Abstract
Approximately 3.1 million women in the US are living with breast cancer and up to 75% of these women experience cancer-related cognitive impairment (CRCI). CRCI is described as impairments in memory, verbal fluency, thought processes, and attention span. Despite the high prevalence of breast cancer, only a few studies have been published on CRCI and most of these studies primarily focused on its pathophysiological mechanism. However, recent evidence has demonstrated that breast cancer patients with CRCI are more likely to have high level of psychologic distress, suggesting a possible relationship between CRCI and psychologic distress. This review aims to examine existing literature that describes CRCI in relation to psychological distress among breast cancer patients. One thousand four hundred and ninety-eight articles were searched using PubMed, CINAHL, and PsycINFO. Thirteen studies met inclusion criteria, and one article was additionally pulled from article reference lists. Of these19 studies, psychologic distress has been operationalized in varied ways such as anxiety (n = 3), depression (n = 2), both anxiety and depression (n = 4), stress (n = 4), worry (n = 2), mental fatigue (n = 1), and undefined psychological distress (n = 2). Except for six studies designed as a longitudinal study, the rest of studies used a cross-sectional design. Twelve studies used both subjective and objective measures to assess cognitive function. We found that the patients with high psychological distress displayed lower performance on cognitive function tests. Our finding indicates that psychological variables contributed to CRCI that breast cancer patients experienced. Areas for further investigation are proposed that will advance the care of breast cancer patients with CRCI.
Keywords: Breast cancer, breast neoplasm, cognitive dysfunction, cognitive impairment, emotional, mood, physiological distress, psychological

Introduction
Breast cancer is the most prevalent type of cancer among women in the US.[1] With advances in medical treatment, breast cancer mortality rates had declined in recent years, resulting in more individuals living with breast cancer. Currently, 3.1 million women in the US are reported as patients living with breast cancer.[2] Often, breast cancer is viewed no longer as an incurable acute disease but follows the trajectory of chronic diseases that is characterized by periods of remission and exacerbation of symptoms.[3] This perspective on breast cancer has broadened the scope of care from treating the disease alone to managing cancer-related symptoms such as cancer-related cognitive impairment (CRCI).[4,5]
CRCI is the most frequent complication reported by breast cancer patients[6] and up to 75% of breast cancer patients experience CRCI during active treatments (e.g., chemotherapy and hormonal treatment).[7,8,9] Individuals with CRCI often characterize their cognitive problems as impairment in memory and verbal fluency, slowing down of thought processes, and a decrease in their attention span.[10,11] If unmanaged, CRCI will impair performance at work and home and eventually diminish an individual's quality of life (QoL).[12,13] CRCI could also progress to long-term cognitive impairment.[14] Older patients with breast cancer and with CRCI have 10% to 15% probability of developing dementia per year compared to about 1% to 2.5% among those without CRCI.[8,15]
Despite the fact that CRCI is influenced by multifactorial variables, most studies on CRCI had been focused primarily on its pathophysiologic mechanism.[10] Psychological factors that affect the variability of CRCIs clinical manifestations are not fully understood.[8,16,17] Moreover, the impact of psychological variables (e.g., anxiety, stress, depression, worry, and mental fatigue) that have received attention in diseases with cognitive impairment such as dementia[18,19,20] has not been explored within the context of CRCI in breast cancer. Only very few studies have been conducted on CRCI in breast cancer.
Background
CRCI is defined as one or more impaired functions of interrelated cognitive process.[21] Patients with CRCI describe their condition as frequent forgetfulness (e.g., names, date, or telephone numbers), slow-progressing speeds, and difficulties in concentration, multitasking, and word retrieval.[8,22] In most cases, breast cancer patients seldom anticipate that they will have to deal with CRCI during the illness trajectory of cancer.[13] This unexpected event leads to frustration and embarrassment in breast cancer patients.[13]
CRCI can be classified as subtle, moderate, and severe using scores obtained from neuropsychological testing. Based on criteria developed by Vardy et al., subtle CRCI is defined as −1 to –1.49 standard deviation (SD) below population normative means; −1.5 to −1.99 SD below the normative means can be defined as moderate CRCI, and equal and below −2 SD is defined as severe CRCI.[23] Using these criteria, 11%–27% breast cancer patients who are recovering from surgical removal for their breast cancer were found to have moderate or severe impairment on verbal fluency and 14%–17% complained of memory impairment.[11]
To assess CRCI in patients, neuropsychological tests and self-reports are commonly used Table 1.[22,24] Neuropsychological tests objectively assess each domain of cognitive function such as memory, verbal fluency, speed of processing, and attention. Examples of neuropsychological tests and subjective measures to assess CRCI are in Table 1.[11,24,25,26] Although arguments exist against the validity of patients' self-reports, subtle decline in patients' cognitive function may be better detected by this method rather than the use of neuropsychological testing.[27]
Table 1.
Neuropsychological tests and self-reports
| Measures | Test | Description | Duration |
|---|---|---|---|
| Neuropsychological tests | AVLT | Assess immediate memory and delayed memory Recall words after listening 15-item word list | 15 min |
| RBMT | Assess immediate memory and delayed memory Recall story after listening a short passage of story | 25-30 min | |
| SDMT | Assess speed of processing Participants substitute geometric figures for numbers | <5 min | |
| WAIS-III Digital span task | Assess attention Repeat a series of digits read by the examiner and to state them in reverse order | 45-55 min | |
| TMT-A | Assess attention Connect a series of randomly placed circles in correct order | 90 s | |
| TMT-B | Assess attention Connect 25 series of randomly placed circles in a certain order as directed | 3 min | |
| Self-reports | FACT-Cog | Assess cognitive complaints in cancer patients 37-item questionnaire | <20 min |
| AFI | Assess the effectiveness of daily cognitive performance requiring attention and working memory 6-item questionnaire | 5 min |
AVLT: Auditory verbal learning test, RBMT: Rivermead Behavioral Memory Test, SDMT: Symbol Digit Modalities Test, WAIS-III: Wechsler Adult Intelligence Scale, TMT: Trail making test, FACT-Cog: Functional Assessment of Cancer Therapy-Cognitive Scale, AFI: Attentional Function Index
CRCI in breast cancer patients varies across the illness trajectory. Approximately 30%–40% patients experienced CRCI before any type of cancer treatments. Factors such as age, menopausal status, education, or psychological variables have been known to contribute toward the incidence of CRCI before the initiation of any treatments.[25,28] Of those who experienced CRCI before cancer treatments, approximately 60% reported persistent CRCI after the completion of cancer treatments.[14,29,30,31,32] Mostly, the phenomenon of persistent CRCI can be explained by psychological variables.[33,34] Patients with high psychological distress were likely to display lower performance on cognitive function tests.
Psychological well-being is easily threatened in life-limiting illnesses such as cancer. While the association of psychological variables with physical[35] and mental health[36] has been studied widely, it has not been studied in relation to cognitive health in breast cancer patients. Therefore, this review paper will describe the findings of several studies on psychological variables and CRCI in breast cancer. Areas for investigation are proposed that will contribute toward the science in the care of breast cancer patients with CRCI.
Methods
Search strategy
A literature search was conducted in PubMed (724 hits), CINAHL (304 hits), and PsycINFO (470 hits) to identify studies reporting CRCI in breast cancer patients. “Breast neoplasm” and “breast cancer” were used for the search in combination with other key terms: cognitive impairment/cognitive dysfunction/cognitive disorders/memory/memory disorders/cognitive/psychological/physiological distress/emotional/mood. Literature published from 1980 to 2017 was included to capture relevant articles to describe CRCI in relation to psychological variables of breast cancer patients.
Selection criteria
Studies which met the following inclusion criteria were included: (1) studies that discussed psychological variables of CRCI in female breast cancer patients aged 18 years and above and (2) academic journals published in English. Of 1498 research studies, those that did not describe the relationship between CRCI and psychological variables were excluded from the literature review. A total of 18 articles were included in this literature review.
Search outcomes
The detailed search procedures are described in Figure 1. Intervention studies were not included for this review as the intervention performed in these studies may confound the association between CRCI and psychological variables which will be difficult to eliminate. Two independent researchers assessed the eligibility based on the selection criteria. Each researcher reviewed titles, abstracts, and full texts individually and compared the search outcomes. To assess the quality of each article, the independent researchers had to agree that research question and study population were properly stated and identified, and study measures were clearly described and pertained to a psychological variable. After title and abstract review, we first excluded 700 studies, 49 studies remained. We further eliminated 32 studies by reviewing full-texts, and 18 studies were retained. One article was pulled from one article's reference list. A total of 19 articles were included in this literature review.
Figure 1.
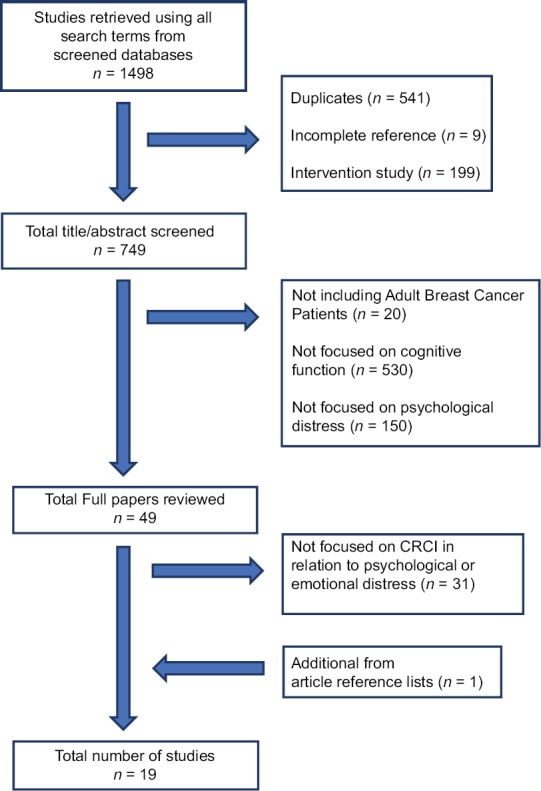
Search flow
Results
Study characteristics
Of the 19 studies that met inclusion criteria, 13 were cross-sectional and 6 were longitudinal. The studies were conducted in the United States (n = 8), Germany (n = 3), the Netherlands (n = 2), Australia (n = 1), Turkey (n = 1), China (n = 1), Singapore (n = 1), Italy (n = 1), and Japan (n = 1). Sample sizes ranged from 23 to 299, and ten studies had sample sizes below 100. More than half of the studies (n = 12) used both subjective and objective measures to assess cognitive function. In studies involving breast cancer patients with CRCI, psychological variables were operationalized in varied ways such as anxiety (n = 3), depression (n = 4), both anxiety and depression (n = 3), stress (n = 4), worry (n = 2), mental fatigue (n = 1), and undefined psychological distress (n = 2). Study characteristics are summarized in Tables 2-8.
Table 2.
Characteristics of studies regarding the association of cognitive function with anxiety
| Authors, date | Purpose | Study design/ sampling | Sample size (n)/sample characteristics | Procedures and instruments | Key findings |
|---|---|---|---|---|---|
| Lehto et al., 1999 | To determine the relationship between anxiety and attention | Cross-sectional/ convenience sampling |
n=45 Female, newly diagnosed with early-stage cancer (I or II), before surgery for breast cancer |
Assessed the anxiety and attention 11 days before surgery Anxiety Self-report: Anxiety subscale of POMS Attention Cognitive function Objective measures: DSF, DSB, SDMT, NCPC Self-report: AFI |
Significant inverse relationship between anxiety and self-perceived attention No significant relationship between anxiety and attention as measured by objective measures |
| Cheung et al., 2012 | To examine CI and its relationship with psychological factors in Asian breast cancer patients receiving chemotherapy | Cross-sectional/ convenience sampling |
n=166 Undergoing chemotherapy (n=85) No chemotherapy (n=81) |
Assessed anxiety and cognitive function in 25-min interview Cognitive function Self-report: FACT-cog Anxiety Self-report: BAI |
A significant and inverse relationship between the level of anxiety and cognitive function in breast cancer patients undergoing chemotherapy |
| Wirkner et al., 2017 | To investigate cognitive function and emotional states in breast cancer patients | Crosssectional/ convenience sampling |
n=51 Breast cancer (n=20) Healthy control (n=31) |
Cognitive function Objective measures: TAP, WMS-R, VLMT Self-report: Questionnaires Anxiety Self-report: STAI |
A significant and inverse relatioanship between anxiety and cognitive function (memory and concentration) in breast cancer patients |
POMS: Profile of Mood States, DSF: Digital Span Forward, DSB: Digital Span Backward, SDMT: Symbol Digit Modalities, NCPC: Necker cube pattern control, AFI: Attention Functional Index, TAP: Test Battery for Assessment of Attention, WMS-R: Wechsler Memory Scale-Revised, VLMT: Verbaler lern- und Merkfahigkeitstest, STAI: State Trait Anxiety Inventory, BAI: Beck Anxiety Index, CI: Cognitive impairment, FACT-cog: Functional Assessment of Cancer Therapy-Cognitive Scale
Table 8.
Characteristics of studies regarding the association of cognitive function with undefined psychological distress
| Authors, date | Purpose | Study design/sampling | Sample size (n)/sample characteristics | Procedures and instruments | Key findings |
|---|---|---|---|---|---|
| Cimprich et al., 2005 | To examine the relationship between cognitive function and pretreatment factors | Cross-sectional/convenience sampling |
n=184 Female, diagnosed with early-stage breast cancer, before surgery for breast cancer |
Cognitive function Objective measures: DSF, DSB, TMA, TMB TS, TW Self-report: AFI Psychological statues Self-report: POMS-SF |
Small but significant relationship between overall mood states and objectively assessed cognitive function A significant relationship between overall mood states and subjectively assessed CI |
| Menning et al., 2015 | To examine pretreatment cognitive function in breast cancer patients | Cross-sectional/convenience sampling |
n=103 Breast cancer (n=65) Female, underwent surgery Prechemotherapy (n=32); postchemotherapy (n=33) Healthy control (n=38) |
Assessed cognitive function, self-reported psychological functioning, and MRI in breast cancer patients who underwent surgery and those without cancer Cognitive function Objective measures: Neurological test, MRI Psychological status Self-report: HSCL-25, POMS |
Performance on verbal memory was associated with depression scores Processing speed was correlated with emotional functioning, overall psychological status, and anxiety |
DSF: Digit span forward, TMA: Trail making test A, TS: Three shape, TW: Three words, AFI: Attentional Function Index, POMS-SF: Profile Mood States-Short Form, DSB: Digit span backward, TMB: Trail making test B, MRI: Magnetic resonance imaging, HSCL-25: Hopkins Symptom Checklist-25, CI: Cognitive impairment
Anxiety
Three studies used anxiety. Anxiety was defined as a sense of fear and tension[37] [Table 2]. A study conducted on 45 women who were waiting for breast cancer surgery investigated how preoperative anxiety was related to directed attention.[38] The participants were divided into three anxiety level groups, and their cognitive performance was measured by objective and subjective tests in each group. There was no significant difference in the mean scores of the objective test; however, statistically significant relationship was found in subjective test in each group (r = −0.63, P < 0.01) with greater cognitive impairment found in patients with high anxiety.
Another study conducted on 166 breast cancer patients examined the association between self-perceived CRCI and anxiety.[39] The findings demonstrated that CRCI was significantly related to the level of anxiety among breast cancer patients (r = −0.58, P < 0.001). The authors stated that anxiety could play a role in increasing the level of CRCI.
A recent cross-sectional study demonstrated a decline in concentration/memory function in breast cancer patients who have completed chemotherapy.[34] Scores on each cognitive test were inversely related to their anxiety level (concentration; r = −0.588, P = 0.006; memory, r = −.523, P = 0.018). Findings suggested that patients who scored lower on neuropsychological tests reported more symptoms of anxiety.
Depression
Depression can be described as a state of feeling sad and hopeless.[40] Three studies focused on the relationship of depression and CRCI in breast cancer patients [Table 3]. Findings of one study that assessed direct attention functioning in 32 breast cancer patients suggested an association between attentional functioning and depression.[41] The participants with negative or depressed mood gave lower scores on self-reported attentional function. Although no detailed information was provided about the relationship between depression and CRCI in the breast cancer patients, the authors concluded that depressed mood could be associated with the severity of patients' CRCI.
Table 3.
Characteristics of studies regarding the association of cognitive function with depression
| Authors, date | Purpose | Study design/ sampling | Sample size (n)/sample characteristics | Procedures and instruments | Key findings |
|---|---|---|---|---|---|
| Cimprich, 1992 | To examine attention following surgery for breast cancer | Cross-sectional/ convenience sampling |
n=32 Female, stage I or II, underwent surgery for breast cancer |
Assessed attention and depression day before discharge from hospital following breast cancer surgery Attention Objective measures: DSF, DSB, SDMT, alphabet backward, letter cancellation Self-report: AFI Depression Self-report: VAMS |
Depression was significantly correlated with attention assessed by AFI No significant relationship between depression and attention measured by objective measures |
| Freeman and Broshek, 2002 | To introduce each neuropsychological test and assess cognitive function in relation to emotional functioning in breast cancer patients | Cross-sectional/ convenience sampling |
n=17 Female, diagnosed with breast cancer In active treatment (n=8) Posttreatment (n=9) |
Cognitive function Objective measures: COWAT, HVLT, PASAT, RBANS, Stroop C-W Self-report: Rating cognitive function on a scale of 0-10 sDepression Self-report: CES-D |
Significant inverse relationship between self-rated cognitive function and depression Significant inverse relationship between depression and visual attention measured by Stroop test No relationship between depression and other objective measures |
| Vearncombe et al., 2009 | To assess whether decline in cognitive function is associated with health, treatment and psychological variables | Longitudinal/ convenience sampling |
n=159 Female, early stage of breast cancer Undergoing chemotherapy (n=138) No chemotherapy (n=21) |
Assessed cognitive function and psychological status before (T0) and 4 months after (T2) chemotherapy Cognitive function Objective measures: AVLT, WMS-III, WAIS-III, SDMT, DKEFS Self-report: Test of everyday attention Anxiety and depression Self-report: HADS |
Association between changes in executive function (T2-T1) and baseline depression |
| Alcalar et al., 2012 | To examine correlation between depression with coping styles and cognitive errors in breast cancer patients who have completed chemotherapy | Cross-sectional/ convenience sampling |
n=110 Female, surgery at least 6 months ago, Completed adjuvant chemotherapy Low depression (n=76) High depression (n=34) |
Categorized into two groups (depression vs. nondepression) and was assessed cognitive errors, coping styles, and automatic thought process Depression Self-report: BDI Cognitive errors Self-report: CEQ |
Relationship between depression and cognitive errors among breast cancer patients |
DSF: Digital Span Forward, DSB: Digital Span Backward, SDMT: Symbol Digit Modalities Test, AFI: Attentional Function Index, VAMS: Visual Analog Mood States, COWAT: Controlled Oral Word Association Test, HVLT: Hopkins Verbal Learning Test, PASAT: Paced Auditory Serial-Addition Task, RBANS: Repeatable Battery for the Assessment of Neuropsychological Status, Stroop C-W: Stroop Interference Trial, CES-D: Center for epidemiological studies depression inventory, AVLT: Auditory verbal learning test, WMS-III: Wechsler Memory Scale, WAIS-III: Wechsler Adult Intelligence Scale, SDMT: Symbol digit modalities test, DKEFS: Delis-Kaplan Executive Function Scale, HADS: Hospital Anxiety And Depression Scale, BDI: Beck’s Depression Index, CEQ: Cognitive Error Questionnaire
Another study examined CRCI measured by objective and subjective tests in breast cancer patients.[42] Except for Stoop test, no correlation was noted between depression and the patients' performance on objective tests. Scores on Stoop test assessing visual attention showed a negative relationship with depression (r = −0.67, P = 0.03), indicating that the patients with depression were more likely to display impairments in visual attention. In addition, patients with depression scored lower on the subjective tests (r = −0.45, P = 0.07).
Depression was also identified as a predictor of the progression of CRCI.[33] Patients reported a decline in executive function after the completion of chemotherapy and this turned out to be associated with patients' baseline level of depression (r = 0.26, P < 0.01). In other words, depression negatively influences the changes in the patients' executive function. This relationship was also supported by one cross-sectional study that found that patients who are depressed performed poorly on the test measuring cognitive errors than those in nondepression group.[43]
Both anxiety and depression
Three studies found an association between both anxiety and depression and CRCI in breast cancer patients [Table 4]. One longitudinal study assessed the association of breast cancer patients' CRCI with both anxiety and depression.[44] While their actual performance on objective tests was not associated with both anxiety and depression, greater decline in self-perceived CRCI was noted when they suffered from high levels of depression and anxiety.
Table 4.
Characteristics of studies regarding the association of cognitive function with both anxiety and depression
| Authors, date | Purpose | Study design/Sampling | Sample size (n)/ sample characteristics | Procedures and instruments | Key findings |
|---|---|---|---|---|---|
| Biglia et al., 2012 | To investigate changes in cognitive function in breast cancer patients undergoing chemotherapy | Longitudinal/convenience sampling |
n=40 Female, aged 18-65, undergoing chemotherapy |
Assessed cognitive function before (T0) and 6 months after (T3) chemotherapy. Emotional status was assessed at T0 during chemotherapy (after 1month; T1, 3 months after; T2) and T3 Cognitive function Objective measures: MMSE, Attention Matrices, DSF, PWF, SSIR, Short Story-delayed recall SSDR, RAVLT, Raven’s Progressive Matrices Self-report: FACT-cog Emotional aspects Self-report: HADS, MADRS |
At T3, self-perceived CI was related to depression and anxiety No relationship between objectively assessed CI and emotional aspects |
| Schilder et al., 2012 | To evaluate cognitive functioning of breast cancer patients and its associations with other factors (e.g., emotional) | Longitudinal/convenience sampling |
n=299 Breast cancer patients (n=179) Undergoing tamoxifen (n=80) and exemestane (n=99) Healthy control (n=120) |
Assessed self-reported and objectively assessed cognitive function and anxiety/depression before (T1) and after 1 year (T2) of adjuvant treatment Cognitive function Objective measures: RAVLT, VWMT, TMT, WAIS-III, FePsy Visual reaction times, FePsY Finger Tapping, Categories animals and professions Self-report: CFQ, interview Anxiety/Depression Self-report: HSCS-25 |
At T1 and T2, a correlation was found between self-perceive CRCI and both of anxiety and depression Weak correlation between cognitive functioning and anxiety/depression |
| Ando-Tanabe et al., 2014 | To evaluate the impact of chemotherapy and psychological distress on cognitive function of breast cancer patients | Longitudinal/convenience sampling |
n=40 Breast cancer (n=18) Female, undergoing chemotherapy, nonmetastatic Healthy control (n=22) |
Assessed three times: at baseline (T1), 8-10 months after T1assessment (T2) and 6months after final chemotherapy or 14-16 months in the control group (T3) Cognitive function Objective measures: WMS-R, WAIS-R, VFT, KWCST, EIQ Psychological test Self-report: HADS |
A significant and negative relationship was found between changes scores for cognitive function and the changes in anxiety/depression |
MMSE: Mini-Mental State Examination, DSF: Digital Span Forward, PWF: Phonemic Word Fluency, SSIR: Short story-immediate recall, SSDR: Short story-delayed recall, RAVLT: Rey auditory verbal learning test, FACT-cog: Functional Assessment of Cancer Therapy Cognitive Scale, HADS: Hospital Anxiety and Depression Scale, MADRS: Montgomery Asberg Depression Rating Scale, VWMT: Verbal Working Memory Task, WAIS-III: Wechsler Adult Intelligence Scale, TMT: Trail making test, CFQ: Cognitive Failure Questionnaire, HSCS-25: High sensitivity cognitive screen, WMS-R: Wechsler Memory Scale-Revised, VFT: Verbal Fluency Test, KWCST: Keio Version wisconsin Card Sorting test, EIQ: Estimated Intelligence Quotient, CRCI: Cancer-Related Cognitive Impairment, CI: Cognitive Impairment, WAIS-R: Wechsler Adult Intelligence Scale-Revised
Similarly, the association of self-perceived CRCI with both anxiety and depression was also reported in another study.[45] Findings demonstrated that self-perceived CRCI was moderately associated with both levels of anxiety and depression before endocrine treatments (T1) (r = 0.22, P < 0.01), and this relationship became stronger 1 year after the completion of treatment (r = 0.34, P < 0.01). Only a weak association was found in the relationship of objectively assessed CRCI and the levels of both anxiety and depression.
Another longitudinal study found that changes in scores of cognitive function tests were significantly associated with the changes in scores for psychological distress (r = −0.50 to −0.48, P < 0.05).[46] In this study, psychological distress was defined as anxiety and depression. The author concluded that higher degree of cognitive impairment was found in patients experiencing higher levels of anxiety and depression.
Stress
Stress is described as a sense of feeling overwhelmed,[47] and four studies in this review examined the relationship of stress with CRCI in breast cancer patients [Table 5]. Of 36 patients who completed neuropsychological testing before chemotherapy, 27% complained of impairments in verbal fluency followed by 14% reporting impairments in memory function.[11] Patients with lower scores from measuring immediate memory, delayed memory, verbal fluency, and attention demonstrated higher scores on stress as measured by self-reported stress measurement (r = −0.37~−0.51, P < 0.01).
Table 5.
Characteristics of studies regarding the association of cognitive function with stress
| Authors, date | Purpose | Study design/sampling | Sample size (n)/sample characteristics | Procedures and instruments | Key findings |
|---|---|---|---|---|---|
| Reid-Arndt and Cox, 2012 | To examine the association among coping, stress, and cognitive difficulties | Cross-sectional/convenience sampling |
n=36 Female, underwent surgery of breast cancer but before other treatments |
Assessed the stress, coping, and cognitive difficulties 1-2 weeks following surgery Cognitive function Objective measures: AVLT, COWAT, animal naming, WAIS-III, DST Stress Self-report: IES-R |
The level of self-reported stress was inversely related to deficits in memory, verbal fluency and attention |
| Hermelink et al., 2015 | To examine the association between pretreatment CI and cancer-related PTSD | Cross-sectional/convenience sampling |
n=226 Breast cancer (n=166) Female, Stage 0-IIIC, aged 18-65 Healthy control (n=60) |
Cognitive function Objective measures: TAP, TMT, DSF, DSB, VLMT Self-report: EORTC-QLQ-C30, FEDA PTSD PTSD diagnostics |
PTSD significantly predicted go/no go errors |
| Li et al. 2015 | To examine the effects of PTSD on perceived CRCI | Cross-sectional/convenience sampling |
n=201 Female, after breast surgery, aged 20-60 |
Cognitive function Self-report: FACT-cog PTSD Self-report: PCL-S |
CRCI was significantly associated with the severity of PTSD symptoms |
| Hermelink et al., 2017 | To test hypothesis that breast cancer patients’ cognitive function is affected by PTSD | Longitudinal/convenience sampling |
n=226 Breast cancer (n=166) Female, newly diagnosed breast cancer, Stage 0-IIIC Healthy control (n=60) |
Assessed at three times: Before chemotherapy or surgery (T1), 1 week after the completion of chemotherapy (T2), 1 year after T1 (T3) Cognitive function Objective measures: VLMT, TAP Self-report: EORTC-QLQ-C30, FEDA PTSD PTSD diagnostics |
CRCI was mediated by PTSD |
AVLT: Auditory verbal learning test, COWAT: Controlled Oral Word Association Test, WAIS-III: Wechsler Adult Intelligence Scale, DST: Digit Span Task, IES-R: Impact of Event Scale-Revised, PTSD: Posttraumatic stress disorder, TAP: Test of Attentional Performance, TMT: Trail making test, DSF: Digital Span Forward, DSB: Digital span backward, VLMT: Verbal learning and memory test, FEDA: Questionnaire of Experienced Deficits of Attendtion, CRCI: Cancer-related cognitive impairment, CI: Cognitive impairment, FACT-cog: Functional Assessment of Cancer Therapy-Cognitive Function, PCL-S: PTSD Checklist-Specific Stressor Version, EORTC-QLQ-C30: European Organization for Research and Treatment of Cancer Quality-of-life Questionnaire C30
This relationship of stress and CRCI was also investigated in other two studies conducted by Hermelink et al.[48,49] Both two studies reported that breast cancer patients could experience posttraumatic stress (PTSD) after cancer diagnosis, and those who experienced PTSD displayed more cognitive errors than others without PTSD (Beta = 0.27, P = 0.004).[48] This relationship was further elaborated in their recent study.[49] The authors identified a positive correlation of PTSD with CRCI (r = 0.26–0.42, P < 0.0001). In addition, poor performance on Go/No-go tasks (one of cognitive tests) could be mediated by PTSD symptom (mediation effect: B = 0.15).
Consistent with prior studies, a study conducted in China reported that PTSD symptom contributed to CRCI among breast cancer patients treated with chemotherapy.[50] Patients with more PTSD symptoms scored lower on self-report cognitive testing. This suggested that PTSD symptoms are associated with CRCI (r = −0.54, P < 0.001).
Worry
While worry may be interpreted as similar to anxiety, worry is more narrowly conceptualized to concerns about the future event.[51] Two studies showed the relationship of worry and CRCI in breast cancer patients [Table 6]. One study reported that a higher level of worry was strongly associated with self-perceived and objectively assessed CRCI in breast cancer patients regardless of their treatment status (e.g., prechemotherapy or preradiation therapy).[28] Breast cancer patients with high levels of worry demonstrated lower accuracy and worse performance on VMWT (r = 0.25, P = 0.08 for low demand, r = 0.25, P = 0.08 for medium, and r = 0.30, P = 0.08 for high). Furthermore, patients produced low scores on a subjective test when they reported high worry (r = −0.57, P < 0.001). In addition, brain MRI in patients with high level of worry showed the inability to deactivate regions of the precuneus/posterior cingulate that regulates cognitive performance.
Table 6.
Characteristics of studies regarding the association of cognitive function with worry
| Authors, date | Purpose | Study design/sampling | Sample size (n)/sample characteristics | Procedures and instruments | Key findings |
|---|---|---|---|---|---|
| Berman et al., 2014 | To examine the influences of worry on neurocognitive responses in breast cancer patients | Cross-sectional/convenience sampling |
n=50 Female, diagnosed with localized breast cancer (Stage 0-IIIa), completed primary surgical treatment Preradiation (n=25) Prechemotherapy (n=25) |
Assessed neurocognitive function before any adjuvant therapy Cognitive performance Objective measures: VWMT during fMRI Self-report: AFI Worry Self-report: TIWI |
A correlation of worry with subjectively and objectively assessed CI was found High level of worry was associated with failure to decrease activation in precuneus/posterior cingulate regions |
| Jung et al., 2017 | To examine cognitive function in relation to physical and psychological symptom | Longitudinal/convenience sampling |
n=92 Breast cancer (n=62) Female, diagnosed with stage 0-IIIa Chemotherapy (n=28); no chemotherapy (n=34) Healthy control (n=30) |
Assessed at four times: baseline (M0), 5 months (M5), 1 year (M12) following baseline Cognitive function Objective measures: VWMT during fMRI scanning Self-report: AFI Worry Self-report: TIWI |
At M12, a significant association between self-reported CRCI and worry |
VWMT: Verbal memory working test, AFI: Attentional Function Index, TIWI: Three-Item Worry Index, fMRI: Functional magnetic resonance imaging, CRCI: Cancer-Related Cognitive Impairment, CI: Cognitive impairment
Similarly, a recent longitudinal study found that, as patients experienced high worry, the greater self-perceived confidence interval (CI) was assessed at 1 year after surgery for breast cancer (M12).[52] In addition, a small but significant relationship of self-perceived CI with worry was assessed at M12 (r = 0.34, P < 0.001). On the contrary, objectively assessed CI at M12 using scores of VMWT was not associated with worry.
Mental fatigue
Mental fatigue refers to a failure to complete mental tasks, contributing to a decrease in both work and study efficiency.[53] One study discussed the relationship of mental fatigue with the alternation of brain mechanism in breast cancer patients who have completed cancer treatments [Table 7].[54] Findings showed that the patients presenting a high level of mental fatigue displayed greater connectivity from left inferior parietal lobule to superior frontal gyrus on MRI images than nonfatigued patients. This increased connectivity was associated with higher levels of mental fatigue (r = 0.82, P = 0.05). Furthermore, the association between fatigue and greater default mode network (DMN) connectivity to the superior frontal gyrus was noted. This enhanced connectivity between DMN to frontal gyrus indicated a decline in cognitive performance.
Table 7.
Characteristics of studies regarding the association of cognitive function with mental fatigue
| Authors, date | Purpose | Study design/sampling | Sample size (n)/sample characteristics | Procedures and instruments | Key findings |
|---|---|---|---|---|---|
| Hampson et al., 2015 | To investigate the impact of mental fatigue on brain connectivity among breast cancer patients who completed cancer treatments | Cross-sectional/convenience sampling |
n=23 Female, breast cancer (Stage 0-IIIA), completed all cancer treatments Nonfatigued (n=8) Fatigued (n=15) |
2 weeks after the initial screening, all eligible participants were divided into two groups based on the results of fatigue questionnaires Cognitive function Objective measures: fcMRI s Fatigue Self-report: BFI, MFI |
Mental fatigue was associated with greater DMN Greater connectivity of DMN was related to CI |
BFI: Brief Fatigue Inventory, MFI: Multidimensional Fatigue Inventory, fcMRI: Functional connectivity magnetic resonance imaging, DMN: Default mode network, CI: Cognitive impairment
Undefined psychological distress
Two studies examined the relationship between CRCI and patients' overall psychological distress [Table 8]. One study found that newly diagnosed breast cancer patients performed poorly on objective test when they suffered high psychological distress[25] (r = −0.21, P < 0.05). Furthermore, patients more often reported self-perceived CRCI when their distress was high (r = −0.62, P < 0.001).
Another study examined each domain of cognitive function (e.g., verbal memory) in postsurgical breast cancer patients and their overall psychological distress.[55] Based on the results, poor performance on verbal memory was correlated with high depression (r = −0.29, P = 0.006); using the same logic, poor performance on processing speed was correlated with high psychological distress (r = −0.418, P = 0.001), low psychological well-being (r = 0.334, P = 0.001), and high anxiety (r = −0.29, P = 0.003). Study authors concluded that depressed patients demonstrated impairment in verbal memory and patients with high levels of anxiety and psychological distress performed poorly on processing speed test.
Discussion
To the best our knowledge, this is the first review paper that examined the association between CRCI and psychological variables in breast cancer patients. Of the 19 studies reviewed, depression (n = 4) and stress (n = 4) were the most frequent variables reported, followed by anxiety (n = 3), both anxiety and depression (n = 3), worry (n = 2), undefined psychological distress (n = 2), and mental fatigue (n = 1). Overall, we found that psychological variables either predicted the progression of CRCI[33,46,48] or simply were associated with the phenomenon of CRCI.[11,25,28,34,38,39,41,42,43,44,45,49,52,54,55] Patients waiting for cancer treatments reported anxiety, stress, and overall psychological distress as factors closely related to CRCI. Majority of those who were actively receiving treatment chose either or both depression and anxiety. Finally, depression was reported as a variable which was associated with CRCI that posttreatment patients experienced.
Results from this review paper showed inconsistent association of CRCI and psychological variables. For example, some studies found a significant relationship between CRCI and psychological variables; however, others found weak relationship only. This inconsistency may be due to a wide variation in sample size, characteristics of sample, study design, and measurements. Importantly, self-perceived CRCI seems to be associated more frequently with psychological variables (anxiety and depression) than objectively measured CRCI. This needs further elaboration as it will influence how to measure CRCI in future studies. In addition, it would be critical to include confounders that potentially affect the association of CRCI with psychological variables as well as having adequate sample size to have confidence in the study results.
We also found that many studies examined the association of CRCI with psychological variable cross-sectionally instead of longitudinally that will allow to assess the trajectory of cognitive function and psychological variables[45,52,54] across time. The manifestations of CRCI could change throughout the trajectory of illness or by the accumulated impacts of psychological variables. Therefore, a longitudinal examination of CRCI and its associated variables may provide more in-depth knowledge about CRCI and its mediators and moderators.
There are limitations in this review paper. Only observational studies were included because the heterogeneity in study designs may cause findings to become difficult to understand. Another limitation of this review was that a total of 19 studies used convenience sampling methods. Samples collected through convenience sampling method are less representative of target population than that of consecutive sampling method.[56] More studies with heterogeneous sample need to be conducted to promote applicability of results to broader breast cancer population. It would also be helpful to expand future search strategy to other cancer population to determine the generalizability of results found in this review.
Conclusion
This review paper presented updated information, showing the relationship between psychological variables and CRCI. Depression was found to be the most frequent variable associated with CRCI followed by anxiety, both anxiety and depression, worry, undefined psychological distress, and mental fatigue. Given these review findings, future research needs to consider the type of measurements used to measure CRCI (objective vs. subjective) and study design (cross-sectional vs. longitudinal). As the number of individuals with cancer continues to rise, there is a need to increase efforts in developing interventions for best practice to advance the care of this patient population. Many breast cancer patients are at high risk for CRCI that negatively affects their QoL. Enhanced information about CRCI will provide the foundation for developing multifactorial interventions for breast cancer patients with CRCI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. Breast Cancer. 2017. [Last accessed on 2017 Jul 14]. Available from: https://www.cdc.gov/cancer/breast/
- 2.American Cancer Society. Cancer Facts & Figures. 2017. [Last accessed on 2018 Mar 18]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-andfigures-2017.pdf .
- 3.American Cancer Society. Managing Cancer as a Chronic Illness. [Last accessed on 2018 Mar 18];2016 Avaialble from: https://www.cancer.org/treatment/survivorship-during-and-after-treatment/whencancer-doesnt-go-away.html . [Google Scholar]
- 4.Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16:772–7. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoemaker LK, Estfan B, Induru R, Walsh TD. Symptom management: An important part of cancer care. Cleve Clin J Med. 2011;78:25–34. doi: 10.3949/ccjm.78a.10053. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt JE, Beckjord E, Bovbjerg DH, Low CA, Posluszny DM, Lowery AE, et al. Prevalence of perceived cognitive dysfunction in survivors of a wide range of cancers: Results from the 2010 LIVESTRONG survey. J Cancer Surviv. 2016;10:302–11. doi: 10.1007/s11764-015-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30:3675–86. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR, et al. An update on cancer- and chemotherapy-related cognitive dysfunction: Current status. Semin Oncol. 2011;38:431–8. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender CM, Ergÿn FS, Rosenzweig MQ, Cohen SM, Sereika SM. Symptom clusters in breast cancer across 3 phases of the disease. Cancer Nurs. 2005;28:219–25. doi: 10.1097/00002820-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Merriman JD, Von Ah D, Miaskowski C, Aouizerat BE. Proposed mechanisms for cancer- and treatment-related cognitive changes. Semin Oncol Nurs. 2013;29:260–9. doi: 10.1016/j.soncn.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid-Arndt SA, Cox CR. Stress, coping and cognitive deficits in women after surgery for breast cancer. J Clin Psychol Med Settings. 2012;19:127–37. doi: 10.1007/s10880-011-9274-z. [DOI] [PubMed] [Google Scholar]
- 12.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: An in-depth look at survivors' reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3:223–32. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers JS. Cancer- and chemotherapy-related cognitive changes: The patient experience. Semin Oncol Nurs. 2013;29:300–7. doi: 10.1016/j.soncn.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–13. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–34. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreotti C, Root JC, Ahles TA, McEwen BS, Compas BE. Cancer, coping, and cognition: A model for the role of stress reactivity in cancer-related cognitive decline. Psychooncology. 2015;24:617–23. doi: 10.1002/pon.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray VJ, Dhillon, HM, Vardy JL. Cancer-related cognitive impairment in adult cancer survivors: A review of the literature. Cancer Forum. 2017;41:46–54. [Google Scholar]
- 18.Holwerda TJ, Deeg DJ, Beekman AT, van Tilburg TG, Stek ML, Jonker C, et al. Feelings of loneliness, but not social isolation, predict dementia onset: Results from the amsterdam study of the elderly (AMSTEL) J Neurol Neurosurg Psychiatry. 2014;85:135–42. doi: 10.1136/jnnp-2012-302755. [DOI] [PubMed] [Google Scholar]
- 19.Ramakers IH, Honings ST, Ponds RW, Aalten P, Sebastian K, Verhey FR, et al. The effect of psychological distress and personality traits on cognitive performances and the risk of dementia in patients with mild cognitive impairment. J Alzheimers Dis. 2015;46:805–12. doi: 10.3233/JAD-142493. [DOI] [PubMed] [Google Scholar]
- 20.Rosness TA, Strand BH, Bergem AL, Nafstad P, Langballe EM, Engedal K, et al. Association of psychological distress late in life and dementia-related mortality. Aging Ment Health. 2016;20:603–10. doi: 10.1080/13607863.2015.1031639. [DOI] [PubMed] [Google Scholar]
- 21.Von Ah D, Jansen C, Allen DH, Schiavone RM, Wulff J. Putting evidence into practice: Evidence-based interventions for cancer and cancer treatment-related cognitive impairment. Clin J Oncol Nurs. 2011;15:607–15. doi: 10.1188/11.CJON.607-615. [DOI] [PubMed] [Google Scholar]
- 22.Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. Eur J Oncol Nurs. 2013;17:236–41. doi: 10.1016/j.ejon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol. 2007;25:2455–63. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 24.Von Ah D, Tallman EF. Perceived cognitive function in breast cancer survivors: Evaluating relationships with objective cognitive performance and other symptoms using the functional assessment of cancer therapy-cognitive function instrument. J Pain Symptom Manage. 2015;49:697–706. doi: 10.1016/j.jpainsymman.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–8. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 26.Cimprich B, Visovatti M, Ronis DL. The attentional function index – A self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 27.Jean-Pierre P. Management of cancer-related cognitive dysfunction-conceptualization challenges and implications for clinical research and practice. US Oncol. 2010;6:9–12. doi: 10.17925/ohr.2010.06.0.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berman MG, Askren MK, Jung M, Therrien B, Peltier S, Noll DC, et al. Pretreatment worry and neurocognitive responses in women with breast cancer. Health Psychol. 2014;33:222–31. doi: 10.1037/a0033425. [DOI] [PubMed] [Google Scholar]
- 29.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123–38. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–52. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–56. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 32.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–56. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 33.Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle G, et al. Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsychol Soc. 2009;15:951–62. doi: 10.1017/S1355617709990567. [DOI] [PubMed] [Google Scholar]
- 34.Wirkner J, Weymar M, Löw A, Hamm C, Struck AM, Kirschbaum C, et al. Cognitive functioning and emotion processing in breast cancer survivors and controls: An ERP pilot study. Psychophysiology. 2017;54:1209–22. doi: 10.1111/psyp.12874. [DOI] [PubMed] [Google Scholar]
- 35.Lauzier S, Maunsell E, Levesque P, Mondor M, Robert J, Robidoux A, et al. Psychological distress and physical health in the year after diagnosis of DCIS or invasive breast cancer. Breast Cancer Res Treat. 2010;120:685–91. doi: 10.1007/s10549-009-0477-z. [DOI] [PubMed] [Google Scholar]
- 36.Saeedi-Saedi H, Shahidsales S, Koochak-Pour M, Sabahi E, Moridi I. Evaluation of emotional distress in breast cancer patients. Iran J Cancer Prev. 2015;8:36–41. [PMC free article] [PubMed] [Google Scholar]
- 37.American Psychological Association. Anxiety. [Last accessed on 2018 Mar 18]. Available from: http://www.apa.org/topics/anxiety/index.aspx .
- 38.Lehto RH, Cimprich B. Anxiety and directed attention in women awaiting breast cancer surgery. Oncol Nurs Forum. 1999;26:767–72. [PubMed] [Google Scholar]
- 39.Cheung YT, Shwe M, Chui WK, Chay WY, Ang SF, Dent RA, et al. Effects of chemotherapy and psychosocial distress on perceived cognitive disturbances in Asian breast cancer patients. Ann Pharmacother. 2012;46:1645–55. doi: 10.1345/aph.1R408. [DOI] [PubMed] [Google Scholar]
- 40.National Institute of Mental Health. [Last accessed on 2018 Mar 18];Depression Basics. 2016 Available from https://www.nimh.nih.gov/health/publications/depression/index.shtml . [Google Scholar]
- 41.Cimprich B. Attentional fatigue following breast cancer surgery. Res Nurs Health. 1992;15:199–207. doi: 10.1002/nur.4770150306. [DOI] [PubMed] [Google Scholar]
- 42.Freeman JR, Broshek DK. Assessing cognitive dysfunction in breast cancer: What are the tools? Clin Breast Cancer. 2002;3(Suppl 3):S91–9. doi: 10.3816/cbc.2002.s.019. [DOI] [PubMed] [Google Scholar]
- 43.Alcalar N, Ozkan S, Kucucuk S, Aslay I, Ozkan M. Association of coping style, cognitive errors and cancer-related variables with depression in women treated for breast cancer. Jpn J Clin Oncol. 2012;42:940–7. doi: 10.1093/jjco/hys119. [DOI] [PubMed] [Google Scholar]
- 44.Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, D'Alonzo M, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: A prospective study. Eur J Cancer Care (Engl) 2012;21:485–92. doi: 10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 45.Schilder CM, Seynaeve C, Linn SC, Boogerd W, Beex LV, Gundy CM, et al. Self-reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: Findings from the neuropsychological TEAM side-study. Psychooncology. 2012;21:479–87. doi: 10.1002/pon.1928. [DOI] [PubMed] [Google Scholar]
- 46.Ando-Tanabe N, Iwamitsu Y, Kuranami M, Okazaki S, Yasuda H, Nakatani Y, et al. Cognitive function in women with breast cancer receiving adjuvant chemotherapy and healthy controls. Breast Cancer. 2014;21:453–62. doi: 10.1007/s12282-012-0405-7. [DOI] [PubMed] [Google Scholar]
- 47.American Psychological Association (n.d.) [Last accessed on 2018 Mar 18];Understanding Chronic Stress. Available from http://www.apa.org/helpcenter/understanding-chronic-stress.aspx . [Google Scholar]
- 48.Hermelink K, Voigt V, Kaste J, Neufeld F, Wuerstlein R, Bühner M, et al. Elucidating pretreatment cognitive impairment in breast cancer patients: The impact of cancer-related post-traumatic stress. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv099. pii: djv099. [DOI] [PubMed] [Google Scholar]
- 49.Hermelink K, Bühner M, Sckopke P, Neufeld F, Kaste J, Voigt V, et al. Chemotherapy and post-traumatic stress in the causation of cognitive dysfunction in breast cancer patients. J Natl Cancer Inst. 2017;109:dxj057. doi: 10.1093/jnci/djx057. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Yu L, Long Z, Li Y, Cao F. Perceived cognitive impairment in chinese patients with breast cancer and its relationship with post-traumatic stress disorder symptoms and fatigue. Psychooncology. 2015;24:676–82. doi: 10.1002/pon.3710. [DOI] [PubMed] [Google Scholar]
- 51.Zebb BJ, Beck JG. Worry versus anxiety. Is there really a difference? Behav Modif. 1998;22:45–61. doi: 10.1177/01454455980221003. [DOI] [PubMed] [Google Scholar]
- 52.Jung MS, Zhang M, Askren MK, Berman MG, Peltier S, Hayes DF, et al. Cognitive dysfunction and symptom burden in women treated for breast cancer: A prospective behavioral and fMRI analysis. Brain Imaging Behav. 2017;11:86–97. doi: 10.1007/s11682-016-9507-8. [DOI] [PubMed] [Google Scholar]
- 53.Mizuno K, Tanaka M, Yamaguti K, Kajimoto O, Kuratsune H, Watanabe Y, et al. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav Brain Funct. 2011;7:17. doi: 10.1186/1744-9081-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hampson JP, Zick SM, Khabir T, Wright BD, Harris RE. Altered resting brain connectivity in persistent cancer related fatigue. Neuroimage Clin. 2015;8:305–13. doi: 10.1016/j.nicl.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menning S, de Ruiter MB, Veltman DJ, Koppelmans V, Kirschbaum C, Boogerd W, et al. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment – The role of fatigue. Neuroimage Clin. 2015;7:547–54. doi: 10.1016/j.nicl.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polit DF, Beck CT. Philadelphia: Wolters-Kluwer Pubs; 2012. Nursing Research: Generating and Assessing Evidence for Nursing Practice. [Google Scholar]


