmidt AM. Soluble levels of receptor for advanced glycation endproducts (sRAGE) and coronary artery disease: The next C-reactive protein? Arterioscler Thromb Vasc Biol. 2005;25:879–82. doi: 10.1161/01.ATV.0000164804.05324.8b. [DOI] [PubMed] [Google Scholar]


