Abstract
Cardiovascular, respiratory, and related disorders (CVRDs) are the leading causes of adult death worldwide, and substantial inequalities in care of patients with CVRDs exist between countries of high income and countries of low and middle income. Based on current trends, the UN Sustainable Development Goal to reduce premature mortality due to CVRDs by a third by 2030 will be challenging for many countries of low and middle income. We did systematic literature reviews of effectiveness and cost-effectiveness to identify priority interventions. We summarise the key findings and present a costed essential package of interventions to reduce risk of and manage CVRDs. On a population level, we recommend tobacco taxation, bans on trans fats, and compulsory reduction of salt in manufactured food products. We suggest primary health services be strengthened through the establishment of locally endorsed guidelines and ensured availability of essential medications. The policy interventions and health service delivery package we suggest could serve as the cornerstone for the management of CVRDs, and afford substantial financial risk protection for vulnerable households. We estimate that full implementation of the essential package would cost an additional US$21 per person in the average low-income country and $24 in the average lower-middle-income country. The essential package we describe could be a starting place for low-income and middle-income countries developing universal health coverage packages. Interventions could be rolled out as disease burden demands and budgets allow. Our outlined interventions provide a pathway for countries attempting to convert the UN Sustainable Development Goal commitments into tangible action.
Introduction
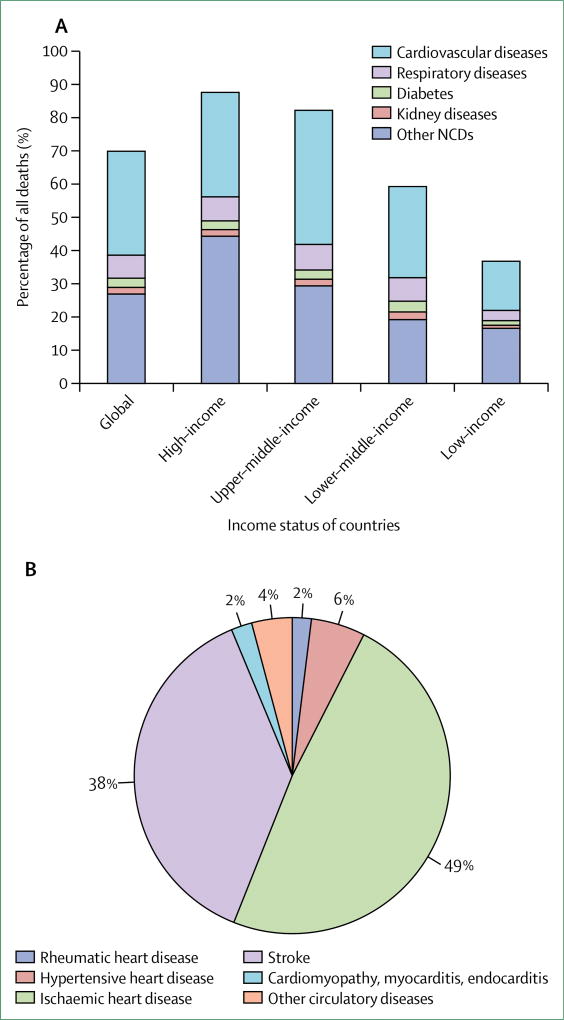
Adults today are most likely to die from a cardiovascular, respiratory, or related disorder (CVRD), with 43% of overall deaths and 49% of adult deaths estimated, by WHO, to be due to CVRDs in 2015 (figure 1).1 Most CVRDs are preventable or, if they do occur, can be treated to improve longevity and reduce disability. The ability to secure resources for optimal risk reduction and treatment, and to ensure consistent and persistent therapeutic compliance, is a challenge even for high-income countries (HICs). In low-income and middle-income countries (LMICs), limited capacity to detect these silent diseases and provide early treatment contributes to the rapid emergence of advanced complications and premature death.
Figure 1.
Share of all deaths caused by cardiovascular, respiratory, or related disorders and other non-communicable diseases, by country income (A), and specific causes of cardiovascular disease mortality in low-income and middle-income countries (B), 2015
Source: WHO Global Health Estimates 2015.1 NCDs=non-communicable diseases.
Cardiovascular, Respiratory, and Related Disorders, the fifth volume of the 3rd edition of Disease Control Priorities (DCP-3 CVRDs), covers three of the four major non-communicable diseases (NCDs) prioritised by the UN high-level meeting on prevention and control of NCDs in 2011:2 cardiovascular diseases (CVDs; ischaemic heart disease and its risk factors—ie, obesity, physical inactivity, tobacco, high blood pressure, and abnormal lipids—and stroke, peripheral artery disease, structural heart disease, and congestive heart failure), respiratory diseases, and diabetes. We also include kidney disease as a related condition (see appendix for complete list of conditions). Cancers and mental health, also typically grouped among NCDs, are covered in other volumes of DCP-3.3–5 CVRDs are closely related, some (eg, hypertension or hyperlipidaemia) serving as precursors, whereas others are sequelae (eg, heart failure or peripheral artery disease), and therefore they share prevention and management measures. Panel 1 summarises the key messages from DCP-3’s volume 5 and provides a framework for systematically addressing CVRDs in LMICs.
Panel 1: Key messages.
Adults living in low-income and middle-income countries (LMICs) face a high risk of death, disability, and impoverishment from cardiovascular, respiratory, and related disorders (CVRDs). The world is experiencing an increase in the number of deaths due to CVRDs.4 Slightly more than 80% of these deaths occur in LMICs.3 40% of the CVRD-related deaths in LMICs occur prematurely—at younger than 70 years of age—as compared with 19% in high-income countries (HICs). In 2015, the UN General Assembly agreed to an array of development goals, including a target to reduce premature mortality from non-communicable diseases by one-third by 2030.6 The world is not on track to achieve this goal. Stronger actions are needed to combat CVRDs in LMICs, where relative risk of death from CVRDs are 1·6–2·4-times higher than in HICs.
Effective prevention strategies are underutilised. HICs and upper-middle-income countries have reduced age-standardised mortality due to cardiovascular disease by more than 25% since 2000.3 This reduction has largely been achieved through the introduction of policy interventions to reduce risk factor levels and methods to strengthen the health system at the primary care level, and improvement of acute care with attention to early initiation of treatment. Residents of LMICs have not benefited from the advances in CVRD risk reduction and treatment seen in HICs. Policies aimed at the reduction of population-wide risk factors (eg, high taxation of tobacco, reduction of salt in processed foods, or bans on trans fatty acids) are effective but have not been widely adopted in LMICs. Targets related to individual-level risk factors (eg, reduction of smoking and obesity, or improvement of physical activity), are harder to achieve; however, when achieved sustainably, improve health in multiple domains.
Primary care centres require strengthening to treat the current and growing burden of CVRDs. Medications crucial for treatment at the individual level have long track records for effectiveness, and in many cases for cost-effectiveness, but have low uptake in LMICs. Most of the disease-specific interventions we recommend are related to long-term use of medications, and should be delivered in primary care centres. Specific needs to shore up this platform include training of primary or non-physician health-care providers in the management of CVRDs using local or regional guidelines, ensuring availability of inexpensive, generic, or combination drugs in clinics, and creating culturally viable strategies to improve patient adherence. These centres can also serve as the entry point for acute treatment, with referral to first-level centres where algorithm-based care could be delivered.
Cost-effective prevention policies and treatments for CVRDs are possible to implement in LMICs. Population-level policies to reduce risk of CVRDs are generally more affordable than treatments because of lower estimated costs. Many cost-effective treatment interventions can be delivered at primary care hospitals, especially related to inexpensive and generic medications, and a selected number at first-level or referral-level hospitals.
Universal health care that includes care for CVRDs provides benefits beyond individual health to financial protection of families. The household financial burden is particularly relevant in economic analyses related to the treatment of CVRDs. On a value-for-health basis, CVRD interventions—particularly ones that incur ongoing, long-term costs—are expensive, even if cost-effective. However, many of the afflicted adults are wage earners, and the potential to improve economic productivity and avert poverty is clear and large.
We discuss the overarching burden of CVRDs, including the reasons LMICs face disproportionately high premature mortality and disability rates. We summarise the effectiveness of and cost-effectiveness evidence for relevant health interventions and policies, and propose a costed essential package of 36 interventions that are feasible for low-income countries (LICs) and lower-middle-income countries to adapt and implement.
High risk of death, disability, and impoverishment
The world’s population is ageing. For most of the 20th century, 5% or fewer people reached the age of 65 years; nowadays, people older than 65 years constitute 10% of the world’s population, and this proportion is expected to increase to more than 15% by 2030.7 Combined with population growth, population ageing has led to an overall increase in the number of people dying from CVRDs, because although the propensity for these diseases starts in utero, their substantive effects are seen in adulthood. From 2000 to 2015, the absolute number of deaths due to CVRDs increased by 23% globally.1
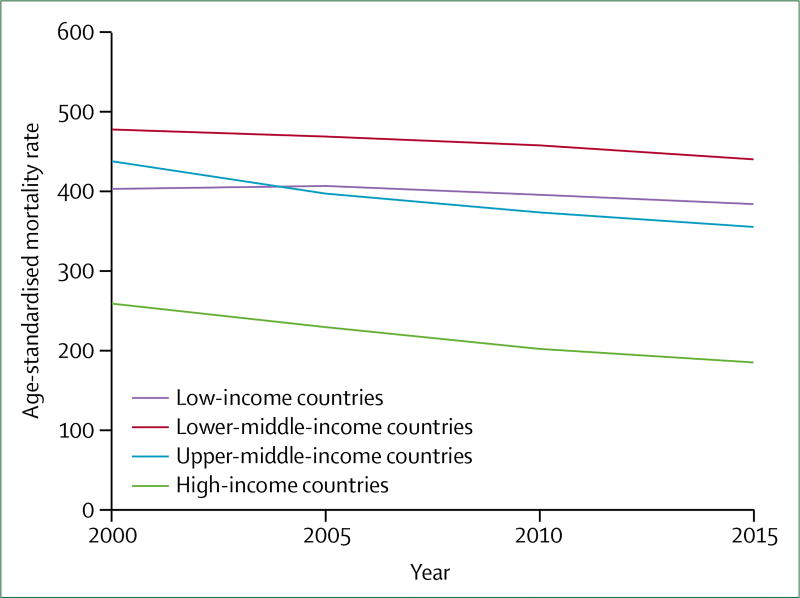
With the implementation of population-level risk reduction measures, and advances in acute and chronic care, age-specific mortality has declined to the extent that it counterbalances the absolute increase in number of deaths associated with population growth and ageing.3,8 Thus, age-standardised mortality rates per 100 000 population for CVDs and respiratory diseases are declining, whereas rates for diabetes and kidney diseases (including kidney disease due to diabetes) are either unchanged or increasing. Compared with HICs, LMICs experienced smaller declines in age-standardised mortality rates from CVRDs from 2000 to 2015; therefore, inequalities in outcomes are worsening (appendix). For CVDs—by far the most common cause of death among the CVRDs—the decline between 2000 and 2015 ranged from 5% in LICs, to 15% in upper-middle-income countries, to 34% in HICs (figure 2). Absolute rates of morbidity and premature mortality, captured in the summary metric of disability-adjusted life-years (DALYs), are increasing rapidly in poor regions: between 2000 and 2015, DALYs from CVD and diabetes increased by 33% and 72% in south Asia, and 26% and 56% in sub-Saharan Africa.9
Figure 2.
Trends in age-standardised mortality rates from cardiovascular disease for both sexes, by country income group, 2000–15
Per year, the decline in age-standardised mortality rates were −0·8% in countries of low income, −0·6% in countries of lower-middle income, −1·0% in countries of upper-middle income, and −2·3% in countries of high income.
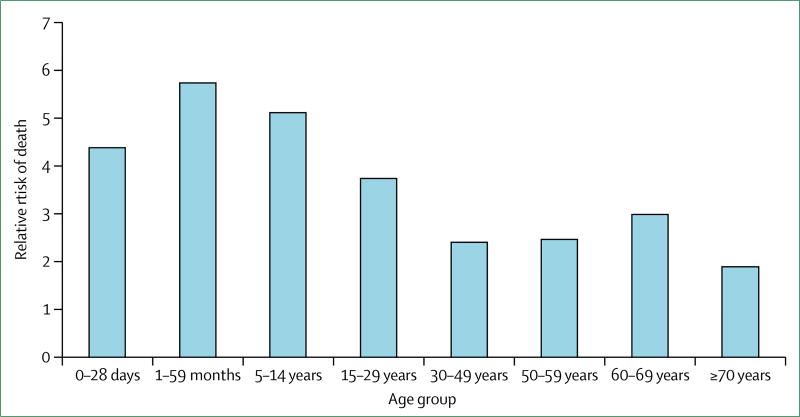
For a person with CVD, where he or she lives predicts risk of death as strongly as being overweight or hypertensive (figure 3).10–12 For adults with CVD, residence in an LMIC also predicts higher likelihood of a serious event—eg, myocardial infarction or stroke—and at a younger age than in HICs.13,14 Acute admissions to hospital are expensive and substantially increase the likelihood of families falling into poverty.15 More than half of people admitted to hospital because of stroke, myocardial infarction, or peripheral artery disease in China, India, or Tanzania experienced catastrophic health spending (ie, annual health expenditure >40% of total non-food household expenditure).16 In the Philippines, even without acute complications, the cost of routine use of generic medications, such as atenolol, would impoverish 5% of the population—20% if brand-name atenolol were used.17
Figure 3.
Relative risk of death due to cardiovascular and respiratory disease in low-income and middle-income countries versus high-income countries, by age group, 2015
Why have LMICs not benefited from advances in CVRD prevention and care? The reasons are many, and vary by region, but we highlight: the frequent absence of population-wide strategies to tackle behavioural risk factors; missed opportunities to identify and treat disease in the early stages; and inability to provide quality care for advanced complications.
Dearth of population-wide strategies to tackle behavioural risk factors
To date, population-wide strategies to encourage behavioural change have focused on tobacco use, poor diet, physical inactivity, and obesity, which have—at least in part—contributed to the substantial decline in age-standardised cardiovascular and respiratory disease mortality in HICs. In LMICs, the burden of these major risk factors for the development and progression of these conditions is increasing. After more than 170 countries signed on to the WHO Framework Convention on Tobacco Control in 2005, experts called for its immediate implementation and some argued for further regulations aimed at achieving a tobacco-free world.18 WHO has set a global target: a 30% reduction in smoking prevalence by 2025.19 Most LMICs are unlikely to meet this goal, because the overall number of smokers is predicted to continue to increase, and many of the evidence-based recommendations of the framework, including taxation, advertising bans, pictorial warnings, and publicly supported smoking cessation assistance, which are described in the WHO MPOWER package,20 have not been implemented in these countries.21 Of all tobacco interventions, taxation is the most effective method of averting tobacco-attributable CVRDs.22 As shown in an extended cost-effectiveness analysis in China,23 taxation also provides financial risk protection for low-income families living in LMICs, addressing concerns about the potential regressivity of taxation on the poor. Yet, in LICs, tobacco taxes are less than 40% of the average price of cigarettes, compared with more than 60% in HICs; thus, cigarettes are more affordable in LMICs and will become even more affordable over time.24 Furthermore, in LMICs, a substantial proportion of people with chronic obstructive pulmonary disease have never smoked;25 in these instances, occupational exposure25 and indoor air pollution because of biomass fuel use26—a common practice in LMICs—contribute to the burden of respiratory disease.
Poor diet, obesity, and physical inactivity are interlinked risk factors that, when addressed early in life, can lead to lifelong protection from CVRDs. Based on current trends, the contribution of these risk factors to death and disability is likely to increase in LMICs and decline in HICs. In HICs, the number of people who partake in physical activity during leisure time is increasing.27 Over the past 30 years, deaths attributed to physical inactivity declined by 15% in HICs and increased by 25% in LMICs.28 In HICs, many stakeholders are working to encourage physical activity: city governments are creating pedestrian plazas, health-care organisations are incorporating physical activity assessments into clinic visits, and employers are offering at-work exercise classes.29–31 By contrast, the rapid and unplanned growth of urban metropolises in LMICs impedes the implementation of cost-effective opportunities for physical activity.32
Missed opportunities to treat CVRDs in the early stages
The scarcity of effective care for people who experience an acute CVRD event, such as myocardial infarction, stroke, or asthma exacerbation, is the first of several missed opportunities for early treatment of CVRDs. A timely emergency response to an acute CVRD event with common, relatively inexpensive medications—eg, aspirin for myocardial infarction and bronchodilators for asthma—can save a life until diagnosis. Yet, in many locations, health workers at primary health centres do not have adequate resources to provide high-quality, evidenced-based preventative care.
Effective long-term management of hypertension and diabetes can reduce complications such as ischaemic heart disease, stroke, peripheral artery disease, retinopathy, and chronic kidney disease. Early use of inhaled corticosteroids decreases the frequency of serious attacks, even for people with mild persistent asthma.33 Medications for long-term disease management overlap across many CVRDs. Statins can reduce the risk of first-time and recurrent myocardial infarctions or strokes by an average of 21%.34 Effective management of hypertension according to standard treatment guidelines affords a similar degree of protection for heart failure, acute coronary events, and strokes;35 however, use of these therapies remains low in LMICs. In a multicountry study, 31% of patients in LICs were aware of their hypertension (vs 49% in HICs), and fewer than a third of patients in LICs were treated, as compared with nearly half of patients in HICs.36 Control of hypertension was achieved in fewer than one in five of the overall population with hypertension, regardless of country-income setting,36 although the trend is towards improved rates of control in HICs.37 The 2003 Doha Declaration on the Trade-Related Aspects of Intellectual Property Rights Agreement and Public Health enabled countries to produce medications for domestic use when necessary for health of their populations.38–40 Generic cardiovascular medications now constitute more than 70% of the market in many LMIC,41 yet affordability remains an issue, with large variations in cost even within the same drug class.42 Although the WHO Model List of Essential Medicines43 attempts to focus resources on selected effective and cost-effective medications, conflicting incentives for physicians lead to highly variable prescribing patterns, which can increase costs without clear health benefits. One example is the heavy provider reliance on insulin analogues, over the cheaper non-analogue form, in Venezuela, Brazil, and Mexico.44
Even if sequelae develop, optimising patient treatment can further delay progression along the disease spectrum to heart failure, limb amputation, blindness or end-stage kidney disease. The gap in care provision between HICs and LMICs is large: fewer than 10% of patients in LICs and fewer than 25% in lower-middle-income countries take β blockers, angiotensin-converting-enzyme inhibitors, or statins after a myocardial infarction or stroke, compared with 50–66% of patients in HICs.45
Inability to provide quality care for advanced complications
Facilities for the care of patients with advanced conditions, including end organ damage, are scarce and, when available, incentives to ensure the delivery of high-quality care are few. The need for specialists or specialised equipment means that some advanced conditions (eg, heart failure, structural heart disease, and end-stage kidney disease) are expensive to treat. Many middle-income countries (MICs) and some LICs do have facilities but are unable to take on the large number of people who require treatment, because of scarcity of resources, patient-level financial constraints, or both. Haemodialysis facilities exist in most countries in the world, but fewer than a quarter of people expected to reach end-stage kidney disease annually have access to therapy.46,47 Even for people who can make the necessary payments, there is little to no oversight of the quality of care delivered. Of six haemodialysis centres surveyed in Lagos, Nigeria, none met accepted standards for microbial decontamination.48 An analysis of patients with rheumatic or congenital heart disease reported that two-thirds of surgical candidates in Uganda did not have access to treatment, and 18% died while on the waiting list for surgery.49 Among those who underwent open-heart surgery, postoperative mortality and loss to follow-up rates were high (19% and 22%, respectively).49
Thus, even as the burden of risk factors for CVRDs increases in LMICs, strategies and facilities to care for people with these diseases are too rarely available. Without oversight, scarce resources are sometimes expended on expensive or low-quality treatments, while cost-effective alternatives go underused.50
Cost-effectiveness of interventions for CVRDs
We reviewed the cost and cost-effectiveness of various CVRD clinical interventions and related policies, with the goal of creating a package of CVRD interventions for LMICs.51 We reviewed the published literature on the cost of the provision of preventive care and treatment for cardiovascular and metabolic diseases,52 and the cost-effectiveness of CVRD interventions in LMICs. Guidelines for reporting cost-effectiveness results were not uniform, and to accurately interpret the results, reference to individual studies was necessary. We extracted cost and cost-effectiveness data from English-language literature published after 2000 through a bibliometric search (appendix), adjusted all reported results to the same currency and year, and ranked the cost and cost-effectiveness outcomes.51 Where necessary, to assess priority interventions when evidence from LMICs was scarce, we referred to evidence from HICs. Some systematic reviews assessed the evidence of cost51 and cost-effectiveness of interventions to tackle CVDs in LMICs specifically,53,54 and found that although the cost-effectiveness evidence on CVD interventions in LMICs is increasing, it is modest in comparison to the evidence in HICs.
Population policies aimed at reducing risk of CVRDs are among the most cost-effective ways to reduce CVRD-related mortality. The leading population policies involve tobacco control strategies that are cost-saving or highly cost-effective, or both; for example, taxation that is cost-saving (Vietnam) and highly cost-effective (US$140 per DALY, Mexico); public smoking bans that cost $2·4–35 per life-year saved (India); advertising bans that cost $2800 per DALY averted (Mexico); and mass media campaigns that range from cost-saving to $3200 per DALY averted.51 Evidence in support of the cost-effectiveness of sugar-sweetened beverage taxes is increasing, but the health effects are still inconclusive.55,56 The strongest evidence to date comes from Mexico, where a sugar-sweetened beverage tax introduced in 2014 reduced consumption of sugary beverages and increased water consumption, especially among the poor.56 Data on changes in obesity, diabetes, or other CVRDs have not yet been reported. Population-level salt reduction strategies range from cost-saving (Argentina) to up to $15 000 per life-year gained.51 Salt reformulation by industry appears to be the most cost-effective approach, and salt reduction campaigns to promote health appear to be the least cost-effective.51
Many disease-prevention or health-promotion programmes have not been assessed for cost-effectiveness. Evidence to indicate the best method to promote physical activity, let alone that which is best value for money, is scarce and largely concentrated on HICs.57 A study from China showed, in school programmes for children, that physical activity combined with a nutrition programme is more effective than either intervention alone.58 Through a systematic review of population-wide interventions in adults and children across multiple countries, Laine and colleagues32 found the most efficient interventions to increase physical activity were community trails built alongside or over abandoned rail tracks ($0·006 per metabolic equivalent of task hours [MET-h]), pedometers ($0·014 per MET-h), and school health education programmes ($0·056 per MET-h). Nearly all physical activity interventions, especially those related to tracking or motivating physical activity, require more detailed evaluation of effectiveness and cost-effectiveness.
Screening and pharmacological treatment of hypertension to reduce the risk of stroke and ischaemic heart disease have been shown to range from cost-saving (China) to cost-effective at $700–5000 per DALY averted or quality-adjusted life-year (QALY) gained in South Africa and Argentina.51 The cost-effectiveness of strategies that use lipid-lowering therapies are slightly higher than screening and pharmacological treatment of hypertension, ranging from $1200 per QALY in most large LMICs when part of a multidrug regimen, to as much as $22 000 per DALY in the Philippines.51 With more statins coming off patent, the price of statins has dropped, and lipid-lowering therapy is becoming more cost-effective.
Opportunistic screening for prediabetes and diabetes in a high-risk population is more cost-effective than mass screening for diabetes alone.59 Structured diabetes self-management education programmes are cost-effective but self-monitoring of blood glucose among people not on insulin or an oral hypoglycaemic is not.59,60 One randomised control trial from a HIC, Denmark, supports comprehensive management (eg, attention to blood glucose, blood pressure, and lipids) as being cost-effective,61 and effectiveness evidence in LMICs is increasing.62 Evidence from both HIC and LMIC settings supports screening for complications of diabetes, with screening for foot ulcers among the most cost-effective methods.63 A study in India suggested that cost-effectiveness of screening for retinopathy via telemedicine ranges from $1200 to $2400 per QALY gained.64
Management of acute ischaemic heart disease and stroke can be divided into the prehospital phase and the hospital phase. Prehospital-phase management requires an established emergency transport system with trained staff and equipment. When available, prehospital thrombolysis was shown to reduce costs in Brazil.65 The use of electrocardiogram machines for patient triage in primary health centres was shown to be cost-effective in India at $12 per QALY gained.51 The use of aspirin and β blockers was shown to cost about $10–20 per DALY averted, streptokinase about $700 per QALY gained, and more fibrin-specific thrombolytics (such as tissue plasminogen activator) about $15 000 per QALY. More advanced treatment includes percutaneous coronary interventions, including stents. In China, the availability of percutaneous coronary interventions or streptokinase for acute myocardial infarction costs between $9000 and $25 000 per QALY gained. Management of people undergoing percutaneous coronary interventions with antiplatelet agents such as prasugrel and clopidogrel is also cost-effective in advanced centres. Data on cost-effectiveness of acute ischaemic stroke management with a thrombolytic agent are sparse in LMICs, but one study recommended home-based rehabilitation.66 Additionally, management of acute ischaemic stroke is particularly challenging in settings without the necessary laboratory and radiographic services to ensure safe administration of thrombolytics.
Management of heart failure with oral agents, such as angiotensin-converting-enzyme inhibitors, β blockers, and aldosterone antagonists, can be either cost-saving or highly cost-effective.51 Advanced therapy with implantable defibrillators and resynchronisation therapy could be cost-effective in MICs, with cost-effectiveness ratios of $17 000–35 000 per QALY gained. The use of low-dose inhaled corticosteroids for mild asthma was cost-effective in a lower-middle-income country.51
Our review of cost-effectiveness has identified multiple cost-effective and even cost-saving interventions for CVRDs in LMICs, particularly for population-level interventions; however, we acknowledge that cost and cost-effectiveness data rarely translate directly across settings, and that each country would need to individually assess its disease burdens and priorities (panel 2). Many highly clinically effective clinical interventions—eg, treatment of hypertension or hyperlipidaemia—are also cost-effective in some LMICs, whereas others requiring greater technology and specialised care are only cost-effective in MICs.
Panel 2: Considerations for the implementation of the Disease Control Priorities, 3rd edition (DCP-3) essential package.
The options we highlight in the DCP-3 essential package take affordability and feasibility in low-income and lower-middle-income countries into account, and address diseases with a high burden. We highlight 13 core interventions that are likely to have the greatest benefit in reduction of risk or treatment of cardiovascular, respiratory, or related disorders. The following important considerations remain:
Affordability is just one consideration when an intervention is chosen. Population-wide preventive interventions are the cheapest; these activities might not have the same efficacy on an individual basis as appropriate treatment of acute myocardial infarction. Between population and individual measures, and between preventive and treatment measures, policy makers should judge which have the best evidence for being both effective and cost-effective in their countries.
Because of the many competing demands on constrained resources, a political commitment to tackling cardiovascular, respiratory, and related disorders is essential. Some countries’ policy makers might feel overwhelmed by the number of diseases requiring attention, and the number of potential methods available to address them. The DCP-3 essential package is a first step towards the identification of relatively affordable and effective strategies, but many countries will need to initiate priority-setting processes guided by political interest. A positive example of the effective use of priority-setting for health—led by strong political will—is Ethiopia, where hypertension and diabetes treatment are being rolled out.67
Regional disease burden varies and should inform policy choices. Low-income countries with high burden, such as in sub-Saharan Africa, might want to consider cost-effective interventions for endemic rheumatic heart disease;68 Central American countries might want to focus on chronic kidney disease and diabetes.69
Budget planning should take the increased demand for health services into account, as life expectancy improves.
Thus, not every country can or should implement all suggested interventions, and some countries will take years to build a health system that can implement some of them. In most countries, the two highest priority areas could be tobacco control, and early detection and management of hypertension. As countries expand and scale up their health benefits, they must ensure interventions are appropriate for the disease burden and feasible given the system capacity.
Pathways to addressing CVRDs in LMICs
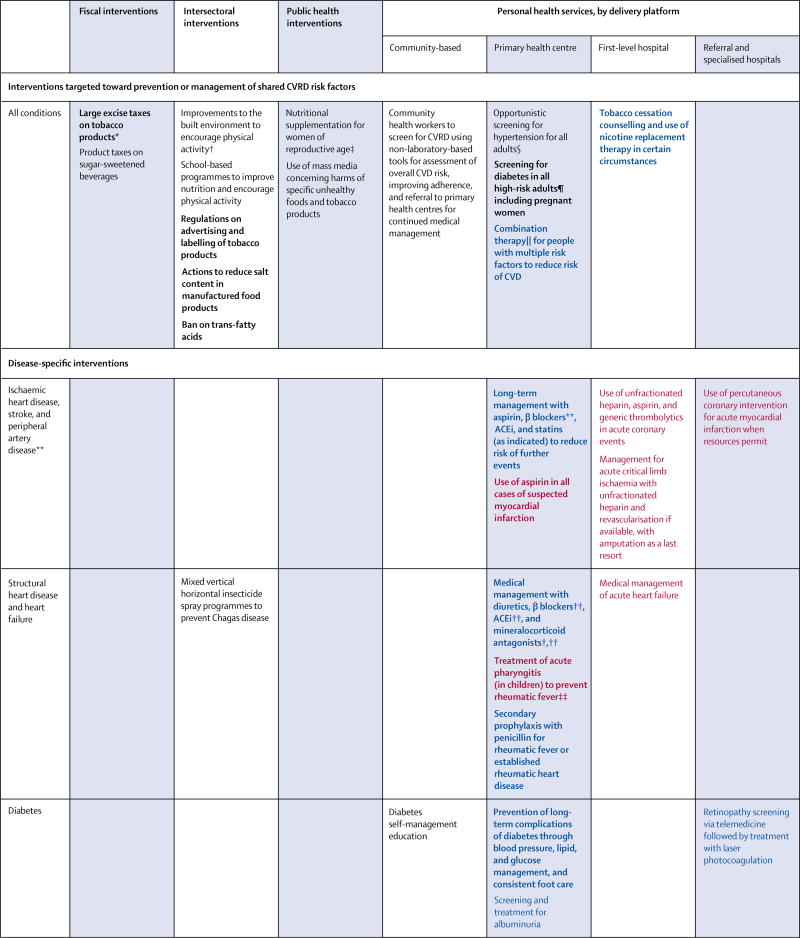
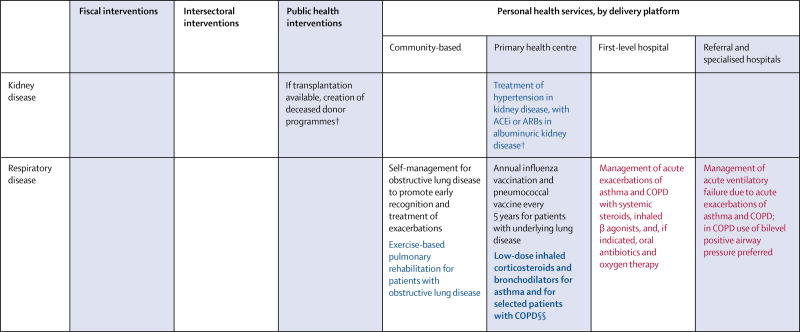
After the UN highlighted the growing and detrimental effect of NCDs on the health and wealth of nations,2 WHO produced a Global Action Plan for the Prevention and Control of NCDs.70 Of the eight voluntary targets set to help countries reduce NCD mortality, six focus on prevention; specifically, interventions that improve diet and reduce smoking, obesity, and physical inactivity. To assist countries in meeting WHO targets, we offer a set of policies and interventions that form an essential package of actions to reduce risk of CVRDs (figure 4). Further, we propose a set of disease-specific individual-level services appropriate for low-resource settings, acknowledging that LMICs struggle with a spectrum of early to advanced cases of CVRDs (figure 4).
Figure 4.
Interventions targeted toward the prevention or management of shared risk factors for cardiovascular and respiratory disease, and disease-specific interventions
Black font denotes routine care, blue font denotes continuing care, and red font denotes urgent care. Recommendations in bold font denote core interventions. CVRD=cardiovascular, respiratory, or related disorder. CVD=cardiovascular disease. ACEi=angiotensin-converting-enzyme inhibitor. ARB=angiotensin receptor blocker. COPD=chronic obstructive pulmonary disease. *For fiscal and intersectoral policies that address CVRD attributable to indoor and outdoor sources of air pollution, see chapter 1 of volume 7 of Disease Control Priorities, 3rd edition.71 †Data from high-income countries only. ‡Aimed at the prevention of gestational diabetes and low birthweight. §Treatment with generic drugs is recommended, guided by the severity of hypertension or the presence of additional risk factors. ¶High risk is typically defined as individuals who are older, have high blood pressure, or are overweight or obese. ‖Where available, fixed-dose combination therapy is preferred. **Not applicable to peripheral artery disease. ††Applicable to heart failure with reduced ejection fraction. ‡‡Use available treatment algorithms to determine appropriate antibiotic use. §§Inhaled corticosteroids indicated in patients with COPD who have severe disease or frequent exacerbations.
These policies and interventions were selected from those deemed most effective and cost-effective by the DCP-3 CVRD author teams, with each combining a literature review with expert judgment to prioritise among those with the strongest evidence. We used the following criteria to include interventions in the essential package: potential to address a significant disease burden; cost-effectiveness evidence from at least one LMIC setting, or strong evidence to suggest LMIC cost-effectiveness; and feasible implementation over the next 10 years in LICs or lower-middle-income countries. The essential package of recommended interventions is organised by delivery level or platform (appendix).
Although the essential package goes beyond the WHO NCD Best Buys,72 it has a high degree of overlap with the more than 100 priority interventions in the recent revision of Appendix 3 of the WHO Global Action Plan (see appendix for a discussion of overlaps and modest differences).70 Since the DCP-3 essential package is designed to be feasible within 10 years in LICs or lower-middle-income countries, it more heavily emphasises multisectoral and primary care interventions. Certain recommendations in the WHO Appendix 3 (eg, subsidies to increase fruit and vegetable intake, measures to reduce illicit trade in tobacco, or medical management of atrial fibrillation) are not included in the essential package because of a scarcity of sufficiently compelling cost-effectiveness data. DCP-3 also gives specific recommendations in areas not covered by WHO Appendix 3—eg, peripheral vascular disease and chronic kidney disease.
Effective risk reduction strategies for most CVRDs
Effective strategies to reduce the risk of CVRDs are available but underused. If implemented, the actions outlined in figure 4 should create an environment that encourages healthy behaviour and reduces involuntary exposure to CVRD risk. Multiple agencies, both public and private, are responsible for establishing this environment.
Fiscal policies recommended in the DCP-3 essential package are among the most effective and affordable actions that governments can take to create a healthy environment. Policies that are not fiscal, such as regulations to reduce salt or tobacco consumption via labelling or bans on advertisements, can also encourage healthy consumer choices. In the UK, when high-salt foods were labelled as such, coupled with a public education campaign, a marked decline in the consumption of these foods was observed.73 In the case of (trans fat) partially hydrogenated oils, the strength of the data demonstrating improvements in cardiovascular mortality warrants bans of trans fats from the food-supply chain.74
Although health promotion activities to improve risk factors on a population level might have similar or better effect sizes to fiscal policies, they generally require more planning or resources to implement. Mass media health campaigns to improve diet are effective when they offer specific actionable health messages, such as increasing fruit and vegetable intake.75 Once a country commits to one aspect of the approach, other strategies can be added with less additional cost. Cities in Brazil and Colombia have committed to providing pedestrian plazas and safe areas for physical activity, allowing them to also offer community exercise classes.76 Many other population-level interventions, such as control of chronic air pollution, chronic infections, and occupational exposure, also have potential to reduce the risk of CVRDs, and these are covered in other volumes of the DCP-3.77
Across many of the CVRD health endpoints, the establishment of absolute risk is a crucial first step—both for matching the intensity of prevention effort to the level of risk and for efficient targeting of health system resources. The use of non-invasive methods to screen for many CVRDs is generally feasible for community health workers.78 The most recent data demonstrate that well-trained community health workers can deliver lifestyle modification advice,79 identify high-risk individuals with similar effectiveness to primary care physicians,80 be cost-effective in helping patients to adhere to hypertension regimens,81 and be trained to use mHealth tools effectively.78,82,83 However, exactly what and how many roles (screening, follow-up, medication prescription) these community health workers should play is unclear. The integration of this workforce into the existing health infrastructure is also a challenge, because their effectiveness depends on the ability to triage the diagnosed cases to appropriate levels of care and to ensure the delivery of medication.
Opportunistic screening for diabetes and hypertension can be done in clinics, especially for high-risk populations, such as pregnant women, obese adults, and people with multiple risk factors. Generic therapy to reduce complications after a diagnosis of CVRD is on the WHO’s Model List of Essential Medicines43 and could be made reliably available in primary health settings. Therapy in combination (eg, as and when fixed-dose combinations are available) or as individual drugs targeting multiple risk factors is especially attractive for high-volume health systems with limited resources for personalisation and titration.84,85 With the wide-spread availability of drug and disease management algorithms, follow-up and titration of medications might again be possible via community health workers, accompanied by broader prescribing rights, but otherwise should be managed by primary providers, rather than specialised physicians.
Central role for primary health centres
Stronger and better-equipped primary health centres are needed to manage the current and growing burden of CVRDs. Health systems in LMICs do not need to be replicas of health systems in HICs. More so than any other set of diseases, CVRDs require screening, long-term follow-up, and reliable medication delivery. The approach in most HICs—that is, individualised interval screening by primary care physicians followed by highly specialised, referral-based care—is unlikely to be viable in most LMICs for a multitude of reasons. Financial and human resource constraints come into play, as does the cultural approach to health; for example, populations in LMICs might be more amenable to peer counselling or community-based health promotion activities.
Therefore, most of the personal interventions recommended in the DCP-3 essential package (figure 4) can be delivered at the community level or primary health-care level. The WHO Health Systems Framework86 provides a comprehensive approach to strengthening health systems. In addition to ensuring availability of key medications, governments can develop national guidelines and targets for specific conditions, which would enable the effective delivery of care (table 1) and, in turn, encourage reliance on the available generic medications and standardisation of follow-up intervals. Structured guidelines for referral to specialised systems could improve efficiency at both the primary and specialised levels of care. If primary health-care centres are the first point of contact in an acute situation, the availability of basic therapy (eg, rapid administration of aspirin or bronchodilators), although limited, could be life-saving. Primary care centres could be empowered to deliver such therapy before facilitating transfer to a first-level hospital.87
Table 1.
Recommendations for improvement of health systems that enable implementation of the recommended interventions
| Platform | |
|---|---|
| Improve access to essential medications, including aspirin, β blockers, diuretics, ACEi, or ARBs, statins, mineralocorticoid agents, non-analogue insulin, bronchodilators, and inhaled corticosteroids | Policy, public health |
| Develop a category of trained (non-physician) health worker | Policy, intersectoral |
| Offer public emergency medical transport services | Policy, intersectoral |
| Create standardised care pathways for first-level hospitals to manage acute episodes for: myocardial infarction, stroke, critical limb ischaemia, heart failure, acute kidney injury, chronic obstructive pulmonary disease, or asthma exacerbation | Policy, public health |
| Issue national targets for secondary prevention to enable primary health centres to manage CVRD effectively | Policy, public health |
ACEi=angiotensin-converting-enzyme inhibitor. ARB=angiotensin receptor blocker. CVRD=cardiovascular, respiratory, or related disorder.
Algorithms for the management of acute events at first-level hospitals might facilitate rapid recognition and treatment, thereby reducing severity and costs of illness. Several international societies have published guidelines for the management of acute asthma exacerbations, which rely on continual administration of β-2 agonists and anticholingerics, and rapid administration of systemic corticosteroids—all of which are inexpensive and highly effective therapies.6,88 Protocol-based implementation in first-level hospitals has been shown to reduce length of stay and use of intensive care units.89
When specialised centres are used for conditions that are rare or costly to treat, two potential strategies merit consideration: (1) the scale-up of one effective treatment from the roster of potential therapies, or (2) the creation of high-volume centres that specialise in specific diseases. After careful analysis, taking into account cost, cultural opinion, and ethics, Thailand has chosen to pay for and scale up the peritoneal form of dialysis (over haemodialysis, which has equivalent efficacy).90 We await the long-term outcomes of this strategy, but preliminary data indicate an increase in treatment availability with similar patient survival to HICs relying mostly on haemodialysis.91,92 In children with congenital heart disease that is amenable to a highly technical but relatively effective surgical procedure (eg, ventricular septal defect), creating centres especially designed to serve these patients might be a viable approach to treatment.93 Similarly, we know that high-volume kidney transplant centres can achieve good outcomes.94,95
The DCP-3 essential package includes a few examples of effective specialised care that are potentially feasible and cost-effective in low-income settings but are not in widespread use.96,97 Especially in countries where the respiratory disease burden is high, equipment such as non-invasive positive pressure ventilation or high-resolution CT for interstitial lung disease could be prioritised. Other effective tertiary care services are not considered feasible or affordable in low-income and most lower-middle-income settings. Advanced treatments such as implantable cardioverter defibrillators or cardiac synchronisation therapy are potentially cost-effective in some places (eg, Brazil), but are expensive.98 The use of thrombolytic therapy to treat ischaemic stroke requires timely access to CT; and, if built only to diagnose stroke, these facilities are as yet unaffordable. As costs drop, and skilled providers are trained, additional capacity to diagnose and manage more complex disease should become available in specialised facilities (panel 2).
Costs of implementing the package
We estimated the potential cost of implementing the DCP-3 essential package in typical low-income and lower-middle-income settings reflecting typical costs, demographic and epidemiological characteristics, and coverage gaps in CVRD care (table 2). The appendix contains more detail on costing methods and results. We estimated, to achieve full implementation of the package (ie, at 80% population coverage), an average LIC would need to spend an additional $21 per person (3·8% of income), and an average lower-middle-income country would need to spend an additional $24 per person (1·3% of income). Most (60%) of the additional investments would need to be in primary health centres that offer preventive services and manage chronic diseases. LICs that are particularly resource constrained could focus on achieving full implementation of the interventions in figure 4 that we have deemed likely to provide the best value for money in these settings. This high-priority subpackage would only cost an additional $11 per person (2·0% of income). This costing exercise suggests that all countries—regardless of resource levels—can begin to put in place at least a few highly effective CVRD interventions at a reasonable cost as they move towards universal health care (panel 3).
Table 2.
Estimated costs of the essential package in a typical low-income country and a lower-middle-income country
| Low-income country |
Lower-middle- income country |
|
|---|---|---|
| Total cost per head | $21 | $37 |
| Total cost as a % current GNI per head | 3·8% | 2·1% |
| Incremental cost per head | $20 | $23 |
| Incremental cost as a % current GNI per head | 3·6% | 1·3% |
GNI=gross national income. GNI estimates taken from the World Bank and deflated to 2012 US dollars. See appendix for details of methods, data sources, and assumptions.
Panel 3: Measuring the benefits from universal health coverage for cardiovascular, respiratory, and related disorders (CVRDs).
When considering whether to expend strained resources on CVRDs, countries can take into account the benefits to individual health, but also outcomes relevant to societal wellbeing, such as poverty aversion, financial risk protection, and equity. Extended cost-effectiveness analyses, developed as part of the Disease Control Priorities effort, attempt to capture some of these outcomes and provide evidence that CVRD care, in particular, offers substantial financial risk protection. Three extended cost-effectiveness analyses relevant to CVRDs—assessing tobacco taxation in China,21 salt reduction in processed foods in South Africa,85 and treatment of hypertension in Ethiopia86—support the cost-effectiveness of these policies, and demonstrate that they could avert thousands of cases of poverty annually.
Treatment of hypertension in Ethiopia illustrates two specific features of universal public finance for CVRD care in low-income and middle-income countries: (1) treatment of CVRDs might be more expensive than interventions in other domains (eg, maternal and child health), but (2) because these health policies and interventions protect wage-earning adults from disability or death, universal coverage could reduce financial risk to a greater degree. Further, poor families spend a much larger proportion of their household income on admissions to hospital or medications for CVRDs than wealthier families, so they could benefit proportionately more.87
For cases of advanced disease, when universal coverage for treatment is not yet affordable or sustainable (eg, complex congenital heart defects, advanced heart failure, or end-stage kidney disease), countries should consider expanding palliative care services. In addition to easing the emotional and physical burden of disease, palliative care might allow families to care for their loved ones without exhausting their financial resources on ultimately unsustainable treatments.
Conclusions
We offer a range of effective and cost-effective policies and interventions to reduce the high and mounting global health burden of CVRDs. We reviewed the evidence for CVRD interventions to assemble a DCP-3 essential package of the most effective policies and services that could be implemented in LMICs. Modelled studies suggest that countries can expect a high return on investment from prevention and control of CVRDs, especially from the implementation of population prevention policies that cost relatively little.99 Countries that have effective and cost-effective choices available to them can, by relying heavily on population-level policies and services that can be delivered at the community and primary health levels (and with an effective referral system for the few specialised interventions that meet the DCP-3 essential package criteria), obtain substantial health gains at reasonable cost.
Many important issues remain uncertain, especially given the scarcity of LMIC economic evidence. Research in areas likely to produce substantial public benefit—eg, further evaluation of the health gains from taxes on sugar-sweetened beverages, agricultural and trade policies to improve fruit and vegetable intake, intersectoral policies to increase physical activity, use of cheaper or faster surveillance techniques, and methods to ensure reliable supply of generic medications, including of fixed-dose combination therapy—could be a specific priority in LMICs. New technologies, medications, and delivery platforms have the potential to disrupt and shift management paradigms. These issues warrant development of strong priority-setting institutions in LMICs to develop a research agenda, assess new technologies, and change disease epidemiology and health system constraints.
Nonetheless, the health benefits of individual medical interventions are clear, and HICs have achieved huge reductions in mortality by making medical treatment widely available. These gains must be extended to LMICs for global goals to be achieved. Since the 2011 UN high-level meeting on prevention and control of NCDs, a Global Action Plan for NCD Prevention and Control has been put in place, and the 2015 UN Sustainable Development Goals have recognised NCDs as a serious threat to development. The DCP-3 essential package provides a pathway to the achievement of substantial reduction in death, disability, and impoverishment from CVRDs in LMICs with evidence-based cost-effective interventions.
Acknowledgments
DP, RN, and DW received funding from the Bill & Melinda Gates Foundation in support of this work. Outside the submitted work, DP’s institutions (Public Health Foundation of India and Centre for Chronic Disease Control) have received grants for chronic disease research and capacity building from multiple sources, including international research funding bodies, government agencies, foundations, United Health and multiple pharmaceutical industries, such as MSD, Johnson and Johnson, Sun Pharmaceuticals, and GlaxoSmithKline. SA is supported by the NIDDK K-23 5K23DK101826-03. DW reports grants from Medtronic Foundation and the American Heart Association, outside the submitted work. TG has received grants for cardiovascular disease research to Brigham and Women’s Hospital from Novartis and United HealthCare Services, outside the submitted work.
The Bill & Melinda Gates Foundation provides financial support for the Disease Control Priorities Network project, of which this volume is a part. The authors acknowledge Jinyuan Qi for her assistance in the preparation of the DCP-3 essential package cost estimates, Dean Jamison for thoughtful review and guidance, Kristen Danforth for assistance with literature reviews and document preparation, Brianne Adderley for project coordination, Jimly Shiju for secretarial assistance, and the World Bank Publisher’s office for excellent production support.
Appendix
Members of the DCP-3 Cardiovascular, Respiratory, and Related Disorders Author Group
Vamadevan S Ajay, Ashkan Afshin, Alma Adler, Mohammed K Ali, Eric Bateman, Janet Bettger, Robert O Bonow, Elizabeth Brouwer, Gene Bukhman, Fiona Bull, Peter Burney, Simon Capewell, Juliana Chan, Eeshwar K Chandrasekar, Jie Chen, Michael H Criqui, John Dirks, Sagar B Dugani, Michael Engelgau, Meguid El Nahas, Caroline H D Fall, Valery Feigin, F Gerald R Fowkes, Amanda Glassman, Shifalika Goenka, Rajeev Gupta, Babar Hasan, Fred Hersch, Frank Hu, Mark D Huffman, Samer Jabbour, Deborah Jarvis, Panniyammakal Jeemon, Rohina Joshi, Jemima H Kamano, Andre Pascal Kengne, Preeti Kudesia, R Krishna Kumar, Kalyanaraman Kumaran, Estelle V Lambert, Edward S Lee, Chaoyun Li, Rong Luo, Matthew Magee, Vasanti S Malik, J Antonio Marin-Neto, Guy Marks, Bongani Mayosi, Helen McGuire, Renata Micha, J Jaime Miranda, Pablo Aschner Montoya, Andrew E Moran, Dariush Mozaffarian, Saraladevi Naicker, Nadraj G Naidoo, K M Venkat Narayan, Irina Nikolic, Martin O’Donnell, Churchill Onen, Clive Osmond, Anushka Patel, Rogelio Perez-Padilla, Neil Poulter, Michael Pratt, Miriam Rabkin, Vikram Rajan, Anis Rassi, Anis Rassi Jr, Ishita Rawal, Giuseppe Remuzzi, Miguel Riella, Greg A Roth, Ambuj Roy, Adolfo Rubinstein, Yuna Sakuma, Uchechukwu K A Sampson, Karen R Siegel, Karen Sliwa, Marc Suhrcke, Nikhil Tandon, Bernadette Thomas, Claudia Vaca, Rajesh Vedanthan, Stephane Verguet, Michael Webb, Mary Beth Weber, Laurie Whitsel, Gary Wong, Lijing L Yan, Clyde W Yancy, Ping Zhang, Dong Zhao, Yishan Zhu.
Footnotes
See Online for appendix
Contributors
DP, SA, TG, and RN created the outline of the Review and of the essential package. DP incorporated input from all authors and finalised the Review. DW performed costing of the essential package and provided input into manuscript structure and drafting. YW and JCM edited the manuscript. The DCP-3 Cardiovascular, Respiratory, and Related Disorders Author Group wrote volume 5 of the DCP-3, which formed the basis for this manuscript and its essential package. All authors approved the final draft.
Declaration of interests
YF and JCM declare no competing interests.
References
- 1.WHO. Global health estimates 2015: deaths by cause, age, sex, by country and by region, 2000–2015. Geneva: World Health Organization; 2017. [Google Scholar]
- 2.UN General Assembly. High-level meeting on prevention and control of non-communicable diseases. New York: Sep 19–20, 2011. [Google Scholar]
- 3.WHO. Global health observatory data for 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 4.Gelband H, Sankaranarayanan R, Gauvreau CL, et al. Costs, affordability, and feasibility of an essential package of cancer control interventions in low-income and middle-income countries: key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387:2133–44. doi: 10.1016/S0140-6736(15)00755-2. [DOI] [PubMed] [Google Scholar]
- 5.Patel V, Chisholm D, Parikh R, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387:1672–85. doi: 10.1016/S0140-6736(15)00390-6. [DOI] [PubMed] [Google Scholar]
- 6.Camargo CAJ, Rachelefsky G, Schatz M. Managing asthma exacerbations in the emergency department: summary of the National Asthma Education and Prevention Program Expert Panel Report 3 guidelines for the management of asthma exacerbations. J Allergy Clin Immunol. 2009;124(suppl 2):S5–14. doi: 10.1016/j.jaci.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Global health and ageing. Geneva: World Health Organization; 2011. [Google Scholar]
- 8.Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–41. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–58. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–27. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 11.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–85. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 12.van den Hoogen PC, Feskens EJ, Nagelkerke NJ, Menotti A, Nissinen A, Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. Seven Countries Study Research Group. N Engl J Med. 2000;342:1–8. doi: 10.1056/NEJM200001063420101. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 14.Sposato LA, Saposnik G. Gross domestic product and health expenditure associated with incidence, 30-day fatality, and age at stroke onset: a systematic review. Stroke. 2012;43:170–77. doi: 10.1161/STROKEAHA.111.632158. [DOI] [PubMed] [Google Scholar]
- 15.Jaspers L, Colpani V, Chaker L, et al. The global impact of non-communicable diseases on households and impoverishment: a systematic review. Eur J Epidemiol. 2015;30:163–88. doi: 10.1007/s10654-014-9983-3. [DOI] [PubMed] [Google Scholar]
- 16.Huffman MD, Rao KD, Pichon-Riviere A, et al. A cross-sectional study of the microeconomic impact of cardiovascular disease hospitalization in four low- and middle-income countries. PLoS One. 2011;6:e20821. doi: 10.1371/journal.pone.0020821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niens LM, Cameron A, Van de Poel E, Ewen M, Brouwer WB, Laing R. Quantifying the impoverishing effects of purchasing medicines: a cross-country comparison of the affordability of medicines in the developing world. PLoS Med. 2010;7:e10000333. doi: 10.1371/journal.pmed.1000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britton J. Progress with the global tobacco epidemic. Lancet. 2015;385:924–26. doi: 10.1016/S0140-6736(15)60498-6. [DOI] [PubMed] [Google Scholar]
- 19.WHO. [accessed March 10, 2017];Noncommunicable diseases and mental health: about 9 voluntary global targets. 2014 http://www.who.int/nmh/ncd-tools/definition-targets/en/
- 20.WHO. [accessed July 12, 2017];Tobacco free initiative (TFI) 2017 http://www.who.int/tobacco/mpower/en/
- 21.Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015;385:966–76. doi: 10.1016/S0140-6736(15)60264-1. [DOI] [PubMed] [Google Scholar]
- 22.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. 2014;370:60–68. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 23.Verguet S, Gauvreau CL, Mishra S, et al. The consequences of tobacco tax on household health and finances in rich and poor smokers in China: an extended cost-effectiveness analysis. Lancet Glob Health. 2015;3:e206–16. doi: 10.1016/S2214-109X(15)70095-1. [DOI] [PubMed] [Google Scholar]
- 24.WHO. WHO technical manual on tobacco tax administration. Geneva: World Health Organization; 2010. [Google Scholar]
- 25.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–63. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 27.Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–57. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 28.The Institute for Health Metrics and Evaluation. GBD data visualizations. Seattle: Institute for Health Metrics and Evaluation; 2013. [Google Scholar]
- 29.Heath GW, Parra DC, Sarmiento OL, et al. Evidence-based intervention in physical activity: lessons from around the world. Lancet. 2012;380:272–81. doi: 10.1016/S0140-6736(12)60816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Cai L, Wu Y, et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes Rev. 2015;16:547–65. doi: 10.1111/obr.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conn VS, Hafdahl AR, Cooper PS, Brown LM, Lusk SL. Meta-analysis of workplace physical activity interventions. Am J Prev Med. 2009;37:330–39. doi: 10.1016/j.amepre.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laine J, Kuvaja-Kollner V, Pietila E, Koivuneva M, Valtonen H, Kankaanpaa E. Cost-effectiveness of population-level physical activity interventions: a systematic review. Am J Health Promot. 2014;29:71–80. doi: 10.4278/ajhp.131210-LIT-622. [DOI] [PubMed] [Google Scholar]
- 33.Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–76. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 34.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 35.Sprint Research Group. Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–68. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 37.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386:801–12. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 38.Correa CM. Implications of bilateral free trade agreements on access to medicines. Bull World Health Organ. 2006;84:399–404. [PMC free article] [PubMed] [Google Scholar]
- 39.Bradford Kerry V, Lee K. TRIPS, the Doha Declaration and paragraph 6 decision: what are the remaining steps for protecting access to medicines? Glob Health. 2007;3:3. doi: 10.1186/1744-8603-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lybecker KM, Fowler E. Compulsory licensing in Canada and Thailand: comparing regimes to ensure legitimate use of the WTO rules. J Law Med Ethics. 2009;37:222–39. doi: 10.1111/j.1748-720X.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan WA, Wirtz VJ, Stephens P. The market dynamics of generic medicines in the private sector of 19 low and middle income countries between 2001 and 2011: a descriptive time series analysis. PLoS One. 2013;8:e74399. doi: 10.1371/journal.pone.0074399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ait-Khaled N, Auregan G, Bencharif N, et al. Affordability of inhaled corticosteroids as a potential barrier to treatment of asthma in some developing countries. Int J Tuberc Lung Dis. 2000;4:268–71. [PubMed] [Google Scholar]
- 43.WHO. [accessed July 25, 2017];Essential medicines and health products: WHO model lists of essential medicines. 2017 http://www.who.int/medicines/publications/essentialmedicines/en/
- 44.Cohen D, Carter P. How small changes led to big profits for insulin manufacturers. BMJ. 2010;341:c7139. doi: 10.1136/bmj.c7139. [DOI] [PubMed] [Google Scholar]
- 45.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–43. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 46.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–82. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 47.Anand S, Bitton A, Gaziano T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS One. 2013;8:e72860. doi: 10.1371/journal.pone.0072860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braimoh RW, Mabayoje MO, Amira CO, Bello BT. Microbial quality of hemodialysis water, a survey of six centers in Lagos, Nigeria. Hemodial Int. 2014;18:148–52. doi: 10.1111/hdi.12070. [DOI] [PubMed] [Google Scholar]
- 49.Grimaldi A, Ammirati E, Karam N, et al. Cardiac surgery for patients with heart failure due to structural heart disease in Uganda: access to surgery and outcomes. Cardiovasc J Afr. 2014;25:204–11. doi: 10.5830/CVJA-2014-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakuma Y, Glassman A, Vaca C. Chapter 21. In: Prabhakaran D, Gaziano T, Anand S, Mbanya J-C, Wu Y, Nugent R, editors. Disease control priorities, 3rd edn. Volume 5: cardiovascular, respiratory, and related disorders. Washington DC: World Bank; 2017. (in press) [PubMed] [Google Scholar]
- 51.Gaziano T, Suhrcke M, Brouwer E, Levin C, Nikolic I, Nugent R. Chapter 19. In: Prabhakaran D, Gaziano T, Anand S, Mbanya J-C, Wu Y, Nugent R, editors. Disease control priorities, 3rd edn. Volume 5: cardiovascular, respiratory, and related disorders. Washington, DC: World Bank; 2017. (in press) [Google Scholar]
- 52.Brouwer ED, Watkins D, Olson Z, Goett J, Nugent R, Levin C. Provider costs for prevention and treatment of cardiovascular and related conditions in low- and middle-income countries: a systematic review. BMC Public Health. 2015;15:1183. doi: 10.1186/s12889-015-2538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shroufi A, Chowdhury R, Anchala R, et al. Cost effective interventions for the prevention of cardiovascular disease in low and middle income countries: a systematic review. BMC Public Health. 2013;13:285. doi: 10.1186/1471-2458-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suhrcke M, Boluarte TA, Niessen L. A systematic review of economic evaluations of interventions to tackle cardiovascular disease in low- and middle-income countries. BMC Public Health. 2012;12:2. doi: 10.1186/1471-2458-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakhimovsky SS, Feigl AB, Avila C, O’Sullivan G, Macgregor-Skinner E, Spranca M. Taxes on sugar-sweetened beverages to reduce overweight and obesity in middle-income countries: a systematic review. PLoS One. 2016;11:e0163358. doi: 10.1371/journal.pone.0163358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704. doi: 10.1136/bmj.h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding D, Lawson KD, Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388:1311–24. doi: 10.1016/S0140-6736(16)30383-X. [DOI] [PubMed] [Google Scholar]
- 58.Meng L, Xu H, Liu A, et al. The costs and cost-effectiveness of a school-based comprehensive intervention study on childhood obesity in China. PLoS One. 2013;8:e77971. doi: 10.1371/journal.pone.0077971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali MK, Siegel KR, Chandrasekar E, et al. Chapter 12. In: Prabhakaran D, Gaziano T, Anand S, Mbanya J-C, Wu Y, Nugent R, editors. Disease control priorities, 3rd edn. Volume 5: cardiovascular, respiratory, and related disorders. Washington, DC: World Bank; 2017. (in press) [PubMed] [Google Scholar]
- 60.Diaz de Leon-Castaneda C, Altagracia-Martinez M, Kravzov-Jinich J, Cardenas-Elizalde Mdel R, Moreno-Bonett C, Martinez-Nunez JM. Cost-effectiveness study of oral hypoglycemic agents in the treatment of outpatients with type 2 diabetes attending a public primary care clinic in Mexico City. Clinicoecon Outcomes Res. 2012;4:57–65. doi: 10.2147/CEOR.S27826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaede P, Valentine WJ, Palmer AJ, et al. Cost-effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care. 2008;31:1510–15. doi: 10.2337/dc07-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali MK, Singh K, Kondal D, et al. Effectiveness of a multicomponent quality improvement strategy to improve achievement of diabetes care goals: a randomized, controlled trial. Ann Intern Med. 2016;165:399–408. doi: 10.7326/M15-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Habib SH, Biswas KB, Akter S, Saha S, Ali L. Cost-effectiveness analysis of medical intervention in patients with early detection of diabetic foot in a tertiary care hospital in Bangladesh. J Diabetes Complications. 2010;24:259–64. doi: 10.1016/j.jdiacomp.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Rachapelle S, Legood R, Alavi Y, et al. The cost-utility of telemedicine to screen for diabetic retinopathy in India. Ophthalmology. 2013;120:566–73. doi: 10.1016/j.ophtha.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Araújo DV, Tura BR, Basileiro AL, et al. Custo-efetividade da trombólise pré-hospitalar vs intra-hospitalar no infarto agudo do miocárdio. Arq Bras Cardiol. 2008;90:100–07. [Google Scholar]
- 66.Sritipsukho P, Riewpaiboon A, Chaiyawat P, Kulkantrakorn K. Cost-effectiveness analysis of home rehabilitation programs for Thai stroke patients. J Med Assoc Thai. 2010;93(suppl 7):S262–70. [PubMed] [Google Scholar]
- 67.Ministry of Health. Guidelines on clinical and programmatic management of major non communicable diseases. Addis Ababa: Federal Democratic Republic of Ethiopia; 2016. [Google Scholar]
- 68.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–22. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 69.Alegre-Díaz J, Herrington W, López-Cervantes M, et al. Diabetes and cause-specific mortality in Mexico city. N Engl J Med. 2017;375:1961–72. doi: 10.1056/NEJMoa1605368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO. Global action plan for the prevention and control of noncommunicable diseases. Geneva: World Health Organization; 2013. [Google Scholar]
- 71.Mock C, Kobusingye O, Nugent R, Smith KR, editors. Disease control priorities, 3rd edn. Volume 7: Injury prevention and environmental health. Washington, DC: World Bank; 2017. (in press) [PubMed] [Google Scholar]
- 72.WHO. From burden to “best buys”: reducing the economic impact of non-communicable diseases in low- and middle-income countries. Geneva: World Health Organization; 2011. [Google Scholar]
- 73.Webster JL, Dunford EK, Hawkes C, Neal BC. Salt reduction initiatives around the world. J Hypertens. 2011;29:1043–50. doi: 10.1097/HJH.0b013e328345ed83. [DOI] [PubMed] [Google Scholar]
- 74.Restrepo BJ, Rieger M. Denmark’s policy on artificial trans fat and cardiovascular disease. Am J Prev Med. 2016;50:69–76. doi: 10.1016/j.amepre.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 75.Afshin A, Micha R, Webb M, et al. Chapter 6. In: Prabhakaran D, Gaziano T, Anand S, Mbanya J-C, Wu Y, Nugent R, editors. Disease control priorities, 3rd edn. Volume 5: cardiovascular, respiratory, and related disorders. Washington, DC: World Bank; 2017. (in press) [PubMed] [Google Scholar]
- 76.Bull F, Goenka S, Lambert V, Pratt M. Chapter 5. In: Prabhakaran D, Gaziano T, Anand S, Mbanya J-C, Wu Y, Nugent R, editors. Disease control priorities, 3rd edn. Volume 5: cardiovascular, respiratory, and related disorders. Washington, DC: World Bank; 2017. (in press) [Google Scholar]
- 77.Mock CN, Kobusingye O, Nugent R, Smith KR, editors. Disease control priorities, 3rd edn. Volume 7: injury prevention and environmental health. Washington, DC: World Bank; 2017. (in press) [PubMed] [Google Scholar]
- 78.Gaziano TA, Abrahams-Gessel S, Denman CA, et al. An assessment of community health workers’ ability to screen for cardiovascular disease risk with a simple, non-invasive risk assessment instrument in Bangladesh, Guatemala, Mexico, and South Africa: an observational study. Lancet Glob Health. 2015;3:e556–63. doi: 10.1016/S2214-109X(15)00143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jafar TH, Islam M, Hatcher J, et al. Community based lifestyle intervention for blood pressure reduction in children and young adults in developing country: cluster randomised controlled trial. BMJ. 2010;340:c2641. doi: 10.1136/bmj.c2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Falbe J, Thompson HR, Becker CM, Rojas N, McCulloch CE, Madsen KA. Impact of the Berkeley excise tax on sugar-sweetened beverage consumption. Am J Public Health. 2016;106:1865–71. doi: 10.2105/AJPH.2016.303362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. 2010;376:1775–84. doi: 10.1016/S0140-6736(10)61514-0. [DOI] [PubMed] [Google Scholar]
- 82.Gaziano T, Abrahams-Gessel S, Surka S, et al. Cardiovascular disease screening by community health workers can be cost-effective in low-resource countries. Health Aff. 2015;34:1538–45. doi: 10.1377/hlthaff.2015.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ajay VS, Jindal D, Roy A, et al. Development of a smartphone-enabled hypertension and diabetes mellitus management package to facilitate evidence-based care delivery in primary healthcare facilities in India: the mPower heart project. J Am Heart Assoc. 2016;5:e004343. doi: 10.1161/JAHA.116.004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaziano TA, Pagidipati N. Scaling up chronic disease prevention interventions in lower- and middle-income countries. Annu Rev Public Health. 2013;34:317–35. doi: 10.1146/annurev-publhealth-031912-114402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lonn E, Yusuf S. Polypill: the evidence and the promise. Curr Opin Lipidol. 2009;20:453–9. doi: 10.1097/MOL.0b013e32833305a3. [DOI] [PubMed] [Google Scholar]
- 86.WHO. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. Geneva: World Health Organization; 2010. [Google Scholar]
- 87.Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112:3547–53. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 88.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 89.Self TH, Usery JB, Howard-Thompson AM, Sands C. Asthma treatment protocols in the emergency department: are they effective? J Asthma. 2007;44:243–48. doi: 10.1080/02770900701246691. [DOI] [PubMed] [Google Scholar]
- 90.Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health. 2007;10:61–72. doi: 10.1111/j.1524-4733.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- 91.Praditpornsilpa K, Lekhyananda S, Premasathian N, et al. Prevalence trend of renal replacement therapy in Thailand: impact of health economics policy. J Med Assoc Thai. 2011;94(suppl 4):S1–6. [PubMed] [Google Scholar]
- 92.Tantivess S, Werayingyong P, Chuengsaman P, Teerawattananon Y. Universal coverage of renal dialysis in Thailand: promise, progress, and prospects. BMJ. 2013;346:f462. doi: 10.1136/bmj.f462. [DOI] [PubMed] [Google Scholar]
- 93.Reddy NS, Kappanayil M, Balachandran R, et al. Preoperative determinants of outcomes of infant heart surgery in a limited-resource setting. Semin Thorac Cardiovasc Surg. 2015;27:331–38. doi: 10.1053/j.semtcvs.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Medina-Pestana JO. Organization of a high-volume kidney transplant program—the “assembly line” approach. Transplantation. 2006;81:1510–20. doi: 10.1097/01.tp.0000214934.48677.e2. [DOI] [PubMed] [Google Scholar]
- 95.Axelrod DA, Guidinger MK, McCullough KP, Leichtman AB, Punch JD, Merion RM. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4:920–27. doi: 10.1111/j.1600-6143.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 96.Wang M, Moran AE, Liu J, et al. Cost-effectiveness of optimal use of acute myocardial infarction treatments and impact on coronary heart disease mortality in China. Circ Cardiovasc Qual Outcomes. 2014;7:78–85. doi: 10.1161/CIRCOUTCOMES.113.000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khan T, Bertram MY, Jina R, Mash B, Levitt N, Hofman K. Preventing diabetes blindness: cost effectiveness of a screening programme using digital non-mydriatic fundus photography for diabetic retinopathy in a primary health care setting in South Africa. Diabetes Res Clin Pract. 2013;101:170–76. doi: 10.1016/j.diabres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 98.Ribeiro RA, Stella SF, Zimerman LI, Pimentel M, Rohde LE, Polanczyk CA. Cost-effectiveness of implantable cardioverter defibrillators in Brazil in the public and private sectors. Arq Bras Cardiol. 2010;95:577–86. doi: 10.1590/s0066-782x2010005000134. [DOI] [PubMed] [Google Scholar]
- 99.Nugent R, Kelly BB, Narula J. An evolving approach to the global health agenda: countries will lead the way on NCD prevention and control. Glob Heart. 2012;7:3–6. doi: 10.1016/j.gheart.2012.02.003. [DOI] [PubMed] [Google Scholar]