Abstract
Background
Ubiquitin-specific peptidase 33 (USP33) is a deubiquitinase that balances the ubiquitin status of proteins. It has been reported to act as a tumor suppressor in colorectal cancer and lung cancer. However, the expression pattern and clinical significance of USP33 have not been investigated in gastric adenocarcinoma (GAC).
Material/Methods
We explored the USP33 protein and RNA levels by immunohistochemistry (IHC), Western blot analysis, and qRT-PCR. The Pearson chi-square test was performed to evaluate the statistical associations between USP33 level and patient characteristics. Additionally, the relationship between USP33 expression and patient survival was investigated. Cellular studies, including proliferation assay, migration assay, and invasion assay, were conducted to demonstrate the underlying mechanisms of USP33 in GAC progression.
Results
This study included 121 patients with GAC. USP33 showed a decreased expression in GAC tissues compared to adjacent normal gastric tissues. Low expression of USP33 was correlated with invasion depth and advanced TNM stage. According to survival analysis, upper location of tumor (P=0.003), invasion depth (P=0.048), advanced TNM stage (P=0.001), and low USP33 level (P=0.001) were all associated with poor overall survival of GAC patients. Cox analysis confirmed the independent role of USP33 in predicting patient survival. Cell experiments showed that USP33 overexpression significantly inhibited the proliferation, migration, and invasion of GAC cells.
Conclusions
USP33 was downregulated in GAC, and was an independent prognostic factor. In vitro results demonstrated the role of USP33 in suppressing tumor progression, suggesting that the developing an agonist of USP33 may be a novel direction for chemotherapy development.
MeSH Keywords: Cell Migration Assays, Cell Proliferation, Neoplasm Invasiveness, Prognosis, Stomach Neoplasms
Background
Gastric cancer is a highly aggressive malignancy, and is the second leading cause of cancer-related deaths worldwide [1]. The most common pathological type of gastric cancer is gastric adenocarcinoma (GAC). In the past decades, great achievements were obtained in diagnosis and management of GAC [2]. However, the overall prognosis varies among patients. For example, patients with local GAC in mucosa or submucosa show an excellent prognosis, while patients with serous invasion depth are less predictable and have poorer clinical outcomes [3]. Besides invasion depth, other clinicopathologic factors are also considered in clinical decisions, such as tumor location, tumor size, and lymph node status. Nevertheless, these conventional parameters usually fail to differentiate patients with more aggressive tumor types, which is a major obstacle to developing targeted treatment and improved prognosis. As a result, identifying novel and clinically applicable molecular biomarkers is of great importance, both for development of new treatments and for predicting prognosis of patients with GACs.
Ubiquitination is a reversible process. On one hand, ubiquitin ligases add ubiquitin moieties onto proteins and induce protein degradation. On the other hand, deubiquitinating enzymes (deubiquitinases, DUBs) catalyze the release of ubiquitin from ubiquitinated substrates [4]. At present, humans are estimated to have 500–1000 E3 ubiquitin ligases [5]. In contrast, there are 102 putative DUB genes in humans, and only 79 of them are predicted to be functional [6]. Therefore, each DUB must have multiple unidentified substrates on average, which is now a focus of enzymatic studies. Among the DUBs, ubiquitin-specific proteases (USPs) is the largest family of cysteine proteases [7]. Since DUBs can modify protein function and degradation, we wondered if some of them have roles in tumor progression.
USP33 is a kind of USP, which has been reported to inhibit cellular migration in breast cancer and colorectal cancer [8,9]. However, its expression and function in GACs have not previously been investigated. Herein, we report our findings on the RNA and protein expression levels of USP33 in GAC tissues. Statistical analysis revealed its significant correlation with tumor progression and patient prognosis. Furthermore, upon transfection with USP33 plasmids, the proliferation and metastatic abilities of GAC cells were remarkably attenuated. Our data not only identifies USP33 as a novel predictive biomarker for GAC, but also provides evidence of its potential in tumor therapy development.
Material and Methods
Patients
This study was approved by the Ethics Committee of Yidu Central Hospital of Weifang. Written informed consents were obtained from all 138 patients enrolled in this study. All patients underwent surgical resection of GAC in Yidu Central Hospital of Weifang between 2009 and 2013. All the diagnoses were based on histomorphology and pathology examinations by the Department of Pathology. None of the patients received any chemotherapy or radiotherapy before surgery. The samples included 121 formalin-fixed paraffin-embedded samples, and 17 fresh-frozen samples stored in −80°C. The paraffin-embedded samples were subjected to immunohistochemistry (IHC) analysis, and the fresh-frozen tissues were subjected to quantitative PCR analysis. The corresponding patients matched with paraffin-embedded samples were followed up for up to 7 years. The median follow-up period was 52.0 months.
IHC
The IHC experiments were performed as described by others [10]. Formalin-fixed and paraffin-embedded samples were cut into 4-μm sections. Slides were first dewaxed and rehydrated using graded ethanol. Second, slides were incubated with 3% H2O2 for 10 min to block endogenous peroxidase, and then the antigen retrieval was achieved using 10 mM of sodium citrate buffer (pH 6.0) in a microwave oven. Thirdly, slides were blocked in 10% nonimmunoreactive goat serum for 30 min at room temperature. Fourth, slides were incubated with a mouse monoclonal primary antibody (sc-100632, Santa Cruz Biotechnology) at 4°C overnight. For negative control, PBS was used instead of primary antibody. Finally, the slides were subjected to a horseradish-peroxidase detection system (Gene Tech) to visualize the immunoreactivities. IHC results were assessed by 2 pathologists according to staining intensity (scored as 0, 1, 2, and 3) and percentage of positive cells (0. 1–10%; 1, 11–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%). The final immunoreactivity score (IRS) was defined by multiplying the intensity score by the percentage score (range 0–12). For the statistical analysis, patients were categorized into 2 groups according to the IRS: 0–4, low expression; 5–12, high expression.
qRT-PCR
The RNA from GAC tissues and adjacent tissues was extracted using Trizol reagent (Life Technologies) and quantified using a spectrophotometer. A total amount of 1 μg RNA was reversely transcribed into cDNA, as described before [11]. The quantitative of RNA was achieved by using the StepOnePlus real-time PCR system (Applied Biosystems, CA, USA) according to the manufacturer’s instructions. Besides USP33, the level of GAPDH was quantified as an internal control. The following primers were synthesized for PCR analysis:
Human USP33 forward TGTGATGCTTAGGCAAGGAG.
Human USP33 reverse GGCCCTCCACCATAAATAGA.
Human GAPDH forward ATGGGGAAGGTGAAGGTCG.
Human GAPDH reverse GGGGTCATTGATGGCAACAATA.
Western blot analysis
Harvested cells were lysed in RIPA buffer supplemented with protease inhibitor cocktail (Roche). After being quantified by a spectrophotometer, 20 μg of proteins were separated on 10% SDS-PAGE and blotted to nitrocellulose membranes (Bio-Rad). The membranes were washed 3 times with TBS for 30 min, blocked with 5% non-fat milk, and incubated with primary antibodies (USP33, sc-100632; GAPDH, sc-47724; Santa Cruz Biotechnology). After being washed again with TBS for 30 min, membranes were then incubated with the secondary IgG-HRP mouse antibody. Immunoreactivity was detected by adding enhanced chemiluminescence (ECL) and developed with X-ray films. The results were semi-quantified using Image J software using GAPDH as the internal control.
Cell culture
Human normal gastric epithelial cells (HGaEpC) was purchased from Cell Applications (San Diego, CA, USA). Human gastric adenocarcinoma cell lines AGS and MKN45 were both obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Both cell lines were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA), 100 U penicillin G, and 100 μg/ml streptomycin sulfate. Cells were maintained in a 37°C humidified incubator with 5% CO2.
Plasmid and transfection
Flag-HA-USP33 was a gift from Wade Harper (Addgene plasmid # 22601) [12]. Transfection was performed by using Lipo2000 transfection reagent (Invitrogen) and Opti-DMEM, following the manufacturer’s instructions.
Proliferation assay
Transfected cells were seeded into 96-well plates with 2×103 cells/well and cultured in 37°C with complete DMEM. At different time points (24 h, 48 h, 72 h, and 96 h), 100 μl of 0.5 mg/ml MTT was added into the wells and cultured for another 4 h. Then, the medium was removed and 150 μl of DMSO was added into wells to dissolve the staining crystals. After 15-min incubation, the absorbance was measured at 570 nm wavelength using a microplate spectrometer.
Migration and invasion assay
The migration assay was performed by using a Boyden chamber (Corning Costar, Rochester, NY, USA). Briefly, 3×103 cells were seeded in the upper compartment and allowed to migrate for 24 h at 37°C. After culturing, cells on the upper surface of the membrane were removed with a cotton swab. Cells that invaded through the membrane were fixed with ethanol and stained with crystal violet. Cells were counted under a microscope from 5 random fields. The invasion assay was performed as described before [13]. In the migration assay, the chamber was pre-coated with 20 μl 0.3 mg/ml Matrigel (BD Biosciences), and the number of seeded cells was 1×104 cells/well instead of 3×103 cells/well. All experiments were performed in triplicate.
Statistics
IBM SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Associations between USP33 expression and pathological characteristics were subjected to crosstabulation and evaluated with the chi-square test. Kaplan-Meier method was used to perform univariate survival analysis, and these were subsequently estimated by log-rank tests. The variables with statistical significance by univariate analysis were further subjected to Cox’s proportional hazards regression analysis. The statistical differences between 2 groups in cell experiments were all compared by Student’s t-test. P<0.05 by two-sided test was considered statistical significance.
Results
Patients’ characteristics
We enrolled 121 GAC patients in our follow-up cohort. Most of the cases (74/121, 61.2%) were older than 55 years old. Similarly, 76 patients (62.8%) were males, while 45 patients (37.2%) were females. The tumor locations in 26 patients were in the upper third gastric (21.5%, UTG), 50 patients in the middle third gastric (41.3%, MTG), and the other 45 patients in the lower third gastric (37.2%, LTG). Most of the patients had moderate or poor differentiation (108/121, 89.3%). Nearly half of the cases (51/121, 42.1%) presented a large tumor size, with diameter larger than 5.0 cm. The tumor invasion depth was evaluated as T1 (invades mucosa or submucosa), T2 (invades muscularis propria), T3 (penetrates subserosal connective tissue), or T4 (invades serosa or adjacent tissues). Accordingly, 41 patients (33.9%) were diagnosed with T1-T2, and the other 80 patients (66.1%) were diagnosed with T3–T4. In addition, the TNM stages of all patients were classified by the WHO criteria [14], and 46 cases (38.0%) were grouped as TNM stage I–II, while 75 cases were grouped as TNM stage III–IV (62.0%). Details are presented in Table 1.
Table 1.
Correlations between clinicopathological parameters and USP33 expression level.
| Clinicopathologic features | Cases (n=121) | USP33 expression level | Chi-square P value | |
|---|---|---|---|---|
| Low (n=63) | High (n=58) | |||
| Age | P=0.583 | |||
| ≤55 yrs | 47 | 23 | 24 | |
| >55 yrs | 74 | 40 | 34 | |
| Gender | P=0.095 | |||
| Female | 45 | 19 | 26 | |
| Male | 76 | 44 | 32 | |
| Location | P=0.548 | |||
| UTG | 26 | 16 | 10 | |
| MTG | 50 | 25 | 25 | |
| LTG | 45 | 22 | 23 | |
| Differentiation | P=0.353 | |||
| Well | 13 | 6 | 7 | |
| Moderate | 56 | 26 | 30 | |
| Poor | 52 | 31 | 21 | |
| Tumor size | P=0.101 | |||
| ≤5.0 cm | 70 | 32 | 38 | |
| >5.0 cm | 51 | 31 | 20 | |
| Invasion depth | P=0.001* | |||
| T1–T2 | 41 | 13 | 28 | |
| T3–T4 | 80 | 50 | 30 | |
| TNM stage | P<0.001* | |||
| I–II | 46 | 12 | 34 | |
| III–IV | 75 | 51 | 24 | |
USP33 – ubiquitin specific peptidase 33; UTG – upper third gastric; MTG – middle third gastric; LTG – lower third gastric.
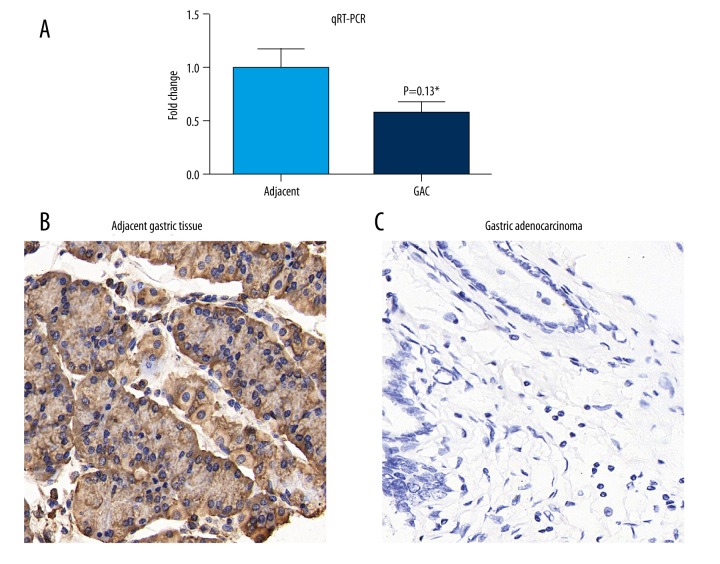
USP33 presented a lower expression in GACs
We first tested the RNA levels of USP33 in 17 pairs of fresh-frozen GACs and adjacent tissues. qRT-PCR results demonstrated that USP33 mRNA level was significantly lower in GACs than in adjacent tissues (Figure 1A). Therefore, we enlarged our study cohort by enrolling 121 GAC patients with available paraffin-embedded samples. The IHC results (Figure 1B, 1C) showed a predominantly cytoplasmic localization of USP33 in the normal gastric epithelial cells and GAC cells. By classifying patients into a low USP33 expression group (n=63) and a high USP33 expression group (n=58), we evaluated the correlations between USP33 expression level and patient pathological characteristics (Table 1). Pearson chi-square tests showed that USP33 expression was negatively correlated with tumor invasion depth (P=0.001). Additionally, patients with advanced TNM stages were more likely to have a lower USP33 level (P<0.001), indicating the possible participation of USP33 in GAC progression.
Figure 1.
USP33 was downregulated in GAC tumor tissues. (A) qRT-PCR results showed a decreased USP33 transcripts in GACs compared with that in adjacent gastric tissues. (B) IHC identified a high positive protein expression of USP33 in adjacent gastric tissues (IRS=12). (C) Representative low expression of USP33 in GAC tumor tissues (IRS=0). * P<0.05, magnification: ×400.
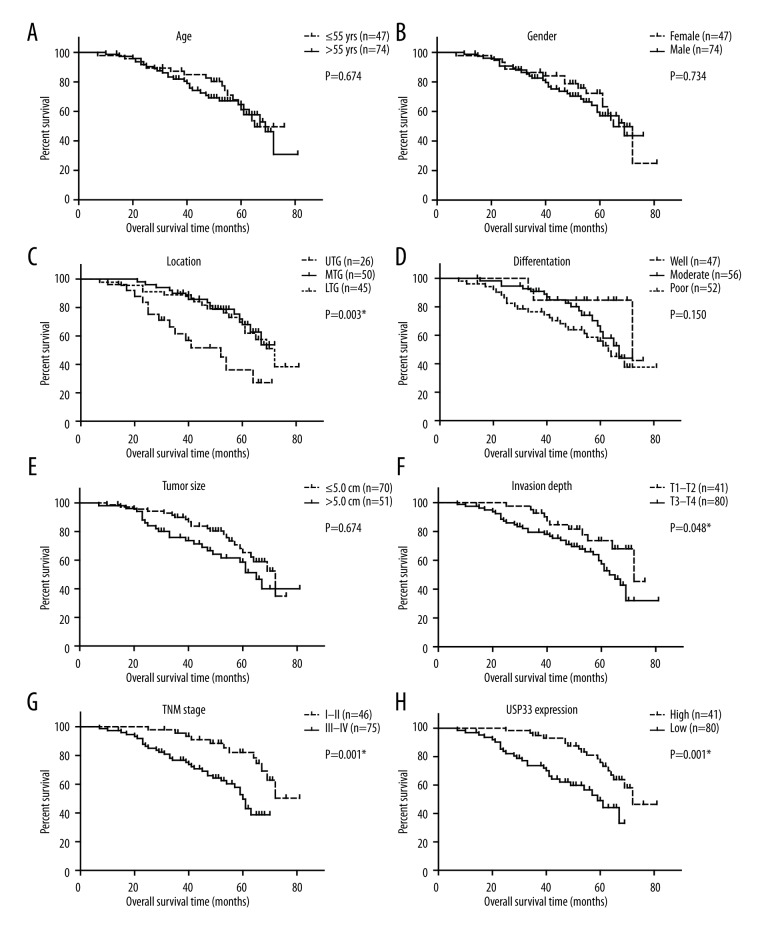
Low USP33 expression was an unfavorable prognostic factor for GACs
By the end of follow-up, 46 patients (38.0%) died, the median survival time was 69.0 months, and the 5-year overall survival was 62.8%. To better investigate the clinical significance of USP33 in GACs, we plotted the overall survival curve of different variables with Kaplan-Meier method (Figure 2). Accordingly, patients with an upper tumor location, for example the upper third gastric (UTG), presented a poor overall survival (estimated OS 46.7±4.3 months, 5-year OS 36.2%) compared with GACs with tumor location in the middle third gastric (MTG) or lower third gastric (LTG). The tumor invasion depth and TNM stage were another 2 prognostic factors, and consistent with conventional opinions, our data revealed that a serous tumor invasion depth or an advanced TNM stage indicated poorer overall survival (P=0.048 and P=0.001, respectively). Of note, we found that USP33 expression level can also affect patient clinical outcome (Table 2). Compared to patients with a high USP33 level, GACs with low USP33 showed a more unfavorable prognosis (estimated OS 51.3±2.6 vs. 69.1±2.3 months, 5-year OS 49.1% vs. 76.0%). We then evaluated the independent role of each predicative factor by using the Cox regression model (Table 3). According to the multivariate analysis results, both tumor location and TNM stage were verified as independent prognostic factors (P<0.001 and P=0.432, respectively). Moreover, data showed that high USP33 can help independently predict a better overall survival in GACs (HR=0.432, 95% confidence interval 0.223–0.839, P=0.013).
Figure 2.
Overall survival curve of GAC patients plotted by Kaplan-Meier method. All data were compared by log-rank test, and * indicated P<0.05 by two-tail test.
Table 2.
Kaplan-Meier univariate survival analysis.
| Clinicopathologic features | OS months (Mean ±S.D.) | 5-year OS (%) | Univariate P value |
|---|---|---|---|
| Age | P=0.674 | ||
| ≤55 yrs | 61.7±2.9 | 61.2% | |
| >55 yrs | 60.8±2.9 | 64.7% | |
| Gender | P=0.734 | ||
| Female | 62.1±3.4 | 72.3% | |
| Male | 59.6±2.4 | 57.0% | |
| Location | P=0.003* | ||
| UTG | 46.7±4.3 | 36.2% | |
| MTG | 62.2±2.2 | 67.9% | |
| LTG | 64.0±3.3 | 69.7% | |
| Differentiation | P=0.150 | ||
| Well | 67.8±4.2 | 84.6% | |
| Moderate | 60.6±2.2 | 62.4% | |
| Poor | 57.5±3.5 | 55.9% | |
| Tumor size | P=0.124 | ||
| ≤5.0 cm | 62.7±2.2 | 65.3% | |
| >5.0 cm | 58.4±3.5 | 58.6% | |
| Invasion depth | P=0.048* | ||
| T1–T2 | 65.7±2.6 | 73.6% | |
| T3–T4 | 58.7±2.8 | 57.5% | |
| TNM stage | P=0.001* | ||
| I–II | 70.4±2.5 | 82.1% | |
| III–IV | 53.1±2.3 | 49.6% | |
| USP33 expression | P=0.001* | ||
| Low | 51.3±2.6 | 49.1% | |
| High | 69.1±2.3 | 76.0% |
OS – overall survival; USP33 – ubiquitin specific peptidase 33; UTG – upper third gastric; MTG – middle third gastric; LTG – lower third gastric.
Table 3.
Multivariate Cox regression analysis.
| Clinicopathologic features | HR | 95% CI | P value |
|---|---|---|---|
| Location (vs. UTG) | 0.282 | 0.146–0.544 | P<0.001* |
| Invasion depth (vs. T1–T2) | 0.955 | 0.382–2.389 | P=0.922 |
| TNM stage (vs. I–II) | 2.726 | 1.052–7.066 | P=0.039* |
| USP33 expression (vs. low) | 0.432 | 0.223–0.839 | P=0.013* |
UTG – upper third gastric; USP33 – ubiquitin specific peptidase 33.
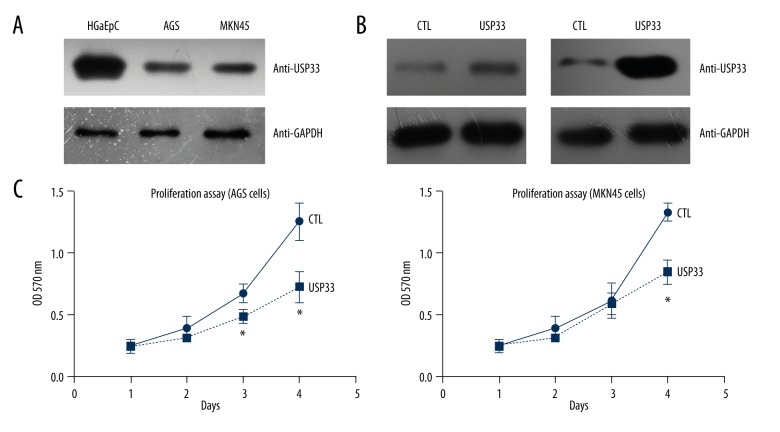
USP33 knockdown significantly upregulated tumor cell proliferation and metastasis
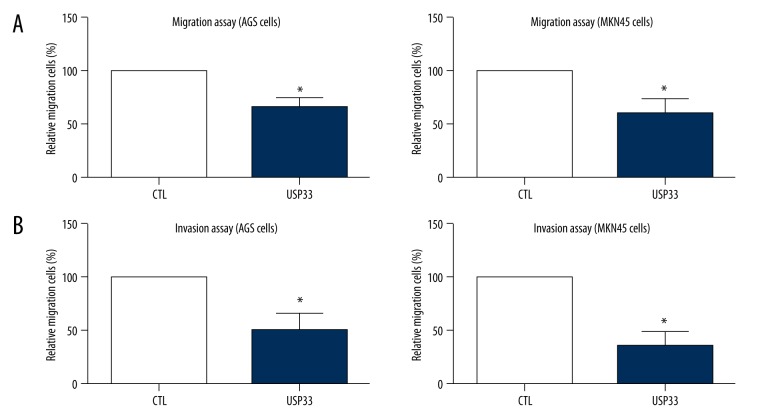
Our clinical results and analyses revealed a possible tumor-suppressing role of USP33 in GACs; therefore, we next wanted to confirm its molecular effect in the GAC cells. Western blot analysis showed that USP33 was downregulated in 2 GAC cell lines, AGS and MKN45, compared with that in human normal gastric epithelial cells (HGaEpC, Figure 3A). We ectopically expressed USP33 in the AGS and MKN45 cells by transiently transfecting the Flag-HA-USP33 plasmids (Figure 3B). MTT results showed that USP33 can inhibit cellular growth (Figure 3C). Consistently, the effects of USP33 on cell migration (Figure 4A) and invasion (Figure 4B) were tested by Transwell assays, which showed an anti-metastasis role of USP33 in both GAC cell lines.
Figure 3.
USP33 inhibited tumor growth of GAC. (A) Immunoblotting showed a lower USP33 level in 2 GAC cell lines, AGS and MKN45, compared to that in human normal gastric epithelial cells (HGaEpC). (B) Transfection efficiency of USP33 was tested by Western blot in both AGS and MKN45 cells. (C) MTT experiments found that USP33 ectopic expression significantly inhibited the cellular proliferation. All experiments were performed at least 3 times. * P<0.05 compared to control (CTL) group.
Figure 4.
USP33 suppressed tumor migration and invasion of GAC cells. (A) Migration capacities of AGS and MKN45 cells were evaluated by Transwell method, which revealed a suppressing role of USP33 in the tumor cell migration. (B) Similarly, by using Matrigel pre-coated Transwell chambers, we confirmed the independent effect of USP33 in inhibiting GAC invasion. Data acquired from 3 independent experiments. * P<0.05 compared to control (CTL) group.
Discussion
Recently, molecular biomarkers have attracted more and more attention due to their possible roles in improving prognostic prediction for cancer patients. In GACs, several biomarkers have been reported as prognostic predictors, such as the well-known EGFR [15], HIF-1α [16], and p53 [17]. Besides basial protein levels, the modification of proteins and corresponding enzymes are also showing evidence in tumor progression [18].
Deubiquitinases are enzymes catalyzing the deubiquitinate process and balancing the ubiquitin status of proteins. Several USPs have been recognized to participate in regulating tumor development [19]. For example, USP7 is overexpressed in colorectal cancer [20], ovarian cancer [21], and lymphocytic leukemia [22]. USP11 is upregulated in female breast cancer and indicates a poor overall survival [23]. USP33 is also a member of the USP family, which can deubiquitinate the centriolar protein CP110 [24], β-arrestins [25], and Robo1 [26]. Recently, several groups demonstrated the tumor-suppressing role of USP33 in various malignancies, including breast cancer [8], colorectal cancer [27], and lung cancer [26]. However, it is completely unknown whether USP33 functions in gastric cancers.
We therefore performed this retrospective study to investigate the expression pattern of USP33 in GACs, and elucidate whether it is associated with tumor progression. According to our data, USP33 was decreased on both RNA and protein levels in GAC tissues compared to normal gastric tissues. Of note, lower USP33 indicates a more aggressive characteristic of gastric cancer, such as severe invasion depth and advanced TNM stage. Therefore, we suspected USP33 functions as a tumor suppressor in the development of GACs. To verify our hypothesis, AGS and MKN45 cell lines were subjected to USP33 silencing by RNAi strategy. As expected, the proliferation, migration, and invasion processes of tumor cells were all significantly suppressed upon USP33-siRNA. Our data revealed the cellular functions of USP33 in the development of GACs, suggesting that activation of USP33 has potential in developing novel GAC therapies.
Moreover, we conducted survival analyses with Kaplan-Meier method and found that the tumor location, tumor invasion depth, TNM stage, and USP33 expression were all prognostic factors for the overall survival of GAC patients. Multivariate regression also verified the independent role of lower USP33 level in predicting a poorer clinical outcome of GACs. Therefore, our data also provide evidence that USP33 functions as a predictor for evaluating patient prognosis.
There are some limitations in this study despite the encouraging results we obtained. Firstly, the cohort in our study only included 121 cases of GACs from 1 hospital, leading to a possible bias in evaluating the patients’ prognosis. It is crucial to confirm the clinical role of USP33 with a larger multi-center sample in the future. Secondly, the details of mechanisms by which USP33 promotes GAC growth and metastasis are still unclear; for example, the effects of USP33 on cell viability may be caused by either suppressing proliferation or enhancing apoptosis. Further studies should focus on identifying the underlying molecular signaling pathways.
Conclusions
USP33 is downregulated in GACs, which indicates more aggressive tumor properties and poor prognosis.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374(9688):477–90. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: Ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228(4):449–61. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997;11(14):1245–56. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama KI, Nakayama K. Ubiquitin ligases: Cell-cycle control and cancer. Nat Rev Cancer. 2006;6(5):369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 6.Nijman SM, Luna-Vargas MP, Velds A, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123(5):773–86. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695(1):189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci USA. 2009;106(34):14530–35. doi: 10.1073/pnas.0801262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z, Wen P, Kong R, et al. USP33 mediates Slit Robo signaling in inhibiting colorectal cancer cell migration. Int J Cancer. 2015;136(8):1792–802. doi: 10.1002/ijc.29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138(2):389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Tian P, Liu Y. P53 and protein phosphorylation regulate the oncogenic role of epithelial cell transforming 2 (ECT2) Med Sci Monit. 2018;24:3154–60. doi: 10.12659/MSM.905388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobin LH, Fleming ID. TNM classification of malignant tumors, (1997) Cancer. 1997;80(9):1803–4. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Lieto E, Ferraraccio F, Orditura M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15(1):69–79. doi: 10.1245/s10434-007-9596-0. [DOI] [PubMed] [Google Scholar]
- 16.Urano N, Fujiwara Y, Doki Y, et al. Overexpression of hypoxia-inducible factor-1 alpha in gastric adenocarcinoma. Gastric Cancer. 2006;9(1):44–49. doi: 10.1007/s10120-005-0356-1. [DOI] [PubMed] [Google Scholar]
- 17.Tamura G, Kihana T, Nomura K, et al. Detection of frequent p53 gene mutations in primary gastric cancer by cell sorting and polymerase chain reaction single-strand conformation polymorphism analysis. Cancer Res. 1991;51(11):3056–58. [PubMed] [Google Scholar]
- 18.Soucy TA, Smith PG, Milhollen MA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458(7239):732–36. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 19.Hussain S, Zhang Y, Galardy P. DUBs and cancer: The role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 2009;8(11):1688–97. doi: 10.4161/cc.8.11.8739. [DOI] [PubMed] [Google Scholar]
- 20.An T, Gong Y, Li X, et al. USP7 inhibitor P5091 inhibits Wnt signaling and colorectal tumor growth. Biochem Pharmacol. 2017;131:29–39. doi: 10.1016/j.bcp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Wang H, Tian L, Li H. Expression of USP7 and MARCH7 is correlated with poor prognosis in epithelial ovarian cancer. Tohoku J Exp Med. 2016;239(3):165–75. doi: 10.1620/tjem.239.165. [DOI] [PubMed] [Google Scholar]
- 22.Carrà G, Panuzzo C, Torti D, et al. Inhibition OF USP7 induces selective cancer cell death in chronic lymphocytic leukemia. Clinical Lymphoma, Myeloma and Leukemia. 2016;16:S49–S50. [Google Scholar]
- 23.Bayraktar S, Barrera AMG, Liu D, et al. USP-11 as a predictive and prognostic factor following neoadjuvant therapy in women with breast cancer. Cancer J. 2013;19(1):10–17. doi: 10.1097/PPO.0b013e3182801b3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, D’Angiolella V, Seeley ES, et al. USP33 regulates centrosome biogenesis via deubiquitination of the centriolar protein CP110. Nature. 2013;495(7440):255–59. doi: 10.1038/nature11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shenoy SK, Modi AS, Shukla AK, et al. β-Arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci USA. 2009;106(16):6650–55. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen P, Kong R, Liu J, et al. USP33, a new player in lung cancer, mediates Slit-Robo signaling. Protein Cell. 2014;5(9):704–13. doi: 10.1007/s13238-014-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: New insights into β-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–40. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]