Abstract
Background:
Since its discovery in 1859, formalin has been considered as the “gold standard” in tissue fixation. As formalin is highly toxic and carcinogenic, the quest for its substitute has started recently. Literature search reveals very sparse studies on natural substitute for formalin. Here, it is an attempt to explore eco-friendly, economical, and readily available natural substance for formalin substitute.
Aim:
The aim of our study was to evaluate the efficacy of natural fixatives such as honey, sugar, jaggery, and water in comparison to the standard fixative used like formalin.
Materials and Methods:
Fresh goat tissues (tongue) were fixed separately with buffered 10% formalin (positive control), honey, sugar syrup, jaggery syrup, and distilled water (negative control). 24 h fixation was done at room temperature followed by conventional processing and routine H and E staining. The stained sections were assessed for cytoplasmic and nuclear detail by three pathologists under light microscope and were graded accordingly.
Results:
The results showed statistically significant differences between jaggery with other natural fixatives for both nuclear details and cytoplasmic staining.
Conclusion:
The preservation of tissue by honey, sugar, and jaggery syrup was comparable to that of formalin. Among the three natural fixatives, jaggery syrup excelled. Hence, it can be considered as an equally effective formalin substitute.
Keywords: Formalin, honey, jaggery, natural fixatives, sugar syrup
INTRODUCTION
All good microscopic preparation requires treatment of the tissue as soon as it is removed from the body. This serves as the foundation for the histopathological techniques. The tissue should be transferred and fixed immediately in an appropriate fixative solution.[1] Fixation is an initial and important step in tissue processing for microscopical examination. The primary aim of fixation is to preserve the tissues in a life-like state, prevent bacterial putrefaction, prevent autolysis, and increase the refractive index of the tissue.[2] In surgical pathology, neutral-buffered formalin has been the “gold standard” fixative for decades.[3] The biological effects of mercury and alcohol as a fixative have been discussed by Hippocrates as early as 400 BC. However, the invention of the microscope led to the curiosity about the histological structure of the tissues. Even then, for the early microscope users, improving their scopes was of top priority and cared little about the specimens.[4] Formaldehyde was first discovered in 1859 by the Russian chemist.[5] Ferdinand Blum in the 19th century accidentally found that it can “fix” the tissue while working on formaldehyde for disinfection and “the rest is history”; formalin became the fixative of choice in just a few years. Hence, he has been credited as the first person to use formaldehyde as a tissue fixative.[6]
Formaldehyde usually in the form of a white hydrated solid polymer consisting of 80–100 methanal units (polyoxymethylene) called paraform or paraformaldehyde. It is used in the manufacture of adhesives, in animal nutrition and agriculture, cosmetics, deodorants, detergents, dyes, explosives, and many more which makes it a substance easy to come in contact with different concentration levels and environments. Incomplete combustion during fires or from hydrocarbon fuels especially from vehicles emissions releases formaldehyde into the atmosphere. Based on the evidences mounting regarding the health risk posed by formaldehyde exposure, the Occupational Safety and Health Administration (OSHA) of the US Department of Labor had introduced exposure standards that are required to be monitored and maintained in areas where formaldehyde is used.[7]
International Agency for Research on Cancer (IARC) classified formaldehyde as “carcinogenic to humans” and therefore represents a risk to anyone handling the solution.[3] It was found that formaldehyde inhalation at 6 ppm and above causes nasal squamous cell carcinoma in rats. Since epidemiological studies have provided only equivocal evidence that formaldehyde is a human carcinogen, quantitative implications of the rat tumors for human cancer risk are of utmost interest. The U. S. OSHA Permissible Exposure Limit is 0.75 ppm as an 8-h time-weighted average (OSHA, 2004), and the American Conference of Governmental Industrial Hygienists Limit is 0.3 ppm (ACGIH, 2004).[8]
It has been found in recent study that a strong evidence that can support a genotoxic and cytotoxic mode of action for the carcinogenesis of inhaled formaldehyde in respiratory nasal epithelium.[8] Guidelines for ambient formaldehyde levels in living spaces have been set in several countries in the range of 0.05–0.4 ppm, with a preference to 0.1 ppm.[7] Second, the chemical action of formalin binds severely to DNA, RNA, and proteins, which makes them difficult or impossible to extract in a useful form for molecular tests.[4,9]
The side effects of formalin are skin and mucous membrane, respiratory system, gastrointestinal tract, cardiovascular system, central nervous system, and eye. Irritation, nausea, vomiting, diarrhea, loss of appetite, burns and ulceration, abdominal pain, gastrointestinal hemorrhage, pharyngeal congestion, and chronic pharyngitis are the few signs and symptoms of formalin vapors.[6]
Considering formalin as a carcinogen and the fact that formalin does not assure a complete DNA and messenger RNA (mRNA) recovery, a quest for formalin substitute began.[7] Bee honey has been shown to preserve tissue morphology similar to that by formalin.[9] Considering that sugar and jaggery are closest in composition with honey, they may also be able to preserve tissues. Thereby, the rationale of this study is to explore the eco-friendly, economical, and readily available substances such as sugar and jaggery as natural alternate solution that can be used for preserving tissues and to find out the best natural substitute.
MATERIALS AND METHODS
The study was conducted in the Department of Oral Pathology and Microbiology, Lucknow. The ethical clearance was taken from the ethical committee for the present study. Single piece of commercially available fresh goat meat (tongue) was bought and cut into 40 small pieces. Equal number of pieces (eight) were placed in each of the five different containers containing 10% buffered formalin, distilled water, 100% honey, 100% sugar syrup, and 100% jaggery syrup. The tissues were allowed to fix for 24 h at room temperature. After fixation, the tissues were water washed thoroughly so that any remaining fixatives get washed. Similarly, fixation was done for 48 h and 72 h. The total number of specimens for each time slot was eight. The tissues were fixed in saturated solution of 20 cubic cm by volume. Hence, saturated solutions of sugar, jaggery, and honey were made by adding each ingredients separately in 20 ml of distill water until saturation was achieved. Cost of 1 kg of jaggery was Rs. 33, sugar was Rs. 39, and honey was Rs. 270. Formalin is used as positive control [Figure 1] and distill water as negative control.
Figure 1.
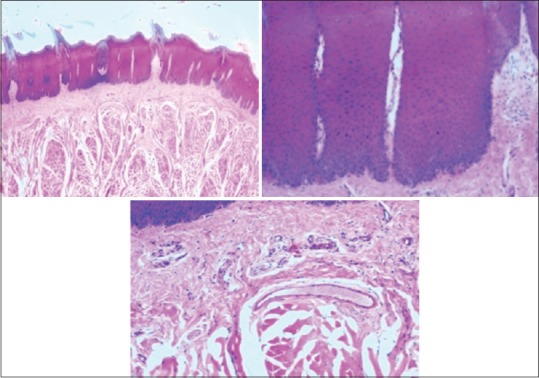
Photomicrograph showing epithelium and connective tissue fixed in formalin
Fixation was followed by conventional method of tissue processing and H and E staining.
The tissue sections were assessed and examined for preservation and staining qualities separately for epithelium and connective tissue by two examiners (oral pathologists) under light microscope and the whole procedure was blinded (single-blinded). The inter- and intra-observer variability was considered in the study.
RESULTS
The values obtained were compiled and analyzed using one-way ANOVA test and post hoc. post hoc analysis was done for intergroup comparison, and one-way ANOVA was done to compare the time period between different samples of the study group. P < 0.001 was considered statistically significant. The histomorphological criteria examined are elaborated in Table 1. The average values of the examiners are tabulated in Table 2. (ANOVA test shows there is significant difference seen in all the time periods herewith. P ≤ 0.001 for Table 1). Tissue fixed in formalin [Figure 1] Post hoc test for epithelium, at 24 h, the difference in preservation, and staining capabilities of jaggery compared to honey and sugar are statistically significant [Figure 2]. With jaggery fixation, the tissue sections had good overall morphology and also good nuclear, cytoplasmic details and staining quality [Figure 3]. In addition, the cellular outlines were clearly discernible. Similarly, for connective tissue area (both preservation and staining) at 24 h of fixations, the differences between jaggery and other fixatives (sugar [Figure 4], water [Figure 5]) have a statistically significant difference. ANOVA test for Graphs 1-4 shows that there is significant difference seen in all the time periods (P ≤ 0.001).
Table 1.
One-way analysis of variance test to see the difference between 5 solutions
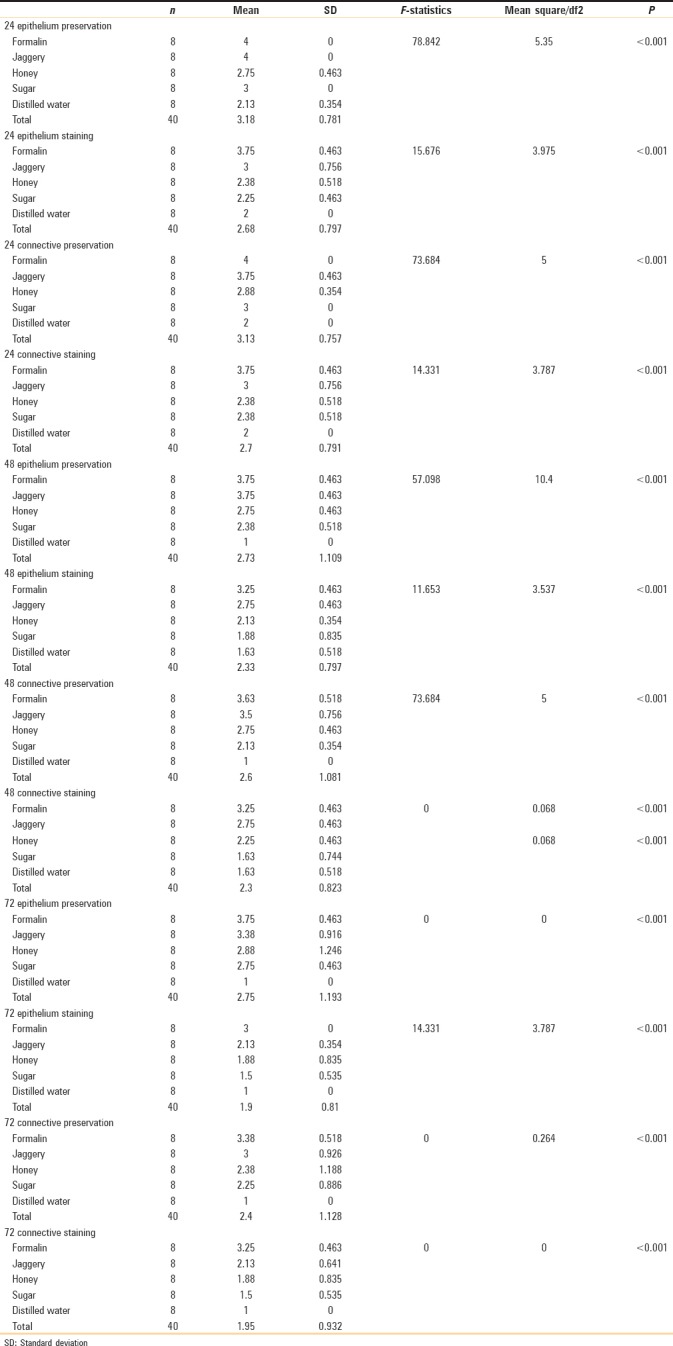
Table 2.
Different fixatives showing mean values of epithelium and connective
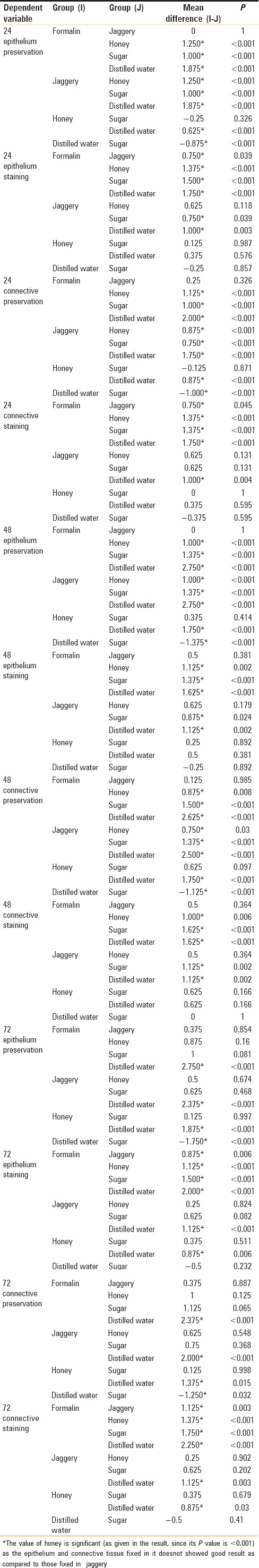
Figure 2.
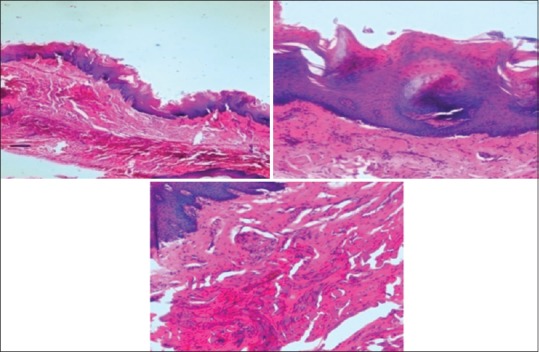
Photomicrograph showing epithelium and connective tissue fixed in honey
Figure 3.

Photomicrograph showing epithelium and connective tissue fixed in tissues fixed in jaggery
Figure 4.
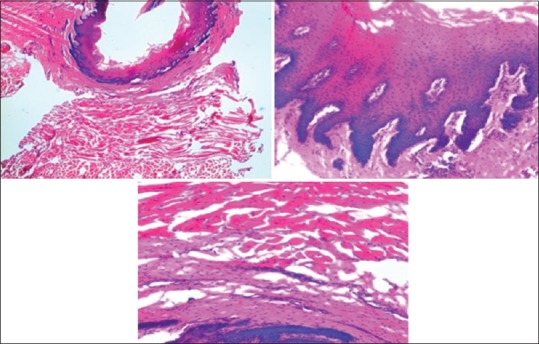
Photomicrograph showing epithelium and connective tissue fixed in tissues fixed in sugar
Figure 5.
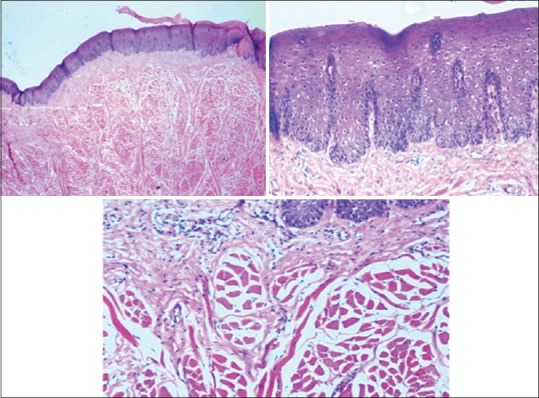
Photomicrograph showing epithelium and connective tissue fixed in tissues fixed in water
Graph 1.
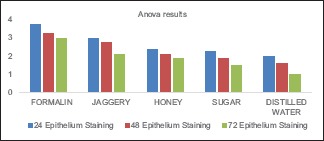
Epithelial staining in formalin, jaggery, honey, sugar, and water
Graph 4.
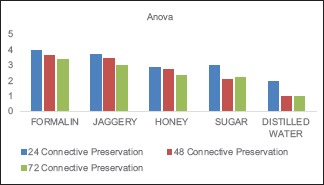
Connective tissue preservation in different fixatives
Graph 2.
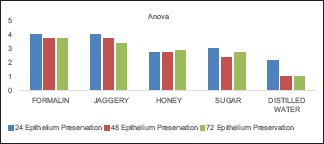
Epithelial preservation in different fixatives
Graph 3.
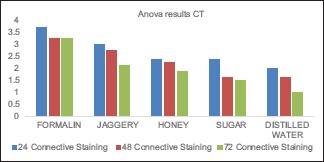
Connective tissue staining in formalin, jaggery, honey, sugar, and water
DISCUSSION
Fixation is an initial and important step in tissue processing for microscopical examination of tissues. The primary aim of fixation is to preserve the tissues in a life-like manner, prevent bacterial putrefaction and autolysis, and increase the refractive index of the tissue.[4]
In surgical pathology, formaldehyde has been the “gold standard” fixative for decades. It enables long-term storage of surgical material and preserves the detailed morphologic features necessary for microscopy. Formaldehyde is, however, toxic and was classified as “carcinogenic to humans” (Group 1) by the IARC, although there is only strong but insufficient evidence for a causal association with myeloid leukemia and a limited association with nasopharyngeal carcinoma.[10] Formalin has two well-known disadvantages. First, formalin is highly toxic. The IARC classifies formaldehyde as a human carcinogen that can cause nasopharyngeal cancer.[11]
A literature search suggested that for several centuries, honey has documented antibacterial, acidic, and dehydrative properties.[11,12] In 2006, Al-Maaini and Bryant showed that tissues fixed in low concentrations of honey at room temperature gave results comparable to formalin-fixed control tissues.[12,13] Properties of honey such as high osmolarity, low pH, and the presence of components such as hydrogen peroxide and phenol inhibine all contribute to its antioxidative and antibacterial effects.[13,14,15,16,17]
Literature search also revealed few studies proving the presence of cytoprotective and antioxidant activity in jaggery.[18]
Patil et al. suggested the use of jaggery for tissue fixation as they are nonhazardous, compatible with routine processing, staining and do not require additional equipments.[6]
All the three natural substances: honey, sugar, and jaggery gave promising results. However, jaggery exceeded our expectations, even surpassing the proven honey.[19,20] There are several advantages of using honey, sugar, and jaggery for tissue fixation: they are nonhazardous, compatible with routine processing, staining, and do not require additional equipments. Jaggery, in addition, is easily available and highly economical when compared to honey.[21] It costs about 1/6th the price of honey. The natural substitutes can be used in rural areas where screening programs, medical camps, and public health service centers were conducted, doctors generally see large number of patients. Few disadvantages of these natural substances are liable to develop molds over time; hence, it is advisable to use thymol crystals as an antimicrobial agent. In addition, jaggery-fixed specimen showed brownish discoloration.[22] The other problems encountered with natural fixatives are breach in continuity of section, intense staining eosin, and folding of tissue sections.[6] Patients with suspicious lesions are advised for immediate biopsy. In certain situations, formalin might not be readily available. In such cases, the biopsied tissues will be discarded or get damaged if left out for drying which poses difficulty in diagnosis.
We used saturated solutions of the natural substitute as it is easy to procure in the rural setting and also because the dilution of the substances was very hard to be standardized.
CONCLUSION
Natural fixatives such as honey, sugar, and jaggery can be used in place of the hazardous formalin with equal competence. Here, jaggery being highly economical and universally available can be employed in large-scale sample size as in screening camp. Natural substitutes can be an advantage whenever the health hazards of formalin are to be considered. In our study, apart from formalin, the preservation of tissues by jaggery was much better as compared to honey and sugar. Large sample size can be used for the more reliability of the result. From this study, we can conclude that the eco-friendly natural fixatives have all the novel traits to substitute formalin. Moreover, jaggery syrup as a replacement for formalin is a step forward in the field of tissue preservation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rajanikanth M, Ravi Prakash A, Sreenath G, Sonia Bai JK, Shyam ND. Transit fixatives: An innovative study. J Clin Diagn Res. 2015;9:ZM01–3. doi: 10.7860/JCDR/2015/11083.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Culling CF. London: Butterworths & Co; 1963. Handbook of Histopathological and Histochemical Techniques: Including Museum Techniques. [Google Scholar]
- 3.Moelans CB, ter Hoeve N, van Ginkel JW, ten Kate FJ, van Diest PJ. Formaldehyde substitute fixatives. Analysis of macroscopy, morphologic analysis, and immunohistochemical analysis. Am J Clin Pathol. 2011;136:548–56. doi: 10.1309/AJCPHH1B0COCBGOM. [DOI] [PubMed] [Google Scholar]
- 4.Sabarinath B, Sivapathasundharam B, Sathyakumar M. Fixative properties of honey in comparison with formalin. J Histotechnol. 2014;37:21–5. [Google Scholar]
- 5.Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. 1985;33:845–53. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 6.Patil S, Premalatha B, Rao RS, Ganavi B. Revelation in the field of tissue preservation – A preliminary study on natural formalin substitutes. J Int Oral Health. 2013;5:31–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Buesa RJ. Histology without formalin? Ann Diagn Pathol. 2008;12:387–96. doi: 10.1016/j.anndiagpath.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Conolly RB, Kimbell JS, Janszen D, Schlosser PM, Kalisak D, Preston J, et al. Human respiratory tract cancer risks of inhaled formaldehyde: Dose-response predictions derived from biologically-motivated computational modeling of a combined rodent and human dataset. Toxicol Sci. 2004;82:279–96. doi: 10.1093/toxsci/kfh223. [DOI] [PubMed] [Google Scholar]
- 9.Ozkan N, Salva E, Cakalaǧaoǧlu F, Tüzüner B. Honey as a substitute for formalin? Biotech Histochem. 2012;87:148–53. doi: 10.3109/10520295.2011.590155. [DOI] [PubMed] [Google Scholar]
- 10.Cogliano VJ, Grosse Y, Baan RA, Straif K, Secretan MB, El Ghissassi F, et al. Meeting report: Summary of IARC monographs on formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2-propanol. Environ Health Perspect. 2005;113:1205–8. doi: 10.1289/ehp.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. 2006;88:1–478. [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Maaini R, Bryant P. The effectiveness of honey as a substitute for formalin in the histological fixation of tissue. J Histochem. 2006;3:173–6. [Google Scholar]
- 13.Rahma A, Bryant P. The effectiveness of honey as a substitute for formalin in the histological fixation of tissue. J Histotechnol. 2006;29:173–6. [Google Scholar]
- 14.White JW, Jr, Subers MH, Schepartz AI. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim Biophys Acta. 1963;73:57–70. doi: 10.1016/0006-3002(63)90359-7. [DOI] [PubMed] [Google Scholar]
- 15.Molan PC. The antibacterial activity of honey 1. The nature of the antibacterial activity. Bee World. 1992;73:15–28. [Google Scholar]
- 16.Molan PC. A brief review of the use of honey as a clinical dressing. Aust J Wound Manage. 1998;6:148–58. [Google Scholar]
- 17.Molan PC. Selection of honey for use as a wound dressing. Prim Intention. 2000;8:87–92. [Google Scholar]
- 18.Al-Jabri AA, Nzeako B, Al Mahrooqi Z, Al Naqdy A, Nsanze H. In vitro antibacterial activity of Omani and African honey. Br J Biomed Sci. 2003;60:1–4. doi: 10.1080/09674845.2003.11783668. [DOI] [PubMed] [Google Scholar]
- 19.Nayaka MA, Sathisha UV, Manohar MP, Chandrashekar KB, Shylaja MD. Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chem. 2009;115:113–8. [Google Scholar]
- 20.Kim KH, Jahan SA, Lee JT. Exposure to formaldehyde and its potential human health hazards. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29:277–99. doi: 10.1080/10590501.2011.629972. [DOI] [PubMed] [Google Scholar]
- 21.Wakefield JC. London: Health Protection Agency (HPA); 2008. Formaldehyde-toxicological overview. [Google Scholar]
- 22.Prioritization of Toxic Air Contaminants – Children's Environmental Health Protection Act October 2001. Office of Environmental Health Hazard Assessment, California Environmental Protection Agency. 2001 [Google Scholar]


