Abstract
Brain energy metabolism relies predominantly on glucose and oxygen utilization to generate biochemical energy in the form of adenosine triphosphate (ATP). ATP is essential for maintaining basal electrophysiological activities in a resting brain and supporting evoked neuronal activity under an activated state. Studying complex neuroenergetic processes in the brain requires sophisticated neuroimaging techniques enabling noninvasive and quantitative assessment of cerebral energy metabolisms and quantification of metabolic rates. Recent state-of-the-art in vivo X-nuclear MRS techniques, including 2H, 17O and 31P MRS have shown promise, especially at ultra-high fields, in the quest for understanding neuroenergetics and brain function using preclinical models and in human subjects under healthy and diseased conditions.
Keywords: In vivo X-nuclear MRS and imaging, Brain energy metabolism, Neuroenergetics, Cerebral metabolic rate of glucose (CMRGlc) and oxygen (CMRO2) consumption, and ATP production (CMRATP), TCA cycle rate (VTCA), NAD redox state, Ultra-high magnetic field (UHF)
Graphical abstract
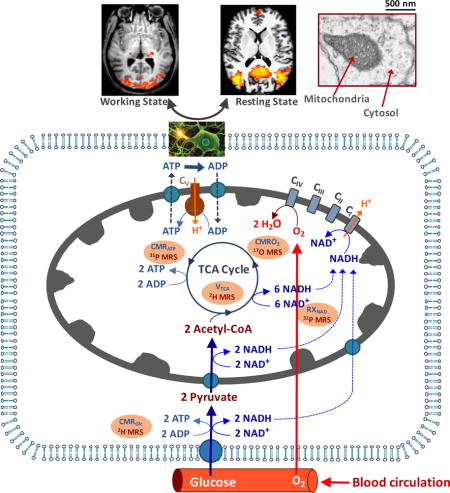
Schematic diagram of major brain network involving energy metabolisms and hemodynamics occurring in the capillary, sub–cellular compartments including mitochondria and cytosol. These metabolic pathways and rates are associated with the cerebral metabolic rates of glucose (CMRGlc), oxygen (CMRO2) and ATP production (CMRATP), TCA cycle rate (VTAC) and NAD redox ratio (RXNAD). They can be noninvasively measured using advanced in vivo X-nuclear 2H, 17O and 31P MRS approaches, respectively.
1. Introduction
The brain is a high-energy consumption organ that relies on glucose as the main carbon substrate to support oxygen metabolism and production of adenosine triphosphate (ATP) molecules via oxidative phosphorylation in the mitochondria. ATP is the primary source of biochemical energy essential for neurophysiology and brain function [1-3]. Approximately, one-fourth of the total brain ATP energy expenditure is devoted to “housekeeping” functions critical for maintaining cell integrity and brain tissue viability [4, 5]; the rest of the ATP energy supports electrophysiological activity and neural processing at either resting or working brain state. A tight coupling between the electrophysiological activity and brain energy consumption holds for a wide range of physiological conditions. To maintain the coupling requires effective metabolic regulations between cellular ATP demand and supply through many key biochemical reactions and metabolic pathways associated with energy metabolisms.
Figure 1 is a schematic illustration of key hemodynamic and metabolic processes at the mesoscopic and sub-cellular level, including capillary blood circulation for providing nutrients, cellular glucose and oxygen metabolisms, oxidative phosphorylation of adenosine diphosphate (ADP) for producing ATP in the mitochondria, and ATP utilization to support neuronal activity and neural processing in a resting or working brain. Oxygen and glucose are supplied by cerebral blood flow (CBF) circulating through the capillary bed. Glucose is transported into the brain cells and converted into two pyruvate molecules via glycolysis in the cytosol. The majority of pyruvate molecules are metabolized in the mitochondria to form Acetyl-CoA, which is subsequently oxidized via the tricarboxylic acid (TCA) cycle to generate the reduced form of nicotinamide adenine dinucleotide (NADH); NADH serves as an electron donor in the electron transport chain reactions and is converted to oxidized NAD (NAD+) via oxygen metabolism. The electron transport chain, in turn, generates an electrochemical potential gradient across the mitochondrial inner membrane via extrusion of H+ ions from the mitochondria. The resulting transmembrane potential is the driving force for the conversion of ADP and inorganic phosphate (Pi) to ATP, a conversion mediated by the F1F0-ATPase (also known as H+-ATPase) enzyme reaction that reversely transports the H+ ions back into the mitochondria [1, 4]. On the other hand, the ATP utilization occurs in the cytosol and cell membrane, resulting in the hydrolysis of ATP. The Na+/K+-ATPase activity utilizes the majority of the ATP energy released in this process to maintain the Na+/K+ ion gradient across the cell membrane, critical in supporting neuronal signaling processes (e.g., action potential propagation, neuronal firing, neurotransmitter cycling, etc.) (see references [1, 6]). The rapid ATP turnover in the brain requires efficient transportation of ATP and ADP molecules between the cytosolic and mitochondrial compartments. This is accomplished, in part, by phosphocreatine (PCr), another high-energy phosphate compound, via the creatine kinase (CK) reaction that catalyzes a rapid, near-equilibrium exchange between (ATP + Creatine (Cr)) and (PCr + ADP) [5, 7]. Because of their role in supporting rapid ATP turnover and ATP homeostasis, the rates of CK reactions are anticipated to be much higher (~ 5 times) than the net rates of ATP synthesis and utilization in the human brain [8, 9].
Fig. 1.
Schematic diagram of major energy metabolism pathways and hemodynamics occurring in the capillary, sub-cellular compartments including mitochondria and cytosol, that are essential for brain function under a resting or working state and can be assessed using the in vivo X-nuclear MRS imaging methods as reviewed in this article. Oxygen and glucose supplied from feeding arteries and blood circulation in the capillary can diffuse (for oxygen) or transport (for glucose) into brain cells. Glucose is first converted to two pyruvates; most pyruvate molecules enter mitochondria and are metabolized oxidatively. The oxygen utilization is, in general, coupled with the ATP production via the oxidative phosphorylation of ADP in the mitochondria. The produced ATP is transported to cytosol for supporting electrophysiological activities and brain functions at resting or activated brain state as demonstrated by the functional MRI (fMRI) map of visual stimulation or resting-state fMRI connectivity map in top panels. The energy metabolic pathways are tightly associated with the NAD redox reactions which are essential for regulating brain energy generation and utilization. The metabolic activities associated with different pathways can be quantitatively determined by the cerebral metabolic rates of CMRGlc, CMRO2, CMRATP, TCA cycle rate (VTAC) and NAD redox ratio (RXNAD) and noninvasively measured using the advanced in vivo X-nuclear MRS approaches as depicted in the shadowed texts with orange background. CI - CV represent five enzyme complexes involving the respiration chain reactions in the mitochondria.
The human brain has an enormous energy demand despite making up only 2% of the body’s total weight. A “resting-state” adult brain receives approximately 15% of the cardiac blood output and accounts for roughly 20% of the body’s total oxygen and glucose consumption [2, 4, 10]. The steady-state ATP concentration of the brain is low (~ 3 mM), which means that the total ATP content in the entire human brain is only about 2 g, assuming an average adult brain weight of 1.4 kg. In contrast, the oxidative ATP synthesis rate through the F1F0-ATPase reaction is very high (8-9 μmole/g/min in the human brain [8, 9, 11]), which translates into 7-8 kg of total ATP synthesized (or utilized) by the human brain in a single day (5-6 times the weight of the average human brain). In addition, the redox reaction for converting intracellular NAD between NAD+ and NADH and its redox state plays a critical role in regulating and balancing the brain energy metabolisms between cytosolic glycolysis and mitochondrial oxidative phosphorylation for producing adequate ATP molecules [12-14]. Abnormal cerebral energy metabolism has been linked to numerous brain disorders and neurodegenerative diseases including Schizophrenia, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, mitochondrial dysfunction as well as the aging process.
There is an urgent need to develop sophisticated and quantitative neuroimaging techniques enabling noninvasive assessment of regional cerebral energy metabolisms under normal and diseased conditions. The brain energy metabolisms associated with glucose and oxygen consumption, oxidative phosphorylation of ADP for producing ATP, and NAD redox state can be quantitatively represented in terms of the cerebral metabolic rates of glucose (CMRGlc) and oxygen (CMRO2), and ATP synthesis (CMRATP), TCA cycle rate (VTCA) and NAD redox ratio (RXNAD=[NAD+]/[NADH]). In vivo X-nuclear magnetic resonance (MR) spectroscopy (MRS) and imaging (MRSI) could play a critical role for determining these neuroenergetic quantities based on newly developed phosphate-31 (31P), oxygen-17 (17O) and deuterium-2 (2H) MRS methods, especially at ultra-high magnetic field (UHF). In this regard, Dr. Joe Ackerman has made remarkable contributions to X-nuclear NMR that can be traced back to his early in vivo 31P work published in Nature (1980) [15] when he invented the surface radiofrequency (RF) coil for studying high-energy phosphate metabolism, as well as his early in vivo 2H NMR work for measuring organ blood perfusion concurrently with 31P MRS measurement [16]. Dr. Chen (Coauthor) was fortunate enough to have joined Professor Joe Ackerman’s lab many years ago and pursued his Ph.D. degree under Joe’s supervision. His thesis work was on the topic of developing spatial localization methods for in vivo 31P and 13C MRS; this unique and memorable experience has benefited his entire academic career and guided his research in developing a variety of X-nuclear MRS techniques for studying brain energy metabolisms, in particular, at UHF.
2. Sensitivity and spatiotemporal limitations of in vivo X-nuclear MRS and benefit at UHF
Biomedical and clinical applications of in vivo X-nuclear MRS face many challenges. The major limitation is the low concentrations of detectable metabolites. For instance, the concentration of phosphate metabolites detected by in vivo 31P MRS in the human brain is in the range of a few millimolars (mM), which is several thousands of times lower than the concentration of tissue water protons commonly detected by 1H MRI. In addition, the gyromagnetic ratios of nuclides such as 2H, 13C, 17O and 31P are ~2 to 7 times lower than that of 1H, and further reduce the intrinsic detection sensitivity and signal-to-noise ratio (SNR); thus, extensive signal averaging is required to achieve reasonable SNR and spectral quality. This has limited the reliability, applicability, and spatiotemporal resolution of in vivo X-nuclear MRS in general, and particularly for clinical applications. One effective way to ameliorate these limitations is to use a high or ultra-high magnetic field MRI scanner, which offers significant SNR spectral resolution improvements. Herein, we describe two examples demonstrating the UHF advantages for in vivo 17O and 31P MRS brain studies.
17O is a stable and NMR detectable nuclide with a spin quantum number of 5/2; in contrast, 16O is the most abundant oxygen isotope with even numbers of protons and neutrons but is NMR invisible. Natural abundance of 17O is only 0.037%, around 30 or 2700 times lower than that of 13C or 1H, respectively. Moreover, the gyromagnetic ratio of 17O is 7.4 times lower than that of 1H. The relaxation mechanism of 17O nuclide is distinct from other nuclides with a quantum number of 1/2 such as 1H and 31P. The 17O nuclide in water possesses a quadrupolar moment, which can interact with local electric field gradients; the temporal fluctuations of this interaction induced by molecular motion dominate the 17O relaxation processes and determine both longitudinal (T1) and transverse (T2) relaxation times of the H217O. The T1, T2 and the apparent T2 (T2*) values of 17O water are extremely short (<7 ms) and insensitive to the magnetic field strength (B0) and B0 inhomogeneity [17], these features have been experimentally examined via the field strength comparison studies from 4.7 Tesla (T), 9.4T to 16.4T [18, 19]. A ~4-fold SNR gain for in vivo 17O MRS of brain water signal at 9.4T vs. 4.7T and ~3-fold SNR gain at 16.4T vs. 9.4T have been demonstrated, indicating an approximated field dependence of B02 for 17O MRS [18, 19] as compared to an approximated linear field dependence for 1H MRI [20]. Figure 2A shows the field dependence of H217O SNR across a wide range of B0, and suggests an SNR improvement of more than 120 times at 16.4T compared to a clinical scanner at 1.5T. The significant sensitivity enhancement at ultrahigh fields makes it possible to acquire three-dimensional (3D) 17O MRSI of the natural abundance tissue H217O (~20 mM) in the animal or human brain with adequate SNR, reasonable voxel MRSI size and ~10 seconds temporal resolution. The sensitivity improvement at UHF is crucial for developing an in vivo 17O MR-based neuroimaging modality in studying brain oxygen metabolism and perfusion.
Fig. 2.
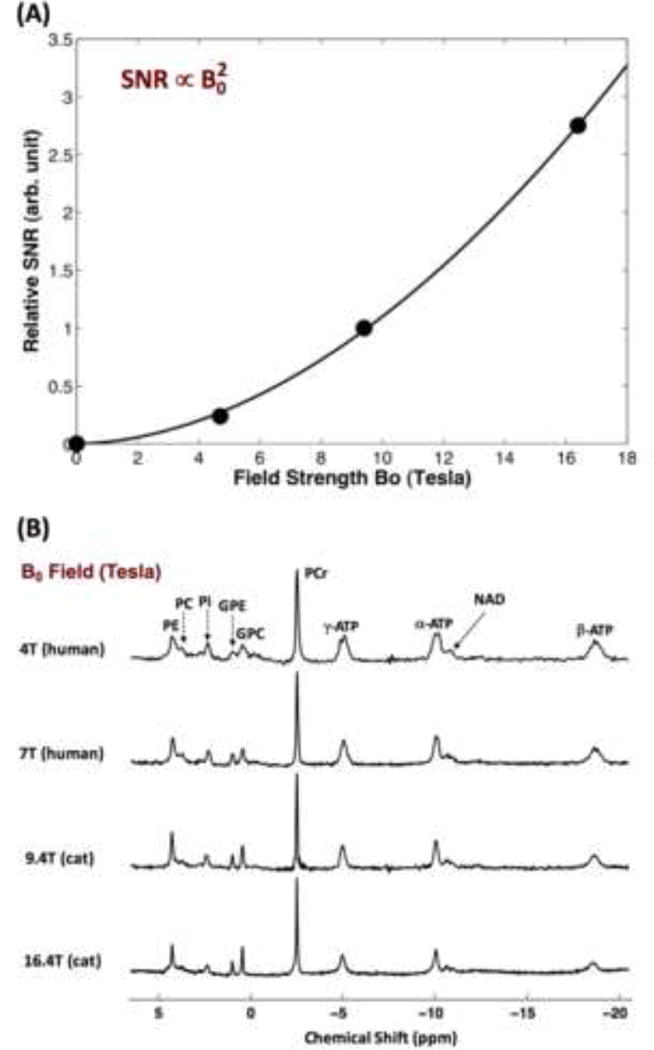
(A) Field dependence of the in vivo 17O signal of brain tissue water. (B) Typical in vivo 31P MR spectra acquired from the human and cat visual cortex at varied magnetic field strength (B0) ranging from 4T to 16.4T. The spectrum at ultrahigh field is characterized by excellent spectral resolution and sensitivity, and a large number of well-resolved resonances including phosphoethanolamine (PE); phosphocholine (PC); inorganic phosphate (Pi); glycerophosphoethanolamine (GPE); glycerophosphocholine (GPC); phosphocreatine (PCr); adenosine triphosphate (ATP); and nicotinamide adenine dinucleotides (NAD).
The 31P nuclide has been extensively studied since the inception of in vivo MRS (e.g., [15, 21]). Its sensitivity dependence on B0 is slightly better than a linear relationship [22, 23]. Interestingly, the PCr T1 in the human brain decreases at higher fields; presumably due to the chemical shift anisotropy (CSA) becoming the dominating relaxation mechanism at UHF [23]. The shorter T1 further improves the SNR per unit sampling time via more signal averaging. The spectral resolution of the brain 31P MRS also improves at higher field. Figure 2B illustrates in vivo 31P spectra acquired in cat or human visual cortex across a wide range of field strengths from 4T to 16.4T, showing substantial improvement at UHFs for resolving the overlapping resonances, which is difficult at lower fields. Besides ATP, PCr and Pi molecules, in vivo 31P MRS can also detect other phosphate metabolites including NAD, glycerophosphoethanolamine (GPE), glycerophosphocholine (GPC), phosphoethanolamine (PE) and phosphocholine (PC), which are actively involved in oxidative phosphorylation, NAD redox reaction and membrane phospholipid metabolisms through phospholipid biosynthetic enzymes. The improvement of spectral resolution at UHF translates into improvements for resolving adjacent phosphate metabolite resonances, for instance, α-ATP versus NAD and NAD+ versus NADH, thus, allowing determination of the NAD redox ratio, as well as intracellular versus extracellular Pi in vivo as shown in Fig. 2B [13, 14, 24].
The advantages of UHF have significantly advanced in vivo X-nuclear MRS technology for quantitatively imaging brain energy metabolisms along the energy metabolic pathways and “roadmap” as illustrated in Fig. 1. In this article, we will briefly describe three X-nuclei MRS imaging techniques for measuring a number of physiological parameters of interest:
in vivo 2H MRSI technique for simultaneous measurement of CMRGlc and VTCA,
in vivo 17O MRSI technique for simultaneous measurement of CMRO2, CBF and oxygen extraction fraction (OEF),
in vivo 31P MRSI technique for measurement of CMRATP and RXNAD.
3. In vivo 2H MRS technique for simultaneous measurement of CMRGlc and VTCA
Quantitative assessment of CMRGlc and VTCA and their relationship in vivo is crucial for understanding neuroenergetics under physiological and pathological conditions. Under normal physiological conditions, CMRGlc and VTCA are coupled with a stoichiometric ratio of approximately two to one since one glucose is converted to two pyruvates before entering the mitochondria (Fig. 1). This relationship could change substantially and even become uncoupled in diseased tissues, e.g. in the case of a brain tumor or stroke. Nevertheless, simultaneous measurement and quantitative imaging of both CMRGlc and VTCA is challenging due to the complexity of brain glucose metabolism and limitations of current in vivo MRS technology. We have recently developed a quantitative in vivo 2H MRS technique for simultaneously measuring CMRGlc and VTCA and tested the technique using a preclinical model at 16.4T [25].
2H contains one proton and one neutron; it is a stable isotope of hydrogen with spin quantum number of 1 and has a very low natural abundance of 0.0156%. Like the 17O nuclide, it has short T1 and T2 values dominated by the quadrupolar relaxation mechanism that allows rapid signal averaging that result in a sizeable SNR gain. High-resolution 2H NMR has been applied to elucidate metabolic pathways in human gluconeogenesis using infusion of D-Glucose-6,6-d2 or oral uptake of deuterated water and analyzing plasma samples [26-29]. In vivo 2H NMR had been employed to measure organ blood perfusion using deuterated water as a tracer [16] or to detect 2H-labeled drug concentrations [30].
The advantage of in vivo 2H MRS for studying brain glucose metabolisms becomes appealing when combined with 2H-isotope labeled glucose infusion at UHF [25, 31]. Deuterium labeled substrates such as glucose, water, and the intermediates of the glycolysis and oxidative pathways including glutamate/glutamine (Glx) and lactate (Lac) in the brain tissue can be monitored and identified using their well-resolved resonance signals and chemical shifts in the dynamic 2H spectra with excellent sensitivity and temporal resolution acquired at UHF. These merits allow for isotope kinetic analysis as well as eventual quantification of both CMRGlc and VTCA.
Figure 3A shows the 2H-isotope labeling scheme detectable by dynamic 2H MRS in the brain tissue and the associated metabolic pathways after an intravenous infusion of D-Glucose-6,6-d2 (d66). Following the infusion, d66 glucose is transported together with non-labeled glucose into the brain and metabolized through glycolysis and oxidative phosphorylation pathways. Dynamics of the cerebral d66 and incorporation of the label into the pools of Lac, Glx and water could be detected by sequential 2H MRS acquisitions. Figure 3B demonstrates excellent 2H spectral quality and fittings of the original spectra not only for the 2H spectrum of d66 phantom solution but also for the in vivo 2H MRS data. Besides the large water signal (assigned the water resonance to 4.8 ppm as a chemical shift reference), three additional well-resolved deuterated resonances (glucose at 3.8 ppm, Glx at 2.4 ppm and lactate at 1.4 ppm) were detected in the rat brain following a brief (2 min) infusion of d66. Excellent SNR and temporal resolution (15 s per spectrum) of the in vivo 2H spectra made it possible to monitor the dynamic changes of deuterated water, glucose and Glx. The dynamic information can be used to determine the values of CMRGlc and VTCA based on the kinetic model as illustrated in Fig. 4A [25]. Figure 4B-C demonstrate fittings of the kinetic model with distinct dynamic changes of deuterated glucose and Glx concentrations in two representative rat brains under deep isoflurane anesthesia (4B) and morphine analgesic conditions (4C), respectively. As a result, CMRGlc and VTCA were found to be reduced significantly in rat brains under isoflurane anesthesia (0.28±0.13 and 0.6±0.2 μmol/g/min) compared to morphine (0.46±0.06 and 0.96±0.4 μmol/g/min) treated brains [25]. This study demonstrates that the in vivo 2H MRS technique is highly sensitive to the changes of CMRGlc and VTCA under various brain conditions.
Fig. 3.
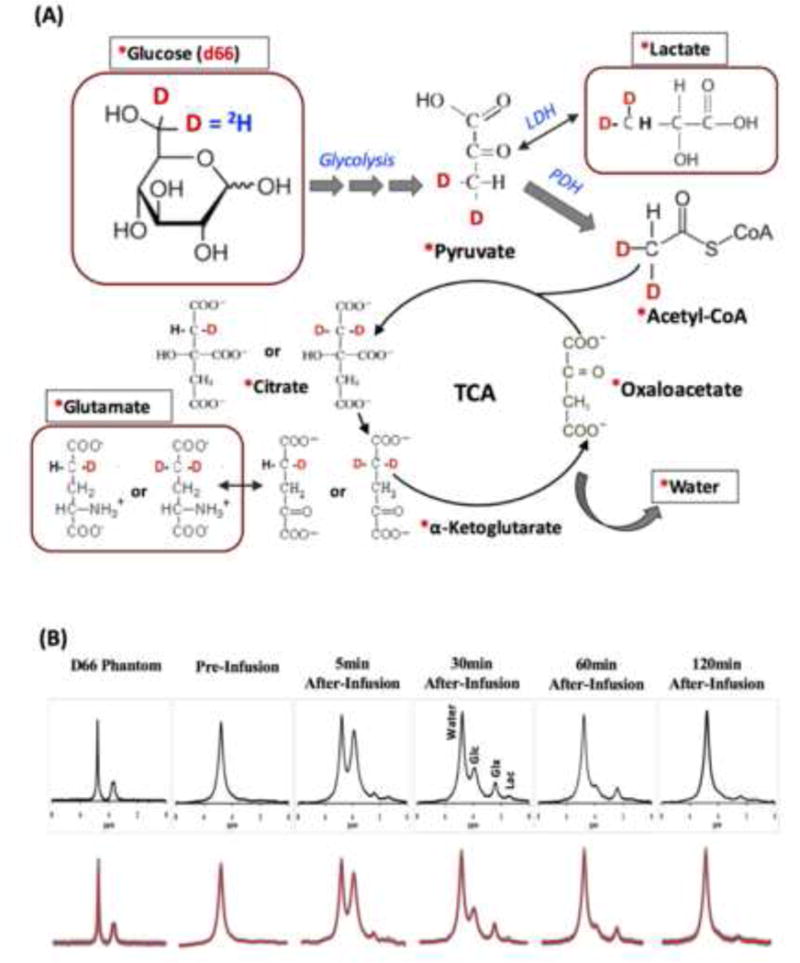
(A) The 2H-isotrope labeling (in red) scheme through the brain glucose metabolisms for in vivo dynamic 2H MRS measurement of cerebral glucose metabolisms using D-Glucose-6,6-d2 (d66) as an isotopic substrate. Labeling firstly incorporates into the pyruvate pool to form [3,3-d2] pyruvate through glycolysis, which is then converted to [3,3-d2] lactate catalyzed by lactate dehydrogenase (LDH). [3,3-d2] Pyruvate transports into mitochondria and transforms into [2,2-d2] Acetyl-CoA via pyruvate decarboxylation by pyruvate dehydrogenase (PDH). By entering the TCA cycle, intermediates of [4-d] or [4,4-d2] citrate and [4-d] or [4,4-d2] α-ketoglutarate will be produced, which could exchange with glutamate to generate [4-d] or [4,4-d2] glutamate. During the following steps in TCA cycle, 2H-labeling may depart from the cycle and exchange with the proton(s) in water molecule to become deuterated water. ‘*’: Pools labeled with 2H; outlined boxes: metabolite pools to be detected by in vivo 2H MRS. (B) Representative original (black trace in upper row and grey trace in bottom row) and fitted (red trace in bottom row) 2H spectra obtained from deuterated glucose (d66) phantom solution (left column), pre- (second left column) and post-deuterated glucose (d66) infusion at different time. The dynamic change of 2H-labeled signals can be used to perform the kinetic analysis and determine the values of CMRGlc and VTCA. 2H resonance assignments: 1) water (4.8 ppm, set as a chemical shift reference and same as the in vivo 1H MRS); 2) glucose (3.8 ppm); 3) Glx (mixed glutamate and glutamine at 2.4 ppm); and 4) lactate (1.4 ppm). Adapted from the reference of Lu et. al. [25].
Fig. 4.
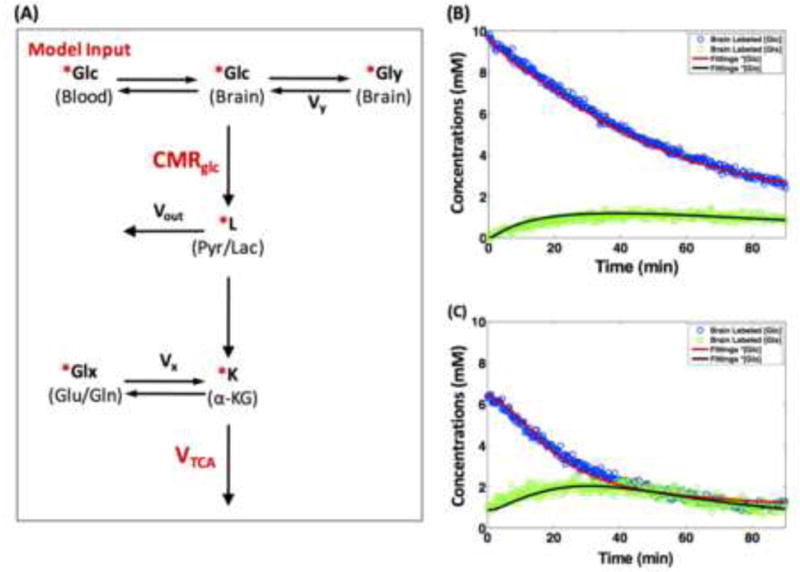
(A) Simplified kinetic modeling for dynamic 2H MRS quantification. Symbols: Glc (glucose); Gly (glycogen); L (combined pool for Pyr (pyruvate) and Lac (lactate)); K (α-KG, α-ketoglutarate); Glx (combined pool for Glu (glutamate) and Gln (glutamine)). Vx stands for the α-KG/Glx exchange rate. Vy is the glycogen synthetic rate. Vout represents an efflux of lactate. ‘*’: 2H-labeled metabolites. Dynamic changes, time courses and model fitting of deuterated brain glucose (d66) and labeled Glx concentrations during sequential 2H MRS acquisitions (15 s temporal resolution) in two representative rat brains under (B) 2% isoflurane (deep) anesthesia and (C) constant morphine sulfate infusion, respectively. Solid lines are the model fittings of labeled glucose (red) and Glx (black) signal changes which are highly sensitive to the brain state. Adapted from the reference of Lu et. al. [25].
In comparison with in vivo 13C MRS [32], in vivo 2H MRS has several merits. The quadrupolar relaxation of the 2H nuclide results in a very short T1 relaxation time (e.g., about 50 ms for the deuterated glucose in the brain at 16.4T [25]) as compared to the T1 values in the range of seconds for 13C or 1H nuclide governed by the dipole-dipole relaxation mechanism; thus, enabling rapid sampling and significantly increasing a SNR for acquiring high quality 2H MRS (see an example in Fig. 3B). The spectral patterns and chemical shift assignments (in ppm) of metabolites detected by in vivo 2H MRS are similar with those measured by in vivo 1H MRS. However, the chemical shift range (in the unit of Hz) covering most deuterated metabolites is almost 7 times lower than that of 1H MRS because of a very low 2H gyromagnetic ratio (6.54 MHz/T versus 42.58 MHz/T for 1H), this will significantly reduce the chemical shift displacement artifacts for spatial localization, especially at UHF [25, 33]. Prior to the infusion of labeled-glucose, an in vivo 2H spectrum only detects the natural abundant water signal in the brain tissue, which could serve as an internal reference for quantifying deuterated metabolites concentration; no other natural abundant metabolites are 2H NMR-detectable owing to their low intracellular concentrations and extremely low natural 2H abundance; thus, it has no background contamination. In contrast, outer-volume suppression technique is commonly required in either 13C or 1H MRS to suppress the intense lipid signals from subcutaneous fat contamination or water signal (for 1H MRS) for achieving high spectral quality for brain application. Therefore, the in vivo 2H MRSI approach could be more robust for quantitatively imaging both CMRGlc and VTCA with anticipated excellent spatial and temporal resolution for human brain application as compared to in vivo 13C MRSI, although its relatively poor spectral resolution makes it hard to resolve the 2H-labeled glutamate and glutamine, thus, posing a technical challenge for studying the neurotransmission cycling between neuron and glia cells [4, 34]. Nevertheless, the 2H MRS technique should be particularly valuable for studying the coupling relationship between brain between glycolysis (CMRGlc) and oxidative (VTCA) metabolisms under a resting or activated state, as well as their impairments associated with brain disorders, and for assessing Warburg effect in brain tumor [35].
4. In vivo 17O MRS technique for noninvasive imaging of CMRO2, CBF and OEF
The 17O MR approach had been employed to assess the cerebral blood perfusion using the 17O-labeled water as a perfusion tracer; however, the most important application of the in vivo 17O MRS imaging technique is to noninvasively imaging CMRO2 through detection of the metabolic end product of H217O water after an inhalation of 17O-labeled O2 gas [36-45] (also see review articles [17, 46, 47] and cited references therein). In this section, we discuss the method and utility of the 17O based imaging for noninvasive and simultaneous measurement of CMRO2, CBF and OEF.
In general, there are similarities between the in vivo 17O based MR imaging method and well-established PET technique [48] for imaging CMRO2. Both apply an inhalation of isotope-labeled oxygen gas: 17O2 for 17O NMR and 15O2 for PET. The inhaled O2 molecules in the lung bind to hemoglobin and are subsequently delivered to the brain cells through blood circulation and diffusion, where they are reduced by cytochrome oxidase to form the isotope-labeled H2O, which can be washed out from the cells and enter the venous system. The entire process is determined by blood perfusion/recirculation and oxygen metabolism, and the dynamic information of the isotopically labeled water provide the vital signal source for determining CMRO2 and CBF.
Although in vivo 17O MR and PET imaging techniques share a common principle in measuring CMRO2, there are also fundamental differences in methodology between these two neuroimaging modalities. PET is unable to distinguish the dissolved or hemoglobin bound 15O2 versus the metabolic product of H215O. To overcome this limitation, the standard PET measurement requires multiple procedures including: i) an inhalation of 15O2 gas (for production of H215O in the brain tissue), ii) an injection of H215O tracer (to measure CBF), and iii) an inhalation of C15O gas (to measure cerebral blood volume) [48]. These requirements substantially increase the total radioactive dose and imaging time, and complicate the CMRO2 modeling and quantification.
In contrast, in vivo 17O MR approach specifically detects only the metabolically generated H217O. 17O2 molecules as substrates, either freely dissolved in the blood or bound to hemoglobin are not visible to the 17O MR detection [17, 46]. 17O-labeled oxygen gas has two unpaired electrons; the strong interaction between the electron and magnetic moments leads to extremely fast relaxation rates and broad resonances, rendering them very difficult to be detected by conventional in vivo or liquid 17O NMR methods. Although 17O2-hemoglobn complex no longer has the unpaired electrons (it is diamagnetic), the slow molecular motion of the large hemoglobin complex leads to very broad 17O2 resonances that again preclude straightforward detection by 17O NMR. The invisibility of the 17O2 molecules is highly advantageous for simplifying the measurement and quantification of CMRO2 using the 17O MR imaging method [17, 49]. In addition, 17O is a non-radioactive and stable isotope, and is safe for human application.
The H217O signal detected in the brain during an 17O2 inhalation experiment reflects an interplay between three concurrent processes: i) oxygen consumption in the mitochondria to produce H217O, ii) the H217O washout from the brain via blood perfusion, and iii) “recirculation” which brings H217O generated in the entire body, including the brain, back to the brain through blood circulation. The contributions from all three processes must be considered for quantification according to the mass balance equation of the isotope labeled H217O in the brain tissue during an 17O2 gas inhalation [17, 41-43, 49]:
| [1] |
where Ca(t), and Cb(t) are the metabolized H217O (via oxygen consumption) concentrations in excess of the natural abundance H217O concentration in the arterial blood, and brain tissue, respectively, as a function of 17O2 inhalation time (t); α(τ) is the 17O enrichment fraction of the blood-contained 17O2; λ is the brain/blood partition coefficient; the factor of 2 in Eq. [1] accounts for the production of two H2O molecules from one O2 molecule [42, 49].
Figure 5A demonstrates the stacked plot of 17O MR spectra of brain H217O signal from one representative 17O MRSI voxel inside the rat brain that were acquired before, during and after a 2-minute inhalation of 17O2 gas at 9.4T [42]. Excellent 17O sensitivity at ultrahigh field is evident, which is essential for detecting the brain H217O signal and its dynamic change during and after the 17O2 inhalation. There are three distinct phases of brain H217O signal: baseline, inhalation and post-inhalation periods. The first phase (natural abundance H217O) signal can serve as an internal concentration reference for quantification, the second and third phases of dynamic signal changes are determined by the oxygen metabolism (the 1st term in the right side of Eq. [1]), blood perfusion in the brain tissue (the 3rd term in Eq. [1]) for washing out the labeled water and the body produced 17O-labeled water being brought into brain by blood circulation (the 2nd term in Eq. [1] associated with the blood recirculation) as governed by Eq. [1], and they can be employed to determine CMRO2 and CBF, then OEF [42, 43, 50, 51]. For small animals, such as rodents, the oxygen gas exchange between the inhaled 17O-labeled gas (with a high enrichment fraction: α) and non-labeled oxygen in the lung as well as between the lung oxygen gas and hemoglobin-bound oxygen are very rapid; and α(t) can quickly approach the level of α within 10 seconds, thus, it can be treated as a constant within the time scale and temporal resolution of 17O image for simplifying the quantification using Eq. [1] [42, 50, 51]. Interestingly, the washing- out and blood recirculation contributions were found to be similar in the rat brain, significantly reducing the net contribution of the 2nd and 3rd terms in Eq. [1], which approaches to a linear equation as the first order approximation for estimating the value of CMRO2 during the inhalation period [42, 50, 51], although a non-linear regression model could slightly improve the fitting accuracy and outcome if SNR is adequate [49]. However, the quantification using Eq. [1] could become more challenging for human brain application. One of major complications is the significantly slow lung gas exchange between the 17O labeled and non-labeled oxygen in human owing to a much large lung volume as compared to redonts. The α(t) term in Eq. [1] becomes time dependent, especially for a relatively short inhalation period (e.g., few minutes), the time variance has to be considered in the modeling and quantification, otherwise, leading to failure in model fitting [43, 52].
Fig. 5.
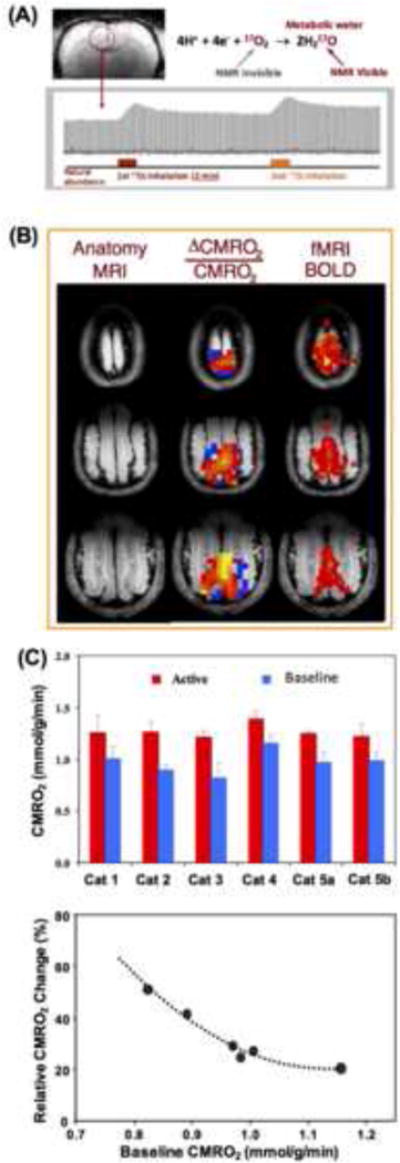
(A) Demonstration of single voxel time course of rat brain H217O signal and change taken from three-dimensional (3D) in vivo 17O MRSI measured before, during and after two inhalations of 17O2 for repeated CMRO2 and CBF imaging measurements. Adapted from the reference of Zhu et. al. [53]. (B) 3D functional CMRO2 activation maps (middle column) obtained from cat visual cortex showing relative increases of CMRO2 elevated by visual stimulation from a representative animal. The corresponding fMRI BOLD maps and anatomic brain images are also shown in the right and left column, respectively. (C) Summary (top panel) of baseline and activated CMRO2 in the visual cortex from five cats (repeated two studies from Cat 5), and the dependence between the baseline CMRO2 level and evoked CMRO2 percent change, indicating a negative correlation. Adapted from the reference of Zhu et. al. [54].
An attractive merit of the 17O-based CMRO2 imaging approach is an ability to perform multiple CMRO2 measurements during the same experimental session in a completely noninvasive manner. The metabolized H217O signal in the brain tissue can quickly reach a new steady-state level (e.g., < 10 minutes in rodent brains) after the end of 17O2 inhalation (see Fig. 5A), this allows to perform repeated CMRO2 measurements in the same subject with excellent reproducibility [53]. This capability is particularly useful for many applications aiming to study CMRO2, CBF and OEF changes induced by physiological or pathological perturbations in which multiple measurements under different conditions (e.g., control versus stimulation for studying brain function) are required. Figure 5B demonstrates functional MRI (fMRI) blood oxygenation level dependent (BOLD) signal and CMRO2 changes in the cat visual cortex in response to visual stimulation [54]. Two three-dimensional (3D) 17O CMRO2 imaging measurements, one with and the other without visual stimulation (each with 2-3 minutes of 17O2 gas inhalation) were performed at 9.4T. The multislice images of functional CMRO2 change are shown side-by-side with the conventional BOLD based fMRI maps in Fig. 5B. The CMRO2 value was found to increase significantly (~32%) in the activated visual cortical regions as depicted by the BOLD- based fMRI maps under anesthetized condition (~1% isoflurane) [54]. The evoked CMRO2 increase in response to visual stimulation in the anesthetized cat visual cortex was signficantly larger than the first reported < 10% increase of CMRO2 in response to visual stimulation in awaked human visual cortex based on the PET measurement [55]. This discrepancy could be explained by the lower baseline CMRO2 level in the cat brain under the isoflurane (1%) anesthesia, thus, having more capacity for increasing the CMRO2 response to visual stimulation. Figure 5C shows a strong dependence of task-evoked percentage increase of CMRO2 on the baseline CMRO2 level between indivisual subjects. This dependence suggests that the anticipated magnitude of functional CMRO2 increase in awaked human visual cortex could be limited within 30% owing to a higher baseline CMRO2 level [55] as compared to the cat visual cortex under anesthesia. This notion is consistent with the reports in the literature [55-59].
Figure 6 demonstrates another preclinical application of the 17O MRSI technique for simultaneously mapping three physiological parameters: CMRO2, CBF and OEF from a single 17O2 inhalation measurement, showing reduced CMRO2 and CBF and elevated OEF (= 0.58±0.11) in the brain region affected by the ischemic insult as compared to the contralaterial and intact brain tissue (= 0.45±0.04) in a preclinical mouse model of middle cerebral artery occlusion (MCAO) [50]. Interestingly, a recent PET study has reported similar OEF results (0.45±0.07 in the contralaterial and intack brain region vs. 0.48±0.08 in the ischemiclly affected brain region) in the human stroke patients with either chronic unilateral MCAO or internal carotid artery occlusion [60]. These studies indicate the feasibility for performing concurrent measurements of BOLD signal using fMRI, CMRO2, CBF and OEF using the quantitative 17O imaging, and such studies are critical to understand the neurophysiological basis of fMRI mapping.
Fig. 6. In vivo.
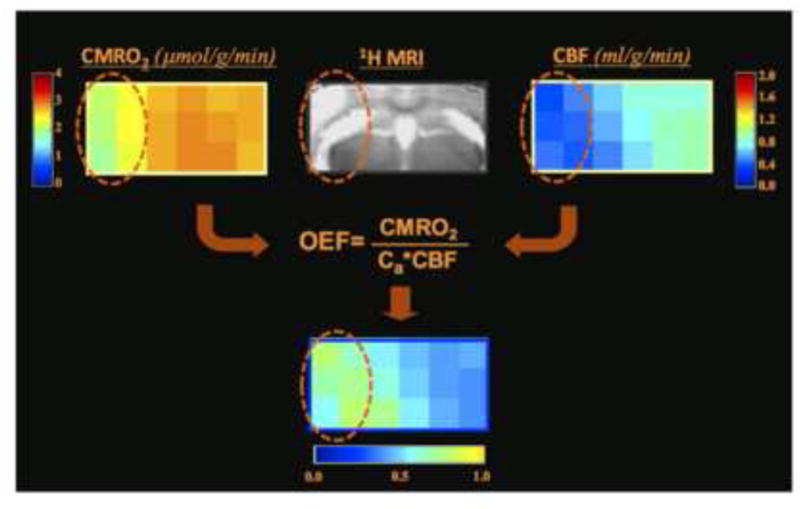
17O MRS imaging demonstration from one representative MCAO ischemic mouse, showing multiple images of CMRO2, CBF and OEF from a selected brain slice. The images clearly demonstrate significant reductions of CMRO2 and CBF in the brain region (cycled) impaired by MCAO as compared to the intact brain tissue in the contralateral hemisphere; in contrast, OEF was increased in the MCAO affected brain region. Adapted from the reference of Zhu et. al. [50].
5. In vivo 31P MRS technique for studying brain ATP energy metabolism and fluxes
In vivo 31P MRS provides an alternative and powerful imaging tool for investigating brain metabolism and bioenergetics without the use of isotope-labeled substrate. As illustrated in Fig. 2B, in vivo 31P MRS detects a number of high-energy phosphate (HEP) compounds (i.e., ATP and PCr) that play a central role in cellular energetics and other phosphate compounds that involve in phospholipid metabolisms; it also provides important physiological parameters such as intracellular pH and free Mg2+ ion concentration in the brain [5, 9, 11, 15, 61, 62]. Furthermore, 31P MRS can provide kinetic information and metabolic fluxes on two crucial chemical reactions associated with the ATP metabolism, namely the reactions that catalyzed by F1F0-ATPase to produce ATP from ADP and Pi in the mitochondria, and catalyzed by CK to form ATP from PCr and ADP. These reactions are coupled together and mediate a three-spin-site 31P chemical exchange system involving the terminal phosphate of ATP (i.e., γ-ATP), PCr, and Pi (i.e., PCr↔γ-ATP↔Pi). The metabolic fluxes involved in this three-spin exchange system can be assessed using in vivo 31P MRS in combination with magnetization transfer (MT) preparation (31P MRS-MT) [8, 9, 63-65].
Figure 7A demonstrates the in vivo 31P spectra acquired from the human visual cortex in the absence (control) and presence (saturated) of γ-ATP saturation using a frequency selected RF pulse train under near fully relaxed acquisition condition, resulting in signal reductions of PCr and Pi. Under this circumstance, the “forward” rate constants and unidirectional fluxes for the CK reaction (i.e., PCr→ATP) and ATPase reaction (i.e. Pi→ATP) can be explicitly determined [8, 9]. The forward metabolic fluxes reflect the cerebral metabolic rates of ATP production catalyzed by CK (CMRCK) and ATPase (CMRATP), respectively. Recently, several quantification methods have been reported to allow efficient acquisition of in vivo 31P MRS-MT in brain or heart with optimal SNR and reliable quantification [66-68].
Fig. 7.
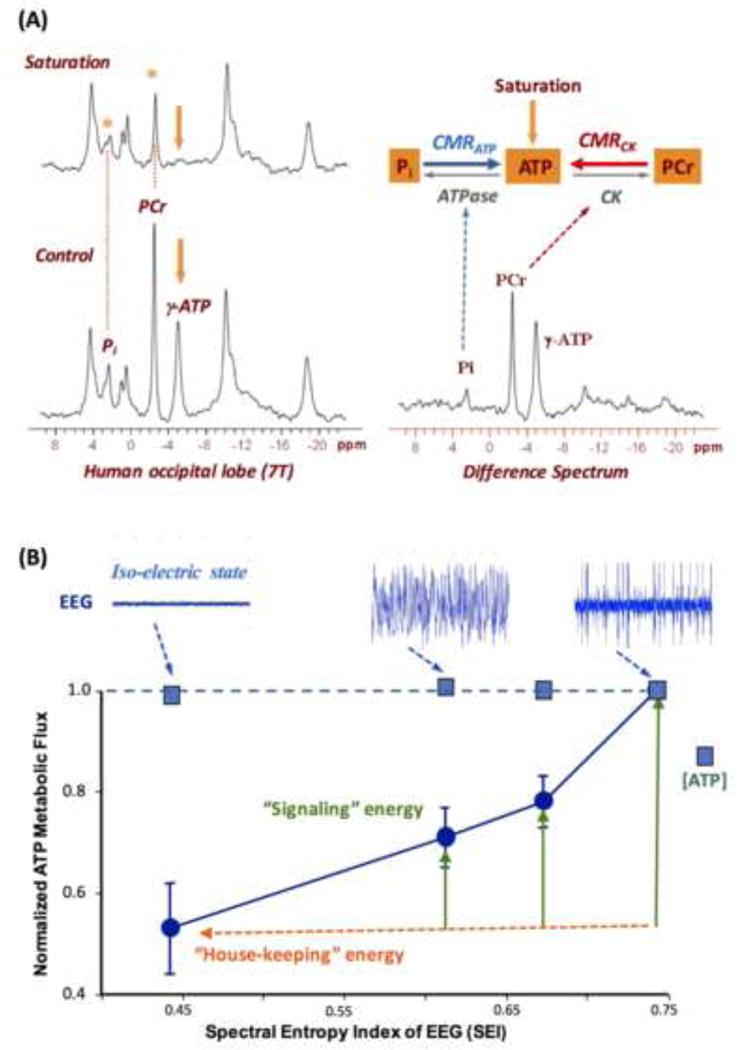
(A) In vivo 31P spectra (left panels) acquired from a healthy human occipital lobe in the absence (control) and presence of γ-ATP resonance saturation, and the difference spectrum (right panel). The intensity reduction in Pi and PCr resonance can be used to calculate the value of CMRATP and CMRCK, respectively. Adapted from the reference of Lei et. al. [8]. (B) Correlation of the rat brain EEG activity level (top tracers) versus normalized CMRATP and cerebral ATP concentration measured under varied brain states using different anesthetics and/or doses. The EEG signal was quantified by the spectral entropy index (SEI). It shows a strong and positive correlation between the EEG amplitude and CMRATP while maintaining the ATP homeostasis across a wide range of neurophysiology condition. Adapted from the reference of Du et. al. [5].
Figure 7B demonstrates one example of using the in vivo 31P MRS-MT method to quantitatively investigate the correlation between the neuronal activity level quantified by electroencephalogram (EEG) and CMRATP across a large range of anesthetic conditions [5]. Several conclusions can be drawn from Fig. 7B. First, there is a strong coupling between the spontaneous brain EEG activity and CMRATP as well as CMRCK [5]. Second, at the iso-electric state when EEG became completely silent, the brain still consumed a significant amount of ATP as the “house-keeping” energy for maintaining the cellular integrity. Third, the cerebral ATP concentration remained constant across all anesthesia conditions despite a ~50% change of CMRATP across a large range of neuronal activities (see Fig. 7B). These findings indicate that the intracellular ATP content in brain is maintained constant under physiological conditions over a large range of ATP turnover rates owing to very effective metabolic regulation for maintaining the ATP homeostasis, thus, CMRATP should be a better measure than the cellular ATP level for assessing brain energetic state and its change under various brain conditions [5].
By taking the advantages of superior sensitivity and improved spectral resolution at ultrahigh field of 7T, in vivo 3D 31P MRS-MT imaging approach covering the entire human brain (as illustrated by Fig. 8) has been employed to assessing and differentiating CMRATP and CMRCK between the human grey matter (GM) and white matter (WM), showing that both CMRATP and CMRCK are approximately three times higher in GM than WM [11]. Moreover, the same study also reveals for the first time that a single neuron in the human cortex under the resting condition could consume ~4.7 billion ATP molecules per second in average [11].
Fig. 8.
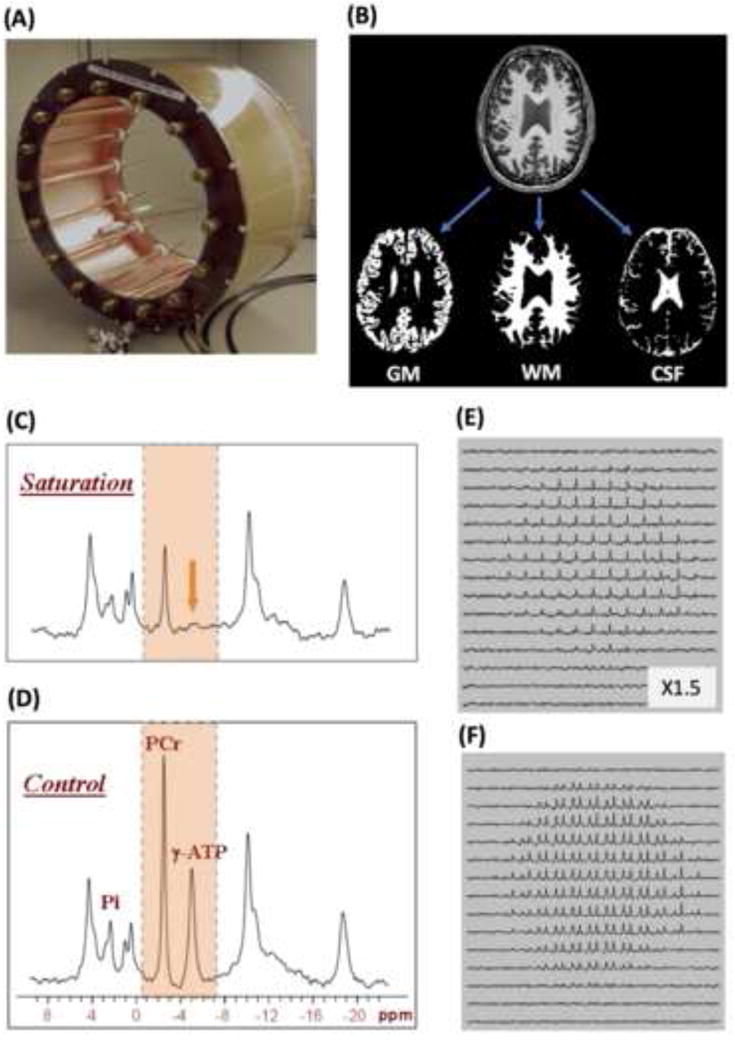
(A) Photo of 31P-1H dual-frequency transverse electromagnetic (TEM) RF head coil allowing concurrent acquisition of brain anatomic images for performing tissue segmentation (B), resulting in separated GM, WM and CSF images, and the in vivo 31P MRS-MT in the presence (C) and absence (D) of γ-ATP resonance saturation, or 3D 31P MRS-MT imaging in the presence (E) and absence (F) of γ-ATP resonance saturation (only PCr and γ-ATP resonances are displayed). These images can be employed to calculate and map CMRATP and CMRCK across the entire human brain and differentiate them between GM and WM. Adapted from the reference of Zhu et. al. [11].
6. In vivo 31P MRS-based NAD assay for measuring brain NAD contents and redox state
Besides the rich information in regard to the HEP metabolism and ATP energetics, in vivo 31P MRS can also provide another important piece of information related to the intracellular NAD contents and redox state. NAD, as an important coenzyme mediating cytosolic and mitochondrial biochemical processes, plays a key role in energy metabolism and regulation in all living cells. The conversion between NADH and NAD+ occurs in all redox reactions catalyzed by NADH-dependent dehydrogenase, involving the major energy metabolic pathways of glycolysis, tricarboxylic acid cycle and electron transport chain as illustrated in Fig. 1. In addition to regulating bioenergetics and maintaining mitochondrial function, accumulating evidence has suggested that NAD+ and NADH are also involved in other biological activities [69] such as calcium homeostasis [70], antioxidation and oxidative stress, gene expression, immunological functions, aging and cell death. The NAD redox ratio RXNAD (= [NAD+]/[NADH]) directly reflects the intracellular redox state; and its change has been linked to alterations in energy metabolism under various physiopathological conditions.
Traditionally, there are two invasive approaches for assessing the intracellular NAD+ and NADH contents and RXNAD: one is based on biochemical assay [71] and another relies on the in vivo auto-fluorescence signal of the intracellular NADH but not NAD+ [72] (see Fig. 9A). We have recently developed an in vivo NAD assay based on in vivo 31P MRS approach enabling the noninvasive assessment and quantification of intracellular NAD+ and NADH contents and RXNAD in animal and human brains across a wide range of field strengths [13, 14, 24]. This new approach is based on a theoretical NMR model for descripting the NAD phosphorus resonance signals and spectral patterns at a given magnetic field strength. By least-square fitting of the model to the partially overlapped resonances of NAD+, NADH and α-ATP in an in vivo 31P spectrum, the NAD+ and NADH concentrations and the RXNAD value can be determined.
Fig. 9.
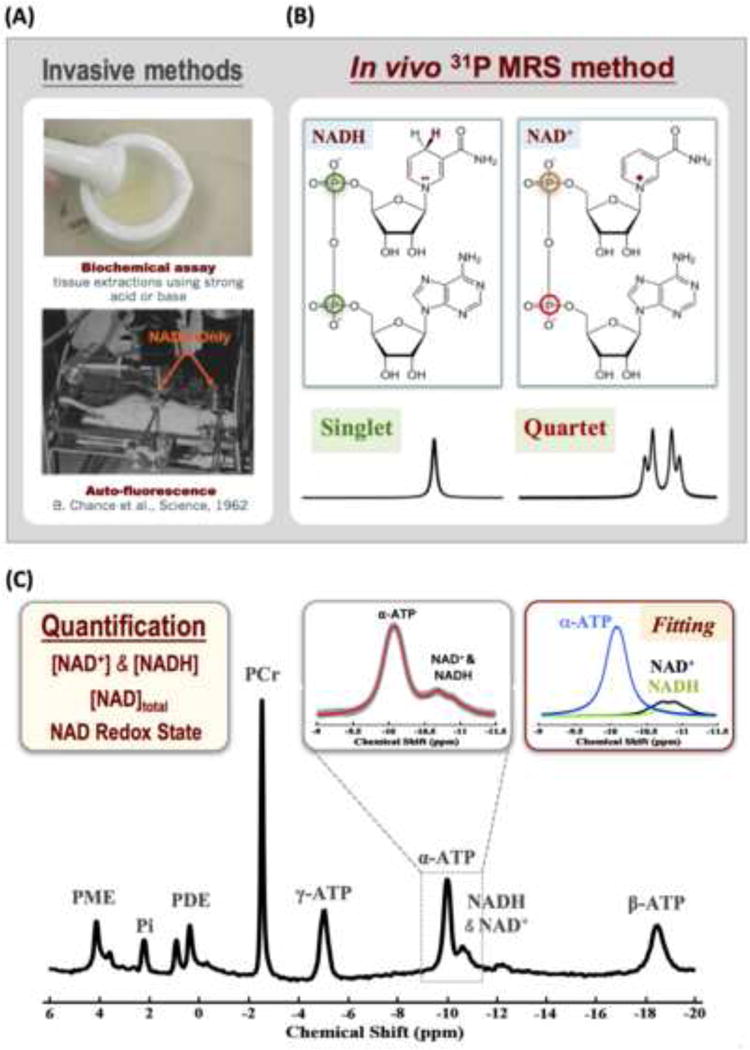
(A) Demonstration of two traditional approaches for assessment of cellular NAD metabolites based on biochemical assay (top) or auto-fluorescence technique. (B) Distinct molecular structures of NAD+ and NADH and their 31P spectral patterns (singlet resonance for NADH and quartet resonances for NAD+). The spectral pattern of the NAD+ quartet resonances depends on the field strength. (C) In vivo 31P spectrum collected from a healthy subject occipital lobe. The inserts display the expanded spectra in the chemical shift range of −9.0 to −11.5 ppm with the original in vivo 31P signals (in gray color) and the total signals (red trace) of α-ATP and NAD determined by the model fitting (center panel). The individual fitting components of α-ATP (blue), NAD+ (black) and NADH (green) for quantification are also showed (right panel). Adapted from the reference of Zhu et. al. [14].
The structure of NAD is similar to that of adenosine triphosphate (ATP), in which the γ–phosphate group on the ATP molecule is replaced by one nucleotide consisting of a ribose ring with nicotinamide attached to the carbon 1 position. The α-phosphate in ATP and the two phosphate groups in NAD share a similar chemical and electronic environment and consequently could have similar 31P spectral pattern and properties such as chemical shifts, relaxation times and resonance linewidths. The molecular structures of NAD+ and NADH are almost identical, whereas NAD+ differs from NADH by one H+ and two electrons (see Fig. 9A). Consequently, NAD+ has an aromatic nicotinamide ring with partially delocalized electrons, while NADH does not. This subtle structural difference makes the nuclear spin shielding of the two phosphorus spins in the NAD+ molecule distinct from NADH. According to the NMR theory, NAD+ is a two-spin system and its 31P spectrum exhibits a quartet consisting of four resonances (Fig. 9B) as a result of the second-order coupling effect. In contrast, the two phosphorus spins in the NADH molecule have similar local magnetic fields induced by the currents of surrounding electrons, leading to a single peak with doubled intensity. Based on the NMR theory of a two-spin system with a strong second-order coupling effect and the chemical shift information of NAD+ in water solution, the spectral pattern of the NAD+ quartet can be precisely predicted and simulated at a given field strength, thus, a quantification model for describing all signals of NAD+, NADH and α-ATP resonances at a given magnetic field strength of interest can be established for quantifying the NAD+ and NADH signals and concentrations using the α-ATP signal as an internal reference. By least-square fitting of the model outputs to the resonance signals of NAD+, NADH and α-ATP obtained from in vivo 31P spectrum, the values of [NAD+], [NADH] and RXNAD can be calculated (see more details in the reference of [13]).
Figure 9C illustrates a representative in vivo 31P spectrum acquired from the healthy human occipital lobe at 7T showing excellent SNR and spectral quality, which ensures the reliable fitting and quantification of NAD+, NADH and α-ATP resonances. We reported for the first time the intracellular concentrations of [NAD+] = 0.30±0.02 mM, [NADH] = 0.06±0.01 mM, total [NAD]total (i.e., [NAD+]+[NADH]) = 0.37±0.02 mM, and the intracellular NAD+/NADH redox ratio (RXNAD) = 4.8±0.9 in the healthy human brain (N=17) [14] (Note that the partial saturation effect was not corrected in quantifying those concentrations, thus, the actual concentrations could be slightly different).
7. Discussion and perspective
7.1 Advantage of X-nuclear MRS imaging at ultrahigh field
Despite the limited inherent MRS detection sensitivity and much lower metabolites concentration, efforts on basic research and technology development in the past decade have significantly advanced in vivo X-nuclear MRS metabolic imaging techniques and their new utilities potentially for biomedical and clinical applications. One major factor contributing to this advancement is the emerging ultrahigh field MR technology for both human (now reaching 10.5T) and animal (>20T) research. The introduction of ultrahigh-field scanners and technology not only benefit structural and functional MRI for the human brain research but also in vivo MRS, in particular, for low gyromagnetic ratio nuclide such as 2H, 13C, 17O, 23Na and 31P. Unlike the in vivo 1H MRS method that requires sophisticated and effective techniques to suppress intense water and lipid contaminations, X-nuclear MRS usually has negligible unwanted background signals especially at UHF, thus, allowing robust detection and 3D imaging of metabolite signals of interest. In addition, it often has a relatively short T1 relaxation time at UHF enabling more signal averaging. With significant sensitivity gain and improved spectral resolution at ultrahigh field, more challenging measurements such as energy metabolic rates of CMRGlc, CMRO2 and CMRATP as well as NAD redox ratio have become possible for both preclinical and human applications, which should be useful for studying cellular energy metabolisms, neuroenergetics and function at the system level under resting and working states or metabolic impairments and dysfunction under diseases conditions. Interestingly, the new developments of X-nuclear MRS imaging technology as discussed in this article could all be related to Joe’s early work thirty years ago and his publications in many scientific journals, including JMR (e.g., from a small assemble of his early publication [15, 16, 73-76]). There is no doubt that Joe’s work will still have an impact in the research field in next decades.
Although in vivo X-nuclear MRS (excluding hyperpolarized in vivo 13C approach) can never match the detection sensitivity and spatiotemporal resolution of the 1H MRI owing to the physical constrain, it provides unique information of tissue physiology and biochemistry at the molecular and cellular population levels with well-defined physiological specificity and meaning beyond the tissue water signal based MRI contrasts. Despite of the limited sensitivity and relatively poor resolution, in vivo X-nuclear MRS is highly reliable and sensitive to detect subtle changes in brain physiology at high/ultrahigh field.
One of the major criticisms we received from the reviewers of our manuscript for reporting the in vivo NAD assay for brain application [13] was that it is hard to believe that the very low concentrations of NAD+ (0.3-0.4 mM) and NADH (<0.1 mM) could be reliably measured in the rat brain in vivo, since their signals were considered below the in vivo detectability; and the determination of RXNAD as a ratio of [NAD+]/[NADH] could be even more challenging owing to the cumulated errors from the two measured concentrations. Nevertheless, the cumulative evidences proved the feasibility and reliability at UHF, showing the consistent results of [NAD+], [NADH] and RXNAD in the cat brains measured at 9.4T and 16.4T despite of a significantly different spectral pattern of NAD+ at these fields [13], and similar results of human brain at 4T and 7T [14, 24]. Strikingly, the measures of [NAD+], [NADH], [NAD]total and NAD redox potential are all age-dependent in healthy human brains as shown in Fig. 10 [14]. The decline in the brain NAD+/NADH redox potential or ratio suggests a significant shift of the glucose-oxygen metabolic balance towards slower oxygen consumption and oxidative phosphorylation in the mitochondria, potentially resulting in deficiency in the mitochondrial capacity for oxidative phosphorylation in aging brains.
Fig. 10.
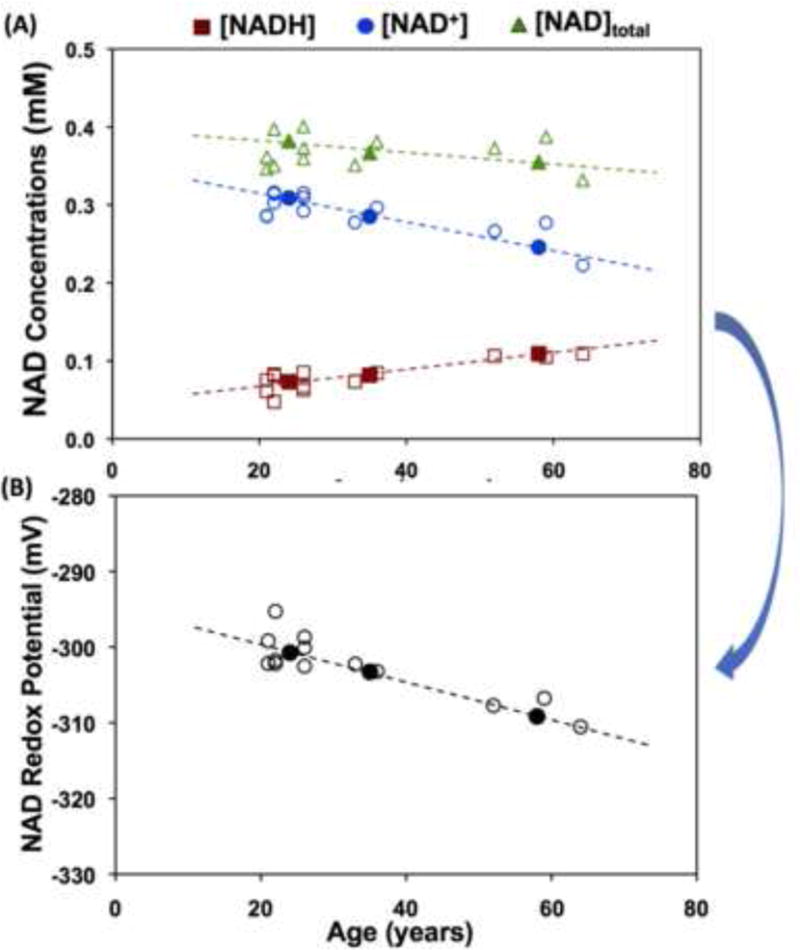
Age dependence of intracellular NAD+, NADH, total NAD concentrations (A) and NAD+/NADH redox potential (B) observed in healthy human occipital lobe. The open symbols represent individual subject data and the filled symbols display the average data from three age groups of young (21-26yr, n=7), middle (33-36yr, n=4) and old (59-68yr, n=6) subjects. Adapted from the reference of Zhu et. al. [14].
7.2 New solutions for further improving the X-nuclei MRS imaging at UHF
Despite of significant advancement in X-nuclear MRS imaging technology at UHF, its spatiotemporal resolution with the current state-of-the-art UHF technology is still largely limited for broad brain application. This is certainly true for imaging CMRATP, which relies on a small Pi signal reduction induced by the magnetization saturation of γ-ATP resonance and chemical exchange between ATP and Pi (Fig. 7A), and/or RXNAD, which depends on the reliable measurements of both [NAD+] and [NADH] with very low concentration.
Two inspiring and specific questions in regard to the future developments of X-nuclear MRS based neuroimaging technology are:
can the spatial resolution of in vivo 17O-based CMRO2-CBF-OEF or 2H-based CMRGlc-VTAC neuroimaging technique at UHF reach or pass that of 15O-based PET (i.e., ~5 mm isotropic resolution)?
can in vivo 31P MRS based neuroimaging technique reach the spatial resolution that enables the differentiation of the human GM (~3 mm thickness) and WM?
To achieve the aforementioned spatial resolution, at least two innovative and promising methods could be considered to further boost the X-nuclear MRS imaging sensitivity and spatiotemporal resolution at UHF: one relies on hardware technology and the other on software development. It has been shown recently that placing the ultrahigh dielectric constant (uHDC) ceramic former(s) between a RF coil and object could significantly enhance the displacement current in the RF coil, thus, increase both RF transmitter ( ) and receive ( ) magnetic fields, resulting in a substantial improvement of detection sensitivity at UHF [77, 78]. We have recently demonstrated the feasibility of uHDC technique for improving in vivo 31P MRS sensitivity (more than two times) and reducing the RF pulse voltage for reaching the same RF pulse flip angle (less than 50%) in the human skeletal muscle [77]. Another interesting software approach called SPectroscopic Imaging by exploiting spatiospectral CorrElation (SPICE) utilizes a unique property known as partial separability of spectroscopic signals to enable special data acquisition and image reconstruction strategies for obtaining high-resolution spatiospectral distributions [79] as well as high temporal resolution [80]. Incorporation of these novel techniques at UHF could break the current technical barriers and significantly advance the in vivo X-nuclear MRS technology for biomedical application.
7.3 Quantification and modeling
Firstly, signal processing of X-nuclear MRS data is critical for quantification. The success depends on the spectral quality that is determined by the SNR and spectral resolution of the MRS data for resolving adjacent resonances; in this regard, UHF should be highly advantageous. Moreover, the spectral fitting requires sophisticated algorisms and models accounting for the fine spectral patterns, for instance, 31P homonuclear coupling, quartet resonance pattern of NAD+ and its field dependence [13].
Secondly, quantifying the metabolites concentration based on in vivo X-nuclear MRS measurement is critical for biomedical application. For 17O and 2H MRS, this is quite simple and robust since the natural abundance signal of brain tissue water (H217O for 17O MRS, and 2H216O for 2H MRS) detected before the introduction of isotope substrate (17O2 for 17O, and d66 for 2H) can serve as an internal reference for calibrating and quantifying the isotope-labeled metabolites and their dynamic changes. However, for in vivo 31P MRS, additional procedure, for instance, a reference phantom with known concentration is needed, and RF pulse flip angle profile, metabolite T1 values and acquisition repetition time need to be considered for correcting the partial saturation effect caused by the short acquisition repetition time (a common practice for gaining a SNR) for quantifying the metabolites concentration.
Thirdly, it is necessary to develop a realistic model for determining physiology parameters of interest such as metabolic fluxes. The information provided by in vivo X-nuclear MRS data is often limited in dealing with complex metabolic pathways. It is challenging to build a sophisticated model for describing a biological system with limited measurements and information. One example is in regards how to quantify CMRO2 based on the dynamic signal of brain tissue H217O measured by 17O imaging before, after and after an inhalation of 17O2 gas, and the quantification can become more challenging for human brain application [43, 52] as compared to rodents [42, 50] owing to the large human body size and slow lung air exchange between the non-labeled and labeled oxygen gases. Significant progress has been made to establish a CMRO2 quantification method in human brain [43, 52], and shown the feasibility for noninvasively imaging and quantifying CMRO2, CBF and OEF from a single measurement with few minutes of inhalation time [81].
In regard to the 2H MRS imaging technique, it is still unclear how to utilize the information of the deuterated water signal metabolically produced in the brain after the d66 infusion for quantifying meaningful metabolic rates. The dynamic deuterated water signal has the best SNR detected from the in vivo 2H MRS from the brain, however, the dilution of the deuterated water during multiple steps of metabolic pathways makes the modeling and quantification difficult. More research efforts are necessary to understand and model the dynamics of deuterated water signal.
7.4 Ultrahigh field versus high field, brain versus other organs
Recently, FDA has approved 7T MRI scanners for clinical application and brain diagnosis. This opens opportunities potentially for translation of the advanced UHF X-nuclear MRS based neuroenergetic imaging techniques for diagnosis and monitoring treatment efficacy. On the other hand, the techniques described herein should not be limited only to UHF application; they could be employed to relatively lower field strength. The in vivo 17O neuroimaging technique has proven successful not only at field strengths ≥ 7T [43, 52, 82] but has also shown promise at 3T [83] or feasibility at 1.5T [44, 45] for human brain application with decent spatial and temporal imaging resolution, indicating a potential for translation and clinical diagnosis, for instance, for brain tumors [84].
The in vivo 31P NAD assay has been applied to measure the intracellular [NAD+], [NADH] and RXNAD in human brain at 4T [24, 85] and 7T [14]. Incorporation of 1H decoupling with the in vivo 31P NAD assay at 4T could significantly improve the spectral resolution and SNR of NAD+, achieving comparable performance as 7T, which has yielded identical result of RXNAD (5.3 ± 0.4, N = 7, age: 23 ± 4 years) as that measured at 7T for the same age group (5.4 ± 0.8, N = 7, age 23 ± 2 years) [14, 24].
Although this review article focuses on the technology development for brain research; the same techniques based on 2H, 17O and 31P MRS imaging should be applicable for other organs such as heart and muscle, though with some precaution about interpretation in considering distinct types of cells, physiology and function between brain and other organs. For example, the in vivo 31P MRS-MT method is highly valuable to quantitatively measure CMRATP which reflects the oxidative phosphorylation of ADP for producing ATP molecules in the brain mitochondria. The measured CMRATP values across varied physiology conditions or different species were found to equal to the net rate of oxidative ATP synthesis calculated as the oxygen consumption rate multiplied by the P:O ratio [86]. However, it has been suggested that conventional 31P MRS-MT measurements of Pi→ATP rate in skeletal and cardiac muscle as well as liver might not yield a quantitative estimation of the oxidative ATP synthesis rate, thus, complicate the physiological interpretation between the measured Pi→ATP rate and mitochondrial functionality for producing ATP via the oxidative ATP synthesis [87]. This indicates the complexity between the in vivo 31P MRS-MT measurement and underlying physiology in other organs beyond the brain and it deserves more research efforts to understand why they are different from the brain in depth.
Ultrahigh field significantly improves in vivo MRS sensitivity and spectral resolution
In vivo 17O MRS for simultaneous imaging of CMRO2, CBF and OEF
In vivo 2H MRS for simultaneous measurement of CMRGlc and TCA cycle rate
In vivo 31P MRS for simultaneous measurement of CMRATP and CMRCK
In vivo 31P MRS for simultaneous measurement of NAD+, NADH and redox state ratio
Acknowledgments
The authors thank Drs. Byeong-Yeul Lee, Fei Du, Nanyin Zhang, Xiaoliang Zhang, Hao Lei, Gregor Adriany, Kamil Ugurbil, Yi Zhang and Mr. Hannes Wiesner, for their support, technical assistance and contribution to the development of the in vivo MRS imaging technologies described in this article. The work as reviewed in this article was partly supported by NIH grants of R01 NS057560, NS070839 and MH111413, R24 MH106049, P41 EB015894, P30 NS5076408; the W.M. Keck Foundation.
Statement
All research involving animals has received the ICUCA approval from the University of Minnesota, and all research involving human has received the IRB approval from the University of Minnesota.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siesjo BK. Brain energy metabolism. Wiley; New York: 1978. [Google Scholar]
- 2.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhu XH, Du F, Zhang N, Lu M, Zhang Y, Liu X, Lei H, Zhang XL, Ugurbil K, Chen W. Study of Brain Bioenergetics and Function using In Vivo MRS. In: Kamil Ugurbil KU, Berliner Lawrence, editors. Biological magnetic Resonance: fMRI: From Nuclear Spins to Brain Function. Springer; New York: 2015. pp. 819–864. [Google Scholar]
- 4.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 5.Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shulman RG, Rothman DL, Hyder F. Stimulated changes in localized cerebral energy consumption under anesthesia. Proc Natl Acad Sci USA. 1999;96:3245–3250. doi: 10.1073/pnas.96.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemp GJ. Non-invasive methods for studying brain energy metabolism: what they show and what it means. Dev Neurosci. 2000;22:418–428. doi: 10.1159/000017471. [DOI] [PubMed] [Google Scholar]
- 8.Lei H, Ugurbil K, Chen W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 Tesla using in vivo 31P magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2003;100:14409–14414. doi: 10.1073/pnas.2332656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57:103–114. doi: 10.1002/mrm.21107. [DOI] [PubMed] [Google Scholar]
- 10.Raichle ME. Circulatory and metabolic correlates of brain function in normal humans. In: Mountcastle VB, Plum F, Geiger SR, editors. Handbook of Physiology-The Nervous System. American Physiological Society; Bethesda: 1987. pp. 643–674. [Google Scholar]
- 11.Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, Ugurbil K, Chen W. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60:2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- 13.Lu M, Zhu XH, Zhang Y, Chen W. Intracellular redox state revealed by in vivo 31P MRS measurement of NAD+ and NADH contents in brains. Magn Reson Med. 2014;71:1959–1972. doi: 10.1002/mrm.24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackerman JJH, Grove TH, Wong GG, Gadian DG, Radda GK. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980;283:167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- 16.Neil JJ, Song SK, Ackerman JJ. Concurrent quantification of tissue metabolism and blood flow via 2H/31P NMR in vivo. II. Validation of the deuterium NMR washout method for measuring organ perfusion. Magn Reson Med. 1992;25:56–66. doi: 10.1002/mrm.1910250106. [DOI] [PubMed] [Google Scholar]
- 17.Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo 17O NMR approaches for brain study at high field. NMR Biomed. 2005;18:83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XH, Merkle H, Kwag JH, Ugurbil K, Chen W. 17O relaxation time and NMR sensitivity of cerebral water and their field dependence. Magn Reson Med. 2001;45:543–549. doi: 10.1002/mrm.1073. [DOI] [PubMed] [Google Scholar]
- 19.Lu M, Zhang Y, Ugurbil K, Chen W, Zhu XH. In vitro and in vivo studies of 17O NMR sensitivity at 9.4 and 16.4 T. Magn Reson Med. 2013;69:1523–1527. doi: 10.1002/mrm.24386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46:24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- 21.Shulman RG, Brown TR, Ugurbil K, Ogawa S, Cohen SM, den Hollander JA. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979;205:160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- 22.Lu M, Chen W, Zhu XH. Field dependence study of in vivo brain 31P MRS up to 16.4 T. NMR Biomed. 2014;27:1135–1141. doi: 10.1002/nbm.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao H, Zhang X, Zhu XH, Du F, Chen W. In vivo 31P MRS of human brain at high/ultrahigh fields: a quantitative comparison of NMR detection sensitivity and spectral resolution between 4 T and 7 T. Magn Reson Imaging. 2006;24:1281–1286. doi: 10.1016/j.mri.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu M, Zhu XH, Chen W. In vivo 31P MRS assessment of intracellular NAD metabolites and NAD+/NADH redox state in human brain at 4 T. NMR Biomed. 2016;29:1010–1017. doi: 10.1002/nbm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu M, Zhu XH, Zhang Y, Mateescu G, Chen W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2017;37:3518–3530. doi: 10.1177/0271678X17706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones JG, Solomon MA, Cole SM, Sherry AD, Malloy CR. An integrated 2H and 13C NMR study of gluconeogenesis and TCA cycle flux in humans. Am J Physiol Endocrinol Metab. 2001;281:E848–856. doi: 10.1152/ajpendo.2001.281.4.E848. [DOI] [PubMed] [Google Scholar]
- 27.Weis BC, Margolis D, Burgess SC, Merritt ME, Wise H, Sherry AD, Malloy CR. Glucose production pathways by 2H and 13C NMR in patients with HIV-associated lipoatrophy. Magn Reson Med. 2004;51:649–654. doi: 10.1002/mrm.20057. [DOI] [PubMed] [Google Scholar]
- 28.Chandramouli V, Ekberg K, Schumann WC, Kalhan SC, Wahren J, Landau BR. Quantifying gluconeogenesis during fasting. Am J Physiol. 1997;273:E1209–1215. doi: 10.1152/ajpendo.1997.273.6.E1209. [DOI] [PubMed] [Google Scholar]
- 29.Kunert O, Stingl H, Rosian E, Krssak M, Bernroider E, Seebacher W, Zangger K, Staehr P, Chandramouli V, Landau BR, Nowotny P, Waldhausl W, Haslinger E, Roden M. Measurement of fractional whole-body gluconeogenesis in humans from blood samples using 2H nuclear magnetic resonance spectroscopy. Diabetes. 2003;52:2475–2482. doi: 10.2337/diabetes.52.10.2475. [DOI] [PubMed] [Google Scholar]
- 30.Evelhoch JL, McCoy CL, Giri BP. A method for direct in vivo measurement of drug concentrations from a single 2H NMR spectrum. Magn Reson Med. 1989;9:402–410. doi: 10.1002/mrm.1910090313. [DOI] [PubMed] [Google Scholar]
- 31.Mateescu GD, Ye A, Flask CA, Erokwu B, Duerk JL. In vivo assessment of oxygen consumption via Deuterium Magnetic Resonance. Adv Exp Med Biol. 2011;701:193–199. doi: 10.1007/978-1-4419-7756-4_26. [DOI] [PubMed] [Google Scholar]
- 32.Gruetter R, Adriany G, Choi IY, Henry PG, Lei HX, Oz GL. Localized in vivo C-13 NMR spectroscopy of the brain. Nmr in Biomedicine. 2003;16:313–338. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W, Zhu XH. Dynamic study of cerebral bioenergetics and brain function using in vivo multinuclear MRS approaches. Concepts in Magnetic Resonance PartA. 2005;27A:84–121. [Google Scholar]
- 34.Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG. In vivo 13C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during. J Neurochem. 2001;76:975–989. doi: 10.1046/j.1471-4159.2001.00074.x. [DOI] [PubMed] [Google Scholar]
- 35.Lu M, Zhu XH, Zhang Y, Low W, Chen W. Proc Intl Soc Mag Reson Med. Singapore: 2016. Simultaneous Assessment of Abnormal Glycolysis and Oxidative Metabolisms in Brain Tumor using In Vivo Deuterium (2H) MRS Imaging; p. 3962. [Google Scholar]
- 36.Mateescu GD, Yvars G, Pazara DI, Alldridge NA, LaManna JC, Lust DW, Mattingly M, Kuhn W. 17O-1H Magnetic Resonance Imaging in Plants, Animals, and Materials. In: Baillie TA, Jones JR, editors. Synthesis and Application of Isotopically Labeled Compounds. Elsevier; Amsterdam: 1989. pp. 499–508. [Google Scholar]
- 37.Mateescu GD, Yvars GM, Dular T. Water, Ions and O-17 Magnetic Resonance Imaging. In: Lauger P, Packer L, Vasilescu V, editors. Water and Ions in Biological Systems. Birkhauser Verlag; Basel-Boston-Berlin: 1988. pp. 239–250. [Google Scholar]
- 38.Ronen I, Merkle H, Ugurbil K, Navon G. Imaging of H217O distribution in the brain of a live rat by using proton-detected 17O MRI. Proc Natl Acad Sci U S A. 1998;95:12934–12939. doi: 10.1073/pnas.95.22.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arai T, Nakao S, Morikawa S, Inubushi T, Yokoi T, Shimizu K, Mori K. Measurement of local cerebral blood flow by magnetic resonance imaging: in vivo autoradiographic strategy using 17O-labeled water. Brain Res Bull. 1998;45:451–456. doi: 10.1016/s0361-9230(97)00369-9. [DOI] [PubMed] [Google Scholar]
- 40.Reddy R, Stolpen AH, Charagundla SR, Insko EK, Leigh JS. 17O-decoupled 1H detection using a double-tuned coil. Magn Reson Imaging. 1996;14:1073–1078. doi: 10.1016/s0730-725x(96)00227-5. [DOI] [PubMed] [Google Scholar]
- 41.Pekar J, Ligeti L, Ruttner Z, Lyon RC, Sinnwell TM, van Gelderen P, Fiat D, Moonen CT, McLaughlin AC. In vivo measurement of cerebral oxygen consumption and blood flow using 17O magnetic resonance imaging. Magn Reson Med. 1991;21:313–319. doi: 10.1002/mrm.1910210217. [DOI] [PubMed] [Google Scholar]
- 42.Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of 17O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci U S A. 2002;99:13194–13199. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson IC, Thulborn KR. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. Neuroimage. 2010;51:723–733. doi: 10.1016/j.neuroimage.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 44.Fiat D, Dolinsek J, Hankiewicz J, Dujovny M, Ausman J. Determination of regional cerebral oxygen consumption in the human: 17O natural abundance cerebral magnetic resonance imaging and spectroscopy in a whole body system. Neurol Res. 1993;15:237–248. doi: 10.1080/01616412.1993.11740143. [DOI] [PubMed] [Google Scholar]
- 45.Fiat D, Hankiewicz J, Liu S, Trbovic S, Brint S. 17O magnetic resonance imaging of the human brain. Neurol Res. 2004;26:803–808. doi: 10.1179/016164104X5156. [DOI] [PubMed] [Google Scholar]
- 46.Zhu XH, Chen W. In vivo oxygen-17 NMR for imaging brain oxygen metabolism at high field. Prog Nucl Magn Reson Spectrosc. 2011;59:319–335. doi: 10.1016/j.pnmrs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu XH, Chen W. In vivo 17O MRS imaging - Quantitative assessment of regional oxygen consumption and perfusion rates in living brain. Anal Biochem. 2016 doi: 10.1016/j.ab.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- 49.Zhang N, Zhu XH, Lei H, Ugurbil K, Chen W. Simplified methods for calculating cerebral metabolic rate of oxygen based on 17O magnetic resonance spectroscopic imaging measurement during a short 17O2 inhalation. J Cereb Blood Flow Metab. 2004;24:840–848. doi: 10.1097/01.WCB.0000125885.54676.82. [DOI] [PubMed] [Google Scholar]
- 50.Zhu XH, Chen JM, Tu TW, Chen W, Song SK. Simultaneous and noninvasive imaging of cerebral oxygen metabolic rate, blood flow and oxygen extraction fraction in stroke mice. Neuroimage. 2013;64:437–447. doi: 10.1016/j.neuroimage.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu XH, Zhang Y, Wiesner HM, Ugurbil K, Chen W. In vivo measurement of CBF using 17O NMR signal of metabolically produced H217O as a perfusion tracer. Magn Reson Med. 2013;70:309–314. doi: 10.1002/mrm.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu XH, Liu X, Lu M, Wiesner HM, Ugurbil K, Chen W. Proc Intl Soc Mag Reson Med. Milan, Italy: 2014. In Vivo 17O MR Imaging and Quantification of CMRO2, CBF and OEF in Human Visual Cortex at Rest and during Activation; p. 3763. [Google Scholar]
- 53.Zhu XH, Zhang Y, Zhang N, Ugurbil K, Chen W. Noninvasive and three-dimensional imaging of CMRO2 in rats at 9.4 T: reproducibility test and normothermia/hypothermia comparison study. J Cereb Blood Flow Metab. 2007;27:1225–1234. doi: 10.1038/sj.jcbfm.9600421. [DOI] [PubMed] [Google Scholar]
- 54.Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 56.Vafaee MS, Meyer E, Marrett S, Paus T, Evans AC, Gjedde A. Frequency-dependent changes in cerebral metabolic rate of oxygen during activation of human visual cortex. J Cereb Blood Flow Metab. 1999;19:272–277. doi: 10.1097/00004647-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med. 1999;41:1152–1161. doi: 10.1002/(sici)1522-2594(199906)41:6<1152::aid-mrm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 58.Chen W, Zhu XH, Gruetter R, Seaquist ER, Adriany G, Ugurbil K. Study of tricarboxylic acid cycle flux changes in human visual cortex during hemifield visual stimulation using 1H-[13C] MRS and fMRI. Magn Reson Med. 2001;45:349–355. doi: 10.1002/1522-2594(200103)45:3<349::aid-mrm1045>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 59.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kudo K, Liu T, Murakami T, Goodwin J, Uwano I, Yamashita F, Higuchi S, Wang Y, Ogasawara K, Ogawa A, Sasaki M. Oxygen extraction fraction measurement using quantitative susceptibility mapping: Comparison with positron emission tomography. J Cereb Blood Flow Metab. 2016;36:1424–1433. doi: 10.1177/0271678X15606713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hetherington HP, Pan JW, Spencer DD. 1H and 31P spectroscopy and bioenergetics in the lateralization of seizures in temporal lobe epilepsy. J Magn Reson Imaging. 2002;16:477–483. doi: 10.1002/jmri.10177. [DOI] [PubMed] [Google Scholar]
- 62.Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn Reson Med. 2003;49:199–205. doi: 10.1002/mrm.10379. [DOI] [PubMed] [Google Scholar]
- 63.Frosen S, Hoffman RA. Study of moderately rapid chemical exchange by means of nuclear magnetic double resonance. J Chem Phys. 1963;39:2892–2901. [Google Scholar]
- 64.Shoubridge EA, Briggs RW, Radda GK. 31P NMR saturation transfer measurements of the steady state rates of creatine kinase and ATP synthetase in the rat brain. FEBS Lett. 1982;140:289–292. doi: 10.1016/0014-5793(82)80916-2. [DOI] [PubMed] [Google Scholar]
- 65.Ugurbil K. Magnetization Transfer Measurements of Individual Rate Constants in the Presence of Multiple Reactions. J Magn Reson. 1985;64:207–219. [Google Scholar]
- 66.Xiong Q, Du F, Zhu XH, Zhang P, Suntharalingam P, Ippolito J, Kamdar FD, Chen W, Zhang J. ATP production rate via creatine kinase or ATP synthase in vivo: a novel superfast magnetization saturation transfer method. Circ Res. 2011;108:653–663. doi: 10.1161/CIRCRESAHA.110.231456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiong Q, Li Q, Mansoor A, Jameel MN, Du F, Chen W, Zhang J. Novel strategy for measuring creatine kinase reaction rate in the in vivo heart. Am J Physiol Heart Circ Physiol. 2009;297:H1010–1019. doi: 10.1152/ajpheart.01195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SY, Chen W, Ongur D, Du F. Rapid and simultaneous measurement of phosphorus metabolite pool size ratio and reaction kinetics of enzymes in vivo. J Magn Reson Imaging. 2018;47:210–221. doi: 10.1002/jmri.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollak N, Dolle C, Ziegler M. The power to reduce: pyridine nucleotides - small molecules with a multitude of functions. Biochemical Journal. 2007;402:205–218. doi: 10.1042/BJ20061638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee HC. Multiplicity of Ca2+ messengers and Ca2+ stores: A perspective from cyclic ADP-ribose and NAADP. Current Molecular Medicine. 2004;4:227–237. doi: 10.2174/1566524043360753. [DOI] [PubMed] [Google Scholar]
- 71.Ying WH. NAD+ and NADH in brain functions, brain diseases and brain aging. Frontiers in Bioscience. 2007;12:1863–1888. doi: 10.2741/2194. [DOI] [PubMed] [Google Scholar]
- 72.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo: The microfluorometry of pyridine nucleotide gives a continuous measurement of the oxidation state. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- 73.Evelhoch JL, Ewy CS, Siegfried BA, Ackerman JJ, Rice DW, Briggs RW. 31P spin-lattice relaxation times and resonance linewidths of rat tissue in vivo: dependence upon the static magnetic field strength. Magn Reson Med. 1985;2:410–417. doi: 10.1002/mrm.1910020409. [DOI] [PubMed] [Google Scholar]
- 74.Evelhoch JL, Sapareto SA, Nussbaum GH, Ackerman JJ. Correlations between 31P NMR spectroscopy and 15O perfusion measurements in the RIF-1 murine tumor in vivo. Radiat Res. 1986;106:122–131. [PubMed] [Google Scholar]
- 75.Kim SG, Ackerman JJ. Quantitative determination of tumor blood flow and perfusion via deuterium nuclear magnetic resonance spectroscopy in mice. Cancer Res. 1988;48:3449–3453. [PubMed] [Google Scholar]
- 76.Reo N, Ewy CS, Siegfried BA, Ackerman JJ. High-field 13C NMR spectroscopy of tissue in vivo. A double-resonance surface-coil probe. Radiat Res. 1984;58:76–84. [Google Scholar]
- 77.Lee BY, Zhu XH, Rupprecht S, Lanagan MT, Yang QX, Chen W. Large improvement of RF transmission efficiency and reception sensitivity for human in vivo 31P MRS imaging using ultrahigh dielectric constant materials at 7T. Magn Reson Imaging. 2017;42:158–163. doi: 10.1016/j.mri.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rupprecht S, Sica CT, Chen W, Lanagan MT, Yang QX. Improvements of transmit efficiency and receive sensitivity with ultrahigh dielectric constant (uHDC) ceramics at 1.5 T and 3 T. Magn Reson Med. 2017 doi: 10.1002/mrm.26943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lam F, Liang ZP. A subspace approach to high-resolution spectroscopic imaging. Magn Reson Med. 2014;71:1349–1357. doi: 10.1002/mrm.25168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma C, Clifford B, Liu Y, Gu Y, Lam F, Yu X, Liang ZP. High-resolution dynamic 31P-MRSI using a low-rank tensor model. Magn Reson Med. 2017;78:419–428. doi: 10.1002/mrm.26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu XH, Wiesner HM, Lee BY, Lu M, Chen W. Proc Intl Soc Mag Reson Med. Toronto, Canada: 2015. Quantitative and Simultaneous Imaging of CMRO2, CBF and OEF in Resting Human Brain; p. 895. [Google Scholar]
- 82.Niesporek SC, Umathum R, Lommen JM, Behl NGR, Paech D, Bachert P, Ladd ME, Nagel AM. Reproducibility of CMRO2 determination using dynamic 17O MRI. Magn Reson Med. 2017 doi: 10.1002/mrm.26952. [DOI] [PubMed] [Google Scholar]
- 83.Kurzhunov D, Borowiak R, Reisert M, Joachim Krafft A, Caglar Ozen A, Bock M. 3D CMRO2 mapping in human brain with direct 17O MRI: Comparison of conventional and proton-constrained reconstructions. Neuroimage. 2017;155:612–624. doi: 10.1016/j.neuroimage.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 84.Hoffmann SH, Radbruch A, Bock M, Semmler W, Nagel AM. Direct 17O MRI with partial volume correction: first experiences in a glioblastoma patient. MAGMA. 2014;27:579–587. doi: 10.1007/s10334-014-0441-8. [DOI] [PubMed] [Google Scholar]
- 85.Kim SY, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, Li T, Du F, Ongur D. Redox Dysregulation in Schizophrenia Revealed by in vivo NAD+/NADH Measurement. Schizophr Bull. 2017;43:197–204. doi: 10.1093/schbul/sbw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu XH, Zhang Y, Ugurbil K, Chen W. Proc Intl Soc Mag Reson Med. Hawaii, USA: 2009. Direct and Noninvasive Measurement of Cerebral Metabolic Rate of ATP in Cat brain and its Physiological Implications; p. 3289. [Google Scholar]
- 87.From AH, Ugurbil K. Standard magnetic resonance-based measurements of the Pi–>ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles. Am J Physiol Cell Physiol. 2011;301:C1–11. doi: 10.1152/ajpcell.00345.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


