Leptin signaling has been found to play an important role in the development of pulmonary hypertension. It was demonstrated that leptin dose- and time-dependently resulted in PPARγ reduction in pulmonary artery smooth muscle cells (PASMC). The study indicates that the ERK1/2 signaling pathway partially mediates leptin-induced PPARγ reduction and PASMC proliferation through up-regulation of Egr-1.
Abstract
Loss of peroxisome proliferator-activated receptor γ (PPARγ) has been found to contribute to pulmonary artery smooth muscle cell (PASMC) proliferation and pulmonary arterial remodeling therefore the development of pulmonary hypertension (PH). Yet, the molecular mechanisms underlying PPARγ reduction in PASMC remain poorly understood. Here, we demonstrated that leptin dose- and time-dependently inducued PPARγ down-regulation and proliferation of primary cultured rat PASMC, this was accompanied with the activation of extracellular regulated kinase1/2 (ERK1/2) signaling pathway and subsequent induction of early growth response-1 (Egr-1) expression. The presence of MEK inhibitors U0126 or PD98059, or prior silencing Egr-1 with small interfering RNA suppressed leptin-induced PPARγ reduction. In addition, activation of PPARγ by pioglitazone or targeting ERK1/2/Egr-1 suppressed leptin-induced PASMC proliferation. Taken together, our study indicates that ERK1/2 signaling pathway-mediated leptin-induced PPARγ reduction and PASMC proliferation through up-regulation of Egr-1 and suggests that targeting leptin/ERK1/2/Egr-1 pathway might have potential value in ameliorating vascular remodeling and benefit PH.
INTRODUCTION
Pulmonary hypertension (PH) is a life-threatening disease characterized by increased pulmonary vascular resistance and pressure, which finally leads to right ventricular failure and death (Bazan and Fares, 2015). Despite various treatments have been used during the past few decades, PH is still incurable (Humbert et al., 2010). Different types of PH share a common pathogenesis including vasoconstriction, pulmonary vascular remodeling, and thrombosis in situ (Humbert et al., 2004). The essential pathological characteristics of PH are excessive proliferation of pulmonary arterial smooth muscle cells (PASMC), leading to medial hypertrophy and vascular remodeling. However, the molecular mechanisms underlying this process are still not well understood.
Leptin is a 16-kDa, 146-amino-acid residue nonglycosylated protein encoded by obese (ob) gene and mainly synthesized and secreted by adipocytes (Zhang et al., 1994) and exerts its actions through its specific receptors present in a variety of tissues (Fruhbeck, 2006). Leptin is primarily known for its role as a hypothalamic modulator of food intake, body weight, and fat stores (Akther et al., 2009). In addition, leptin is also implicated in the modulation of other physiological processes, such as angiogenesis, wound healing, central and peripheral endocrine actions, and renal and pulmonary functions (Mantzoros et al., 2011). Recently, leptin signaling has been found to play an important role in the development of PH by stimulating PASMC proliferation (Schroeter et al., 2013; Chai et al., 2015; Huertas et al., 2015, 2016). However, the exact mechanisms underlying leptin-induced PASMC proliferation are still largely unknown.
Peroxisome proliferator-activated receptor γ (PPARγ) is ubiquitously expressed in pulmonary vascular endothelial and smooth muscle cells and belongs to the nuclear hormone receptor superfamily with increasingly diverse functions as a transcriptional regulator (Tian et al., 2009; Gong et al., 2011). Mounting evidence has shown that activation of PPARγ attenuates PASMC proliferation and suppresses the development of PH in several animal models (Crossno et al., 2007; Li et al., 2010; Zhang et al., 2014; Xie et al., 2015), while PPARγ expression is reduced in the lungs and pulmonary vascular tissue of patients with PH and in several experimental models of PH (Ameshima et al., 2003; Tian et al., 2009; Gong et al., 2011; Lu et al., 2013). Several lines of evidence indicate that the increased leptin level is associated with the down-regulation of PPARγ and promotes cell proliferation in various cell types of nonpulmonary artery smooth muscle cell (Zhou et al., 2009a; Jain et al., 2011; Wang et al., 2012). However, it is still unclear whether leptin also causes the down-regulation of PPARγ and implicates in PASMC proliferation. To clarify this, primary cultured PASMC were stimulated with leptin, the expression of PPARγ and phosphorylation of ERK1/2 were determined, and the molecular mechanisms underlying these changes were further investigated.
RESULTS
Leptin stimulates PASMC proliferation
To examine whether leptin induces PASMC proliferation, time course and dose–response of leptin on cells proliferation were investigated. Cell proliferation was determined using the BrdU incorporation assay. As shown in Figure 1A, leptin dose-dependently stimulated PASMC proliferation at 24 h, and the maximal BrdU incorporation was a 2.30-fold increase over control at 100 ng/ml leptin (p < 0.01). Figure 1B demonstrates that leptin induced PASMC proliferation in a time-dependent manner; 100 ng/ml leptin caused a significant increase in BrdU incorporation over control after 24 h, and BrdU incorporation was a 2.81-fold increase compared with control at 48 h (p < 0.01).
FIGURE 1:
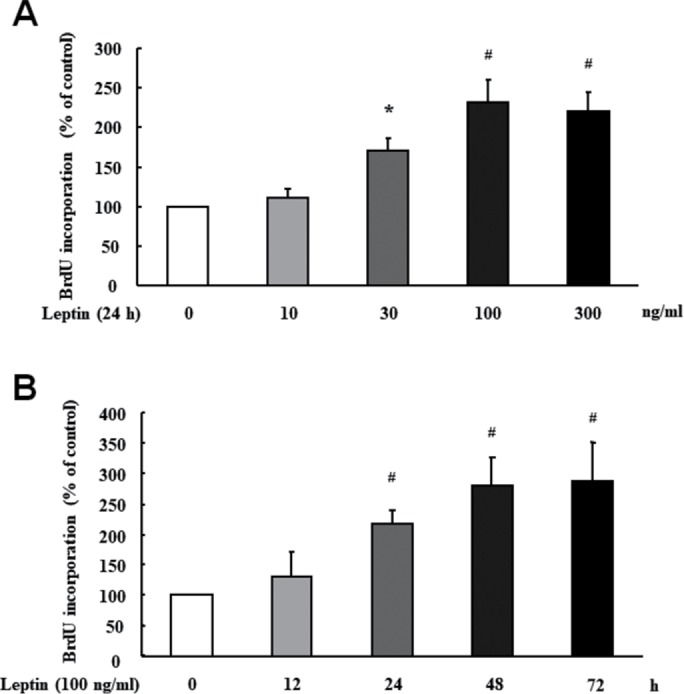
Leptin stimulates PASMC proliferation. (A) PASMC were stimulated with different concentration of leptin ranging from 0–300 ng/ml for 24 h, and the rate of BrdU incorporation in cells was determined using the BrdU ELISA Kit (n = 5 each group). (B) Cells were exposed to 100 ng/ml for the indicated times, BrdU incorporation in cells was measured (n = 5 each group). *p < 0.05 vs. control; #p < 0.01 vs. control.
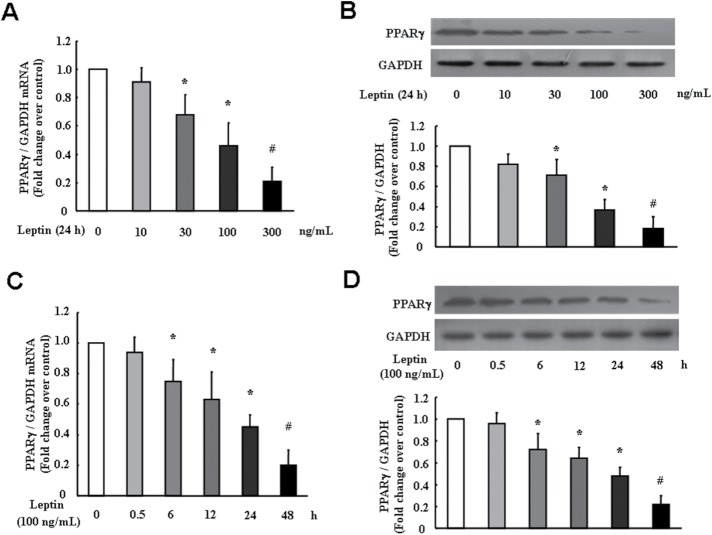
Leptin down-regulates PPARγ expression in PASMC
It has been shown that leptin down-regulates PPARγ expression in several types of nonPASMC (Zhou et al., 2009a; Jain et al., 2011; Wang et al., 2012). To clarify whether leptin also reduces PPARγ expression in PASMC, cells were treated with different concentrations of leptin over different time periods, and the expression of PPARγ was determined using quantitative real-time reverse transcription PCR (qRT-PCR) and immunoblotting. As shown in Figure 2, A and B, leptin down-regulated PPARγ expression in PASMC in a dose-dependent manner at 24 h; 100 ng/ml leptin reduced PPARγ mRNA and protein levels to 0.46-and 0.37-fold compared with control, respectively (both p < 0.05). Figure 2, C and D, shows that leptin down-regulated PPARγ expression in PASMC in a time-dependent manner after 6 h treatment, and 100 ng/ml leptin for 24 h incubation reduced PPARγ mRNA and protein levels to 0.45- and 0.42-fold compared with control, respectively (both p < 0.05). These results suggest that leptin also suppresses PPARγ expression in PASMC.
FIGURE 2:
Leptin dose- and time-dependently reduces PPARγ expression in PASMC. Cells were treated with different concentrations of leptin ranging from 0 to 300 ng/ml for 24 h, and the levels of PPARγ mRNA (A) and protein (B) were determined using RT-PCR and immunoblotting (n = 5 each group). Cells were treated with 100 ng/ml leptin for the indicated times, and the levels of PPARγ mRNA (C) and protein (D) were determined using RT-PCR and immunoblotting (n = 4 each group). *p < 0.05 vs. control and #p < 0.01 vs. control.
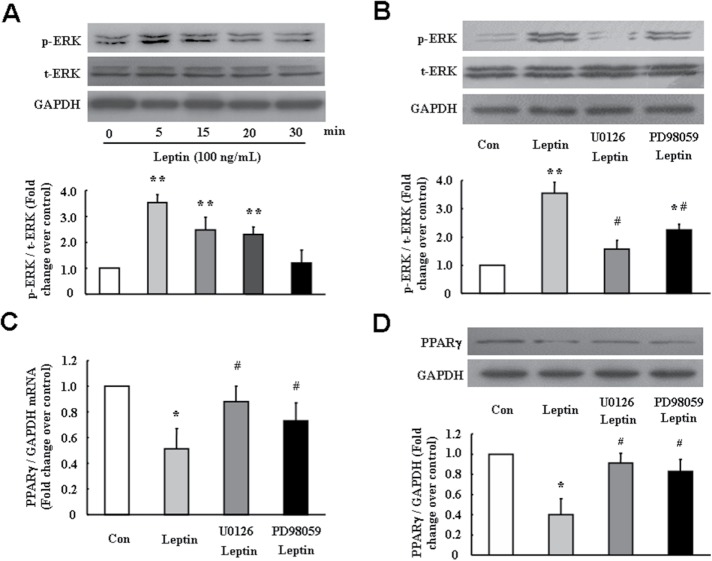
Activation of ERK1/2 signaling mediates leptin-induced PPARγ reduction in PASMC
To investigate the mechanisms of leptin-induced PPARγ reduction, cells were treated with leptin (100 ng/ml) for different times; phosphorylation of ERK1/2 was determined using immunoblotting. As shown in Figure 3A, ERK1/2 phosphorylation was time dependent on 100 ng/ml leptin stimulation. Peak phosphorylation occurred at 5 min, which increased 3.54-fold over control (p < 0.01). To further examine whether ERK1/2 signaling mediated leptin-induced PPARγ down-regulation in PASMC, cells were pretreated with MEK inhibitor U0126 (10 μM) or PD98059 (10 μM) for 30 min followed by leptin (100 ng/ml) stimulation for 5 min or 24 h. The phosphorylation of ERK1/2 was measured after leptin stimulation for 5 min, and mRNA and protein levels of PPARγ were determined at 24 h. Figure 3B indicates that leptin induced a significant ERK1/2 phosphorylation, and this effect was suppressed by either MEK inhibitor U0126 or PD98059, which decreased from a 3.3-fold increase over control in leptin-treated cells to a 1.57- and a 2.25-fold increase over control, respectively (both p < 0.05 vs. leptin-treated cells). As shown in Figure 3C, the presence of U0126 or PD98059 dramatically blocked leptin-induced reduction of PPARγ mRNA level, which increased from 0.51-fold over control in leptin-treated cells to 0.88- and 0.73-fold over control, respectively (both p < 0.05). Similarly, pretreatment of cells with U0126 or PD98059 also suppressed leptin-induced reduction of PPARγ protein level, which increased from 0.40-fold over control in leptin stimulated cells to 0.91- and 0.83-fold over control, respectively (both p < 0.05) (Figure 3D). These results suggest that ERK1/2 signal pathway particularly mediated leptin-induced PPARγ down-regulation in PASMC.
FIGURE 3:
ERK1/2 signaling pathway mediates leptin-induced PPARγ reduction in PASMC. (A) Cells were treated with 100 ng/ml leptin for indicated times. The levels of p-ERK1/2 and t-ERK1/2 were determined using immunoblotting. GAPDH was used as the loading control (n = 5 each group). (B) Cells were pretreated with MEK inhibitor U0126 (10 μM) or PD98059 (10 μM) for 30 min followed stimulation with leptin (100 ng/ml) for 5 min, and the levels of p-ERK1/2 and t-ERK1/2 were determined using immunoblotting. GAPDH was used as the loading control (n = 4 each group). Cells were pretreated with MEK inhibitor U0126 (10 μM) or PD98059 (10 μM) for 30 min and then stimulated with leptin (100 ng/ml) for 24 h. The levels of PPARγ mRNA (C) and protein (D) were determined using RT-PCR and immunoblotting. GAPDH served as loading control (n = 5 each group). *p < 0.05 vs. control; **p < 0.01 vs. control; #p < 0.05 vs. leptin group.
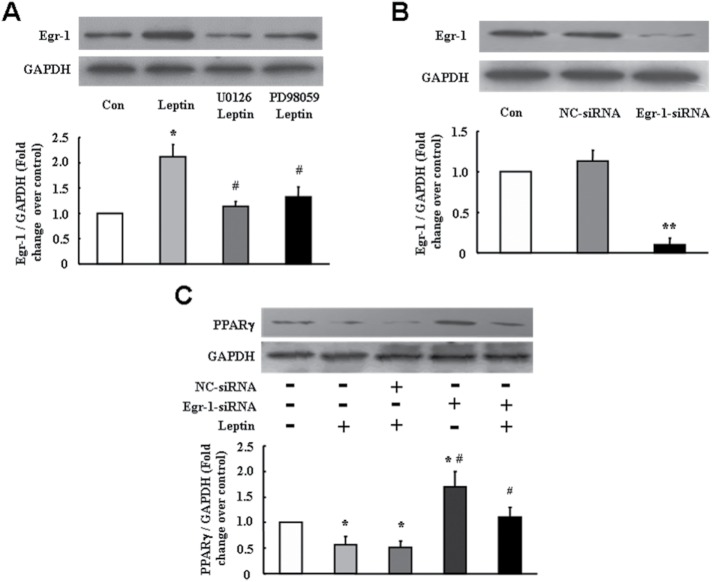
Up-regulation of Egr-1 by ERK1/2 signaling mediates leptin-induced PPARγ reduction
It has been shown that activation of ERK1/2 signaling up-regulates Egr-1 expression in several types of nonPASMC (Hartney et al., 2011; Lee et al., 2015; Huynh et al., 2016; Simo-Cheyou et al., 2016; Sysol et al., 2016; Wang et al., 2016). Previous studies have reported that the PPARγ proximal promoter contains an overlapping binding site for Egr-1, which is involved in the down-regulation of PPARγ (Zhou et al., 2009b; Nebbaki et al., 2012). It is therefore interesting to examine whether induction of Egr-1 by ERK1/2 activation mediates leptin-induced PPARγ down-regulation in PASMC. Cells were incubated with MEK inhibitor U0126 (10 μM) or PD98059 (10 μM) for 30 min followed by leptin (100 ng/ml) stimulation for 24 h. Figure 4A shows that PASMC treated with 100 ng/ml leptin for 24 h exhibited a 2.11-fold increase in Egr-1 protein level compared with control (p < 0.01), while pretreatment of cells with MEK inhibitor U0126 or PD98059 dramatically suppressed leptin-induced up-regulation of Egr-1, which reduced to a 1.14- and a 1.32-fold increase over control, respectively (p < 0.05).
FIGURE 4:
Up-regulation of Egr-1 by ERK1/2 signaling mediates leptin-induced PPARγ reduction. (A) Cells were pretreated with MEK inhibitor U0126 (10 μM) or PD98059 (10 μM) for 30 min and then stimulated with leptin (100 ng/ml) for 24 h. The protein level of Egr-1 was determined using immunoblotting, and GAPDH served as loading control (n = 5 each group). (B) Protein level of Egr-1 was examined by immunoblotting in cells transfected with indicated siRNA for 48 h (n = 5 each group). (C) PASMC were transfected with negative control siRNA or Egr-1-siRNA for 24 h followed by leptin stimulation for 24 h; PPARγ protein expression was analyzed using immunoblotting (n = 5 each group). *p < 0.05 vs. control; **p < 0.01 vs. control; #p < 0.05 vs. leptin or NC-siRNA + leptin.
To verify the involvement of Egr-1 in leptin-induced PPARγ reduction in PASCM, knockdown of Egr-1 was applied. Figure 4B indicates that transfection of 100 nM Egr-1 specific small interfering RNA (siRNA) for 48 h reduced Egr-1 protein level to 21% of control (p < 0.01), while nontargeting siRNA did not affect Egr-1 protein expression. Figure 4C shows that PPARγ protein level decreased to 0.40-fold over control in cells treated with 100 ng/ml leptin for 24 h (p < 0.01 vs. control), while presilencing Egr-1 increased PPARγ protein level to 0.93-fold over control in leptin-stimulated cells (p < 0.05). In addition, PPARγ protein level was elevated in cells loss of Egr-1, which was a 1.70-fold increase over control (p < 0.05), suggesting that Egr-1 also suppressed PPARγ expression in basal condition. The above results indicate that up-regulation of Egr-1 by ERK1/2 signaling pathway specifically mediated leptin-induced PPARγ reduction in primary cultured rat PASMC.
ERK1/2/Egr-1/PPARγ signaling pathway mediates leptin-stimulated PASMC proliferation
To clarify whether ERK1/2/Egr-1/PPARγ pathway was involved in leptin-induced PASMC proliferation, cells were previously treated with MEK inhibitor U0126 (10 μM) or PD98059 (10 μM) for 30 min and PPARγ activator pioglitazone (10 μM) for 30 min and pretransfected with either 100 nM nontargeting siRNA or Egr-1 siRNA for 24 h and then stimulated with 100 ng/ml leptin for 24 h. As shown in Figure 5, prior treatment of cells with MEK inhibitor U0126 or PD98059 significantly suppressed leptin-induced PASMC proliferation, and BrdU incorporation rate decreased from a 1.98-fold increase over control in leptin-treated cells to a 1.19- and a 1.30-fold increase over control, respectively (both p < 0.05). In addition, prior depletion of Egr-1 reduced PASMC proliferation induced by leptin, and BrdU incorporation rate decreased from a 1.98-fold increase over control in leptin-treated cells to a 1.35-fold increase over control (p < 0.05). Furthermore, pretreatment of cells with PPARγ activator pioglitazone dramatically suppressed leptin-induced PASMC proliferation, which reduced from a 1.98-fold increase over control in leptin stimulated cells to a 1.23-fold increase over control (p < 0.05). Collectively, these results suggest that up-regulation of Egr-1 by ERK1/2 signaling cascade and subsequent PPARγ down-regulation mediates the effect of leptin on PASMC proliferation, while activation of PPARγ inhibits leptin-induced proliferation of PASCM.
FIGURE 5:
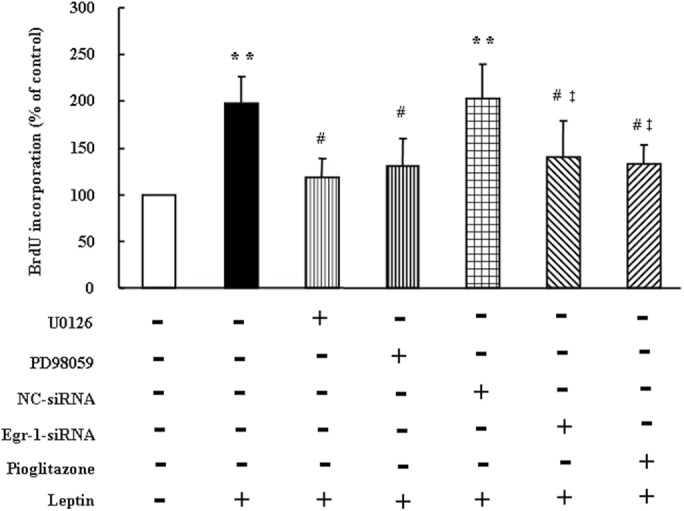
Inhibition of ERK1/2/Egr-1 signaling or activation of PPARγ inhibits leptin-induced PASMC proliferation. PASMC were treated with MEK inhibitor U0126 (10 μM) or PD98059 (10 μM) for 30 min, PPARγ activator pioglitazone (10 μM) for 30 min, or transfected with 100 nM nontargeting siRNA or Egr-1 siRNA for 24 h followed by 100 ng/ml leptin stimulation for 24 h, cell proliferation was measured using BrdU incorporation assay (n = 5 each group). **p < 0.01 vs. control, #p < 0.01 vs. Leptin, ‡p < 0.05 vs. NC-siRNA + Leptin.
DISCUSSION
In the present study, we have provided direct evidence that leptin causes PPARγ reduction in primary cultured PASMC; this effect is coupled to leptin-induced ERK1/2 activation and subsequent induction of Egr-1, which further down-regulates PPARγ expression and results in PASMC proliferation. Our study provides novel molecular mechanisms underlying the down-regulation of PPARγ in pulmonary vasculature in the development of PH.
Leptin has been shown to be involved in the regulation of many pathophysiological processes such as energy balance, hypertension, coronary atherosclerosis, myocardial hypertrophy, diabetes, reproduction, bone homeostasis, and immune function (Mantzoros et al., 2011). Leptin modulates various signaling pathways through the interaction with its receptors, such as janus-activated kinase/signal transducers and activators of transcription (JAK/STAT), mitogen-activated protein kinases (MAPK)/ERK, suppressors of cytokine signaling (SOCS), phosphatidylinositol-3-kinase and insulin receptor substrate proteins (PI3K/IRS) (Sweeney, 2002), and nitric oxide/cyclic GMP/protein kinase G (NO/cGMP/PKG) signal pathways (Rodriguez et al., 2010; Garcia-Juarez et al., 2012). Interestingly, leptin activates both pro-proliferative cascades (Shan et al., 2008; Chavez et al., 2012; Trovati et al., 2014; Yu et al., 2017) and anti-proliferative pathway (Rodriguez et al., 2007, 2010) in vascular smooth muscle cells (VSMC); these conflicting phenomena might be due to cell type specificity, concentration of leptin and cell conditions. Numerous studies have also shown that leptin plays an important role in the pathophysiology of PH (Schroeter et al., 2013; Chai et al., 2015; Huertas et al., 2015, 2016). The results of the present study demonstrate that leptin induced PASMC proliferation and PPARγ down-regulation accompanied with ERK1/2 MAPK phosphorylation. Inhibition of ERK1/2 MAPK markedly suppressed leptin-induced PPARγ reduction and PASMC proliferation, suggesting that ERK1/2 signal pathway particularly mediated leptin-induced PPARγ down-regulation and PASMC proliferation. In addition, activation of the ERK1/2 signaling pathway has been shown to cause PPARγ nuclear export, resulting in its transcriptional activity reduction (Burgermeister et al., 2007), and to promote proteasomal-dependent PPARγ degradation (Floyd and Stephens, 2002). Therefore, further studies are still needed to investigate the fully mechanisms responsible for ERK1/2 signaling pathway regulating PPARγ reduction/inactivation in the development of PH. We also detected PKG activity in leptin-treated cells, and the result showed that activity of PKG was slightly but significantly increased compared with control cells (data not shown). We speculated that activation of the PKG pathway was considered a counteracting action to the PASMC proliferation induced by leptin, which was still not sufficient to suppress PASMC proliferation caused by leptin.
Egr-1 is a critical transcriptional factor regulating cell proliferation and differentiation, which is rapidly and transiently induced in response to a heterogenic group of stimuli, such as growth factors (Cao et al., 1990), shear stress (Ni et al., 2010), oxygen deprivation (Chang et al., 2008), and oxidative stress (Zhang et al., 2015). Induction of Egr-1 has been observed in various malignant tumors (Jacob et al., 2016; Park et al., 2016), inflammation (Ho et al., 2016), and cardiovascular diseases (Khachigian, 2006; Shin et al., 2009). Recently, overexpression of Egr-1 has been found in several animal models of PH (van Albada et al., 2010; Dickinson et al., 2011, 2014) and in patients with PH (van der Feen et al., 2016). Mounting evidence suggests that stimuli such as growth factors (i.e., platelet-derived growth factor and brain-derived neurotrophic factor) (Kwapiszewska et al., 2012; Sysol et al., 2016) and hypoxia (Nozik-Grayck et al., 2008; Hartney et al., 2011) induce PASMC proliferation by increasing Egr-1 expression. ERK1/2 signaling cascade has been shown to increase the expression and activity of Egr-1 in several cell types (Zhou et al., 2009b; Hartney et al., 2011). It has been further reported that PPARγ proximal promoter contains a binding site of Egr-1, which is involved in the down-regulation of PPARγ (Zhou et al., 2009b; Nebbaki et al., 2012). The present study confirmed that ERK1/2 signaling cascade mediated leptin-induced PPARγ reduction by up-regulation of Egr-1 in PASMC. We also found that pretreatment with PPARγ activator pioglitazone significantly inhibited leptin-stimulated PASMC proliferation. Taken together, this study provided novel molecular mechanisms by which leptin induced PPARγ down-regulation and PASMC proliferation, suggesting that targeting leptin/ERK1/2/Egr-1/PPARγ pathway might have potential value in ameliorating vascular remodeling and benefit PH.
MATERIALS AND METHODS
Cell preparation and culture
Primary PASMC from pulmonary arteries were prepared from 40 male Sprague-Dawley rats (4-wk-old, 70–80 g) according to the method of our previous studies (Wu et al., 2014; Ke et al., 2016). All animal care and experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the Xi’an Jiaotong University Animal Experiment Center. All protocols used in this study were approved by the Laboratory Animal Care Committee of Xi’an Jiaotong University. Briefly, pulmonary arteries were rapidly isolated from killed rats, washed in phosphate-buffered saline (PBS; 4°C), and dipped into DMEM (Life Technologies, Grand Isle, NY) containing 10% fetal bovine serum (FBS; Sijiqing, Hangzhou, China), 100 U/ml penicillin, and 100 μg/ml streptomycin. A thin layer of the adventitia was gently stripped off with a forceps, and the endothelium was carefully removed by scratching the intima surface with an elbow tweezers. The remaining smooth muscle was cut into 1-mm pieces and placed into a culture flask and then incubated in a 37°C, 5% CO2 humidified incubator. PASMC were passaged using 0.25% trypsin (Invitrogen, Carlsbad, CA) until reaching 70–80% confluence. All experiments were performed using cells between passages 4 and 6. The purity of PASMC was determined by immunostaining with α-actin as previously described (Wu et al., 2014). Leptin (Peprotech, Rocky Hill, NJ) was used to stimulate PASMC. U0126 (Selleckchem, Houston, TX) or PD98059 (Calbiochem, La Jolla, CA) was applied to inhibit ERK1/2 signaling pathway. PPARγ activator pioglitazone was purchased from Takeda Pharmaceutical Co. (Tianjin, China).
siRNA transfection
To silence protein expression, PASMC were transfected with sequence-specific or nontargeting control siRNA (GenePharm, Shanghai, China) using Lipofectamine 2000 reagent (Invitrogen), the following sequences were used: Egr-1 siRNA, sense 5′-CAGGACUUAAAGGCUCUUATT-3′, anti-sense 5′-UAAGAGCCUUUAAGUCCUGTT-3′; NC-siRNA, sense 5′-UUCUCCGAACGUGUCACGUTT-3′, anti-sense 5′-ACGUGACACGUUCGGAGAATT-3′. Briefly, cells were cultured until reaching 30–40% confluence; siRNA and Lipofectamine were diluted in serum-free DMEM, separately, and incubated for 5 min at room temperature. Diluted siRNA was mixed with diluted Lipofectamine and incubated at room temperature for 20 min. Then the complex of siRNA and Lipofectamine was added into cells, and cells were cultured for 48 h at 37°C, 5% CO2 in a humidified incubator. The working concentration of siRNA in cell experiments was 100 nM. Effects of siRNA transfection were analyzed using immunoblotting.
Cell proliferation assay
To determine PASMC proliferation, the rate of BrdU incorporation was examined using a BrdU ELISA Kit (Maibio, Shanghai, China) following the established protocol. PASMC were seeded on 96-well plates at 5 × 103 cells per well, allowed to adhere for at least 24 h, and then serum starved overnight (1% FBS in DMEM) before the start of experiments. After different treatments, BrdU labeling reagent was added to the wells and incubated for 2 h at 37°C. Cells were then denatured with FixDenat solution for 30 min and incubated with anti-BrdU mAbs conjugated to peroxidase for 90 min at room temperature. After incubation, antibody conjugate was removed and substrate solution was added for reaction for 10 min. Finally, the reaction product was quantified by measuring the absorbance at 370 nm using a microplate reader (Bio-Rad, Richmond, CA). The blank corresponded to 100 μl of culture medium with or without BrdU.
Quantitative real-time reverse transcription PCR
Total RNA was extracted from PASMC using the RNeasy Micro plus Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Isolated RNAs were polyadenylated using the Thermo Scientific RevertAid First Strand cDNA Synthesis Kit (Logan, UT). The cDNA synthesized was used to perform quantitative PCR on an IQ5 Real-Time PCR Detection System (Bio-Rad) using the Bio-Rad SsoAdvanced Universal SYBR Green kit. Primers specific for PPARγ and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Sangon Biotech (Shanghai, China), and the following primer sets were used: rat PPARγ, 5′-CGGTTGATTTCTCCAGCATT-3′ and 5′-TCGCAC TTTGGTATTCTTGG-3′; rat GAPDH, 5′-CCTGGAGAAACCTGCCAAGTAT-3′ and 5′-CTCGGCCGCCTGCTT-3′. The fold increase relative to control samples was determined by the 2−∆∆Ct method (Livak and Schmittgen, 2001). GAPDH was used as internal control for PPARγ. Amplification was performed at 95°C for 1 min, followed by 40 cycles of 95°C for 5 s, 60°C for 20 s, and 72°C for 30 s.
Immunoblotting
The cultured cells were washed twice with ice-cold PBS and then lysed in radioimmunoprecipitation assay (RIPA) lysis buffer containing 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.1% SDS, 150 mM NaCl, 0.5% sodium deoxycholate, 1 mM EDTA, 1 mM phenylmethanesulfonyl fluoride (PMSF), 1 mM Na3VO4, 1 mM NaF, and proteinase inhibitors. Lysates were centrifuged at 13,000 rpm at 4°C for 15 min, and the supernatant was collected as total protein. Protein concentration was determined with a BCA protein assay kit (Pierce). Protein was separated on an SDS–PAGE gel and transferred to a nitrocellulose (NC; Bio-Rad) membrane via semidry transfer. The membrane was then blocked with 5% (wt/vol) nonfat dry milk in PBS containing 0.1% (vol/vol) Tween-20. Polyclonal or monoclonal antibodies were used against phosphor-ERK1/2 (p-ERK1/2; Cell Signaling Technology), total-ERK1/2 (t-ERK1/2; Cell Signaling Technology), early growth response-1 (Egr-1; Cell Signaling Technology), PPARγ (Proteintech Group, Chicago, IL), and glyceraldehyde-3-phosphate dehydrogenase (Chemicon International, Billerica, MA) (1:1000 dilution) according to the manufacturer’s protocols. Horseradish peroxidase (HRP)-conjugated goat anti-mouse or anti-rabbit immunoglobulin G was used as the secondary antibodies (Sigma, St. Louis, MO) (1:5000 dilution). Reactions were developed with the SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL) and then exposed to the autoradiographic film. Signaling was quantified from scanned films using Quality One software (Bio-Rad).
Statistical analysis
All values are presented as mean ± SD. Data were analyzed using one-way analysis of variance with Tukey post hoc test by SPSS13.0 software. Probability values of p < 0.05 were considered to represent a statistically significant between groups.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 81330002).
Abbreviations used:
- Egr-1
early growth response-1
- ERK1/2
extracellular regulated kinase1/2
- PASMC
pulmonary artery smooth muscle cell
- PH
pulmonary hypertension
- PPARγ
peroxisome proliferator-activated receptor γ
- RT-PCR
real-time PCR
- siRNA
small interfering RNA.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E17-03-0141) on December 6, 2017.
REFERENCES
- Akther A, Khan KH, Begum M, Parveen S, Kaiser MS, Chowdhury AZ. Leptin: a mysterious hormone; its physiology and pathophysiology. Mymensingh Med J. 2009;18:S140–S144. [PubMed] [Google Scholar]
- Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- Bazan IS, Fares WH. Pulmonary hypertension: diagnostic and therapeutic challenges. Ther Clin Risk Manag. 2015;11:1221–1233. doi: 10.2147/TCRM.S74881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M, Seger R. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2007;27:803–817. doi: 10.1128/MCB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XM, Koski RA, Gashler A, McKiernan M, Morris CF, Gaffney R, Hay RV, Sukhatme VP. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990;10:1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai S, Wang W, Liu J, Guo H, Zhang Z, Wang C, Wang J. Leptin knockout attenuates hypoxia-induced pulmonary arterial hypertension by inhibiting proliferation of pulmonary arterial smooth muscle cells. Transl Res. 2015;166:772–782. doi: 10.1016/j.trsl.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Chang JS, Wendt T, Qu W, Kong L, Zou YS, Schmidt AM, Yan SF. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res. 2008;102:905–913. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- Chavez RJ, Haney RM, Cuadra RH, Ganguly R, Adapala RK, Thodeti CK, Raman P. Upregulation of thrombospondin-1 expression by leptin in vascular smooth muscle cells via JAK2- and MAPK-dependent pathways. Am J Physiol Cell Physiol. 2012;303:C179–C191. doi: 10.1152/ajpcell.00008.2012. [DOI] [PubMed] [Google Scholar]
- Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L885–L897. doi: 10.1152/ajplung.00258.2006. [DOI] [PubMed] [Google Scholar]
- Dickinson MG, Bartelds B, Molema G, Borgdorff MA, Boersma B, Takens J, Weij M, Wichers P, Sietsma H, Berger RM. Egr-1 expression during neointimal development in flow-associated pulmonary hypertension. Am J Pathol. 2011;179:2199–2209. doi: 10.1016/j.ajpath.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson MG, Kowalski PS, Bartelds B, Borgdorff MA, van der Feen D, Sietsma H, Molema G, Kamps JA, Berger RM. A critical role for Egr-1 during vascular remodelling in pulmonary arterial hypertension. Cardiovasc Res. 2014;103:573–584. doi: 10.1093/cvr/cvu169. [DOI] [PubMed] [Google Scholar]
- Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Juarez M, Beyer C, Gomora-Arrati P, Lima-Hernandez FJ, Dominguez-Ordonez R, Eguibar JR, Etgen AM, Gonzalez-Flores O. The nitric oxide pathway participates in lordosis behavior induced by central administration of leptin. Neuropeptides. 2012;46:49–53. doi: 10.1016/j.npep.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, Nozell SE, Oparil S, Chen YF. Hypoxia induces downregulation of PPAR-gamma in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-beta signaling. Am J Physiol Lung Cell Mol Physiol. 2011;301:L899–L907. doi: 10.1152/ajplung.00062.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartney T, Birari R, Venkataraman S, Villegas L, Martinez M, Black SM, Stenmark KR, Nozik-Grayck E. Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PLoS One. 2011;6:e27531. doi: 10.1371/journal.pone.0027531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LC, Sung JM, Shen YT, Jheng HF, Chen SH, Tsai PJ, Tsai YS. Egr-1 deficiency protects from renal inflammation and fibrosis. J Mol Med (Berl) 2016;94:933–942. doi: 10.1007/s00109-016-1403-6. [DOI] [PubMed] [Google Scholar]
- Huertas A, Phan C, Bordenave J, Tu L, Thuillet R, Le Hiress M Avouac J, Tamura Y, Allanore Y, Jovan R, et al. Regulatory T cell dysfunction in idiopathic, heritable and connective tissue-associated pulmonary arterial hypertension. Chest. 2016;149:1482–1493. doi: 10.1016/j.chest.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Huertas A, Tu L, Thuillet R, Le Hiress M, Phan C, Ricard N, Nadaud S, Fadel E, Humbert M. Leptin signalling system as a target for pulmonary arterial hypertension therapy. Eur Respir J. 2015;45:1066–1080. doi: 10.1183/09031936.00193014. [DOI] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- Huynh VT, Lim YS, Tran SC, Pham TM, Nguyen LN, Hwang SB. Hepatitis C virus nonstructural 5A protein interacts with Abelson interactor 1 and modulates epidermal growth factor-mediated MEK/ERK signaling pathway. J Biol Chem. 2016;291:22607–22617. doi: 10.1074/jbc.M116.727081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Nayak S, Kakar R, Chaudhari UK, Joshi D, Vundinti BR, Fernandes G, Barai RS, Kholkute SD, Sachdeva G. A triad of telomerase, androgen receptor and early growth response 1 in prostate cancer cells. Cancer Biol Ther. 2016;17:439–448. doi: 10.1080/15384047.2016.1156255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Budinger GR, Lo A, Urich D, Rivera SE, Ghosh AK, Gonzalez A, Chiarella SE, Marks K, Donnelly HK, et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-gamma. Am J Respir Crit Care Med. 2011;183:1490–1498. doi: 10.1164/rccm.201009-1409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke R, Liu L, Zhu Y, Li S, Xie X, Li F, Song Y, Yang L, Gao L, Li M. Knockdown of AMPKalpha2 promotes pulmonary arterial smooth muscle cells proliferation via mTOR/Skp2/p27(Kip1) signaling pathway. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachigian LM. Early growth response-1 in cardiovascular pathobiology. Circ Res. 2006;98:186–191. doi: 10.1161/01.RES.0000200177.53882.c3. [DOI] [PubMed] [Google Scholar]
- Kwapiszewska G, Chwalek K, Marsh LM, Wygrecka M, Wilhelm J, Best J, Egemnazarov B, Weisel FC, Osswald SL, Schermuly RT, et al. BDNF/TrkB signaling augments smooth muscle cell proliferation in pulmonary hypertension. Am J Pathol. 2012;181:2018–2029. doi: 10.1016/j.ajpath.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Lee H, Bae HC, Kim J, Jeong SH, Ryu WI, Son SW. Chloroform upregulates early growth response-1-dependent thymic stromal lymphopoietin expression via the JNK and ERK pathways in human keratinocytes. Int J Dermatol. 2015;54:e521–e526. doi: 10.1111/ijd.12946. [DOI] [PubMed] [Google Scholar]
- Li M, Li Z, Sun X, Yang L, Fang P, Liu Y, Li W, Xu J, Lu J, Xie M, et al. Heme oxygenase-1/p21WAF1 mediates peroxisome proliferator-activated receptor-gamma signaling inhibition of proliferation of rat pulmonary artery smooth muscle cells. Febs J. 2010;277:1543–1550. doi: 10.1111/j.1742-4658.2010.07581.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu X, Bijli KM, Ramirez A, Murphy TC, Kleinhenz J, Hart CM. Hypoxia downregulates PPARgamma via an ERK1/2-NF-kappaB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radic Biol Med. 2013;63:151–160. doi: 10.1016/j.freeradbiomed.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbaki SS, El Mansouri FE, Afif H, Kapoor M, Benderdour M, Duval N, Pelletier JP, Martel-Pelletier J, Fahmi H. Egr-1 contributes to IL-1-mediated down-regulation of peroxisome proliferator-activated receptor gamma expression in human osteoarthritic chondrocytes. Arthritis Res Ther. 2012;14:R69. doi: 10.1186/ar3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Waldman A, Khachigian LM. c-Jun regulates shear- and injury-inducible Egr-1 expression, vein graft stenosis after autologous end-to-side transplantation in rabbits, and intimal hyperplasia in human saphenous veins. J Biol Chem. 2010;285:4038–4048. doi: 10.1074/jbc.M109.078345. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van Rheen Z, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:L422–L430. doi: 10.1152/ajplung.90293.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Kim JY, Lee SM, Chung JO, Lee KH, Jun CH, Park CH, Kim HS, Choi SK, Rew JS, et al. Expression of early growth response gene-1 in precancerous lesions of gastric cancer. Oncol Lett. 2016;12:2710–2715. doi: 10.3892/ol.2016.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Fortuno A, Gomez-Ambrosi J, Zalba G, Diez J, Fruhbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–331. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Gomez-Ambrosi J, Catalan V, Fortuno A, Fruhbeck G. Leptin inhibits the proliferation of vascular smooth muscle cells induced by angiotensin II through nitric oxide-dependent mechanisms. Mediators Inflamm. 2010;2010:105489. doi: 10.1155/2010/105489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter MR, Leifheit-Nestler M, Hubert A, Schumann B, Gluckermann R, Eschholz N, Kruger N, Lutz S, Hasenfuss G, Konstantinides S, et al. Leptin promotes neointima formation and smooth muscle cell proliferation via NADPH oxidase activation and signalling in caveolin-rich microdomains. Cardiovasc Res. 2013;99:555–565. doi: 10.1093/cvr/cvt126. [DOI] [PubMed] [Google Scholar]
- Shan J, Nguyen TB, Totary-Jain H, Dansky H, Marx SO, Marks AR. Leptin-enhanced neointimal hyperplasia is reduced by mTOR and PI3K inhibitors. Proc Natl Acad Sci USA. 2008;105:19006–19011. doi: 10.1073/pnas.0809743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin IS, Kim JM, Kim KL, Jang SY, Jeon ES, Choi SH, Kim DK, Suh W, Kim YW. Early growth response factor-1 is associated with intraluminal thrombus formation in human abdominal aortic aneurysm. J Am Coll Cardiol. 2009;53:792–799. doi: 10.1016/j.jacc.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Simo-Cheyou ER, Vardatsikos G, Srivastava AK. Src tyrosine kinase mediates endothelin-1-induced early growth response protein-1 expression via MAP kinase-dependent pathways in vascular smooth muscle cells. Int J Mol Med. 2016;38:1879–1886. doi: 10.3892/ijmm.2016.2767. [DOI] [PubMed] [Google Scholar]
- Sweeney G. Leptin signalling. Cell Signal. 2002;14:655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- Sysol JR, Natarajan V, Machado RF. PDGF induces SphK1 expression via Egr-1 to promote pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2016;310:C983–C992. doi: 10.1152/ajpcell.00059.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Smith A, Nechtman J, Podolsky R, Aggarwal S, Snead C, Kumar S, Elgaish M, Oishi P, Goerlach A, et al. Effect of PPARgamma inhibition on pulmonary endothelial cell gene expression: gene profiling in pulmonary hypertension. Physiol Genomics. 2009;40:48–60. doi: 10.1152/physiolgenomics.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovati M, Doronzo G, Barale C, Vaccheris C, Russo I, Cavalot F. Leptin and vascular smooth muscle cells. Curr Pharm Des. 2014;20:625–634. doi: 10.2174/13816128113199990022. [DOI] [PubMed] [Google Scholar]
- van Albada ME, Bartelds B, Wijnberg H, Mohaupt S, Dickinson MG, Schoemaker RG, Kooi K, Gerbens F, Berger RM. Gene expression profile in flow-associated pulmonary arterial hypertension with neointimal lesions. Am J Physiol Lung Cell Mol Physiol. 2010;298:L483–L491. doi: 10.1152/ajplung.00106.2009. [DOI] [PubMed] [Google Scholar]
- van der Feen DE, Dickinson MG, Bartelds B, Borgdorff MA, Sietsma H, Levy M, Berger RM. Egr-1 identifies neointimal remodeling and relates to progression in human pulmonary arterial hypertension. J Heart Lung Transplant. 2016;35:481–490. doi: 10.1016/j.healun.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Wang A, Zhang H, Liang Z, Xu K, Qiu W, Tian Y, Guo H, Jia J, Xing E, Chen R, et al. U0126 attenuates ischemia/reperfusion-induced apoptosis and autophagy in myocardium through MEK/ERK/EGR-1 pathway. Eur J Pharmacol. 2016;788:280–285. doi: 10.1016/j.ejphar.2016.06.038. [DOI] [PubMed] [Google Scholar]
- Wang L, Shao YY, Ballock RT. Leptin antagonizes peroxisome proliferator-activated receptor-gamma signaling in growth plate chondrocytes. PPAR Res. 2012;2012:756198. doi: 10.1155/2012/756198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Liu L, Zhang Y, Wang G, Han D, Ke R, Li S, Feng W, Li M. Activation of AMPK inhibits pulmonary arterial smooth muscle cells proliferation. Exp Lung Res. 2014;40:251–258. doi: 10.3109/01902148.2014.913092. [DOI] [PubMed] [Google Scholar]
- Xie X, Wang G, Zhang D, Zhang Y, Zhu Y, Li F, Li S, Li M. Activation of peroxisome proliferator-activated receptor gamma ameliorates monocrotaline-induced pulmonary arterial hypertension in rats. Biomed Rep. 2015;3:537–542. doi: 10.3892/br.2015.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YM, Tsai CC, Tzeng YW, Chang WC, Chiang SY, Lee MF. Ursolic acid suppresses leptin-induced cell proliferation in rat vascular smooth muscle cells. Can J Physiol Pharmacol. 2017;95:811–818. doi: 10.1139/cjpp-2016-0398. [DOI] [PubMed] [Google Scholar]
- Zhang D, Wang G, Han D, Zhang Y, Xu J, Lu J, Li S, Xie X, Liu L, Dong L, et al. Activation of PPAR-gamma ameliorates pulmonary arterial hypertension via inducing heme oxygenase-1 and p21(WAF1): an in vivo study in rats. Life Sci. 2014;98:39–43. doi: 10.1016/j.lfs.2013.12.208. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liao H, Zhong S, Gao F, Chen Y, Huang Z, Lu S, Sun T, Wang B, Li W, et al. Effect of N-n-butyl haloperidol iodide on ROS/JNK/Egr-1 signaling in H9c2 cells after hypoxia/reoxygenation. Sci Rep. 2015;5:11809. doi: 10.1038/srep11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jia X, Wang G, Wang X, Liu J. PI-3 K/AKT and ERK signaling pathways mediate leptin-induced inhibition of PPARgamma gene expression in primary rat hepatic stellate cells. Mol Cell Biochem. 2009a;325:131–139. doi: 10.1007/s11010-009-0027-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jia X, Zhou M, Liu J. Egr-1 is involved in the inhibitory effect of leptin on PPARgamma expression in hepatic stellate cell in vitro. Life Sci. 2009b;84:544–551. doi: 10.1016/j.lfs.2009.01.018. [DOI] [PubMed] [Google Scholar]