Abstract
Sorting of soluble proteins for transport to intracellular compartments and for secretion from cells is essential for cell and tissue homeostasis. The trans-Golgi network (TGN) is a major sorting station that sorts secretory proteins into specific carriers to transport them to their final destinations. The sorting of lysosomal hydrolases at the TGN by the mannose 6-phosphate receptor is well understood. The recent discovery of a Ca2+-based sorting of secretory cargo at the TGN is beginning to uncover the mechanism by which cells sort secretory cargoes from Golgi residents and cargoes destined to the other cellular compartments. This Ca2+-based sorting involves the cytoplasmic actin cytoskeleton, which through membrane anchored Ca2+ ATPase SPCA1 and the luminal Ca2+ binding protein Cab45 sorts of a subset of secretory proteins at the TGN. We present this discovery and highlight important challenges that remain unaddressed in the overall pathway of cargo sorting at the TGN.
INTRODUCTION
Biosynthetic transport of soluble proteins
Soluble proteins delivered to the secretory pathway include resident proteins such as endoplasmic reticulum (ER) chaperones, lysosomal hydrolases, and secretory proteins. Most soluble proteins contain a signal sequence that targets them to the ER (Blobel, 1980). In the ER these proteins are folded, glycosylated, and if properly folded, packaged into coat protein II (COP II)-coated vesicles for transport to the Golgi apparatus (GA) (Barlowe et al., 1994; Schekman et al., 1995; Malkus et al., 2002). Subsequently, these proteins passage through the GA and they are sorted at the trans-Golgi Network (TGN) for transport to their final destinations (Anitei and Hoflack, 2011). These destinations include endosomes, lysosomes, secretory storage granules and the plasma membrane (De Matteis and Luini, 2008; Guo et al., 2014; Kienzle and Blume, 2014). Furthermore, an additional level of complexity is the transport of proteins to different directions for delivery to various cell domains (Mellman and Nelson, 2008) for instance in mature epithelial cells and neurons that have functional and morphological polarization. Moreover, migrating cells, which develop a leading edge for forward movement, require polarized vesicular transport (Miller et al., 2009; Veale et al., 2010). To achieve high accuracy of protein transport into distinct exit routes, cells employ elaborate cargo sorting machineries to package cargo into the right transport carriers for targeting to the right destinations.
Sorting of transmembrane proteins at the TGN.
The mechanism of TGN sorting of many transmembrane proteins has been well studied in the past three decades. Most of these proteins contain cytosolic domains that are recognized by adapter proteins that recruit clathrin triskelia, thereby forming a coating structure that concentrates the cargo molecules into a clathrin-coated vesicle. These sorting motifs have been identified for proteins directed to the endosomal system and for some basolateral-directed cargoes (Fölsch et al., 2003; Ang and Fölsch, 2012; Bonifacino, 2014). It has also been postulated that glycosylphosphatidylinositol (GPI)-anchored proteins have a particular affinity for sphingolipid and cholesterol-rich membrane domains. This feature allows them to coalesce with these lipids and accumulate in TGN microdomains (Keller and Simons, 1997; Simons and Ikonen, 1997; Harder et al., 1998; Paladino et al., 2004; Lingwood and Simons, 2010; Simons and Gerl, 2010; Surma et al., 2012).
Since soluble cargoes do not contain bona fide membrane binding domains, their sorting is less well understood; only a few mechanisms have been proposed and studied.
Sorting of soluble lysosome hydrolases.
Sorting lysosomal hydrolases as well as secretory storage granules targeted proteins have been well studied in past three decades, and we refer to excellent reviews for more detailed information (Kornfeld and Mellman, 1989; Borgonovo et al., 2006). Kornfeld and colleagues described the first sorting receptor-dependent route for acid hydrolases from the TGN to endolysosomes that is mediated by mannose 6-phosphate (M6P) recognition. Most newly synthesized lysosomal hydrolases acquire these M6P moieties on their N-linked oligosaccharide chains as a unique marker that is recognized by the M6P-receptor (MPR) at the TGN. MPRs then bind to luminal hydrolases and to cytoplasmic adaptors that recruit clathrin coats on the cytosolic face of the TGN membrane. These clathrin-coated vesicles deliver their contents to late endosomes via early endosomes (Reitman and Kornfeld, 1981; Hoflack and Kornfeld, 1985; Griffiths et al., 1988; Kornfeld and Mellman, 1989; Le Borgne and Hoflack, 1997; Traub and Kornfeld, 1997; Ghosh and Kornfeld, 2004; Niehage et al., 2014). There are also M6P-independent sorting pathways that include sortilin, a member of the vacuolar sorting protein 10p (VPS10p) family of sorting receptors. Sortilin mediates the sorting and lysosomal trafficking of sphingolipid activator proteins prosaposin and acid sphingomyelinase (Braulke and Bonifacino, 2009). Furthermore, sorting of the acid hydrolase β-glucocerebrosidase (GC) by lysosomal integral membrane protein type 2 (LIMP-2) to lysosomes has been reported to be M6P-independent (Reczek et al., 2007). In 2014, a study of Zhao et al. questioned the view on LIMP-2 as an M6P-independent trafficking receptor. LIMP-2 crystal structure revealed a M6P residue at N325 suggesting MPR binding to LIMP-2 (Zhao et al., 2014). By contrast, studies in living cells showed that LIMP-2 and GC localize to lysosomes independently of the M6P pathway (Blanz et al., 2015). Whether GC sorting is facilitated by LIMP-2 in an M6P-independent manner remains to be elucidated. These studies underline the complexity of cargo sorting at the TGN.
Sorting of large and rigid extracellular matrix proteins.
Sorting, packaging, and export of fibrillar rigid procollagens (PC) that contain rod-like triple helical domains and can reach 450 nm in length is challenging since procollagens are too large to fit into canonical COPII-coated vesicles that reach a diameter of 60–90 nm. Transport and Golgi organization protein 1 (Tango1) has been described to be essential for collagen export at the ER (Bard et al., 2006; Saito et al., 2009). These results suggest a unique mechanism to modulate COPII vesicle size to support the exit of rigid and bulky cargo such as for PC VII. However, the mechanism how these bulky cargoes are sorted at the TGN remains unknown. Interestingly, it has been shown that collagen IV and laminin require a low pH, for their sorting to the basolateral membrane in cultured glomerular epithelial cells (Natori et al., 1992). Furthermore, it has been demonstrated that polarized secretion of laminin and heparan sulfate proteoglycans in Madin–Darby canine kidney cells requires an acidic pH (Caplan et al., 1987). To date, the mechanistic features of the sorting of these proteins have not been investigated.
Sorting by formation of protein complexes or aggregates.
Professional secretory cells store peptide hormones in secretory storage granules that localize in the cytosol closed to the plasma membrane. In contrast to constitutive secretion, these proteins are released upon an extracellular stimulus that induces the fusion of the granule with the cell membrane. The prohormone VGF is an important factor regulating animal metabolism including insulin secretion in pancreatic β cells. Pro-VGF is sorted into dense core secretory granules and is proteolytically processed into secreted peptides (Possenti et al., 1999; Trani et al., 2002; Stephens et al., 2012). It has been shown that the secretion of the C-terminal VGFP peptide leads to increase of glucose-stimulated insulin secretion (GSIS) and promotes β cell survival (Stephens et al., 2012). Loss of VGF in isolated islet β cells and conditional knockout mice leads to a decrease of GSIS and to the accumulation of granule cargo chromograninA (CgA) at the TGN, indicating that VGF also facilitates efficient exit of granule cargo thereby controlling granule biogenesis and insulin biosynthesis in islet β cells (Stephens et al., 2017). The formation of protein complexes or aggregates has been postulated to segregate these soluble cargo proteins, by clustering-induced sorting (Arvan and Castle, 1998; Arvan et al., 2002; Borgonovo et al., 2006; Bartolomucci et al., 2011; Fargali et al., 2014). VGF, CgA, and secretograninII (SGCII), are sorted by aggregation that depends on millimolar Ca2+ concentrations and on a mildly acidic pH (TGN pH is 6.2). Ca2+/pH dependent aggregation of proteins is mediated by structural features in the cargoes that often contain numerous acidic amino acids distributed over vast areas of the folded polypeptide chains (Gerdes et al., 1989; Bartolomucci et al., 2011; Fargali et al., 2014). This mechanism has been proposed for cargoes destined for regulated secretion that need to be sorted away from the cargo of the conventional sorting pathway. Therefore, the exact mechanism how these cargoes are packaged into storage granules is still poorly understood. The following section will describe a novel sorting process sharing similar features.
Discovery of Ca2+-based sorting at the TGN.
A genome-wide screen demonstrated the requirement for the actin-severing protein twinstar, for secretion of signal sequence horseradish peroxidase (ss-HRP) from Drosophila S2 cells (Bard et al., 2006). Further examination of the process revealed that twinstar and its orthologues, in both yeast (cof1) and mammalian cells (ADF and cofilin), are required to sort a subset of cargo molecules at the TGN (Blume et al., 2009; Curwin et al., 2012). We showed that a pool of cofilin localizes transiently to TGN membranes and regulates the Ca2+ influx into the TGN by interacting with the Secretory Pathway Calcium ATPase1 (SPCA1) (Blume et al., 2011).
SPCA1 pumps Ca2+, as well as Mn2+, into the lumen of the TGN in an ATP-dependent manner (Van Baelen et al., 2004). The concentration of Ca2+ in the Golgi apparatus is heterogeneous, and it was suggested that there is a Ca2+ gradient across the secretory pathway from the ER to the TGN (Pizzo et al., 2011). At steady state, The ER has the highest concentration (400 µM), while the cis Golgi contains 250 µM and the TGN around 100 µM. However, the TGN Ca2+ level oscillates over time (unpublished data) (Dolman and Tepikin, 2006). Tulio Pozzan and colleagues have shown that TGN Ca2+ uptake relies solely on SPCA1 (Lissandron et al., 2010). Using purified proteins, we found that the SPCA1 phosphorylation domain (P-domain), crucial for pump activation, interacts with F-actin in a cofilin dependent manner (Kienzle et al., 2014). When expressed in HeLa cells, the P-domain inhibits Ca2+ entry into the TGN and causes missorting of secretory cargo. Furthermore, mutation of four amino acids in the SPCA1 cofilin binding site impairs Ca2+ import into the TGN and affects secretory cargo sorting (Kienzle et al., 2014).
The next major question was how luminal Ca2+ facilitates the sorting process. Interestingly, cells that are depleted of ADF/cofilin or SPCA1 mis-sort secretory proteins and also secrete the soluble Golgi-resident protein, 45 kDa calcium-binding protein (Cab45) (Blume et al., 2012). Cab45 is evolutionary conserved in higher eurkaryotic organisms with highest sequence homology in vertebrates. There are no Cab45 homologues reported in fungi, indicating a specialized enhancement in vertebrates due to expanded secretory cargo complexity such as the emerging of an increased variety of extracellular matrix proteins that require sorting to be secreted in order to support cell adhesion and migration (Tabach et al., 2013a, b).
Lodish and colleagues identified Cab45 as a Golgi-resident protein with 6 Ca2+ binding EF hand domains (Scherer et al., 1996). Consistent with this observation, we recently demonstrated that Cab45 localization in the Golgi is sensitive to Ca2+ levels, and disrupting Golgi Ca2+ gradients induces Cab45 secretion by cells (Blume et al., 2009, 2011). The knockdown of Cab45 affects cargo sorting similar to ADF/cofilin or SPCA1 depletion. Also, Cab45 binds several secretory proteins in a Ca2+-dependent manner, and this binding appears to be required for cargo sorting at the TGN (Blume et al., 2012). Taken together these results indicated that Cab45 is a component of the cofilin/F-actin/SPCA1 sorting machinery. What is the role of Cab45 in this process?
Cab45 forms oligomers in the presence of Ca2+ in vitro and living cells (Crevenna et al., 2016). Furthermore, Cab45 changes its secondary structure upon Ca2+ binding, possibly to enable it to interact with its target cargo proteins. Moreover, we observed that only the oligomeric form of Cab45 binds selectively to specific cargo molecules such as Cartilage Oligomerizing Matrix Protein (COMP) and LysozymeC (LyzC), but not to cathepsin D in vitro. Finally, three-dimensional structured illumination microscopy showed that Cab45, SPCA1, and cargo colocalize in specific clusters at the TGN. We conclude from this data that upon SPCA1-dependent Ca2+ influx into the lumen of the TGN, Cab45 binds Ca2+, triggering a conformational change and allowing oligomerization. These oligomers then bind specific proteins, thereby sorting cargo from noncargo (Crevenna et al., 2016).
Taken together, cofilin binds to SPCA1 at the TGN and recruits F-actin (Figure 1), resulting in pump activation, thereby inducing Ca2+ influx into a specific domain of the TGN. This transient, local increase in Ca2+ recruits Cab45, which has a high affinity for Ca2+ and oligomerizes and binds cargo. Subsequent dissociation of the Cab45-cargo complex occurs either upon a decrease in Ca2+ or by a signal such as phosphorylation, resulting in the segregation of cargo for sorting into a particular class of transport carrier. We have named this Cab45 sorting oligomer a cernosome, from the Latin cernere, which means to choose, sift, separate, decide, or distinguish. Thus we suggest that this is a unique way to export cargo molecules independent of a bona fide cargo receptor.
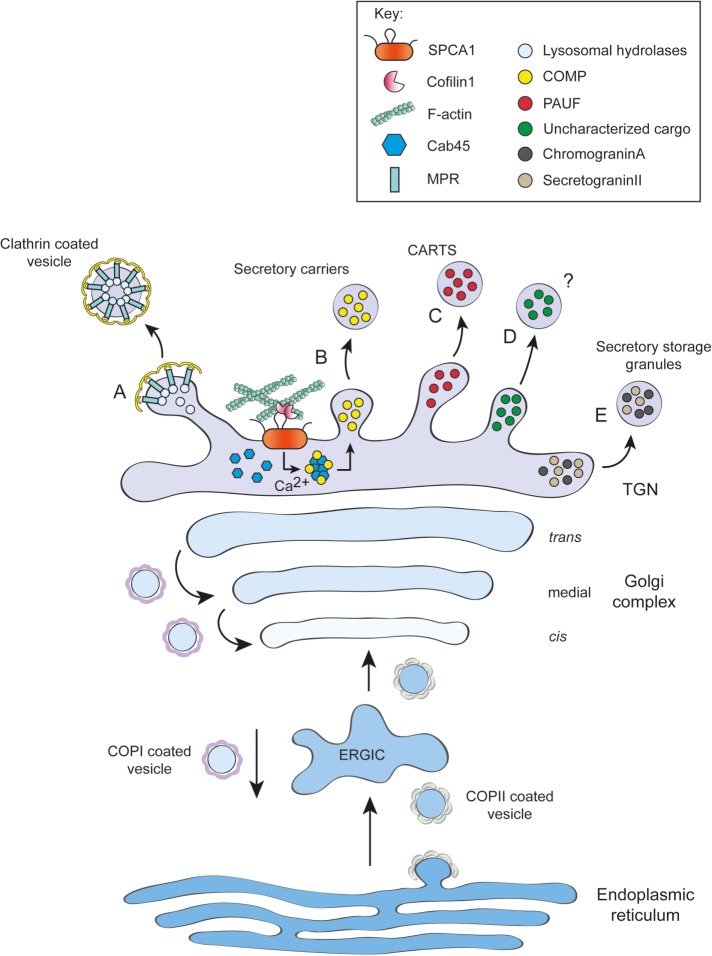
FIGURE 1:
Protein transport and cargo sorting in the secretory pathway. Proteins containing a signal sequence are cotranslationally inserted into the endoplasmic reticulum (ER). Secretory proteins leave the ER in coat protein complex II (COPII)-coated vesicles and are transported via the ER Golgi intermediate compartment (ERGIC) to the Golgi apparatus (GA). After transport through the cis- and medial Golgi compartments, proteins enter the trans-Golgi network (TGN) and are sorted to their correct destination. (A) Mannose 6-phosphate (M6P) modified lysosomal hydrolases are captured by M6P-receptor (MPR) and packaged into clathrin-coated vesicles. (B) The Secretory Pathway Calcium ATPase 1 (SPCA1) pumps Ca2+ into the TGN in a cofilin and F-actin dependent manner. Ca2+ influx leads to calcium binding protein 45 (Cab45) oligomerization and sorting of soluble secretory cargo such as cartilage oligomerizing matrix protein (COMP) into secretory carriers. (C) Carriers from the TGN to the cell surface (CARTS) transport pancreatic adenocarcinoma up-regulated factor (PAUF). (D) Alternative cargo sorting mechanisms of yet uncharacterized cargoes remain only poorly understood. (E) In specialized cells, secretory storage granule proteins chromograninA (CgA) and secretograninII (SGCII) are sorted by aggregation into secretory storage granules under high Ca2+ concentrations and midly acidic pH. ER resident proteins are retrograde transported in COPI-coated vesicles.
OPEN QUESTIONS
SPCA1 and Cab45-dependent sorting have evolved as a unique pathway to sort proteins such as LyzC, tissue inhibitor of matrix proteinases (TIMP1), Thrombospondin (TSP) 1, and 3, Matrix Metalloproteinase9 (MMP9) and COMP while, for instance, Carboxpeptidase A4 (CPA4), Fibulin1, Fibrillin1, and Fibronectin are sorted via a different pathway (Blume et al., 2009; Kienzle and Blume, 2014). However many open questions remain to be elucidated to understand the mechanism of this process.
Does actin control SPCA1 activation?
SPCA1 Ca2+ uptake- and sorting activity requires its binding to F-actin via cofilin. However, the precise role of this interaction has not yet been elucidated. The TGN has a low Ca2+ concentration, and we hypothesize that SPCA1 pumps high Ca2+ only at specific subdomains of the TGN (Blume et al., 2011; Aulestia et al., 2015). Previous work has shown that specific lipids such as cholesterol regulate clustering of proteins at the plasma membrane (Goswami et al., 2008). Also, the activity of SPCA1 is determined by the cholesterol and sphingolipid composition in living cells and in a reconstituted system (Baron et al., 2010; Chen et al., 2017). F-actin and cofilin might mediate the clustering of SPCA1 into a specific lipid environment rich in cholesterol and sphingomyelin. Furthermore, SPCA1 clustering could favor a model of high local Ca2+ influx, leading to spatially regulated Cab45 oligomer formation and cargo sorting at distinct lipid domains that could promote secretory vesicle formation. In this respect, Burd and colleagues have described a new class of unknown TGN derived sphingomyelin-rich vesicles (Deng et al., 2016). Inhibition of sphingomyelin synthesis has been shown to affect the trafficking to the plasma membrane of several proteins including vesicular stomatitis virus G protein, influenza hemagglutinin, and pancreatic adenocarcinoma up-regulated factor (PAUF) (Subathra et al., 2011; Tafesse et al., 2013; Wakana et al., 2015). Sphingomyelin has structural functions by decreasing membrane fluidity (Barenholz and Thompson, 1980; Van Blitterswijk et al., 1981). Furthermore, it serves as a source of important signaling molecules (Hla and Dannenberg, 2012). How Sphingomyelin signaling and structural features are potentially involved during secretory cargo sorting, remains to be elucidated.
Another important question is whether the dynamics of F-actin polymerization versus depolymerization regulates SPCA1 Ca2+ pumping cycles. It has been shown that expressed LIM kinase (LIMK) localizes to the Golgi and regulates cofilin activity by phosphorylation at serine3 (Arber et al., 1998; Rosso et al., 2004). LIMK is activated by p21-activated kinase (Pak1) through a cell division control protein 42 homologue (Cdc42) signaling cascade (Edwards et al., 1999). This process might also be directly linked to vesicle generation at the TGN since others (Almeida et al., 2011; Pylypenko et al., 2016) and we (unpublished results) have already identified the involvement of myosins in this pathway. The question remains whether cofilin activation at the Golgi by LIMK is temporally regulated by upstream stimuli or cofilin is activated in a stochastic manner leading to Ca2+ influx cycles.
How is cargo recognized by Cab45?
Our work has shown that oligomeric Cab45 binds to secretory proteins and we propose that these clusters sort cargo. It is not yet clear how Cab45 recognizes its target proteins. One possibility would be that there is a sorting sequence present in Cab45 dependent cargoes, such KDEL that has been shown for escaped ER resident chaperones (Munro and Pelham, 1987). So far a defined consensus sequence for Cab45 cargo could not be identified. It might be also the case that Cab45 recognizes different classes of cargoes through multiple interaction surfaces as it has been shown for Calmondulin (Tidow and Nissen, 2013). Similarly, Cab45 oligomers could bind to intrinsically disordered cargo binding sites that fold up upon Cab45 binding. In contrast, since lysosomal hydrolases such as cathepsin D do not interact with Cab45, they are captured with high affinity by MPR and targeted to clathrin-coated vesicles.
To solve this problem we need to increase our repertoire about Cab45 target proteins. In addition, the binding surfaces in Cab45 mono- and oligomers have to be identified biochemically and by structural biology to finally characterize the mechanism of binding.
How and where does Cab45 dissociate from the cargo?
Since Cab45 is a Golgi resident, it must somehow separate from cargo before being packaged into a transport carrier. This process might occur upon a drop of Ca2+ after complex formation of the oligomer with cargo. Furthermore, we imagine that Cab45 cargo dissociation occurs by a posttranslational modification such as phosphorylation. The serine/threonine kinase family with sequence similarity 20, member C (Fam20C) (Tagliabracci et al., 2012, 2015) and the extracellular tyrosine-protein kinase PKDCC (Bordoli et al., 2014) were reported to phosphorylate several resident as well as secreted proteins throughout the secretory pathway.
CONCLUSIONS AND FUTURE DIRECTIONS
Secretory proteins are essential for many crucial cellular events. Cells secrete signaling molecules such as hormones or neurotransmitters, digestive enzymes, antibodies, mucus, and extracellular matrix proteins such as collagens that provide mechanical strength and tissue integrity. For instance, matrix metalloproteinases (MMPs) in monocytes are specifically secreted to invadosomes that are cell matrix contacts with an actin-rich core. This local MMP secretion facilitates the lysis of extracellular matrix components at invadosomes being key features in both physiological and pathological cell invasion (Linder et al., 2011). The general view of secretory protein sorting into the constitutive pathway in the TGN was that proteins traverse and exit the Golgi independent of sorting signals (Pfeffer and Rothman, 1987). In contrast, research in recent years has shown that at least a subset of secretory proteins such as LyzC, COMP, TSP1, TSP5, TIMP1 and MMP9 are actively sorted at the TGN. Malhotra and colleagues have identified carriers from the TGN to the cell surface (CARTS) that transport PAUF but not collagen I (Wakana et al., 2012). We have proposed a mechanism that involves F-actin/cofilin/SPCA1/Ca2+ and Cab45 that form a functional sorting module in a particular TGN subdomain to direct LyzC, TIMP1, TSP1 and 5, Matrix MMP9 and COMP to the cell surface (Kienzle and Blume, 2014; Blank and Blume, 2017). Importantly, we found that there are other cargoes such as interleukins (unpublished data) and other proteins (Blume et al., 2009) that are sorted in a Cab45 independent manner for instance Carboxpeptidase A, Fibulin1, Fibrillin1, Fibronectin (Blume et al., 2009). This highlights the fact that there are additional alternative sorting events that remain to be elucidated. These may also be cell type specific and differ during development of an organism.
The TGN, as a highly dynamic organelle, is challenging to study. Nevertheless, tremendous progress in technology will help to elucidate the mechanism of SPCA1 dependent sorting as well as the identification these new exit routes. Genome editing now allows monitoring sorting and transport of tagged endogenous proteins by live-cell microscopy with high temporal resolution as well as super-resolution microscopy. Furthermore, biochemical approaches such as proximity dependent biotin identification (BioID) and engineered ascorbate peroxidase (APEX) show promise for the identification of new protein-protein interactions in the highly dynamic TGN environment. These interactions can be reconstituted in vitro to give a comprehensive understanding of the mechanism of protein sorting at the TGN. Future studies should therefore be aimed at answering remaining questions about secretory cargo sorting at the TGN.
Acknowledgments
J. v. Blume’s group is funded by the Plus 3 Perspective Program (Boehringer Ingelheim Foundation), CRC914 (TP A09), and a project grant (BL 1186/4-1) from the Deutsche Forschungsgemeinschaft (DFG), J. v. Blume is supported by the Max Planck Institute of Biochemistry and by the department led by Reinhard Fässler.
Abbreviations used:
- APEX
engineered ascorbate peroxidase
- BioID
proximity dependent biotin identification
- Cab45
45 kDa calcium-binding protein
- Cdc42
cell division control protein 42 homologue
- CgA
chromograninA
- COMP
Cartilage Oligomerizing Matrix Protein
- COP II
coat protein II
- CPA4
Carboxpeptidase A4
- ER
endoplasmic reticulum
- ERGIC
ER Golgi intermediate compartment
- Fam20C
family with sequence similarity 20, member C
- GC
β-glucocerebrosidase
- GPI
glycosylphosphatidylinositol
- GSIS
glucose-stimulated insulin secretion
- LIMP-2
lysosomal integral membrane protein type 2
- LyzC
LysozymeC
- M6P
mannose 6-phosphate
- MMP9
Matrix Metalloproteinase9
- MPR
mannose 6-phosphate-receptor
- P-domain
phosphorylation domain
- Pak1
p21-activated kinase
- PAUF
pancreatic adenocarcinoma up-regulated factor
- PC
procollagen
- SGCII
secretograninII
- SPCA1
Secretory Pathway Calcium ATPase1
- ss-HRP
signal sequence horseradish peroxidase
- Tango1
Transport and Golgi organization protein 1
- TGN
trans-Golgi network
- TIMP1
tissue inhibitor of matrix proteinases
- TSP
Thrombospondin
- VPS10p
vacuolar sorting protein 10p
Footnotes
REFERENCES
- Almeida CG, Yamada A, Tenza D, Louvard D, Raposo G, Coudrier E. Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat Cell Biol. 2011;13:779–789. doi: 10.1038/ncb2262. [DOI] [PubMed] [Google Scholar]
- Ang SF, Fölsch H. The role of secretory and endocytic pathways in the maintenance of cell polarity. Essays Biochem. 2012;53:29–39. doi: 10.1042/bse0530029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitei M, Hoflack B. Exit from the trans-Golgi network: from molecules to mechanisms. Curr Opin Cell Biol. 2011;23:443–451. doi: 10.1016/j.ceb.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332(Pt 3):593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Zhang B-Y, Feng L, Liu M, Kuliawat R. Lumenal protein multimerization in the distal secretory pathway/secretory granules. Curr Opin Cell Biol. 2002;14:448–453. doi: 10.1016/s0955-0674(02)00344-7. [DOI] [PubMed] [Google Scholar]
- Aulestia FJ, Alonso MT, Garcia-Sancho J. Differential calcium handling by the cis and trans regions of the Golgi apparatus. Biochem J. 2015;466:455–465. doi: 10.1042/BJ20141358. [DOI] [PubMed] [Google Scholar]
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439:604–607. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- Barenholz Y, Thompson TE. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta. 1980;604:129–158. doi: 10.1016/0005-2736(80)90572-6. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Baron S, Vangheluwe P, Sepúlveda MR, Wuytack F, Raeymaekers L, Vanoevelen J. The secretory pathway Ca(2+)-ATPase 1 is associated with cholesterol-rich microdomains of human colon adenocarcinoma cells. Biochim Biophys Acta. 2010;1798:1512–1521. doi: 10.1016/j.bbamem.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SRJ. The extended granin family: structure, function, and biomedical implications. Endocr Rev. 2011;32:755–797. doi: 10.1210/er.2010-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank B, Blume von J. Cab45-Unraveling key features of a novel secretory cargo sorter at the trans-Golgi network. Eur J Cell Biol. 2017;96:383–390. doi: 10.1016/j.ejcb.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Blanz J, Zunke F, Markmann S, Damme M, Braulke T, Saftig P, Schwake M. Mannose 6-phosphate-independent Lysosomal Sorting of LIMP-2. Traffic. 2015;16:1127–1136. doi: 10.1111/tra.12313. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci USA. 1980;77:1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume von J, Alleaume A-M, Cantero-Recasens G, Curwin A, Carreras-Sureda A, Zimmermann T, van Galen J, Wakana Y, Valverde MA, Malhotra V. ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev Cell. 2011;20:652–662. doi: 10.1016/j.devcel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Blume von J, Alleaume A-M, Kienzle C, Carreras-Sureda A, Valverde M, Malhotra V. Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network. J Cell Biol. 2012;199:1057–1066. doi: 10.1083/jcb.201207180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume von J, Duran JM, Forlanelli E, Alleaume A-M, Egorov M, Polishchuk R, Molina H, Malhotra V. Actin remodeling by ADF/cofilin is required for cargo sorting at the trans-Golgi network. J Cell Biol. 2009;187:1055–1069. doi: 10.1083/jcb.200908040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS. Adaptor proteins involved in polarized sorting. J Cell Biol. 2014;204:7–17. doi: 10.1083/jcb.201310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoli MR, Yum J, Breitkopf SB, Thon JN, Italiano JE Jr, Xiao J, Worby C, Wong SK, Lin G, Edenius M, et al. A secreted tyrosine kinase acts in the extracellular environment. Cell. 2014;158:1033–1044. doi: 10.1016/j.cell.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgonovo B, Ouwendijk J, Solimena M. Biogenesis of secretory granules. Curr Opin Cell Biol. 2006;18:365–370. doi: 10.1016/j.ceb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim Biophys Acta. 2009;1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Caplan MJ, Stow JL, Newman AP, Madri J, Anderson HC, Farquhar MG, Palade GE, Jamieson JD. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987;329:632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Chen J, De Raeymaecker J, Hovgaard JB, Smaardijk S, Vandecaetsbeek I, Wuytack F, Møller JV, Eggermont J, De Maeyer M, Christensen SB, et al. Structure/activity relationship of thapsigargin inhibition on the purified Golgi/secretory pathway Ca(2+)/Mn(2+)-transport ATPase (SPCA1a) J Biol Chem. 2017;292:6938–6951. doi: 10.1074/jbc.M117.778431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevenna AH, Blank B, Maiser A, Emin D, Prescher J, Beck G, Kienzle C, Bartnik K, Habermann B, Pakdel M, et al. Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J Cell Biol. 2016;213:305–314. doi: 10.1083/jcb.201601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin AJ, von Blume J, Malhotra V. Cofilin-mediated sorting and export of specific cargo from the Golgi apparatus in yeast. Mol Biol Cell. 2012;23:2327–2338. doi: 10.1091/mbc.E11-09-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- Deng Y, Rivera-Molina FE, Toomre DK, Burd CG. Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc Natl Acad Sci USA. 2016;113:6677–6682. doi: 10.1073/pnas.1602875113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolman NJ, Tepikin AV. Calcium gradients and the Golgi. Cell Calcium. 2006;40:505–512. doi: 10.1016/j.ceca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Fargali S, Garcia AL, Sadahiro M, Jiang C, Janssen WG, Lin WJ, Cogliani V, Elste A, Mortillo S, Cero C, et al. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure. Faseb J. 2014;28:2120–2133. doi: 10.1096/fj.13-239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes HH, Rosa P, Phillips E, Baeuerle PA, Frank R, Argos P, Huttner WB. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989;264:12009–12015. [PubMed] [Google Scholar]
- Ghosh P, Kornfeld S. The cytoplasmic tail of the cation-independent mannose 6-phosphate receptor contains four binding sites for AP-1. Arch Biochem Biophys. 2004;426:225–230. doi: 10.1016/j.abb.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, Vishwakarma R, Rao M, Mayor S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Guo Y, Sirkis DW, Schekman R. Protein sorting at the trans-Golgi network. Annu Rev Cell Dev Biol. 2014;30:169–206. doi: 10.1146/annurev-cellbio-100913-013012. [DOI] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T, Dannenberg AJ. Sphingolipid signaling in metabolic disorders. Cell Metab. 2012;16:420–434. doi: 10.1016/j.cmet.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoflack B, Kornfeld S. Lysosomal enzyme binding to mouse P388D1 macrophage membranes lacking the 215-kDa mannose 6-phosphate receptor: evidence for the existence of a second mannose 6-phosphate receptor. Proc Natl Acad Sci USA. 1985;82:4428–4432. doi: 10.1073/pnas.82.13.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110(Pt 24):3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Kienzle C, Blume von J. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 2014;24:584–593. doi: 10.1016/j.tcb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Kienzle C, Basnet N, Crevenna AH, Beck G, Habermann B, Mizuno N, von Blume J. Cofilin recruits F-actin to SPCA1 and promotes Ca2+-mediated secretory cargo sorting. J Cell Biol. 2014;206:635–654. doi: 10.1083/jcb.201311052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B. Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Lissandron V, Podini P, Pizzo P, Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci USA. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkus P, Jiang F, Schekman R. Concentrative sorting of secretory cargo proteins into COPII-coated vesicles. J Cell Biol. 2002;159:915–921. doi: 10.1083/jcb.200208074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM, Folkmann AW, Maia ARR, Efimova N, Efimov A, Kaverina I. Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nat Cell Biol. 2009;11:1069–1080. doi: 10.1038/ncb1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Natori Y, O’Meara YM, Manning EC, Minto AW, Levine JS, Weise WJ, Salant DJ. Production and polarized secretion of basement membrane components by glomerular epithelial cells. Am J Physiol. 1992;262:F131–F137. doi: 10.1152/ajprenal.1992.262.1.F131. [DOI] [PubMed] [Google Scholar]
- Niehage C, Stange C, Anitei M, Hoflack B. Liposome-based assays to study membrane-associated protein networks. Methods Enzymol. 2014;534:223–243. doi: 10.1016/B978-0-12-397926-1.00013-5. [DOI] [PubMed] [Google Scholar]
- Paladino S, Sarnataro D, Pillich R, Tivodar S, Nitsch L, Zurzolo C. Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J Cell Biol. 2004;167:699–709. doi: 10.1083/jcb.200407094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR, Rothman JE. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Lissandron V, Capitanio P, Pozzan T. Ca(2+) signalling in the Golgi apparatus. Cell Calcium. 2011;50:184–192. doi: 10.1016/j.ceca.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Possenti R, Rinaldi AM, Ferri GL, Borboni P, Trani E, Levi A. Expression, processing, and secretion of the neuroendocrine VGF peptides by INS-1 cells. Endocrinology. 1999;140:3727–3735. doi: 10.1210/endo.140.8.6920. [DOI] [PubMed] [Google Scholar]
- Pylypenko O, Welz T, Tittel J, Kollmar M, Chardon F, Malherbe G, Weiss S, Michel CIL, Samol-Wolf A, Grasskamp AT, et al. Coordinated recruitment of Spir actin nucleators and myosin V motors to Rab11 vesicle membranes. Elife. 2016;5:213. doi: 10.7554/eLife.17523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, Brondyk W, Van Patten S, Edmunds T, Saftig P. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Reitman ML, Kornfeld S. Lysosomal enzyme targeting. N-Acetylglucosaminylphosphotransferase selectively phosphorylates native lysosomal enzymes. J Biol Chem. 1981;256:11977–11980. [PubMed] [Google Scholar]
- Rosso S, Bollati F, Bisbal M, Peretti D, Sumi T, Nakamura T, Quiroga S, Ferreira A, Caceres A. LIMK1 regulates Golgi dynamics, traffic of Golgi-derived vesicles, and process extension in primary cultured neurons. Mol Biol Cell. 2004;15:3433–3449. doi: 10.1091/mbc.E03-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Schekman R, Barlowe C, Bednarek S, Campbell J, Doering T, Duden R, Kuehn M, Rexach M, Yeung T, Orci L. Coat proteins and selective protein packaging into transport vesicles. Cold Spring Harb Symp Quant Biol. 1995;60:11–21. doi: 10.1101/sqb.1995.060.01.004. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Lederkremer GZ, Williams S, Fogliano M, Baldini G, Lodish HF. Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J Cell Biol. 1996;133:257–268. doi: 10.1083/jcb.133.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Stephens SB, Edwards RJ, Sadahiro M, Lin W-J, Jiang C, Salton SR, Newgard CB. The Prohormone VGF Regulates β Cell Function via Insulin Secretory Granule Biogenesis. Cell Rep. 2017;20:2480–2489. doi: 10.1016/j.celrep.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SB, Schisler JC, Hohmeier HE, An J, Sun AY, Pitt GS, Newgard CB. A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet β-cell survival and function. Cell Metab. 2012;16:33–43. doi: 10.1016/j.cmet.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subathra M, Qureshi A, Luberto C. Sphingomyelin synthases regulate protein trafficking and secretion. PLoS One. 2011;6:e23644. doi: 10.1371/journal.pone.0023644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surma MA, Klose C, Simons K. Lipid-dependent protein sorting at the trans-Golgi network. Biochim Biophys Acta. 2012;1821:1059–1067. doi: 10.1016/j.bbalip.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Tabach Y, Billi AC, Hayes GD, Newman MA, Zuk O, Gabel H, Kamath R, Yacoby K, Chapman B, Garcia SM, et al. Identification of small RNA pathway genes using patterns of phylogenetic conservation and divergence. Nature. 2013a;493:694–698. doi: 10.1038/nature11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabach Y, Golan T, Hernández-Hernández A, Messer AR, Fukuda T, Kouznetsova A, Liu J-G, Lilienthal I, Levy C, Ruvkun G. Human disease locus discovery and mapping to molecular pathways through phylogenetic profiling. Mol Syst Biol. 2013b;9:692–692. doi: 10.1038/msb.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafesse FG, Sanyal S, Ashour J, Guimaraes CP, Hermansson M, Somerharju P, Ploegh HL. Intact sphingomyelin biosynthetic pathway is essential for intracellular transport of influenza virus glycoproteins. Proc Natl Acad Sci USA. 2013;110:6406–6411. doi: 10.1073/pnas.1219909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336:1150–1153. doi: 10.1126/science.1217817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabracci VS, Wiley SE, Guo X, Kinch LN, Durrant E, Wen J, Xiao J, Cui J, Nguyen KB, Engel JL, et al. A single kinase generates the majority of the secreted phosphoproteome. Cell. 2015;161:1619–1632. doi: 10.1016/j.cell.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidow H, Nissen P. Structural diversity of calmodulin binding to its target sites. Febs J. 2013;280:5551–5565. doi: 10.1111/febs.12296. [DOI] [PubMed] [Google Scholar]
- Trani E, Giorgi A, Canu N, Amadoro G, Rinaldi AM, Halban PA, Ferri GL, Possenti R, Schininà ME, Levi A. Isolation and characterization of VGF peptides in rat brain. Role of PC1/3 and PC2 in the maturation of VGF precursor. J Neurochem. 2002;81:565–574. doi: 10.1046/j.1471-4159.2002.00842.x. [DOI] [PubMed] [Google Scholar]
- Traub LM, Kornfeld S. The trans-Golgi network: a late secretory sorting station. Curr Opin Cell Biol. 1997;9:527–533. doi: 10.1016/s0955-0674(97)80029-4. [DOI] [PubMed] [Google Scholar]
- Van Baelen K, Dode L, Vanoevelen J, Callewaert G, De Smedt H, Missiaen L, Parys JB, Raeymaekers L, Wuytack F. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004;1742:103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk WJ, Van Hoeven RP, Van der Meer BW. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim Biophys Acta. 1981;644:323–332. doi: 10.1016/0005-2736(81)90390-4. [DOI] [PubMed] [Google Scholar]
- Veale KJ, Offenhäuser C, Whittaker SP, Estrella RP, Murray RZ. Recycling endosome membrane incorporation into the leading edge regulates lamellipodia formation and macrophage migration. Traffic. 2010;11:1370–1379. doi: 10.1111/j.1600-0854.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- Wakana Y, Kotake R, Oyama N, Murate M, Kobayashi T, Arasaki K, Inoue H, Tagaya M. CARTS biogenesis requires VAP-lipid transfer protein complexes functioning at the endoplasmic reticulum-Golgi interface. Mol Biol Cell. 2015;26:4686–4699. doi: 10.1091/mbc.E15-08-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana Y, van Galen J, Meissner F, Scarpa M, Polishchuk RS, Mann M, Malhotra V. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. Embo J. 2012;31:3976–3990. doi: 10.1038/emboj.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ren J, Padilla-Parra S, Fry EE, Stuart DI. Lysosome sorting of β-glucocerebrosidase by LIMP-2 is targeted by the mannose 6-phosphate receptor. Nat Commun. 2014;5:4321. doi: 10.1038/ncomms5321. [DOI] [PMC free article] [PubMed] [Google Scholar]