Abstract
Interleukin-10 (IL-10), interleukin-17A (IL-17A) and transforming growth factor α (TGF-α) have been implicated in the progression of breast cancer. However, the diagnostic and prognostic roles of these cytokines in ductal carcinoma remain unclear. The present study therefore aimed to determine the serum levels of IL-10, IL-17 and TGF-α in subjects with benign and malignant breast diseases and to evaluate the clinical significance of these cytokines in ductal carcinoma. Pre-operative serum samples were collected from 378 patients with breast disease and 70 healthy subjects. IL-10, IL-17A and TGF-α levels were measured using ELISA. Serum levels of these cytokine in patients with different breast diseases were evaluated. Furthermore, correlations between levels of these cytokines and the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) in ductal carcinoma were determined. The results demonstrated that serum levels of IL-10 and IL-17A were significantly increased in subjects with atypical hyperplasia and ductal carcinoma. Furthermore, IL-10 and IL-17A levels were increased in patients with a more serious clinical tumor stage and tumors that were ER− and PR−. Furthermore, high serum levels of TGF-α were associated with HER2+ tumors. A strong positive correlation was identified between TGF-α and IL-17A levels. Therefore, the results of the current study revealed that elevated serum IL-10, IL-17A and TGF-α levels are strongly associated with ductal carcinoma, specifically with tumor stage. High serum levels of IL-10 and IL-17A were also associated with the negative expression of ER and PR in ductal carcinoma, and high serum levels of TGF-α were associated with the positive expression of HER2 in ductal carcinoma. Thus, serum cytokine levels may be measured to identify patients with a poor prognosis who may benefit from more aggressive management and treatment.
Keywords: interleukin-10, interleukin-17A, transforming growth factor-α, human benign and malignant breast diseases
Introduction
Human benign and malignant breast diseases, including atypical hyperplasia, papilloma, fibroma and ductal carcinoma, are usually associated with inflammation (1,2). Benign breast conditions are common and the majority of breast changes are not cancer; however, certain benign diseases increase the risk of breast cancer, including atypical hyperplasia, which is a potential precursor of cancer (3). Cells involved in the inflammatory response are attracted by cytokines and chemokines and may promote the onset and progression of breast diseases (4–7). Inflammation mediates the initiation and growth of breast cancer and cytokines serve a very important role during this process (5,8).
Previous studies have indicated that pro-inflammatory cytokines, including interleukin (IL) 17A and transforming growth factor α (TGF-α) are involved in the initiation and progression of mammary diseases (5,9,10). IL-17A is produced by activated T cells (9). The number of IL-17A-producing tumor-infiltrating lymphocytes (TILs) is increased in patients with breast cancer, particularly in estrogen receptor-negative (ER−) tumors, these cells are indicative of a poor prognosis for the patient (9,11,12). TGF-α is secreted by various types of cells and stimulates their proliferation, differentiation and development (10). Previous studies have demonstrated that TGF-α levels are correlated with different types of tumor, including mammary, squamous and renal tumors (10,13). Anti-inflammatory cytokines are also very important in tumor development; for example IL-10, which is a potent anti-inflammatory cytokine, promotes the formation of a microenvironment, which inhibits anti-tumor immune response and promotes the growth of cancer cells (14–16). It has been demonstrated that serum levels of IL-10 are higher in patients with breast cancer than in healthy subjects and IL-10 is also overexpressed in ER− breast cancer, compared with ER+ breast cancer (17). Several studies have identified the presence of these cytokines in breast, liver and gastric cancer (13,17–19); however, it remains unclear whether serum levels of these cytokines are correlated with clinical tumor stage. Additionally, it is unclear whether the expression of human epidermal growth factor receptor 2 (HER2) in tumors is correlated with the serum levels of these cytokines. HER2 is a tumor antigen that is overexpressed in breast cancer and is a very important biomarker for predicting the prognosis of patients with breast cancer.
To fully understand the significance of IL-10, IL-17A and TGF-α during the progression of breast cancer, the serum levels of these cytokines in patients with mammary diseases, including atypical hyperplasia, papilloma, fibroma and ductal carcinoma, were compared to those in healthy women. It was also determined whether there was a correlation between levels of these cytokines and clinical tumor stage, as well as with ER, progesterone receptor (PR) and HER2 antigen expression.
Materials and methods
Samples
Serum samples were collected from 378 patients with benign (atypical hyperplasia, papilloma, fibroma and ductal carcinoma) and malignant breast diseases (mean age, 45±11 years) and 70 healthy subjects (mean age, 38±9 years), all female, who were recruited from the Huai'an First People's Hospital (Huai'an, China; Table I). All patients were diagnosed by histopathology as blood samples were collected from the elbow vein of each participant. The expression of ER, PR and HER2 were analyzed if patients were suspected of having cancer. The blood was placed in a glass container, left to clot at room temperature for 1 h and stored overnight at 4°C. Serum was collected and centrifuged at 150 × g for 5 min at room temperature (to sediment erythrocytes) and then at 350 × g for 15 min at room temperature. The serum (straw-colored supernatant) was transferred to containers suitable for long-term storage and heated at 56°C for 30 min to destroy the heat-labile components of the complement.
Table I.
Demographic data of recruited patients with mammary diseases and healthy subjects.
| Demographic | Ductal cancer (n=110) | Atypical hyperplasia (n=95) | Papilloma (n=85) | Fibroma (n=88) | Healthy subjects (n=70) | P-value |
|---|---|---|---|---|---|---|
| Mean age, years | 46±19 | 40±16 | 39±13 | 37±17 | 38±9 | P>0.05a |
P>0.05 in patients with benign and malignant breast diseases (ductal cancer or atypical or hyperplasia or papilloma or fibroma) vs. healthy subjects. Data are presented as the mean ± standard error of the mean.
Patients were excluded if breast cancer was present alongside other malignancies, or if they had other serious conditions, including advanced organ failure or active infections. The control group consisted of 70 healthy women. Preoperative serum samples were collected from the patients with ductal breast cancer and stored at −80°C prior to further analysis. The protocol of the present study conformed to the ethical standards of the Declaration of Helsinki and was approved by the Ethics Committee of Huai'an First People's Hospital Faculty of Medicine. Informed consent was obtained from each patient, according to the committee's regulations.
Classification of ductal carcinoma patients
Data regarding the tumor node metastasis (TNM) classification and ER, PR, and HER2 expression in cancer tissues of the ductal carcinoma patients were acquired from the patient's medical records. The patients were classified by TNM classification and the status of ER, PR and HER2 (Table II).
Table II.
Demographic data of patients with ductal carcinoma.
| Variable | Classification | No. of patients with ductal carcinoma (n=110) |
|---|---|---|
| ER | Negative | 55 |
| Positive | 55 | |
| PR | Negative | 50 |
| Positive | 60 | |
| HER-2/Neu | Overexpressed | 45 |
| Not overexpressed | 65 | |
| TNM | I | 25 |
| II | 50 | |
| III | 35 |
TNM, tumor node metastasis; ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal growth factor receptor 2.
Measurement of serum cytokine levels
Human IL-10 (cat. no. S1000B), IL-17A (cat. no. DY5194-05) and TGF-α (cat. no. DTGA00) ELISA kits were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Levels of serum IL-10, IL-17A and TGF-α from patients and healthy subjects were determined by ELISA following the manufacturer's protocol.
Statistical analysis
Mean ± standard error of the mean were calculated from data. Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad, Inc., La Jolla, CA, USA). Differences among groups were evaluated using the one-way analysis of variance, followed by a post hoc multiple comparisons test (Tukey's test). Pearson's correlation coefficient was used to assess correlations between IL-10, IL-17A and TGF-α levels in patients with ductal carcinoma. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of recruited patients with mammary diseases and healthy subjects
The age difference between patients and healthy groups was compared to exclude the factor of age on the production of cytokines. There were no significant differences in the mean age of all recruited patients with mammary diseases (ductal cancer, atypical hyperplasia, papilloma, and fibroma) and healthy subjects (Table I).
Serum IL-10 levels in benign and malignant breast diseases
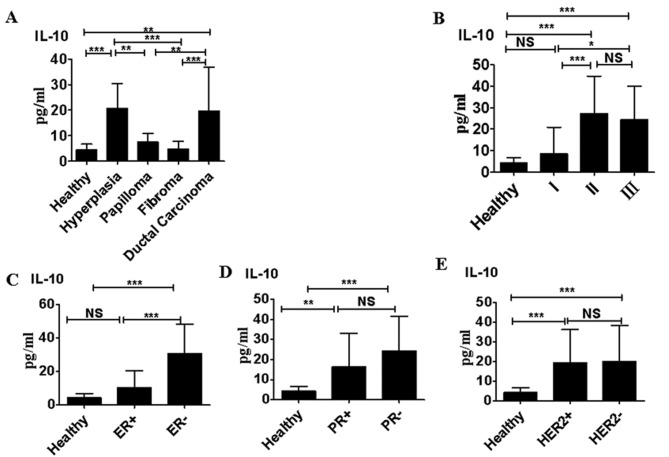
Serum levels of IL-10 in subjects with atypical hyperplasia and ductal carcinoma were significantly higher than those in healthy women, and in patients with papilloma or fibroma (P<0.01; Fig. 1A). Patients with TNM stage II and III ductal carcinoma exhibited significantly higher serum IL-10 levels than those with stage I ductal carcinoma (P<0.0001; Fig. 1B). Furthermore serum IL-10 levels were significantly higher in patients with ductal carcinoma that were ER− compared with those that were ER+ (P<0.0001; Fig. 1C). IL-10 levels were significantly higher in sera collected from patients with ductal carcinoma that were PR+ than in the sera of healthy controls (P<0.01); however, IL-10 levels did not differ significantly between patients with ductal carcinoma that were PR+ and those that were PR− (Fig. 1D). Furthermore, there was no significant difference between IL-10 levels in patients with ductal carcinoma that were HER2+ compared with those that were HER2− (Fig. 1E).
Figure 1.
Serum IL-10 levels in benign and malignant breast diseases. (A) IL-10 levels in healthy women (n=70) and patients with atypical hyperplasia (n=95), papilloma (n=85), fibroma (n=88) or ductal carcinoma (n=110). (B) Serum IL-10 levels in patients with different clinical stages of ductal carcinoma. (C) Serum IL-10 levels in patients with ductal carcinoma that were ER+ compared with those that were ER−. (D) Serum IL-10 levels in patients with ductal carcinoma that were PR+ compared with those that were PR−. (E) Serum IL-10 levels in patients with ductal carcinoma that were HER2+ positive compared with those that were HER2−. *P<0.05; **P<0.01 and ***P<0.0001. Data are representative of three different experiments and presented as the mean ± standard error of the mean. IL, interleukin; NS, not significant; ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal growth factor receptor 2.
Serum IL-17A levels in benign and malignant breast diseases
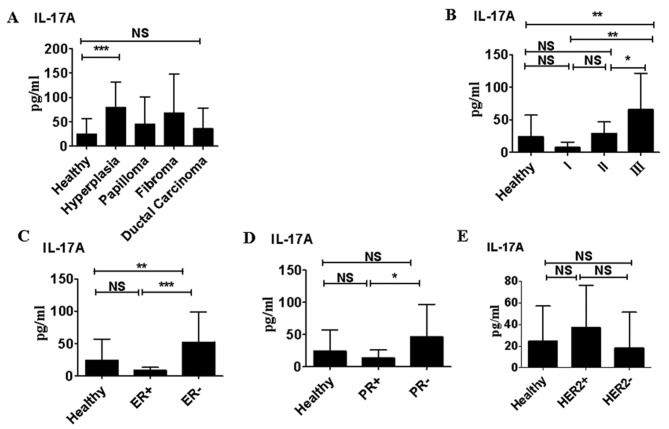
Only in patients with atypical hyperplasia were the serum IL-17A levels significantly higher than those in healthy women (P<0.05). IL-17A levels were slightly higher in patients with papilloma and fibroma compared with healthy volunteers; however, this difference was not significant (Fig. 2A). Although IL-17A levels did not differ significantly between patients with ductal carcinoma and healthy subjects, IL-17A levels in patients with stage III ductal carcinoma were significantly higher than those in healthy volunteers (P<0.01; Fig. 2B). IL-17A levels were significantly higher in patients with ductal carcinoma that were ER− compared with those that were ER+ (P<0.0001; Fig. 2C). This was also the case in patients that were PR− compared with those that were PR+ (P<0.05; Fig. 2D). However, IL-17A levels did not differ significantly in between patients with ductal carcinoma that were HER+ and those that were HER− (Fig. 2E).
Figure 2.
Serum IL-17A levels in benign and malignant breast diseases. (A) In healthy women (n=70) and patients with atypical hyperplasia (n=95), papilloma (n=85), fibroma (n=88) or ductal carcinoma (n=110). (B) By clinical tumor stage in patients with ductal carcinoma. (C) In patients that were ER+ vs. those that were ER−. (D) Patients who were PR+ vs. those that were PR−. (E) In patients that were HER2+ vs. those that were HER2−. *P<0.05; **P<0.01 and ***P<0.0001. Data are representative of three different experiments and presented as the mean ± standard error of the mean. IL, interleukin; ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal growth factor receptor 2; NS, not significant.
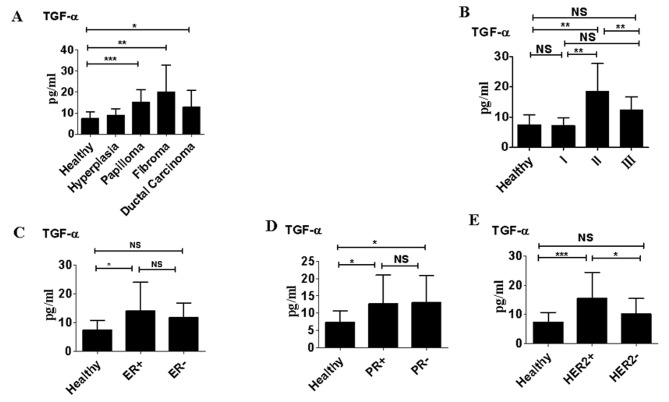
Serum TGF-α levels in benign and malignant breast diseases. Serum levels of TGF-α were significantly higher in patients with papilloma, fibroma and ductal carcinoma than in healthy women (P<0.05; Fig. 3A). However, there were no significant differences in levels of TGF-α between patients with atypical hyperplasia and healthy women. Patients with ductal carcinoma classified as TNM stage II also exhibited significantly higher serum TGF-α levels than those classed as TNM stage I (P<0.01; Fig. 3B); however there were no significant differences in TGF-α levels between patients with stage III ductal carcinoma and those with stage I ductal carcinoma (Fig. 3B). TGF-α levels did not differ significantly between patients that were ER− compared with those that were ER+ and there were no significant differences between TGF-α levels between patients that were PR+ and those that were PR− (Fig. 3C and D). However, TGF-α levels were significantly higher in patients that were HER2+ compared with those that were HER2− (P<0.05; Fig. 3E).
Figure 3.
Serum TGF-α levels in benign and malignant breast diseases (A) In healthy women (n=70) and patients with atypical hyperplasia (n=95), papilloma (n=85), fibroma (n=88) or ductal carcinoma (n=110). (B) By clinical tumor stage in patients with ductal carcinoma. (C) In patients that were ER+ vs. those that were ER−. (D) In patients that were PR+ vs. those that were PR−. (E) In patients that were HER2+ vs. those that were HER2−. *P<0.05; **P<0.01; ***P<0.0001. Data are representative of three different experiments and presented as the mean ± standard error of the mean. TGF, transforming growth factor α; ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal growth factor receptor 2; NS, not significant.
Correlations between IL-10, IL-17A, and TGF-α levels in patients with ductal carcinoma
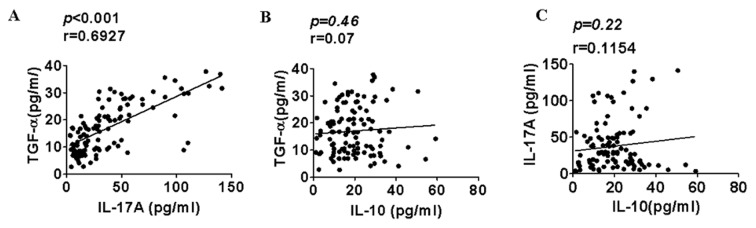
In samples isolated from ductal carcinoma patients, a significant positive correlation was identified between TGF-α and IL-17A levels (P<0.001; Fig. 4A). However, there was no correlation between TGF-α and IL-10 levels (Fig. 4B) and no correlation between IL-17A and IL-10 levels in patients with ductal carcinoma (Fig. 4C).
Figure 4.
Correlation between levels of the cytokines IL-10, IL-17A and TGF-α in patients with ductal carcinoma. (A) Correlation between IL-17A and TGF-α levels. (B) Correlation between TGF-α and IL-10 levels. (C) Correlation between IL-10 and IL-17A levels. IL, interleukin; TGF-α, transforming growth factor α.
Discussion
Despite wide interest in the development of novel diagnostic biomarkers for breast cancer (8,10,20), limited data have been collected regarding whether IL-10, IL-17A and TGF-α levels are associated with the clinical stage of breast cancer, as well as ER, PR and HER2 status. The results of the present study revealed that elevated serum levels of IL-10, IL-17A and TGF-α are strongly associated with ductal carcinoma and are associated with a more severe clinical stage of this disease. IL-10 and IL-17A levels were also associated with ER and PR status in ductal carcinoma. Thus, these biomarkers may be used to diagnose women with breast cancer and to identify patients with a poor prognosis that may benefit from more aggressive forms of treatment and management.
IL-10 is a pleiotropic cytokine able to suppress and stimulate the immune response (16,21). It has been suggested that patients with atypical hyperplasia may have an increased risk of tumorigenesis (22). In the current study, serum IL-10 levels in patients with atypical hyperplasia were significantly higher compared with healthy subjects and those with papilloma or fibroma. It is therefore possible that these increased IL-10 levels in patients with atypical hyperplasia may promote the development of breast cancer. The results also demonstrated that IL-10 levels were higher in sera collected from patients with stage II and III ductal carcinoma than in the sera of patients with stage I carcinoma. These results were not consistent with those reported by Ikeguchi et al (19), who reported that serum IL-10 levels were not correlated with tumor stage in patients with gastric cancer. Therefore, it was speculated that the intensity of the immune response varies among patients with different types of cancer. For example, the antigens of ER and HER2 are highly expressed on breast cancer cells but not gastric cancer cells, and these antigens may promote or suppress cytokine production (23). The results of the current study indicated that high serum IL-10 levels were associated with negative ER expression, but not with PR and HER2 expression.
IL-17A-producing TILs are found in greater numbers in breast tumor tissue than in healthy mammary tissue (9,12). The results of the current study revealed that serum IL-17A levels did not differ significantly between healthy women and patients with ductal carcinoma; however, IL-17A levels in patients with stage III ductal carcinoma were significantly higher than those in patients with stages I and II ductal carcinoma and healthy volunteers. This suggests that IL-17A serves a critical role in promoting the progression of cancer (9,14) The results of the current study demonstrated that serum IL-10 and IL-17A levels are increased in patients with atypical hyperplasia. These results may not actually be contradictory, as IL-10 and IL-17A may promote tumorigenesis. Furthermore, in the current study, serum IL-17A levels were associated with the absence of ER in tumor tissue. Specifically, IL-17A levels were significantly elevated in patients that were ER− and PR−. The mechanisms underlying this association between IL-17A and ER− remain unknown. One possibility is that ER deficiency in breast cancer may directly promote the expansion of IL-17A-producing TILs (9).
In breast cancer, TGF-α may promote the growth and progression of tumors via an autocrine/paracrine loop involving the epidermal growth factor receptor (10,13,24). In the current study, serum TGF-α levels in patients with papilloma, fibroma and ductal carcinoma were significantly higher than those in healthy subjects; this suggests that TGF-α may also promote tumor development. High serum TGF-α levels were also associated with a more severe tumor stage and were significantly increased in patients that were HER2+; however there was no association between serum TGF-α levels and ER or PR expression. Since mammary gland epithelial cells are able to secrete TGF-α, it was hypothesized that HER2 expression in tumor cells may enhance TGF-α production.
It was then assessed whether there were correlations between serum IL-17A and TGF-α, IL-10 and TGF-α, and between IL-10 and IL-17A. The results demonstrated that IL-10 levels increased as IL-17A levels increased; however, there appeared to be no significant association between IL-10 and IL-17/TGF-α levels. However, a strong positive correlation between TGF-α and IL-17A was identified. Therefore, TGF-α and IL-17A may function synergistically during the initiation and development of tumors.
In conclusion, the results of the present study suggest that increased serum levels of IL-10, IL-17 and TGF-α are associated with ductal carcinoma. Increased levels of these cytokines are also associated with a more severe clinical stage of ductal carcinoma, and with the negative expression of ER and HER2. Thus, these cytokines may be developed as potential diagnostic and prognostic cancer biomarkers.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- IL
interleukin
- TGF-α
transforming growth factor α
- ER
estrogen receptor
- PR
progesterone receptor
- HER2
human epidermal growth factor receptor 2
- TILs
tumor-infiltrating lymphocytes
Funding
The current study was supported by grants from the National Natural Science Foundation of China (grant nos. 81472822, and 81501377), Natural Science Foundation of Shaanxi Province (grant no. 2015JM8385), China Postdoctoral Science Foundation (grant no. 2014M560787), Shanxi Postdoctoral Science Foundation and Fundamental Research Funds for the Central Universities (grant no. 2015gjhz16).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZL and ML performed the immunoassays. JS collected the patient and control samples. DX performed the data analysis. YM drafted the manuscript. YM and YJ designed the study and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The protocol of the present study conformed to the ethical standards of the Declaration of Helsinki. All procedures were approved by the Ethics Committee of Huai'an First People's Hospital Faculty of Medicine (Huai'an, China). Informed consent was obtained from each patient, according to the committee's regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Amin AL, Purdy AC, Mattingly JD, Kong AL, Termuhlen PM. Benign breast disease. Surg Clin North Am. 2013;93:299–308. doi: 10.1016/j.suc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Shapiro DJ. The immune system and inflammation in breast cancer. Mol Cell Endocrinol. 2014;382:673–682. doi: 10.1016/j.mce.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEvoy MP, Coopey SB, Mazzola E, Buckley J, Belli A, Polubriaginof F, Merrill AL, Tang R, Garber JE, Smith BL, et al. Breast cancer risk and follow-up recommendations for young women diagnosed with atypical hyperplasia and Lobular Carcinoma In Situ (LCIS) Ann Surg Oncol. 2015;22:3346–3349. doi: 10.1245/s10434-015-4747-1. [DOI] [PubMed] [Google Scholar]
- 4.Standish LJ, Sweet ES, Novack J, Wenner CA, Bridge C, Nelson A, Martzen M, Torkelson C. Breast cancer and the immune system. J Soc Integr Oncol. 2008;6:158–168. [PMC free article] [PubMed] [Google Scholar]
- 5.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson FM, Ross MS, Tober KL, Long BW, Oberyszyn TM. Inhibition of pro-inflammatory cytokine gene expression and papilloma growth during murine multistage carcinogenesis by pentoxifylline. Carcinogenesis. 1996;17:1719–1728. doi: 10.1093/carcin/17.8.1719. [DOI] [PubMed] [Google Scholar]
- 7.Schmid BC, Rudas M, Rezniczek GA, Leodolter S, Zeillinger R. CXCR4 is expressed in ductal carcinoma in situ of the breast and in atypical ductal hyperplasia. Breast Cancer Res Treat. 2004;84:247–250. doi: 10.1023/B:BREA.0000019962.18922.87. [DOI] [PubMed] [Google Scholar]
- 8.Chin AR, Wang SE. Cytokines driving breast cancer stemness. Mol Cell Endocrinol. 2014;382:598–602. doi: 10.1016/j.mce.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Cochaud S, Giustiniani J, Thomas C, Laprevotte E, Garbar C, Savoye AM, Curé H, Mascaux C, Alberici G, Bonnefoy N, et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci Rep. 2013;3:3456. doi: 10.1038/srep03456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth BW, Smith GH. Roles of transforming growth factor-alpha in mammary development and disease. Growth Factors. 2007;25:227–235. doi: 10.1080/08977190701750698. [DOI] [PubMed] [Google Scholar]
- 11.Bastid J, Bonnefoy N, Eliaou JF, Bensussan A. Lymphocyte-derived interleukin-17A adds another brick in the wall of inflammation-induced breast carcinogenesis. Oncoimmunology. 2014;3:e28273. doi: 10.4161/onci.28273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 13.Kornasiewicz O, Grąt M, Dudek K, Lewandowski Z, Gorski Z, Zieniewicz K, Krawczyk M. Serum levels of HGF, IL-6, and TGF-α after benign liver tumor resection. Adv Med Sci. 2015;60:173–177. doi: 10.1016/j.advms.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 15.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–27. doi: 10.1016/j.coi.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igietseme JU, Ananaba GA, Bolier J, Bowers S, Moore T, Belay T, Eko FO, Lyn D, Black CM. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: Potential for cellular vaccine development. J Immunol. 2000;164:4212–4219. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 17.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- 18.Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35:1–16. doi: 10.1089/jir.2014.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S. Serum interleukin-6 and −10 levels in patients with gastric cancer. Gastric Cancer. 2009;12:95–100. doi: 10.1007/s10120-009-0509-8. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: Mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11:1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- 21.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: Influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HZ, Li XH, Zhang X, Zhang ZY, Meng YL, Xu SW, Zheng Y, Zhu ZL, Cui DS, Huang LX, et al. PINCH protein expression in normal endometrium, atypical endometrial hyperplasia and endometrioid endometrial carcinoma. Chemotherapy. 2010;56:291–297. doi: 10.1159/000319953. [DOI] [PubMed] [Google Scholar]
- 23.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissière F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, Matsushima-Nishiwaki R, Kuroyanagi G, Suzuki N, Takamatsu R, Furui T, Yoshimi N, Kozawa O, Morishige K. Regulation by heat shock protein 22 (HSPB8) of transforming growth factor-α-induced ovary cancer cell migration. Arch Biochem Biophys. 2015;571:40–49. doi: 10.1016/j.abb.2015.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.