Abstract
Purpose
Optical coherence tomography reveals retinal ganglion cell layer (GCL) and retinal nerve fiber layer (RNFL) thinning in chronic optic nerve injury. With acute optic nerve injury, as in acute nonarteritic anterior ischemic optic neuropathy (NAION), swelling obscures early demonstration of RNFL thinning, which might be used to evaluate therapies. We hypothesized that measurement of GCL plus inner plexiform layer (IPL) thickness and trajectory of thinning would show it is an earlier and more accurate biomarker of early permanent neuronal injury.
Methods
We prospectively studied 29 acute NAION eyes with standard automated perimetry and spectral domain (SD) optical coherence tomography for 6 months. We used a three-dimensional layer segmentation (method 1) and a commercial proprietary (method 2), to compute the combined thickness of macular GCL+IPL and method 2 to compute peripapillary RNFL thickness.
Results
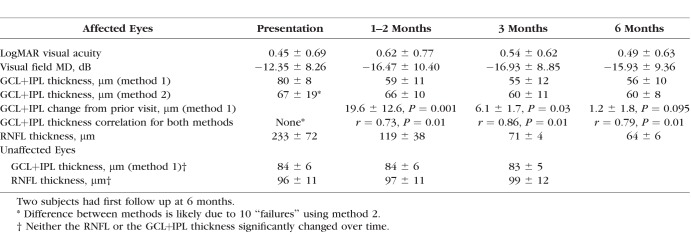
At presentation, the mean GCL+IPL thickness (78.7 μm ± 8.9) for NAION eyes, did not differ from unaffected fellow eyes (83 μm ± 6.4), using method 1 while method 2 (66.8 μm ± 18.7) failed in 34% of NAION eyes. At 1 to 2 months, 12% had RNFL loss compared to baseline, while 68% of NAION eyes had GCL+IPL thinning. The ganglion cell layer plus inner plexiform layer reduction was greatest at 1 to 2 months (19.6 μm ± 12.6) and was minimally worse after month 3. Ganglion cell layer plus inner plexiform layer thinning showed moderate to strong significant correlation with the visual acuity and mean deviation at each exam time. The retinal nerve fiber layer was not thinned until month 3.
Conclusions
Ganglion cell layer plus inner plexiform layer is acutely unaffected and provides a reliable measure of retinal neuronal structure using three-dimensional segmentation. Thinning develops within 1 to 2 months of onset, which is prior to RNFL swelling resolution. This suggests GCL+IPL measurement is better than the RNFL thickness to use as biomarker of early structural loss in NAION.
Keywords: retinal ganglion cell layer, OCT, non-arteritic anterior ischemic optic neuropathy
Acute optic nerve injury due to nonarteritic anterior ischemic optic neuropathy (NAION) typically causes significant visual field defects and reduced visual acuity. Significant vision loss is typically permanent with NAION. Optic nerve head swelling can last a month or more, making it difficult for optical imaging techniques, particularly optical coherence tomography (OCT), to determine if and when permanent structural injury to the optic nerve and optic nerve axons in the retinal nerve fiber layer (RNFL) has occurred.
Optical imaging is extensively used to evaluate the structure of the peripapillary RNFL in disorders affecting the optic nerve. Peripapillary RNFL thinning or loss correlates with visual field loss in glaucoma and after an episode of optic neuritis or NAION.1–9 In the most common optic neuropathy, glaucoma, retinal ganglion cell layer (GCL) thinning parallels the thinning of RNFL,10,11 and reflects progressive injury and field loss.12 Given that approximately 50% of retinal ganglion cells are located in the human macula region13 and NAION tends to frequently affect some portion of the central 10° of visual field, exploring the macula region GCL might be useful for detecting early structural loss, particularly when optic nerve head and RFNL swelling is present.
Methods that measure the GCL thickness, typically combined with the inner plexiform layer (IPL), reveal thinning or loss of these retinal layers in patients with long standing multiple sclerosis14,15 or months after optic nerve injury due to NAION.16 Thinning of the macula region, using whole retina thickness measurement is also found in longstanding multiple sclerosis (MS).17 A prior prospective case series showed GCL+IPL thinning in approximately 70% of eyes after a minimum of 6 months following an episode of NAION, but the method used might have underestimated the frequency and amount of loss due to the inclusion of the macula RNFL in the measurement.16 We postulated: (1) at acute presentation, GCL+IPL measurement would not be obscured or confounded by optic nerve head or RNFL swelling; (2) GCL+IPL thinning or loss would be demonstrated at 1 to 2 months, even while RNFL swelling was still present; and (3) at 6 months, the amount of GCL+IPL thinning would correlate with visual deficits. The goals of this study were to:
Demonstrate the trajectory of the macula region GCL+IPL thinning relative to RNFL thinning and whether GCL+IPL thickness could be a structural biomarker for early injury due to NAION;
Correlate macula region GCL+IPL thinning with vision performance at 1 to 2, 3, and 6 months;
Determine whether a three-dimensional segmentation (method 1) and the commercially available two-dimensional segmentation (method 2) for computing GCL+IPL thickness have clinical value and are comparable for NAION analysis.
Methods
Over a 3-year interval, we prospectively studied eyes with new onset NAION (n = 29), within 15 days of patient-reported vision loss, at 1 to 2 months (30 to 60 days from reported vision loss), and at 3 and (90 to 120 days from reported vision loss) and 3 months (approximately 180 days from vision loss). Each subject had complete clinical evaluation and standard automated threshold perimetry performed using the Humphrey Field Analyzer (Zeiss-Meditec, Inc., Dublin, CA, USA) with SITA 24-2 standard perimeter strategy using size III (expressed as mean deviation [MD] in decibels), and OCT of the optic disc and macula regions at each visit. Visual acuity was reported as logMAR values. This research was conducted with New York Eye and Ear Infirmary Institutional Review Board approval and adhered to the tenets of the Declaration of Helsinki.
The inclusion criteria included: (1) having acute painless unilateral vision loss within 15 days of the presentation evaluation; (2) unilateral swelling of the affected optic disc with RNFL thicker than the 95% limit of the control database provide for Cirrus; (3) visual field loss typical of NAION (not just enlarged blindspot); (4) relative afferent pupillary defect unless fellow eye affected in the past; and (5) no toxic or systemic infectious or inflammatory cause suggested by history or blood tests (complete hemogram, CRP, ESR, RPR, and FTA) performed in all patients.
Optical coherence tomography imaging was performed following pupillary dilation. For this study, we used one OCT machine (high definition, Cirrus spectral domain [SD] OCT, Zeiss-Meditec, Inc.) with laser scanned 6 × 6 mm area, capturing of a cube of data consisting of 200 A-scans from 200 linear B- scans for the optic nerve and macula regions. At least two volume scans were performed for each region on each eye and only images centered on the optic disc or macula with signal strength scores 6 or greater were analyzed. The Zeiss-Meditec, Inc., average peripapillary RNFL thickness was calculated in microns from values at 256 points in the peripapillary circumference.
For method 1, the GCL+IPL thickness (excluding the RNFL) in microns was calculated from macula data acquired from an area with dimensions of 6 × 6 mm with 128 b-scans, each with 512 pixels of horizontal width and 1024 pixel of vertical height (2 mm). Eleven intraretinal surfaces of each macula-centered volumetric scan were first segmented using a previously published graph-theoretic approach developed at the University of Iowa.18 The following surfaces were retained to enable computation of the fovea center and the combined ganglion cell layer and inner plexiform layer (GCL+IPL) thickness: (1) the internal limiting membrane, (2) the interface between the RNFL and the GCL, (3) the interface between the IPL and the inner nuclear layer (INL), and (4) the posterior surface of the retinal pigment epithelial layer. For each A-scan location, the GCL+IPL thickness was defined as the distance between the second surface (the interface between the RNFL and the GCL) and the third surface (the interface between the IPL and the INL). The mean GCL+IPL thickness was computed within an elliptical annulus (with a vertical inner and outer radius of 0.5 mm and 2.0 mm, respectively; and horizontal inner and outer radius of 0.6 mm and 2.4 mm, respectively) centered at the fovea.
Method 2, developed by Zeiss-Meditec, Inc., utilizes a GCL+IPL algorithm (part of Cirrus software 6.0.2) and finds the distance between the outer boundary of the RNFL and the outer boundary of the IPL to report the combined thickness of the GCL+IPL in microns.10 The average, minimum (lowest GCL+IPL thickness over a single meridian crossing the annulus), and sector thicknesses of the GCL+IPL are measured in an elliptical annulus (dimensions: vertical inner and outer radius of 0.5 mm and 2.0 mm, horizontal inner and outer radius of 0.6 and 2.4 mm, respectively) around the fovea. The size of the inner ring was chosen to exclude the area where the GCL is thin and difficult to detect, whereas the dimension of the outer ring was selected to conform closely to the real anatomy of the macular region, where the GCL is thickest in a normal eye. The input image data are initially segmented using the inner limiting membrane (ILM) and RPE segmentation algorithms to create a region of interest within which lie within the intraretinal layers. The algorithm continues in such a hierarchical approach, segmenting first the outer boundary of the outer plexiform layer (OPL), followed by the outer boundary of the IPL, and last the outer boundary of the RNFL.
We also measured the peripapillary RNFL thickness and the average thickness of the macula region GCL+IPL in the fellow eyes of study subjects. We calculated the variability in the average GCL+IPL in normal eyes (based on no history or clinical evidence by visual field, ophthalmoscopy, or OCT RNFL measurement suggestive of prior or current optic nerve pathology) opposite study eyes, by retesting on at least three visits over several months. These data provided the variability data for method 1 and the 95th percentile limit for control measurements. For normal eyes, the mean change between visits was 0.3 ± 2.0 μm and the 95th percentile for change was 4.6 μm. Reduction in the affected eye average GCL+IPL measurement greater than the 95th percentile limit was considered thinning of the GCL+IPL in study eyes.
We analyzed the data for all eyes using scan images that were centered on the optic disc for RNFL and macula for GCL+IPL determinations with signal strength ≥6. Although not ideal, we evaluated the central six points corresponding to the Garway-Heath organization of the papillomacular region19 and the central four-point corresponding to the macula region mapping for the GCL+IPL measurement by Cirrus.20 We determined these points were abnormal if the total deviation for a point was worse than −3 dB for the Humphrey database. The paired t-test was used to compare GCL+IPL values and RNFL values between visits (Table). Spearman nonparametric correlations were used to compare the GCL+IPL values of the two methods and to compare the RNFL and GCL+IPL findings to the visual acuity and MD (Table). The number of abnormal central four points in the baseline visual field was correlated with the GCL+IPL thickness using method 1 calculations and adjusted using the Bonferroni method.
Table.
Vision and OCT Findings in NAION Eyes Over Time
Results
We report results for 29 eyes with NAION in 18 males and 11 females, with a mean age 65 ± 12 years, who were evaluated on average at 8 ± 4 days from the reported onset of vision loss. The visual acuity and visual field loss at presentation and over time were typical of NAION (Table). The baseline visual field was abnormal for the central four points in 26 eyes and was abnormal for the six central points in 28 eyes. At 1 to 2 months, five eyes had worse MD (>4 dB less), three of which had worse visual acuity (>3 lines).
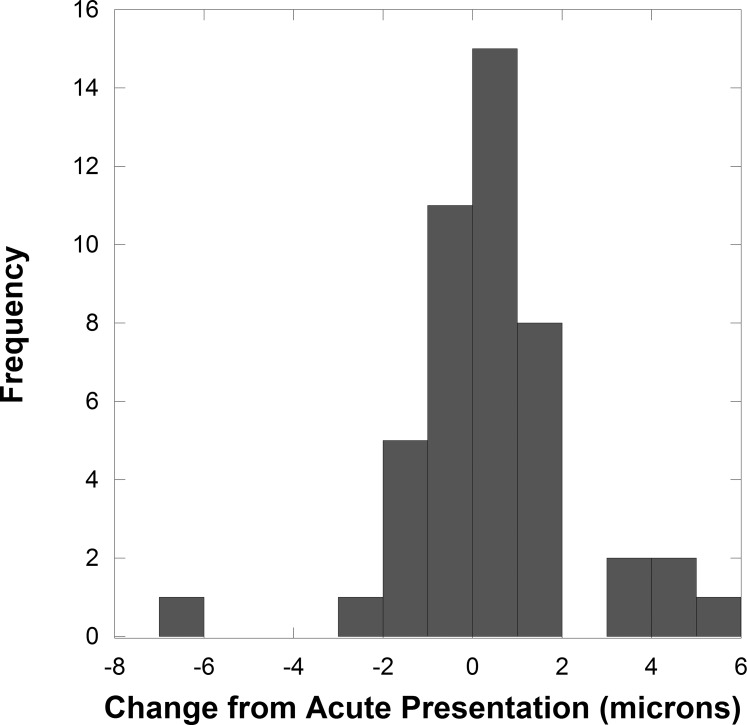
In general, methods 1 and 2 gave similar results for GCL+IPL measurement in the unaffected fellow eyes. For the same dates, the values from both methods showed a correlation with r = 0.96, P = 0.001. However, two additional eyes without optic neuropathy had pathological macula thickening (average total macula thickness ILM to RPE was >350 μm) due to an epiretinal membrane and method 2 calculated GCL+IPL values were spuriously reduced by approximately 35 μm compared with method 1 values in both eyes for four visits. Using method 1, all fellow eyes without optic nerve injury showed minor variability for repeat testing from baseline over 3 to 6 months (Fig. 1) and the 21 unaffected fellow eyes did not have a change in RNFL or GCL+IPL thickness over time (Table).
Figure 1.
The histogram shows a low frequency of GCL+IPL thickness measurement change >2 μm from baseline over three to four visits in 21 unaffected normal fellow eyes.
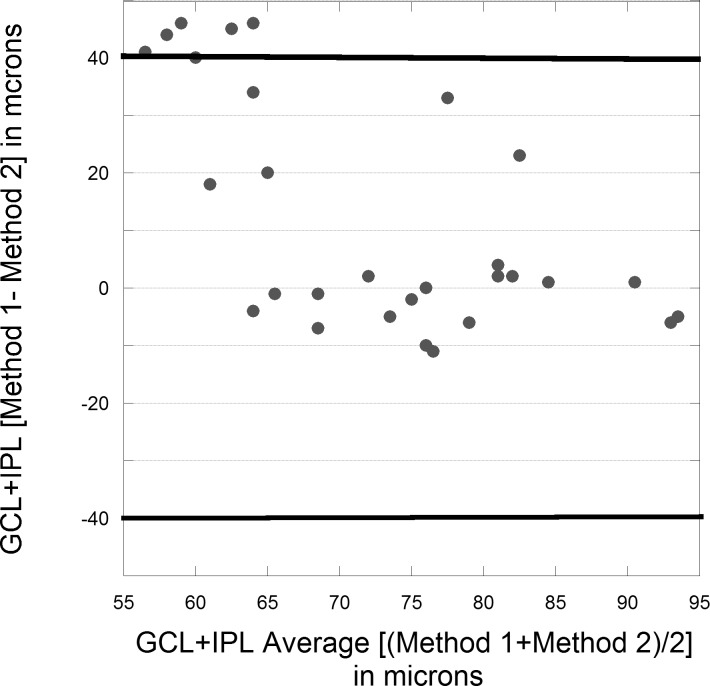
At presentation, the average RNFL was thickened (>95th percentile for Zeiss-Meditec, Inc., 65-year-olds, 103.5 μm) in all 29 NAION eyes, and the GCL+IPL thickness was normal in all affected eyes using method 1 (mean 78.7 ± 8.9 μm, and no eyes were < fifth percentile for method 1, 70.0 μm). The ganglion cell layer plus inner plexiform layer thickness at presentation for methods 1 and 2 did not correlate. The ganglion cell layer plus inner plexiform layer thickness appeared reduced by method 2 (66.8 ± 18.7 μm; Fig. 2). By method 2, 10 eyes (34%) had GCL+IPL values more than 20 μm less than by method 1 Supplementary Fig. S1 and 13 eyes < fifth percentile for method 2 (70.2 μm). The average total macula thickness was greater in these 10 eyes (303 ± 25 μm, P = 0.008) than in the remaining eyes (284 ± 14 μm). This difference in GCL+IPL determination between the two methods was primarily seen in eyes with more optic nerve head swelling as these 10 eyes had average RNFL mean 257 ± 48 μm at presentation. This is contrasted with a mean average RNFL of 213 ± 71 μm (P = 0.06) in the remaining 19 eyes with similar GCL+IPL measurements for both methods at presentation. Excluding the 10 eyes with apparent erroneous GCL+IPL reduction by method 2 at presentation, the GCL+IPL values derived from both methods were strongly correlated at presentation (r = 0.80, P = 0.01). The initial lower GCL+IPL values from method 2 rebounded and were thicker by more than 10 μm at the next visit (1 to 2 months) as the optic nerve head swelling as measured by the RNFL thickness was reduced by a mean of 108 ± 47 μm in all 10 eyes (which was comparable to 111 ± 62 μm reduction in RNFL thickness for other affected eyes).
Figure 2.
Bland-Altman plot of baseline GCL+IPL comparing method 1 and method 2. Dark horizontal lines represent two standard deviations from the average difference between both methods. Eleven eyes had measurement markedly less (plus direction) for method 2 and no eyes had a similar reduction for method 1 (minus direction).
At presentation method 1 showed no significant thinning or thickening of GCL+IPL thickness in eyes with NAION compared with normal fellow eyes. The mean difference for affected eye minus unaffected fellow eye GCL+IPL thickness was 3.3 ± 6.4 μm for NAION eyes. The number of abnormal four central points in the presentation visual field strongly correlated with the GCL+IPL thickness by method 1 at 1 to 2 (r = −0.72, P = 0.01), 3 (r = −0.64, P = 0.01), and 6 (r = −0.66, P = 0.01) months.
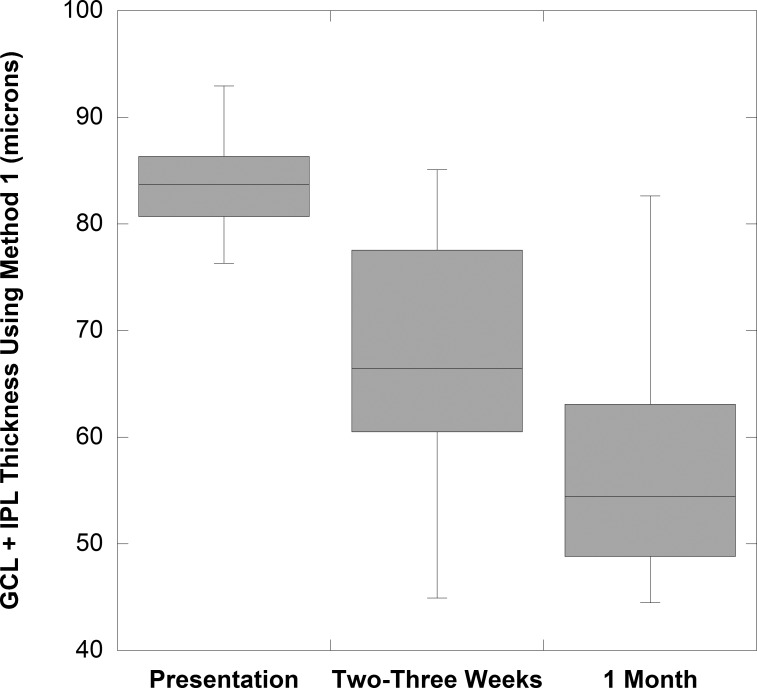
We evaluated 12 eyes with NAION (mean 15.9 ± 4.9 days) of patients who returned earlier than 1 month for evaluations (within 21 days of presentation) for numerous reasons. GCL+IPL thinning, compared with values at presentation, was seen in 10 of 12 NAION eyes (Fig. 3).
Figure 3.
Box plots of GCL+IPL thickness for 12 study NAION eyes evaluated within 2 to 3 weeks of acute presentation shows thinning before 1 month.
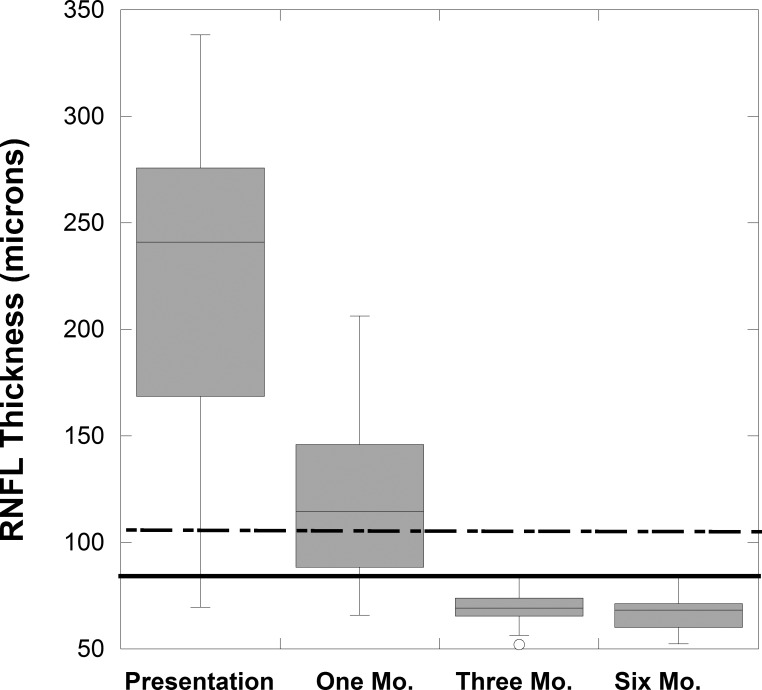
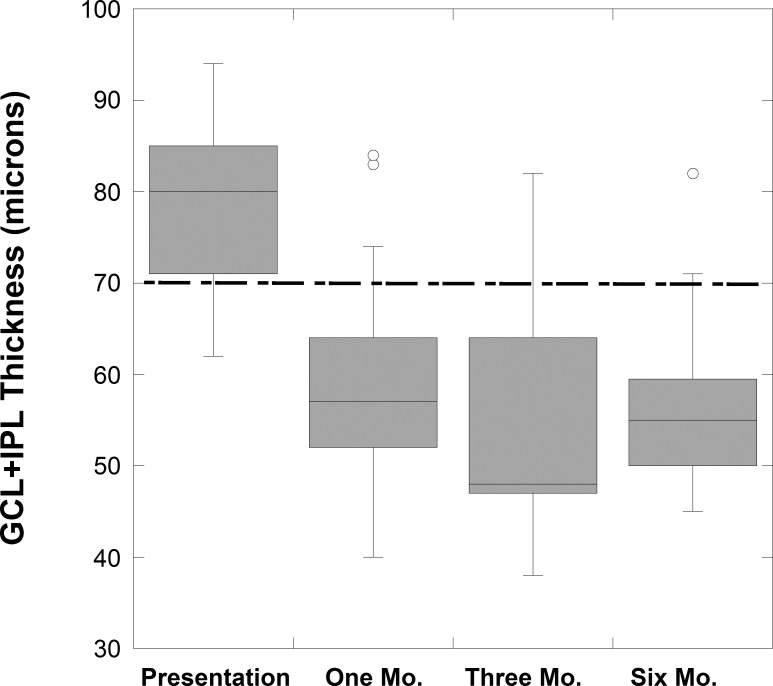
At 1 to 2 months, the mean RNFL was 119 ± 38 μm for 25 eyes with NAION (two patients did not returned until 6 months). The average RNFL was thinner than at presentation (Fig. 4) in all the NAION eyes (mean reduction was 110 ± 57 μm) but only three eyes had significant thinning (RNFL < fifth percentile for 65-year-old controls, 76 μm). For 23 of 25 NAION eyes (92%) at 1 to 2 months, the GCL+IPL measurements by method 1 were considered thinned (defined as reduced by > 4.6 μm) compared to the baseline measurement (Table; Fig. 5). The five eyes with worse MD did not have significantly worse GCL+IPL thickness. By method 1, the mean GCL+IPL thickness was significantly thinned and 17 of these eyes had thickness < fifth percentile.
Figure 4.
Box plots of RNFL thickness change for study NAION eyes over 6 months. The 95th limit (dashed line) and fifth limit (solid line) are shown for 65-year-old control eyes.
Figure 5.
Box plots for GCL+IPL thickness (by method 1) for study NAION eyes over 6 months. The fifth percentile limit for 65 years is shown (dashed line).
At 1 to 2 months, the GCL+IPL thickness values for all affected eyes using method 1 and 2 were similar (Table; r = 0.77, P = 0.001). The 1 to 2 months GCL+IPL thinning (by method 1) correlated with the visual acuity (r = 0.61, P = 0.01) and MD (r = −0.51, P = 0.011) at 1 to 2 months. The amount of GCL thinning at 1 to 2 months did not correlate with duration of vision loss or MD but did show moderate correlation (r = 0.45, P = 0.024) with the visual acuity at presentation.
After 1 to 2 months, RNFL and GCL+IPL (by both methods) thickness reduction continued but GCL+IPL thinning was minimal after 1 to 2 months compared with reduction in RNFL thickness, which was due to both loss and resolution of swelling (Table; Fig. 4). However, at both 3 and 6 months, all affected eyes had thinning of the GCL+IPL. The retinal nerve fiber layer was minimally but significantly less at 6 months compared to at 3 months (P = 0.025). At 6 months, the GCL+IPL measurements for both methods were similar to the values at 3 months (no statistical difference). The ganglion cell layer plus inner plexiform layer thickness values for all affected eyes using method 1 and 2 were strongly correlated at 3 (r = 0.73, P = 0.01) and at 6 months (r = 0.77, P = 0.001; Table). The 3-month GCL+IPL thinning from baseline measurement strongly correlated with the 3-month visual acuity (r = 0.68, P = 0.002), and MD (r = −0.61, P = 0.007). The 6-month GCL+IPL thinning from baseline thickness measurement correlated with the 6-month visual acuity (r = 0.42, P = 0.003), but less well with MD (r = −0.56, P = 0.07). The retinal nerve fiber layer thickness at 3 months did not correlate with either measure of vision, but the 6-month RNLF thickness did correlate (r = 0.54, P = 0.024) with the MD at this time point.
Discussion
This study shows that GCL+IPL thickness is normal at presentation of acute NAION. Thinning, which represents permanent loss of inner retinal neurons, retinal ganglion cell atrophy, or reduction of dendritic arborization, occurs at 1 month and in some cases even earlier. Retinal ganglion cell layer and IPL loss is more profound with NAION than reported for optic neuritis.21 Ganglion cell layer plus inner plexiform layer thinning below the fifth percentile of normal eyes occurred while the OCT still showed RNFL thickening, which confounds the ability of OCT to show RNFL loss early in an episode. Generally, the amount of GCL+IPL thinning correlated with the visual acuity and visual field MD deficit at 1 to 2 months to 6 months. Although it appears the trajectories for GCL+IPL and RFNL thickness reduction were similar, the RNFL thinning below the normal fifth percentile did not occur until 3 months and early reduction from baseline was likely due to less swelling. In contrast, affected eyes had the greatest amount of reduction in GCL+IPL thickness between presentation and 1 to 2 months. Ganglion cell layer plus inner plexiform layer thinning in all of the affected eyes at 3 and 6 months shows that NAION affects the macula region even if the visual field does not initially appear to affect the macula region. However, at presentation the visual field spared the macula in only 10% of affected eyes.
Using three-dimensional segmentation, the GCL+IPL thickness was normal and not thinned at presentation in the majority of eyes with NAION, when compared with the normal fellow eyes. However, method 2 for determining GCL+IPL thickness frequently failed when the peripapillary RNFL or macula was considerably thickened, presumably due to processes that distort normal retinal layer architecture. The method 2 algorithm failure caused GCL+IPL thickness at presentation to appear significantly thinned compared with the corresponding values obtained using method 1 for NAION affected eyes. However, in eyes without algorithm failure, the two methods gave similar results. At the 1- to 2-month follow up evaluation, the GCL+IPL thickness measurement showed no algorithm failures, and the mean values were similar between the two methods and correlated to each other. The likely explanation for failure in the setting of optic nerve head and retina swelling is due to the fact that method 2 utilizes an algorithm that assumes a quantitative relationship between the internal limiting membrane and the other layers of the retina. Thus, method 2 would be more susceptible to failure with any process, such as edema due to swelling of the peripapillary RNFL and adjacent retina, which disrupts the regular retinal layer position or shape or boundaries. In contrast, method 1 uses an algorithm that incorporates three-dimensional contextual information into the optimization process, which in general helps to reduce failures due to local distortions in retinal layers. In clinical practice, it is important to carefully evaluate algorithm performance in OCT scans, since methodological failures may lead to false interpretations of data and may adversely influence clinical decisions.
Optical coherence tomography demonstration of thinning of the RNFL and GCL+IPL is clinically important in determining irreversible neuronal loss and correlates with measures of vision loss in glaucoma,22 NAION,6,16,23 optic neuritis, and MS.10,11,15–17,24 Ganglion cell layer loss has long been shown on histopathology in glaucoma22 and recently in eyes of patients with MS, even without a history of optic neuritis.24,25 Further, optical coherence tomography shows that even after months, NAION spares the other retinal layers, such as the inner and outer nuclear, outer plexiform, retinal pigment epithelium, and photo receptor layers.26 Studies are needed to show the earliest signs of structural injury with all optic neuropathies. For example, with NAION, scanning layer polarimetry at presentation shows a loss of retinal birefringence suggesting that early axonal damage can be detected and is likely to be irreversible.27 Our study suggests that permanent structural loss or atrophy or size reduction of retina ganglion cells can occur prior to the 1-month time point with NAION. Further, we showed 92% of NAION eyes had reduction of the macula region average GCL+IPL thickness at 1 to 2 months and majority of the loss for also occurred during this interval. In contrast to the GCL+IPL measurement, at 1 to 2 months the RNFL thickness cannot be used to demonstrate consistent axonal loss due to the persistence of edema and swelling.
In a small group of our patients, who happened to return for follow up evaluation earlier than scheduled, GCL+IPL thinning developed within 2 to 3 weeks after presentation. These cases suggest that retinal ganglion cell body loss or atrophy or reduced dendritic arborizations could occur prior to the 1-month time point in NAION.
After 1 month, the RNFL swelling lessens, and the RNFL thickness measurement in part reflects the loss and thinning of affected axons. By 3 months, most eyes show RNFL thinning while the GCL+IPL thinning had essentially stopped. These data suggest that the subclinical edema or swelling persists in the RNFL for at least 3 months.
The early GCL+IPL thinning appears to be greatest in NAION eyes with worse presentation visual acuity, a possible reflection of greater initial injury. This is consistent with a profound ischemic injury of axons that are close to their cell bodies and the persistent, likely permanent vision loss in almost all cases of NAION. GCL+IPL thickness measurement appears to be a better biomarker of early structural injury with acute optic nerve injury than OCT measured peripapillary RNFL thickness, particularly when optic nerve head or RNFL swelling is present. The amount of GCL+IPL thinning correlates with the amount of visual acuity and visual field loss better than the actual thickness measurements.26 Given that the most profound GCL+IPL thinning occurs early, potential neural preservation or protection therapy must be delivered before 1 month to reduce retinal ganglion cells loss.
Supplementary Material
Acknowledgments
Supported, in part, by 3U10EY017281-04AS1, NEI subcontract R009040554, NEI R01 EY018853, R01 EY023279, the Department of Veterans Affairs Rehabilitation Research and Development Division (Iowa City Center for the Prevention and Treatment of Visual Loss and Career Development Award 1IK2RX000728 for MKG), and Research to Prevent Blindness (New York, NY, USA).
Disclosure: M.J. Kupersmith, None; M.K. Garvin, None; J.-K. Wang, None; M. Durbin, Zeiss-Meditec, Inc. (E); R. Kardon, None
References
- 1. Raza A, Jungsuk C, de Moraes C,et al. . Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol. 2011. ; 129: 1529– 1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takagi S, Kita Y, Fumihiko Y, Tomita G. . Macular retinal ganglion cell complex damage in the apparently normal visual field glaucomatous eyes with hemifield defects. J Glaucoma. 2012. ; 21: 318– 325. [DOI] [PubMed] [Google Scholar]
- 3. Costello F, Coupland S, Hodge W,et al. . Quantifying axonal loss after optic neuritis with ocular coherence tomography. Ann Neurol. 2006. ; 59: 963– 969. [DOI] [PubMed] [Google Scholar]
- 4. Trip A, Schlottmann P, Jones S,et al. . Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005. ; 58: 383– 391. [DOI] [PubMed] [Google Scholar]
- 5. Danish-Meyer H, Carroll S, Ku J,et al. . Correlation of retinal nerve fiber layer measured by scanning laser polarimeter to visual field in ischemic optic neuropathy. Arch Ophthalmol. 2006. ; 124: 1720– 1726. [DOI] [PubMed] [Google Scholar]
- 6. Contrearas I, Noval S, Rebolleda G, Munoz-Negrete F. . Follow-up of nonarteritic anterior ischemic optic neuropathy with optical coherence tomography. Ophthalmology. 2007. ; 114: 2338– 2344. [DOI] [PubMed] [Google Scholar]
- 7. Hood D, Anderson S, Rouleau J,et al. . Retinal nerve fiber structure versus visual field function in patients with ischemic optic neuropathy. Ophthalmology. 2008. ; 115: 904– 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeLeon-Ortega J, Carrol K, Arthur S, Girkin C. . Correlations between retinal nerve fiber layer and visual field in eyes with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2007. ; 143: 288– 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellusci C, Savini G, Carbonelli M, Carelli V, Sadaun A, Barboni P. . Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefs Arch Clin Exp Ophthalmol. 2008. ; 246: 641– 647. [DOI] [PubMed] [Google Scholar]
- 10. Mwanza JC, Durbin M, Budenz D,et al. . Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012. ; 119: 1151– 1158. [DOI] [PubMed] [Google Scholar]
- 11. Koh V, Tham YC, Cheung C,et al. . Determinants of ganglion cell-inner plexiform layer thickness measured by high-definition optical coherence tomography. Invest Ophthalmol Vis Sci. 2012. ; 53: 5853– 5859. [DOI] [PubMed] [Google Scholar]
- 12. Na JH, Kook M, Lee Y, Baek S. . Structure-function relationship of the macular visual field and the ganglion cell complex thickness in glaucoma. Invest Ophthalmol Vis Sci. 2012. ; 53: 5044– 5051. [DOI] [PubMed] [Google Scholar]
- 13. Curcio C, Allen K. . Topography of ganglion cells in human retina. J Comp Neurol. 1990. ; 300: 5– 25. [DOI] [PubMed] [Google Scholar]
- 14. Fisher JB, Jacobs DA, Markowitz CE,et al. . Relation of visual function to retinal nerve fiber. Brain. 2012. ; 135: 521– 533. [DOI] [PubMed] [Google Scholar]
- 15. Walter S, Ishikawa H, Galetta K,et al. . Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012. ; 119: 1250– 1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aggarwal D, Tan O, Huang D, Sadun A. . Patterns of ganglion cell complex and nerve fiber layer loss in nonarteritic ischemic optic neuropathy by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012. ; 53: 4539– 4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burkholder B, Osborne B, Loguice M,et al. . Macular volume determined by optic coherence tomography as a measure of neuronal loss in multiple sclerosis. Arch Neurol. 2009. ; 66: 1366– 1372. [DOI] [PubMed] [Google Scholar]
- 18. Garvin MK, Abràmoff MD, Kardon R, Russell SR, Wu X, Sonka M. . Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search. IEEE Trans Med Imaging. 2008. ; 27: 1495– 1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garway-Heath D, Poinoosawmy D, Fitzke F, Hitchings R. . Mapping the visual field to the optic disc in normal tension glaucoma. Ophthalmology. 2000. ; 107: 1809– 1815. [DOI] [PubMed] [Google Scholar]
- 20. Hood D, Raza A, de Moraes C, Liebmann J, Ritch R. . Glaucomatous damage of the macula. Prog Retin Eye Res. 2013. ; 32: 1– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kupersmith M, Garvin M, Wang JK, Durbin M, Kardon R. . Retinal ganglion cell layer thinning within one month of presentation of optic neuritis. Mult Scler. 2016. ; 22: 641– 648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lei Y, Garrahan N, Hermann B,et al. . Quantification of retinal transneuronal degeneration in human glaucoma: a novel multiphoton-DAPI approach. Invest Ophthalmol Vis Sci. 2008. ; 49: 1940 –194. [DOI] [PubMed] [Google Scholar]
- 23. Gonul S, Koktekir BE, Bakbak B, Gedik S, . Comparison of the ganglion cell complex and retinal nerve fibre layer measurements using Fourier domain optical coherence tomography to detect ganglion cell loss in non-arteritic ischaemic optic neuropathy. Br J Ophthalmol. 2013. ; 97: 1045– 1050. [DOI] [PubMed] [Google Scholar]
- 24. Syc S, Saidha S, Newsome S,et al. . Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012. ; 135: 521– 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Green A, McQuaid S, Hauser S, Allen I, Lyness R. . Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010. ; 133: 1591– 1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rebolleda G, Sanchez-Sanchez C, Gonzalez-Lopez J, Contreras I, Munoz-Negrete F. . Papillomacular bundle and inner retinal thickness correlate with visual acuity in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2015. ; 56: 682– 692. [DOI] [PubMed] [Google Scholar]
- 27. Kupersmith M, Kardon R, Durbin M, . Horne MK, Schulman J. Scanning laser polarimetry reveals status of axon integrity in areas of optical coherence tomography revealed thickened retinal nerve fiber layer (RNFL) with optic nerve head swelling. Invest Ophthalmol Vis Sci. 2012. ; 53: 1962– 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.