Abstract
Background
Programmed cell death-1 (PD-1) inhibitor-related hematologic toxicities are a category of rare but clinically serious and potentially life-threatening adverse events; however, little is known about their risks across different treatment regimens and tumor types. The objective of this study was to compare the incidences of PD-1 inhibitor-related hematologic toxicities among different therapeutic regimens and tumor types.
Methods
Twenty-six original articles on PD-1 inhibitor trials were identified based on a PubMed search completed on September 26, 2017. The incidences of hematologic toxicities were collected.
Results
A total of 26 studies containing 5,088 patients were included in the meta-analysis. PD-1 inhibitor monotherapy was associated with an increased risk of all-grade anemia in cancer patients (5%, 95% CI 4%–6%), particularly in patients with renal cell carcinoma (RCC) (8%, 95% CI 6%–12%), compared with all-grade thrombocytopenia (2%, 95% CI 1%–5%), leukopenia (2%, 95% CI 1%–3%), and neutropenia (1%, 95% CI 0–1%). However, low incidences of high-grade hematologic toxicities were observed in cancer patients treated with PD-1 inhibitor monotherapy. The use of PD-1 inhibitors in combination with ipilimumab, peptide vaccines, or chemotherapy had significantly higher risks than PD-1 inhibitor monotherapy for all-grade anemia (13%, 95% CI 5%–31%), thrombocytopenia (6%, 95% CI 2%–18%), leukopenia (5%, 95% CI 1%–35%), neutropenia (4%, 95% CI 1%–26%), and only high-grade thrombocytopenia (4%, 95% CI 1%–15%). In addition, all-grade and high-grade hematologic toxicities in chemotherapy and everolimus treatment arms were more frequent than in PD-1 inhibitor monotherapy arms.
Conclusion
The risks of PD-1 inhibitor-related hematologic toxicities were higher in RCC than in other cancers, and during combination therapy. These results may contribute toward enhancing awareness among clinicians about frequent clinical monitoring when managing PD-1 inhibitors.
Keywords: nivolumab, pembrolizumab, immunotherapy, hematological adverse events
Introduction
In recent years, programmed cell death-1 (PD-1) inhibitors have shown remarkable efficacy in the clinic, thus accelerating US Food and Drug Administration (FDA) approval of these agents for cancer therapy. Nivolumab and pembrolizumab are the human PD-1-blocking antibodies that have been approved for treatment of non-small-cell lung cancer (NSCLC),1–9 melanoma,10–13 renal cell carcinoma (RCC),14,15 urothelial carcinoma,16,17 and head and neck squamous cell carcinoma (HNSCC).18 Furthermore, anti-PD-1 antibodies in combination with other drugs have also shown significant effects in many refractory cancers.19–23
As a result of widespread prescribing of PD-1 inhibitors in the clinic, the profile of immune-related adverse events (irAEs) started to raise many concerns. These irAEs are characterized by T-cell response hyperactivation (overproduction of CD4+ T-helper-cell cytokines and abnormal migration of cytolytic CD8+ T cells) leading to normal tissue damage or organ system failure. Among them, hematologic toxicity during PD-1 inhibitor therapy is of particular interest owing to its causing potentially life-threatening events (severe hypoxia, infection, and bleeding) if not promptly recognized and adequately treated.
Although many clinical trials have reported hematologic toxicities associated with PD-1 inhibitors, these are a series of relatively rare adverse events, and hence the knowledge based on the individual cohort data from each trial is limited. Given the increasing number of published PD-1 inhibitor trials, a systematic assessment may provide important knowledge on these categories of rare but clinically significant and potentially serious irAEs. In this study, we conducted a meta-analysis to address the incidences of PD-1 inhibitor-related hematologic toxicities among cohorts with different treatment regimens and tumor types.
Methods
Search methods and study selection
The literature published in the English language up to September 26, 2017, that included the results of prospective trials of PD-1 inhibitor therapy in cancer patients using nivolumab or pembrolizumab, including monotherapy and combination therapy, was identified by a PubMed search and by examining the references of published trials, review articles, editorials, and other relevant articles. The following keywords or corresponding Medical Subject Heading terms were used: “nivolumab”, “pembrolizumab”, and “PD-1 inhibitor”. Articles that were published online “ahead of print” were included. Meeting abstracts without published full-text original articles were excluded. The search focused on trials of nivolumab and pembrolizumab because of their having being approved by the US FDA as well as being widely available on prescription in the clinic.
Data extraction
The data were extracted by one primary investigator (J-D Sui) and then reviewed independently by 2 secondary investigators (Y Wang and Y Wan). The following information was collected from the eligible articles: principal author’s name; year of issuance; type of treatments; type of tumors; trial phase; clinical trial information; number of enrolled patients; and number of events with all-grade and high-grade (grades 3–5) anemia, thrombocytopenia, leucopenia, and neutropenia. Hematologic toxicities in each trial were defined according to the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute.
Statistical analysis
Review Manager version 5.3 was used for statistical analysis. The data type “dichotomous” was selected to compare the incidences of hematologic toxicities between PD-1 inhibitor monotherapy and chemotherapy or everolimus monotherapy. A value of p<0.05 was considered statistically significant.
The data type “generic inverse variance” was selected for the outcome of incidence; if the p-value was close to neither 0 nor 1, and both n×p and n×(1 − p) were more than 5, p could be calculated by n/X, in which n and X refer to the number of patients with hematologic toxicities and the total number of patients in treatment arm, respectively. SE was calculated using the formula (p(1 − p)/n)1/2, in which p and n have the same meanings as above. On the other hand, if these conditions could not be met, p and SE were calculated by the following formulas: p = ln(odds) = ln[X/(n − X)], SE(p) = SE[ln(odds)] = [1/X + 1/(n − X)]1/2. Significantly, this is the method used for categorical data; thus, the calculations for ORs should be transformed using the following formula: Pf = OR/(1 + OR), lower limit (LL) of 95% CI = LLOR/(1 + LLOR), upper limit (UL) = ULOR/(1 + ULOR). In our study, the latter method was adopted owing to the low incidences of adverse effects.
Heterogeneity was evaluated by the Cochran chi-square test and the I2 test. Heterogeneity was considered statistically significant if p<0.05. If substantial heterogeneity was not observed, the pooled estimate was calculated based on the fixed effects model. If substantial heterogeneity existed, data were analyzed using a random effects model. I2<30% represented a slight level of heterogeneity, 30%–60% was moderate while I2>60% meant that the heterogeneity was high. Publication bias was assessed using funnel plots showing the relationship between OR and SE (log[OR]). Publication bias existed if the funnel plots lacked symmetry.
Results
Eligible studies and characteristics
Based on our search strategy, a total of 485 records were identified for screening. Exclusion criteria are presented in Figure 1. Accordingly, a total of 26 full-text articles were considered eligible for our analysis, including 9 phase III trials,1–3,7,8,11,13,17,18 5 phase II trials,9,10,15,16,23 2 phase I/II trials19,24 and 10 phase I trials.4–6,12,14,20–22,25,26 Of these included studies, 10 trials evaluated PD-1 inhibitor monotherapy (6 nivolumab5,6,9,12,14,24 and 4 pembrolizumab4,16,25,26), 10 trials evaluated PD-1 inhibitor monotherapy versus chemotherapy control (6 nivolumab vs chemotherapy1–3,11,13,18 and 4 pembrolizumab vs chemotherapy7,8,10,17), 1 trial evaluated nivolumab monotherapy versus everolimus,15 3 trials evaluated nivolumab/ipilimumab combinations,19,21,23 1 trial evaluated nivolumab/chemotherapy combination,22 and 1 trial evaluated nivolumab/peptide vaccine combination.20 Tumor types tested in these studies included NSCLC (n=11),1–9,21,22 melanoma (n=6),10–13,20,23 RCC (n=2),14,15 urothelial carcinoma (n=2),16,17 small-cell lung cancer (SCLC) (n=1),19 hepatocellular carcinoma (n=1),24 HNSCC (n=1),18 triple-negative breast cancer (n=1),26 and malignant pleural mesothelioma (n=1).25 The characteristics of all the included trials are summarized in Table 1.
Figure 1.
Flow diagram of study inclusion.
Abbreviations: mAbs, monoclonal antibodies; PD-1, programmed cell death-1.
Table 1.
Characteristics of all included studies
| Study | Drug | Tumor type | Phase | No of treated patientsa | Median age (years) | Clinical trial information |
|---|---|---|---|---|---|---|
| Alley et al (2017)25 | Pembrolizumab | Malignant pleural mesothelioma | 1b | 25 | 65 | NCT02054806 |
| Antonia et al (2016)19 | Nivolumab and ipilimumab | SCLC | 1/2 | 115 | 63 | NCT01928394 |
| Balar et al (2017)16 | Pembrolizumab | Urothelial carcinoma | 2 | 370 | 74 | NCT02335424 |
| Bellmunt et al (2017)17 | Pembrolizumab | Urothelial carcinoma | 3 | 266 | 66 | NCT02256436 |
| Borghaei et al (2015)1 | Nivolumab | NSCLC | 3 | 287 | 62 | NCT01673867 |
| Brahmer et al (2015)2 | Nivolumab | NSCLC | 3 | 131 | 63 | NCT01642004 |
| Carbone et al (2017)3 | Nivolumab | NSCLC | 3 | 267 | 64 | NCT02041533 |
| El-Khoueiry et al (2017)24 | Nivolumab | Hepatocellular carcinoma | 1/2 | 48 | 62 | NCT01658878 |
| Ferris et al (2016)18 | Nivolumab | HNSCC | 3 | 236 | 60 | NCT02105636 |
| Garon et al (2015)4 | Pembrolizumab | NSCLC | 1 | 495 | 64 | NCT01295827 |
| Gettinger et al (2015)6 | Nivolumab | NSCLC | 1 | 129 | 65 | NCT00730639 |
| Gettinger et al (2016)5 | Nivolumab | NSCLC | 1 | 52 | 67 | NCT01454102 |
| Gibney et al (2015)20 | Nivolumab and peptide vaccine | Melanoma | 1 | 33 | 47 | – |
| Hellmann et al (2017)21 | Nivolumab and ipilimumab | NSCLC | 1 | 77 | 65 | NCT01454102 |
| Herbst et al (2016)7 | Pembrolizumab | NSCLC | 3 | 682 | 63 | NCT01905657 |
| McDermott et al (2015)14 | Nivolumab | RCC | 1 | 34 | 58 | NCT0730639 |
| Motzer et al (2015)15 | Nivolumab | RCC | 2 | 406 | 62 | NCT01668784 |
| Nanda et al (2016)26 | Pembrolizumab | Triple-negative breast cancer | 1b | 32 | 50.5 | NCT01848834 |
| Reck et al (2016)8 | Pembrolizumab | NSCLC | 3 | 154 | 64.5 | NCT02142738 |
| Ribas et al (2015)10 | Pembrolizumab | Melanoma | 2 | 357 | 62 | NCT01704287 |
| Rizvi et al (2015)9 | Nivolumab | NSCLC | 2 | 117 | 65 | NCT01721759 |
| Rizvi et al (2016)22 | Nivolumab and chemotherapy | NSCLC | 1 | 56 | 64 | NCT01454102 |
| Robert et al (2015)11 | Nivolumab | Melanoma | 3 | 206 | 65 | NCT01721772 |
| Topalian et al (2014)12 | Nivolumab | Melanoma | 1 | 107 | 61 | NCT00730639 |
| Weber et al (2015)13 | Nivolumab | Melanoma | 3 | 268 | 59 | NCT01721746 |
| Weber et al (2016)23 | Nivolumab and ipilimumab | Melanoma | 2 | 138 | 62 | NCT01783938 |
Note:
Includes the number of patients treated in PD-1 inhibitor arms but does not include patients treated in the control arms without PD-1 inhibitors.
Abbreviations: HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; PD-1, programmed cell death-1; RCC, renal cell carcinoma; SCLC, small cell lung cancer.
Overall incidence of hematologic toxicities
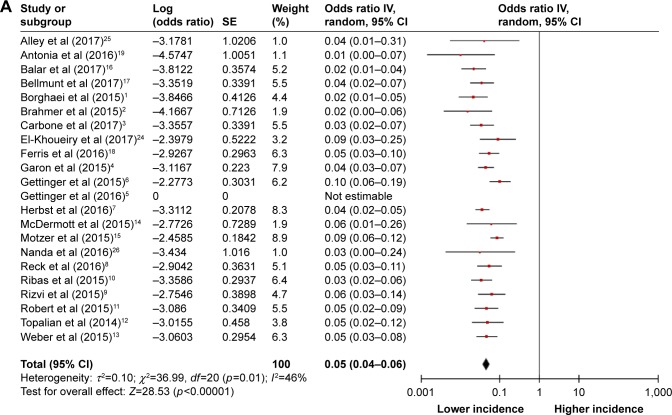
For the incidences of hematologic toxicities, all PD-1 inhibitor arms were considered. The summary incidences of all-grade anemia, thrombocytopenia, leukopenia, and neutropenia were 5% (95% CI 4%–6%), 2% (95% CI 1%–5%), 2% (95% CI 1%–3%), and 1% (95% CI 0–1%), respectively (Figure 2). The test for heterogeneity was significant for anemia and thrombocytopenia, so the random effects model was used to minimize the influence of heterogeneity. Regarding high-grade toxicities, there were relatively low incidences of anemia, thrombocytopenia, leukopenia, and neutropenia (Figure S1).
Figure 2.
Forest plots of incidences of all-grade hematologic toxicities during PD-1 inhibitor monotherapy: (A) anemia, (B) thrombocytopenia, (C) leukopenia, and (D) neutropenia.
Abbreviations: PD-1, programmed cell death-1; IV, inverse variance.
Incidence of hematologic toxicities in PD-1 inhibitor monotherapy versus combination therapy
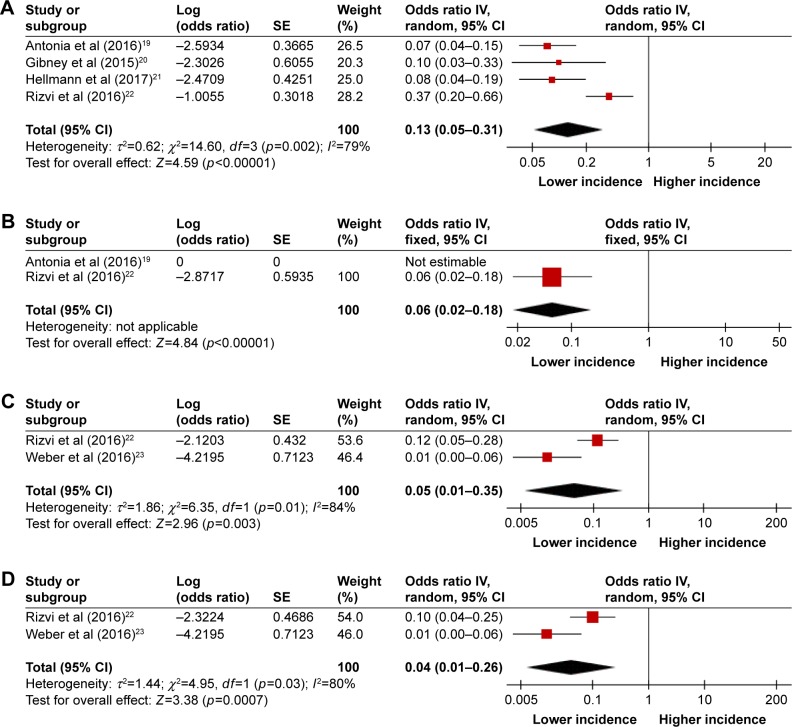
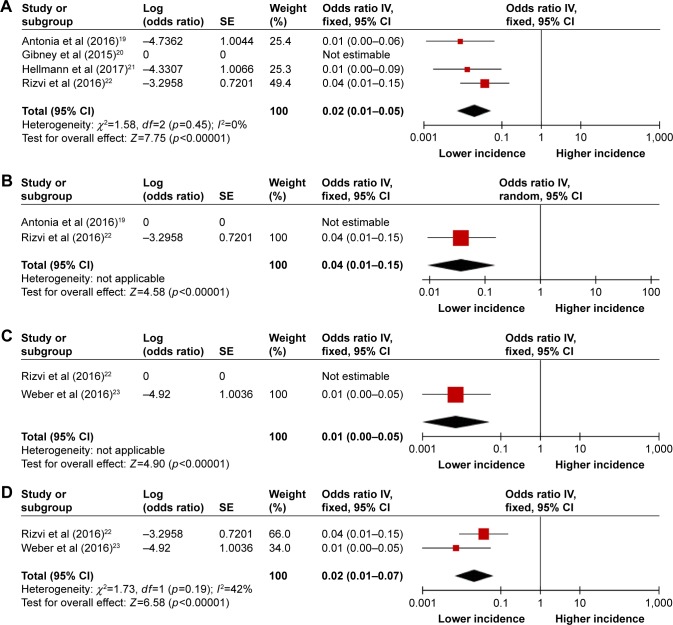
The incidences of hematologic toxicities during PD-1 inhibitor monotherapy versus combination therapy were compared in the studies of NSCLC, melanoma, and SCLC because the included studies of combination therapy were conducted in patients with these 3 tumor types.19–23 Combination regimens included nivolumab and ipilimumab given concurrently or sequentially,19,21,23 and nivolumab plus peptide vaccines20 or chemotherapy.22 The incidences were significantly higher in the combination therapy group compared with the monotherapy group for all-grade toxicities (13% vs 5% for anemia; 6% vs 2% for thrombocytopenia; 5% vs 2% for leukopenia; 4% vs 1% for neutropenia) (Figures 2 and 3) and high-grade thrombocytopenia (4% vs 1%) (Figures 4 and S1). However, there was no significant difference between the monotherapy group and combination therapy group for high-grade anemia, leukopenia, and neutropenia (Figures 4 and S1).
Figure 3.
Forest plots of incidences of all-grade hematologic toxicities during PD-1 inhibitor combination therapy: (A) anemia, (B) thrombocytopenia, (C) leukopenia, and (D) neutropenia.
Abbreviations: PD-1, programmed cell death-1; IV, inverse variance.
Figure 4.
Forest plots of incidences of high-grade hematologic toxicities during PD-1 inhibitor combination therapy: (A) anemia, (B) thrombocytopenia, (C) leukopenia, and (D) neutropenia.
Abbreviations: PD-1, programmed cell death-1; IV, inverse variance.
Incidence of hematologic toxicities in PD-1 inhibitor monotherapy versus chemotherapy or everolimus control
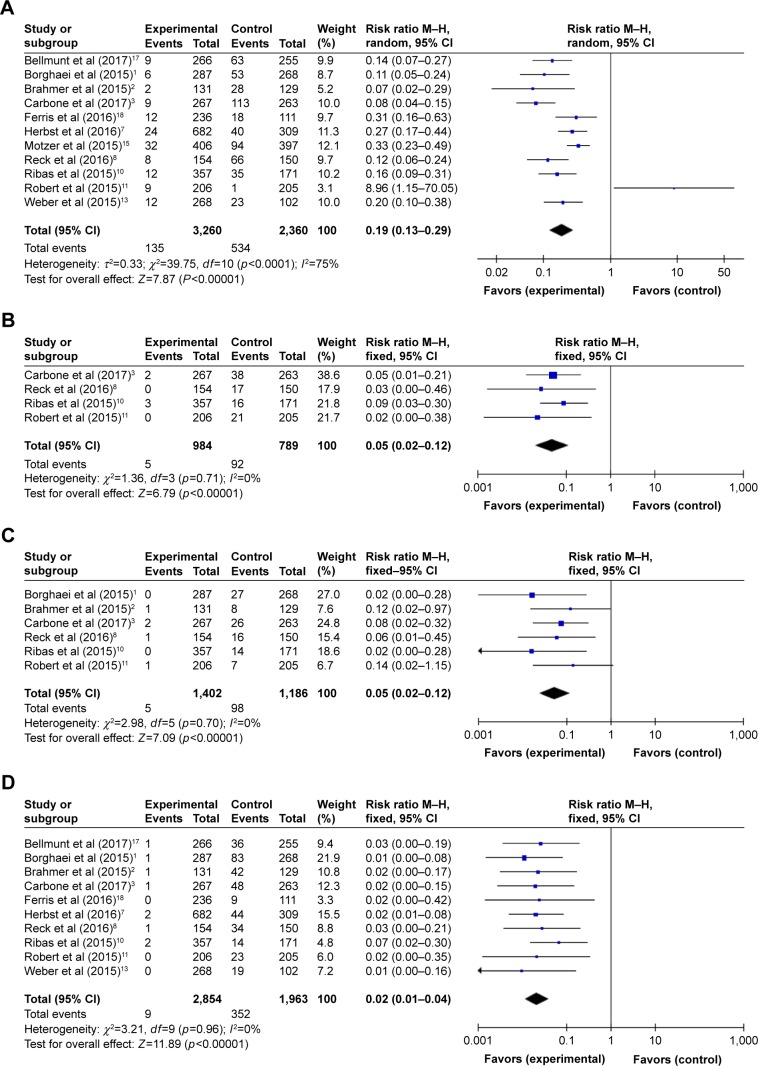
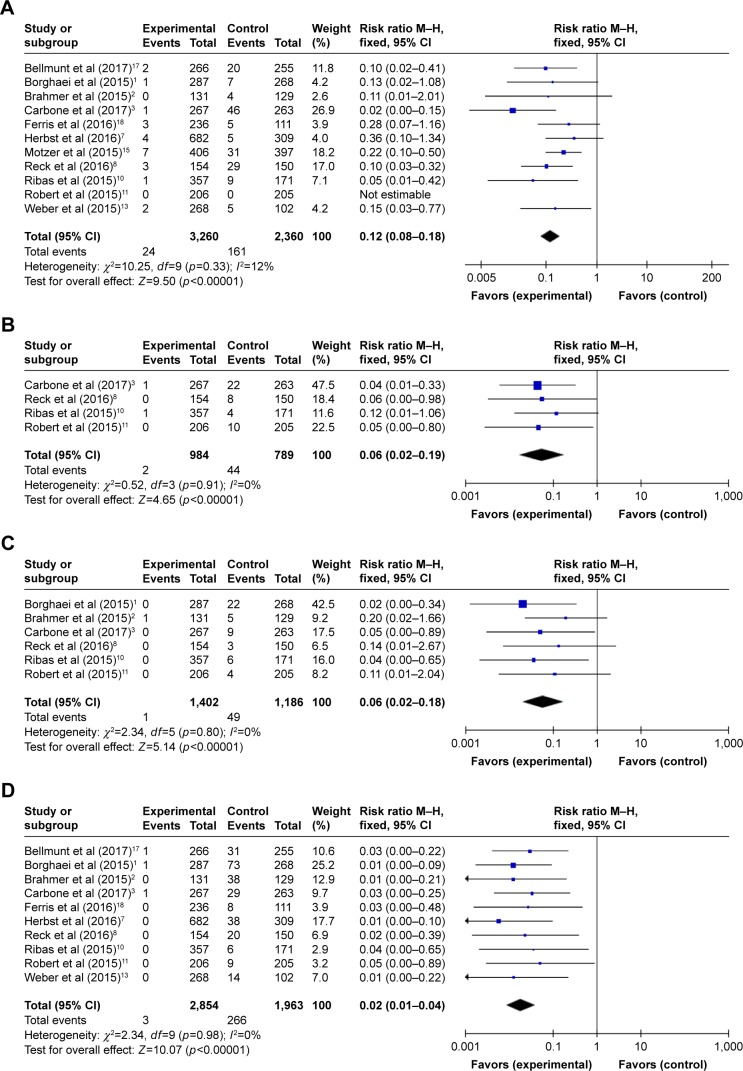
The relative risks (RRs) of hematologic toxicities were calculated by comparing the development of toxicities from the PD-1 inhibitor arm to those from the control arm in the same trial. The RRs of all-grade anemia, thrombocytopenia, leukopenia, and neutropenia were 0.19 (95% CI 0.13–0.29; p<0.00001), 0.05 (95% CI 0.02–0.12; p<0.00001), 0.05 (95% CI 0.02–0.12; p<0.00001), and 0.02 (95% CI 0.01–0.04; p<0.00001), respectively (Figure 5). The random effects model was used for calculating the RR of all-grade anemia owing to the remarkable heterogeneity. Moreover, the RRs of high-grade toxicities were 0.12 for anemia (95% CI 0.08–0.18; p<0.00001), 0.06 for thrombocytopenia (95% CI 0.02–0.19; p<0.00001), 0.06 for leukopenia (95% CI 0.02–0.18; p<0.00001), and 0.02 for neutropenia (95% CI 0.01–0.04; p<0.00001) (Figure 6). The combined results demonstrated that the use of PD-1 inhibitor monotherapy is associated with a decreased risk of developing all-grade and high-grade hematologic toxicities.
Figure 5.
Forest plots of relative risk of all-grade hematologic toxicities associated with PD-1 inhibitor monotherapy (experimental) versus chemotherapy or everolimus monotherapy (control): (A) anemia, (B) thrombocytopenia, (C) leukopenia, and (D) neutropenia.
Abbreviations: PD-1, programmed cell death-1; M–H, Mantel–Haenszel (dichotomous).
Figure 6.
Forest plots of relative risk of high-grade hematologic toxicities associated with PD-1 inhibitor monotherapy (experimental) versus chemotherapy or everolimus monotherapy (control): (A) anemia, (B) thrombocytopenia, (C) leukopenia, and (D) neutropenia.
Abbreviations: PD-1, programmed cell death-1; M–H, Mantel–Haenszel (dichotomous).
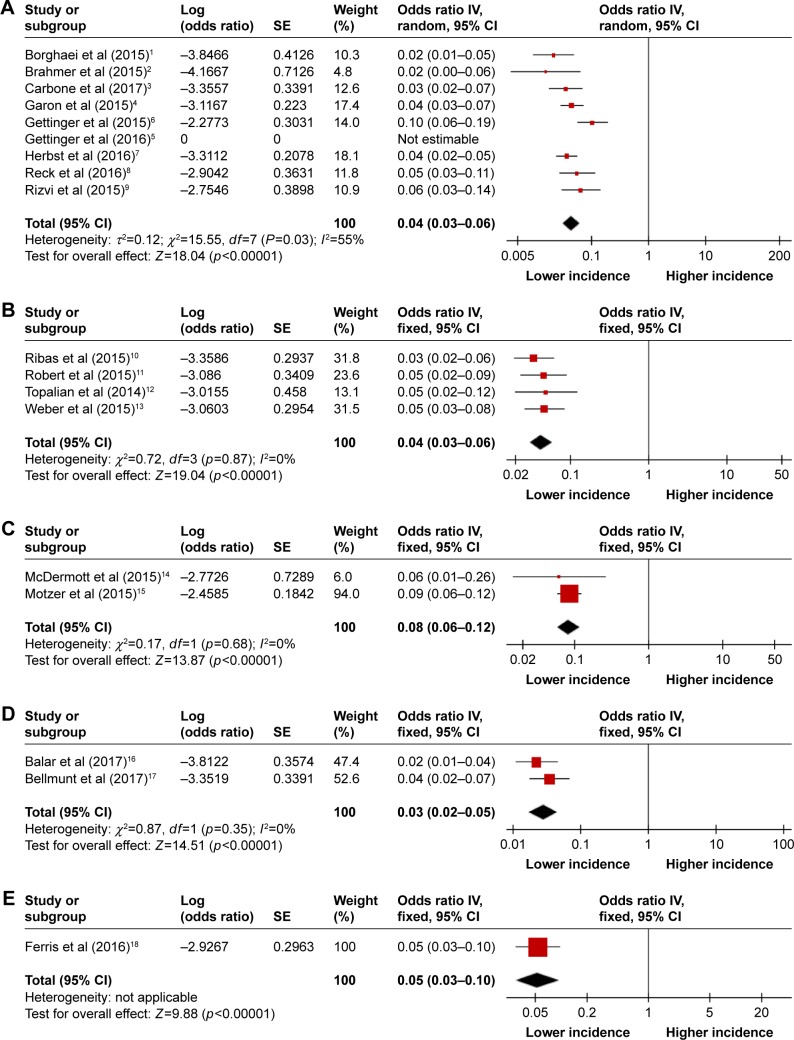
Incidence of hematologic toxicities during PD-1 inhibitor monotherapy in diverse tumor types
Subgroup analysis was conducted to investigate the incidences of hematologic toxicities by distinct tumor types. It is notable that the incidence of all-grade anemia appeared to occur somewhat more frequently in RCC (8%, 95% CI 6%–12%) compared with NSCLC (4%, 95% CI 3%–6%), melanoma (4%, 95% CI 3%–6%), urothelial carcinoma (3%, 95% CI 2%–5%), and HNSCC (5%, 95% CI 3%–10%), but the incidences of high-grade anemia among these tumor types could not be compared with each other because of the rare incidences (Figures 7 and S2). Moreover, no significant differences were noted between NSCLC and melanoma for all-grade leukopenia, neutropenia, and thrombocytopenia (Figure S3). However, this conclusion requires more trials for it to be further verified.
Figure 7.
Forest plots of incidence of all-grade anemia during PD-1 inhibitor monotherapy by tumor type: (A) NSCLC, (B) melanoma, (C) RCC, (D) urothelial carcinoma, and (E) HNSCC.
Abbreviations: PD-1, programmed cell death-1; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; HNSCC, head and neck squamous cell carcinoma; IV, inverse variance.
Publication bias
The heterogeneity of some studies was indicated by the assessment of all-grade anemia in the monotherapy data (I2=46%), which appeared to be concentrated in the studies on NSCLC (I2=55%). Funnel plots for the monotherapy data were shown to visually demonstrate publication bias (Figure S4).
Discussion
To date, the risks of developing hematologic toxicities from PD-1 inhibitors have not adequately assessed. Herein, we report the results of a meta-analysis including data from 26 clinical trials and 5,088 cancer patients, focused on PD-1 inhibitor-related hematologic toxicities. Our results demonstrated that the overall risk of all-grade anemia is significantly higher than the risk of thrombocytopenia, leukopenia, and neutropenia during PD-1 inhibitor monotherapy (Figure 2). Combination therapy was associated with a significantly increased risk of all-grade hematologic toxicities and only high-grade thrombocytopenia compared with monotherapy (Figures 2–4 and S1). Our analysis also revealed that compared with chemotherapy or everolimus control arms, the use of PD-1 inhibitor monotherapy was associated with a significantly lower risk of developing all-grade and high-grade hematologic toxicities (Figures 5 and 6). Furthermore, there was a nearly double incidence of all-grade anemia in RCC compared with NSCLC, melanoma, urothelial carcinoma, and HNSCC (Figure 7), implying a potential tumor-specific relationship that needs to be further clarified. To our knowledge, this is the first meta-analysis report to focus on PD-1 inhibitor-related hematologic toxicities in cancer patients that compares the risks among different therapeutic regimens and tumor types.
The incidence of hematologic toxicities was significantly higher in the combination therapy group compared with the monotherapy group for all-grade hematologic toxicities but only high-grade thrombocytopenia, indicating the additive effects of 2 agents in developing myelosuppression, particularly reducing platelet counts and potentially leading to severe bleeding. Of the agents used in combination regimens, ipilimumab is another immune-checkpoint inhibitor which acts via the cytotoxic T-lymphocyte antigen-4 pathway and is known to be associated with a variety of irAEs.27–30 Although less clinically apparent, and thus less recognized than PD-1 inhibitor-related hematologic toxicities, ipilimumab-associated myelosuppression has been previously reported in a clinical trial.27 Although the detailed role and effects of ipilimumab on the development and severity of hematologic toxicities when used in combination with PD-1 inhibitors remain to be further investigated, clinicians should be alerted to the significantly higher incidences of hematologic toxicities and perform regular hematologic monitoring in cancer patients during combination therapy.
A higher incidence of anemia among patients with RCC compared with patients with other tumor types may be due to these patients being more prone to developing drug-related erythrocytopenia because of underlying kidney conditions, including frequency of hematuria and incomplete deficiency of erythropoietin. Existing tumor burden in the kidney may also impair the capability for renal elimination of metabolites from the blood, leading to the accumulation of toxic metabolites, some of which are potent inhibitors of erythropoiesis. Although the underlying reasons remain to be further investigated, these observations emphasize a need for careful monitoring of hemoglobin concentration and red blood cell count in patients with RCC during PD-1 inhibitor therapy, because of the possibility of anemia. However, our results indicated that there is no different susceptibility to developing PD-1 inhibitor-related leukopenia, neutropenia, and thrombocytopenia among patients with different tumor types; this is probably due to the limited data from current trials.
Our meta-analysis has some limitations. First, the analysis was performed at the study level rather than analyzing data on the individual patient, meaning that potential variables at the patient level, such as age, prior chemotherapy, and palliative irradiation, were not included in the analysis. Second, different doses of therapeutic agents, frequencies of administration of agents, and types of malignancies may be sources of heterogeneity among the included studies. Third, we did not include hepatocellular carcinoma, triple-negative breast cancer, or malignant pleural mesothelioma for subgroup analysis because there was only one published report on each of these, with relatively small sample sizes. Likewise, other tumor types, such as lymphoma or ovarian cancer, were not included because of the paucity of published reports at the time of data collection. Finally, publication bias was present in the monotherapy data for all-grade anemia, which was particularly concentrated in NSCLC studies, probably reflecting underreporting of small, negative, or non-significant data in the published literature.
Conclusion
Our meta-analysis has demonstrated that PD-1 inhibitor monotherapy increases the risk of anemia in cancer patients, particularly in patients with RCC, compared with thrombocytopenia, leukopenia, and neutropenia. However, the risks of hematologic toxicities are lower in patients treated with PD-1 inhibitor monotherapy compared with combination therapy and chemotherapy or everolimus treatment. Moreover, the use of PD-1 inhibitors in combination with ipilimumab, peptide vaccines, or chemotherapy is associated with an increased risk of developing severe bleeding that requires platelet transfusions. The motive of this meta-analysis was to identify what is known and what remains uncertain based on systematic investigations of the published clinical data. We believe that with more data sharing from multiple studies in the future, we will move closer toward the united goal of maximizing the benefits of cancer immunotherapy.
Acknowledgments
This study was funded by National Natural Science Foundation of China (grant number 11575038), Natural Science Foundation of Chongqing City (grant numbers 2015ZDXM041 and 2016MSXM090), and Science and Technology Foundation of Chongqing City (grant number cstc2017jcyj-yszx0001).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage iv or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 5.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2980–2987. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 8.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 14.McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33(18):2013–2020. doi: 10.1200/JCO.2014.58.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 17.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 20.Gibney GT, Kudchadkar RR, DeConti RC, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21(4):712–720. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2969–2979. doi: 10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber JS, Gibney G, Sullivan RJ, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 2016;17(7):943–955. doi: 10.1016/S1470-2045(16)30126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 26.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312(17):1744–1753. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirumani SH, Ramaiya NH, Keraliya A, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2015;3(10):1185–1192. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]