Abstract
Pulmonary ventilation (V̇I) in awake and sleeping goats does not change when antagonists to several excitatory G protein-coupled receptors are dialyzed unilaterally into the ventral respiratory column (VRC). Concomitant changes in excitatory neuromodulators in the effluent mock cerebral spinal fluid (mCSF) suggest neuromodulatory compensation. Herein, we studied neuromodulatory compensation during dialysis of agonists to inhibitory G protein-coupled or ionotropic receptors into the VRC. Microtubules were implanted into the VRC of goats for dialysis of mCSF mixed with agonists to either μ-opioid (DAMGO) or GABAA (muscimol) receptors. We found: 1) V̇I decreased during unilateral but increased during bilateral dialysis of DAMGO, 2) dialyses of DAMGO destabilized breathing, 3) unilateral dialysis of muscimol increased V̇I, and 4) dialysis of DAMGO decreased GABA in the effluent mCSF. We conclude: 1) neuromodulatory compensation can occur during altered inhibitory neuromodulator receptor activity, and 2) the mechanism of compensation differs between G protein-coupled excitatory and inhibitory receptors and between G protein-coupled and inotropic inhibitory receptors.
Keywords: breathing, neuromodulation, μ-opioids, GABAA, ventral respiratory column, sleep
Introduction
From invertebrates to mammals, the excitability of neural circuits that govern motor outputs depends largely upon the local levels of excitatory and inhibitory neuromodulators. However, the mechanisms that regulate the local release of neuromodulators, or compensatory shifts in second messengers downstream of neuromodulatory receptors remain unclear. Gaining insight into regulation of neuromodulation is important to improve treatment of disorders such as epilepsy, depression, and Parkinson’s disease, as well as opioid induced respiratory depression; thus, there is a need for studies on control of neuromodulators.
Data from rodent models suggests there is “neuromodulator interdependence”, whereby changes in the activity of one or more neuromodulatory receptors are compensated by changes in the release of other neuromodulators to maintain stable ventilation (Doi and Ramirez, 2008, 2010). This concept has also been studied in awake and sleeping goats by dialyzing atropine (50 mM) unilaterally into the ventral respiratory column (VRC) to reduce or block excitatory muscarinic receptor activity (Muere et al., 2013). Rather than a hypothesized decrease in pulmonary ventilation (V̇I) and breathing frequency (f), atropine dialysis increased f and V̇I (Muere et al., 2013) which could have been due to increases in the excitatory neuromodulators substance P (SP) and serotonin (5-HT) as measured in the effluent dialysate. Subsequent studies in this goat model found that a lower dose of atropine (5 mM), or selective M2 (methoctramine) or M3 (4-DAMP) receptor antagonists had no effect on f and V̇I, but increased SP in the effluent dialysate to a lesser extent than 50 mM atropine (Muere et al., 2015a). Moreover, dialysis into the VRC of antagonists to excitatory SP receptors (neurokinin-1; NK-1) or 5-HT receptors (5-HT2A) alone or in combination with 5 mM atropine did not affect f and V̇I, but increased SP and decreased GABA in effluent dialysate (Muere et al., 2015b).These findings suggest that presumptive decreases in activity of one or more excitatory receptors are accompanied by changes in other neuromodulators to maintain f and V̇I in awake and NREM sleep states which supports the concept of neuromodulator interdependence (Doi and Ramirez, 2008). In other words, there is “compensation” defined as no change in ventilation with dialysis of excitatory antagonists and that this may be due to the observed concomitant increase in excitatory (SP) and decrease in inhibitory neurotransmitters (GABA).
The studies above examined the effects of blocking excitatory, G protein-coupled neuromodulatory receptors. It is unknown whether increased activation of inhibitory receptors in the VRC will also elicit compensatory changes in neuromodulators and/or affect f and V̇I. Widely expressed in the VRC are μ-opioid inhibitory receptors (Krause et al., 2009a; Pattinson, 2008), which are also G protein-coupled receptors (GPCRs). When activated, μ-opioid receptors act through second messenger cascades to reduce intracellular calcium and increase potassium efflux to decrease cellular activity (Pattinson, 2008). Administration of μ-opioid receptor agonists to the preBötzinger Complex (preBötC) region of the VRC has, in some studies, caused respiratory depression (Montandon and Horner, 2014; Montandon et al., 2011; Pattinson, 2008). However, other studies found that eupneic breathing is unchanged (Krause et al., 2009a) or increased with μ-opioid receptor agonists administration to the preBötC/VRC (Mustapic et al., 2010). The absence of a depression of breathing with increased μ-opioid receptor-mediated inhibition could be due to neuromodulatory compensation.
Another endogenous inhibitory neuromodulator controlling breathing is γ-aminobutyric acid (GABA) acting at GABAB metabotropic receptors to regulate neuronal membrane potential. However, acting through GABAA ionotropic receptors, GABA also directly affects neuronal activity by altering intracellular chloride (Tillakaratne et al., 1995). It is unknown whether neuromodulator interdependence occurs when the activity of ionotropic receptors is altered, and whether compensation occurs by similar or different mechanisms to increases in inhibitory signaling through GPCRs.
Herein we studied whether neuromodulatory compensation occurs in response to increased inhibitory receptor activity by unilaterally dialyzing the μ-opioid receptor agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) or the GABAA receptor agonist muscimol into the VRC while measuring breathing and neurochemicals in the effluent dialysate of awake and sleeping goats. We chose these inhibitory agonists due to lack of agreement among several previous studies on effects on respiratory control of these agonists (Curran et al., 2000; Gatti et al., 1987; Krause et al., 2009a; Lalley, 2003, 2008; Montandon et al., 2011; Mustapic et al., 2010; Nattie and Li, 2000; Taylor et al., 2006; Yamada et al., 1981; Yamada et al., 1982). We hypothesized that f and V̇I would not change during DAMGO or muscimol dialysis, but that there would be changes in neurochemicals in the effluent dialysate. We also hypothesized that the mechanism of compensation would differ between antagonism of GPCR and ionotropic receptors.
Through bilateral dialysis of DAMGO, we also tested the hypothesis that increased activity of the contralateral VRC may contribute to the stable f and V̇I during unilateral DAMGO dialysis. We did not test this same hypothesis for muscimol because of the large ventilatory and behavioral responses observed in some goats with unilateral muscimol dialysis (see Results).
Methods
Goats
Fifteen adult female, non-pregnant goats with an average body weight of 51.0 +/− 8.5kg were housed and studied in an environmental chamber with a fixed ambient temperature and alternating 12-hour light-dark cycles set between 7am and 7pm. There was free access to food and water except for study periods and the 24 hours preceding surgeries. All protocols and procedures utilized in this study were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Surgical Procedures
The goats were anesthetized with ketamine (IV), intubated, and mechanically ventilated with isoflurane in 100% oxygen for the duration of the surgery. Flunixin meglumine (2mg/kg, IM) was administered once pre-operatively and for two days post-operatively for pain. To minimize infection, ceftriaxone (25mg/kg, IV) in 100mL saline was administered over 30 minutes twice daily at 8-12 hour intervals for three days starting the morning of a craniotomy surgery. Subsequently, ceftiofur sodium (4mg/kg, IM) and gentamycin (6mg/kg, IM) were administered once daily for the remainder of the study period. To minimize intracranial edema following the craniotomy, dexamethasone (4mg/mL, IV) was administered three times daily and tapered for a week following surgery. Surgical sites were treated daily for 7 days with triple antibiotic ointment. Rectal temperature (TR), heart rate (HR), and oxygen saturation were continuously monitored during surgery. Over the following 24 post-operative hours these variables were monitored at predetermined intervals.
Under sterile conditions, two surgeries were performed separated by two weeks. During one surgery, an incision was made in the goat’s neck to locate the carotid artery which was subsequently dissected out, elevated to beneath the skin, and sutured to the overlaying skin before closing the incision. Also, electroencephalographic (EEG) and electrooculographic (EOG) electrodes were inserted into the midline cranium and the superior orbital ridge for recordings to score wakefulness/sleep during night studies. The electrodes were attached to screws, secured with dental acrylic, and the wires were pulled through the skin prior to closure of the incision.
In a second surgery two weeks later, stainless steel microtubules (70.0 mm length, 1.27 mm outer diameter, 0.84 mm inner diameter) were chronically implanted unilaterally (n=3) or bilaterally (n=12) into or near the preBötC of the VRC (Figure 1A & 1B). An incision was made that bisected the nuchal ligament along the dorsal midline of the skull and neck. A rotary drill was used to create an occipital craniotomy for visualization of the dura which, when opened, exposed the dorsal medulla. Obex served as an anatomical reference for micromanipulator controlled microtubule placement into the VRC. The target for placement was the preBötC which in goats is approximately 2.5 to 3.5 mm rostral from obex, 4.0 to 5.0 mm lateral from the midline and 4.0 to 6.0 mm from the dorsal surface of the medulla (Krause et al., 2009a; Wenninger et al., 2004). However, in some cases adjustments in microtubule placement had to be made to avoid blood vessels. Following microtubule placement, screws were inserted into the skull to which the microtubules were chronically secured using dental acrylic. Stainless steel stylets, slightly shorter than the microtubules, were inserted into the microtubules and the incisions sutured closed. Following the procedure, post-operative monitoring and medications were administered as described above.
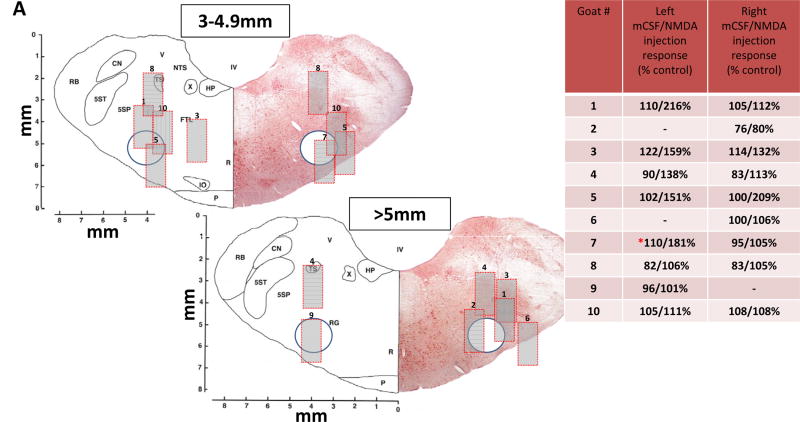
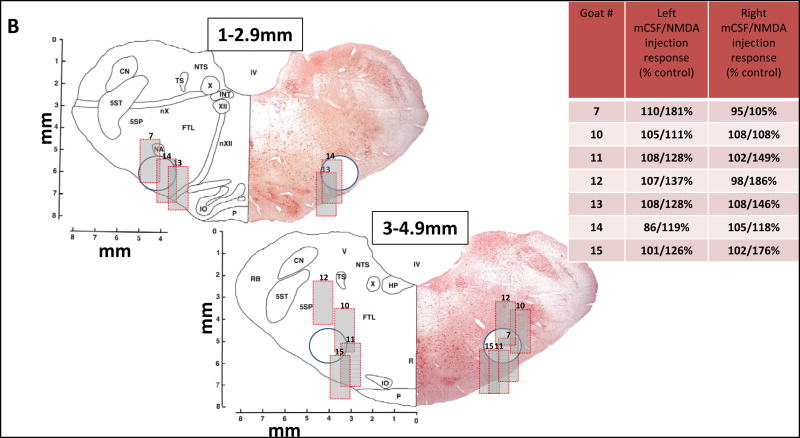
Figure 1.
Figures 1A and 1B show sections of the goat medulla at two rostral to caudal distances from obex depicting the microtubule location in which DAMGO or muscimol were dialyzed unilaterally (1A) and bilaterally (1B). Each goat was numbered for unilateral and bilateral studies, and the numbers for the left and right microtubule are located in the images where the distal end of the microtubule was identified by post mortem histological analysis. The 0.5 mm wide porous membrane of the dialysis probe extended 2 mm beyond the distal end of the microtubule; thus, we inserted a 2 mm long, 1 mm wide gray filled rectangle for each number to represent a conservative estimate of the region of diffusion of the dialyzed substances (see text for basis of minimal diffusion). The circle in each image is the approximate location of the VRC in goats. The inset in 1A and 1B is the quantitation of the breathing frequency responses to mCSF and NMDA (mCSF/NMDA) injections displayed as percent of control. Goats number 7 and 10 were included in both groups of studies.
Physiological Studies
The goats were acclimated to study protocols and equipment during the weeks of recovery from surgery. To obtain ventilatory data, a custom fitted mask was secured to the goat’s muzzle. A two-way breathing valve was inserted into the front of the mask with hoses attached to the inlet and outlet of the valve. For all studies, normal room air was inhaled though the inlet hose which was attached to a pneumotachograph connected to a computer. Windaq data acquisition software was utilized for breath-by-breath recording of inspiratory flow for computation of inspiratory (TI) and expiratory (TE) time, breathing frequency (f), tidal volume (VT), mean inspiratory flow rate (VT/TI) and inspiratory minute ventilation (V̇I). The outlet hose was attached to a Tissot spirometer for collection of expired air (the volume of which was measured at 5 minute intervals). Expired air was analyzed for O2 and CO2 concentration and those values were used to calculate O2 consumption and CO2 excretion. In several goats, heart rate (HR) and blood pressure (BP) were recorded via an indwelling catheter secured in the elevated carotid artery (which also facilitated blood sampling). Arterial blood samples were analyzed for pH, paCO2, paO2, and O2 saturation using a Siemens RapidLab248 blood gas analyzer. For night/sleep studies, the only change was the addition of EEG and EOG wires attached to the implanted electrodes. Rectal temperature was routinely recorded.
Dialysis probes (Harvard Apparatus, formerly CMA microdialysis, Holliston MA) inserted into the microtubules were 72 mm in length, 70 mm of which is a stainless-steel tube and the final 2 mm is a semi-permeable membrane (membrane diameter 0.5 mm, 20kda cut-off, 3μl internal volume). Only the 2mm membrane penetrated the brain tissue. During studies, the perfusate was either mock cerebral spinal fluid (mCSF: 124mM NaCl, 2.0mM KCL, 2.0mM MgCl2, 1.3mM K2PO4, 2.0mM CaCl2, 11mM glucose, 26mM NaHCO3− and pH 7.32 in sterile distilled H2O) alone or with the μ-opioid agonist DAMGO (10, 50 or 100μM, Sigma-Aldrich: E7384) or the GABAA agonist muscimol (0.5, 1.0, or 10.0mM, Abcam: ab120094). The solutions were prepared in a tonometer flask, warmed to 39°C, and equilibrated with 6.4% CO2 and 12% O2 balance N2. A syringe pump (Harvard Apparatus) delivered the dialysate to the dialysis probe at a flow rate of 25μL/min. To minimize distraction of the goat, the pump was outside of the chamber; thus, a 150 cm length of polypropylene tubing (PE50) was needed to connect the syringe to the probe. The length of the tubing caused a delay of about 20 minutes between the start of dialysis and the arrival of perfusate at the probe tip. Moreover, during the initial 15-20 minutes of hour three, drug delivery to the tissue would continue as in hour 2. In addition, thereafter washout of the drug from the tissue would be time-dependent. As a result, the temporal pattern of ventilatory responses (shown in Figures and Tables) likely reflect these drug delivery and washout characteristic. To collect effluent dialysate, a short length of tubing was attached to the outlet end of the probe which was then attached to a modified cryotube. Separate tubes were used for effluent collection during each hour of dialysis. The effluent was then aliquoted and frozen at − 80°C for subsequent analysis by department biochemical core lab facilities.
We performed uni- and bilateral dialysis of DAMGO, but dialysis of muscimol was only unilateral for reasons stated on page 6. Provided in Table 1 is a list of studies which were completed on each of the 15 goats. Thirty minutes after probe insertion, we collected data over a fifteen-minute pre-dialysis control period which was followed by three continuous hours of dialysis. Hour 1 was mCSF alone, hour 2 was mCSF alone or mCSF mixed with a drug, and hour 3 was again mCSF alone. DAMGO dialysis was at concentrations of 10, 50, or 100 μM which is over the range and duration others used in dialyses studies on rats or dogs (Montandon et al., 2011; Mustapic et al., 2010). Muscimol dialysis was at concentrations of 0.5, 1.0, or 10.0 mM which is over the range and duration others used in studies on awake and sleeping rats and piglets (Curran et al., 2001; Nattie and Li, 2008; Taylor et al., 2006). Day studies were completed between 9 AM and 2 PM. Night studies were completed between 7 PM and 2AM. A minimum of 36 hours was allowed between consecutive studies on individual goats.
Table 1.
This table summarizes which studies each of the fifteen goats completed. Abbreviations are as follow: u denotes unilateral dialysis, b denotes bilateral dialysis, L and R denote dialysis on the left and right side respectively, and mCSF denotes mock cerebral spinal fluid.
| DRUG | Goat 1 |
Goat 2 |
Goat 3 |
Goat 4 |
Goat 5 |
Goat 6 |
Goat 7 |
Goat 8 |
Goat 9 |
Goat 10 |
Goat 11 |
Goat 12 |
Goat 13 |
Goat 14 |
Goat 15 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAY | mCSF | u (R) | u (L) | u (L) | u (L) | u (R) | u (L) | u (L × 2) |
b | b | b | b | b | b | ||
| 10uM DAMGO |
u (L) | u (R) | u (L) | u (L) | u (L) | u (R) | u (L) | u (L) | ||||||||
| 50uM DAMGO |
u (L) | u (R) | u (L) | u (L) | u (L) | u (R) | u (L) | u (L) | ||||||||
| 100uM DAMGO |
u (L&R) |
u (R) | u (L & R) |
u (L) | u (L & R) |
u (R) | u (L) & b |
u (R) | u (L) | b | b | b | b | b | b | |
| 0.5mM MUSCIMOL |
u (R) | u (L) | u (R) | u (R) | u (R) | u (L) | u (L) | |||||||||
| 1.0mM MUSCIMOL |
u (R) | u (L) | u (L) | u (R) | u (R) | u (L) | u (L) | |||||||||
| 10.0mM MUSCIMOL |
u (R) | u (L & R) |
u (L) | u (R) | u (R) | u (L) | u (L) | u (L) | ||||||||
| NIGHT | 100uM DAMGO |
u (L&R) |
u (R) | u (L) | u (L) | u | u (R) | u (L) & b |
u (L) | b | b | b | b | b × 2 | b |
Neurochemical Analyses
To measure the amino acids glutamine (GLN), glycine (GLY) and GABA present in effluent dialysate reverse phase high performance liquid chromatography was used as published by Muere et al. with fluorescent detection using: Waters Resolve C18 column (150X 3.9) and a fluorescent detector with excitation at 229nm and emission at 470nm, β-alanine internal standard was used with O-phthaldialdehyde derivatization (Muere et al., 2013). To measure 5-HT and its metabolite HIAA, an identical column was used but the potential was set at 0.6 volts vs. an Ag/AgCl reference electrode and a N-methylserotonin internal standard was used. A commercially available assay (Enzo Life Sciences, Assay Designs 900-018, range 9.76-10,000 pg/mL) and a microplate reader at 405 nm were used to determine SP levels. Measurement of norepinephrine, dopamine and their metabolites (DOPAC and HVA) was accomplished by using a Waters μBondapak C18 column (300 × 3.9), potential setting of 0.65 volts vs Ag/AgCL reference electrode, and a 2, 5-dihydroxybenzylamine hydrobromide internal standard.
Microtubule location was determined both functionally and histologically. Functional determination was accomplished via injections of N-methyl-D-aspartic acid (NMDA). The rationale for NMDA injections was based on previous findings that glutamate receptor agonist injections into the preBötC elicits a tachypneic ventilatory response (Krause et al., 2009b; McCrimmon et al., 2000; Solomon et al., 1999; Wenninger et al., 2004) thus, to approximate microtubule placement relative to the preBötC, 500 nL injections were made of mCSF alone or mixed with NMDA (100 mM). These injections were made in the awake state at least two weeks after microtubule implantation when the goat had recovered from the surgery. Injection tubes were inserted only to the distal end of the microtubule to avoid tissue damage. Breathing was monitored continuously during a 30-minute control period and over two hours during which, at 30 minute intervals, either mCSF or NMDA was injected unilaterally.
A post-mortem histological determination of microtubule location was also performed in each animal. The cerebral circulation was isolated under deep anesthesia before flushing the brainstem with phosphate buffered saline, followed by 4% paraformaldehyde in PBS following euthanasia. The brainstems were then extracted, dehydrated in successive 20 and 30% sucrose solutions at 4ºC and then frozen (-80ºC). The brainstems were serially sectioned at 25μM in the transverse plane. One series was used for Nissl (cresyl violet) staining to determine microtubule (MT) placement. Nissl-stained images (scanned resolution of 4,000 dots/inch) of the entire microtubule tract were captured (Nikon Super Coolscan 9000). Image software (Metamorph) was used to calibrate and measure microtubule placement in millimeters (relative to the midline and the ventral medullary surface) near the middle of the rostral-caudal MT damage range.
Data and statistical Analyses
A pump with a known airflow rate was used to calibrate the inspiratory flow signal for breath by breath calculation of V̇I, f, VT, TI, TE and VT/TI, using custom designed software programs. The EEG and EOG signals were utilized by a single investigator to score sleep state for each breath for night studies. Individual breath ventilatory data were averaged into 5 and 15 minute bins. For all ventilatory variables, metabolic rate (V̇O2), arterial blood gases, arterial blood pressure (BP), heart rate (HR), and rectal temperature (TR), a two-way repeated (RM) measures ANOVA compared all drug (DAMGO or muscimol) doses to mCSF alone (one factor repetition with dose and time as factors). Statistical analyses were over different portions of the predialysis period and 3-hour dialysis protocol. The interaction P values indicated whether there were statistically significant differences between mCSF alone and mCSF mixed with drugs. For night studies, interaction term P values from two-way RM ANOVAs (two factor repetition, state and time as factors) were performed to determine the effect of sleep state on ventilatory variables. Statistical analyses were on absolute values for each variable and again for each variable during hours 2 and 3 of the protocol expressed as a percent of the variable over the last 15 minutes of hour 1. Variability in ventilatory parameters was quantified using the coefficient of variation (CV). The CV was determined for all breaths during 5 minute intervals. A two-way RM ANOVA (with dose and time as factors) was used to determine if significant interactions occurred resulting from DAMGO or muscimol dialysis. Holm-Sidak post hoc tests were used where appropriate after each ANOVA.
Neurochemical Analysis
For statistical analyses of neurochemical data, two-way RM ANOVAs (one factor repetition, dose and time as factors) were used. The P values of the interaction term of ANOVA indicated whether the effect on effluent neurochemical concentration over time of antagonist dialysis was significantly different from that of the time-control studies. This analysis was performed for SP, 5-HT, GLY, GABA, and norepinephrine for both muscimol and DAMGO studies. Also, we computed the change in each neurochemical between pre and post dialysis of DAMGO and muscimol, and then used a one-way ANOVA (drug as factor) to determine whether the neurochemicals significantly changed.
Results
Placement of microtubules (MTs)
Shown in Figures 1A and 1B are representative transverse sections of the goat medulla in which the location of the distal end of the histologically identified microtubule tract is identified for each animal (represented by number). The dialysis membrane extended 2 mm beyond (ventral) the dorsal-most aspect of the microtubule implantation site. We did not measure drug diffusion in this study, but conservatively estimated an affected region (Figure 1) based on our previous dialysis studies in which we measured brain pH changes at two distances that resulted from dialysis of CO2-enriched mCSF (Hodges et al., 2004). In that study, we measured a dose-dependent decrease in extracellular fluid pH ~220 μm from the dialysis probe when 25% or 80% CO2 was dialyzed, but simultaneous measurements of pH at a distal brainstem site (~1.4 mm) from the site of focal acidosis indicated no detectable change in pH. Accordingly, we conservatively estimate a minimal diffusion of DAMGO to be ~0.25 mm from the dialysis probe recognizing that others in rats have found greater DAMGO diffusion distance (1.5 – 2.0 mm) from the dialysis probe (Montandon et al., 2011). We included a 2×1 mm gray box which represents a minimal estimate of the affected brainstem region relative to the estimated location of the VRC (circle). However, our model of pH changes with dialysis of acidified mCSF may underestimate the diffusion area as the in vivo brainstem has great capacity to buffer protons but there is no comparable uptake of DAMGO.
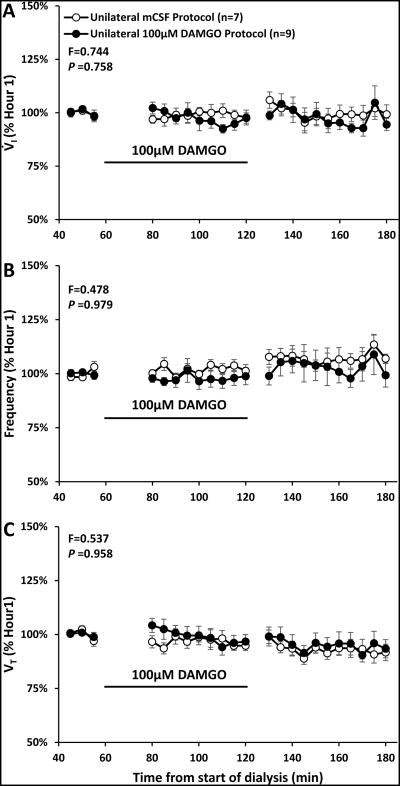
Figure 2.
Unilateral dialysis during the day of 100μM DAMGO (n=9, filled circles) did not significantly (P ≥0.758) alter ventilation (V̇I, panel A), breathing frequency (f, panel B) or tidal volume (VT, panel C) compared to dialysis of mCSF alone (n=7, open circles). Data are means ± SE. The x Axis is time from start of 180 minutes of dialysis. As indicated by the black line, 100μM DAMGO was dialyzed between 60 and 120 minutes. The values shown during and after DAMGO dialyses are the percent of the last 15 minutes prior to DAMGO dialyses. F and P values are the interaction term obtained from two-way repeated measures ANOVA using time and dose as factors. Note the trend of a transient reduction in V̇I and f over the last 30 minutes of DAMGO dialysis.
As indicated in Table 1, we unilaterally dialyzed on both sides of the VRC in six goats and in four other goats, unilateral dialysis was only on one side; thus, there were 16 sites of unilateral dialysis. Of these sixteen, eleven were in the estimated affected regions. (Figure 1A). Bilateral dialysis was in seven goats. Thirteen of the fourteen estimated affected regions for the bilateral studies (Figure 1B) reached the VRC.
As in past studies, we used the breathing frequency response to the glutamate receptor agonist NMDA as a functional marker of proximity to the preBötC. We found breathing frequency increased at least 10% after twenty-two of thirty-one NMDA injections made in the fifteen goats (insets Figure 1A and 1B). In contrast, when only mCSF was injected, breathing frequency increased more than 10% in only two of the thirty-one injections. The variation in the responses to NMDA and the variation in histologically identified MT placement suggests that the dialyzed receptor agonists likely were not restricted to the targeted preBötC or the VRC in all goats. This possibility is a limitation of our study and will subsequently be discussed.
DAMGO Microdialysis
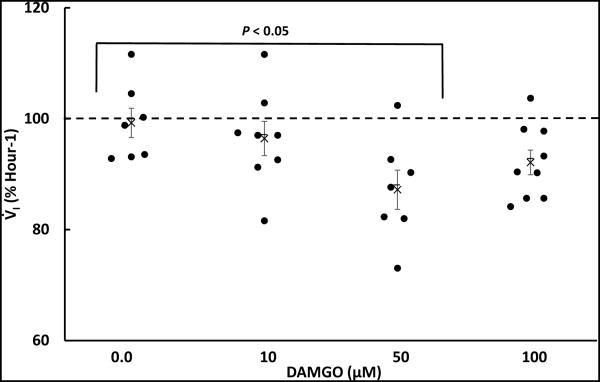
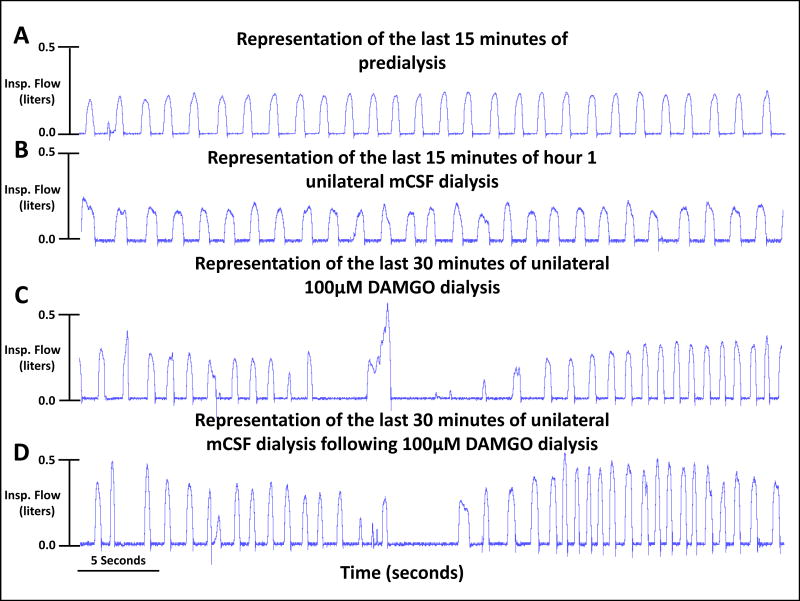
There were no effects of time or dose (no significant interaction term; P>0.05) in the two-way repeated (RM) measures ANOVA comparing absolute values measured during unilateral dialyses of mCSF alone or three doses of DAMGO (Table 2). Moreover, when the physiologic responses during and after unilateral DAMGO dialysis were normalized to Hour 1 control values (immediately prior to DAMGO dialysis), there also was no significant effects of time and dose for V̇I, f, VT, (Figure 2) or any other measured physiologic parameter. However, we noted a consistent trend for a transient reduction in V̇I during the final 30 minutes of 50 μM and 100 μM DAMGO dialyses. The individual goat data suggested there were differences between goats in the time within this period when V̇I was reduced; thus, a moving time average was computed for 3 successive 5 minute intervals, which is shown in Figure 3. Using this approach, there was a significant difference in the V̇I responses among mCSF dialysis and the 3 doses of DAMGO (P = 0.042) (one-way ANOVA, dose as factor). The post hoc test indicated that the response to mCSF dialysis differed significantly from 50 μM DAMGO dialysis. Finally, dialysis of DAMGO destabilized breathing (Figure 4), where the coefficient of variation in f during and after the last 30 minutes of DAMGO dialysis was significantly (P < 0.042) increased compared to mCSF dialysis.
Table 2.
Average of raw values for physiologic variables obtained during studies testing the effect of unilateral dialysis of DAMGO into the ventral respiratory column of awake goats. Presented are data for four, fifteen minute time periods for each of four studies (A, B, C, D). The first period of each study was predialysis control. During the subsequent periods of all studies, mock cerebral spinal fluid (mCSF) was dialyzed. During period 3 of studies B-D, DAMGO was added to the mCSF to provide concentration of 10 μM, 50 μM or 100 μM. Abbreviation of physiologic variables are defined in the text. For all variables, there was no statistically significant (P>0.05) interaction value from the two-way repeated measures ANOVA (time and study as factors) comparisons of all 4 studies.
| Study A |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/mi n) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mm Hg) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=7) |
7.73 | 25.03 | 0.94 | 1.47 | 0.32 | 0.33 | 287.71 | 39. 04 |
- | - | - | - | - |
| Predial ysis |
Std. Error |
0.57 | 1.86 | 0.06 | 0.09 | 0.04 | 0.03 | 36.34 | 0.2 1 |
- | - | - | - | - |
| 2 | Ave. (n=7) |
7.39 | 26.84 | 0.88 | 1.44 | 0.29 | 0.34 | 273.81 | 39. 07 |
- | - | - | - | - |
| mCSF | Std. Error |
0.71 | 1.89 | 0.07 | 0.11 | 0.03 | 0.04 | 28.49 | 0.1 6 |
- | - | - | - | - |
| 3 | Ave. (n=7) |
7.25 | 27.14 | 0.81 | 1.41 | 0.28 | 0.34 | 265.38 | 39. 12 |
- | - | - | - | - |
| mCSF | Std. Error |
0.59 | 2.48 | 0.06 | 0.10 | 0.03 | 0.04 | 26.72 | 0.1 6 |
- | - | - | - | - |
| 4 | Ave.(n =7) |
7.65 | 29.47 | 0.79 | 1.28 | 0.28 | 0.35 | 256.14 | 39. 25 |
- | - | - | - | - |
| mCSF | Std. Error |
0.61 | 3.03 | 0.07 | 0.10 | 0.02 | 0.04 | 28.52 | 0.2 1 |
- | - | - | - | - |
| Study B |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/mi n) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mm Hg) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=8) |
7.90 | 24.76 | 0.93 | 1.78 | 0.34 | 0.38 | 264.52 | 39. 17 |
- | - | - | - | - |
| Predial ysis |
Std. Error |
0.53 | 2.19 | 0.07 | 0.23 | 0.03 | 0.02 | 31.50 | 0.1 5 |
- | - | - | - | - |
|
| ||||||||||||||
| 2 | Ave. (n=8) |
7.03 | 22.67 | 0.96 | 1.92 | 0.33 | 0.36 | 250.86 | 39. 17 |
- | - | - | - | - |
| mCSF | Std. Error |
0.63 | 1.97 | 0.09 | 0.25 | 0.03 | 0.02 | 29.79 | 0.1 5 |
- | - | - | - | - |
|
| ||||||||||||||
| 3 | Ave. (n=8) |
7.10 | 23.69 | 0.85 | 1.79 | 0.31 | 0.37 | 233.62 | 39. 17 |
- | - | - | - | - |
| 10μM DAMG O |
Std. Error |
0.63 | 2.13 | 0.05 | 0.25 | 0.02 | 0.02 | 28.72 | 0.1 5 |
- | - | - | - | - |
|
| ||||||||||||||
| 4 | Ave.(n =8) |
7.48 | 24.98 | 0.79 | 1.75 | 0.32 | 0.39 | 240.76 | 39. 17 |
- | - | - | - | - |
| mCSF | Std. Error |
0.62 | 2.62 | 0.07 | 0.26 | 0.03 | 0.02 | 29.58 | 0.1 5 |
- | - | - | - | - |
| Study C |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/mi n) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mm Hg) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=8) |
7.28 | 23.43 | 0.95 | 1.79 | 0.33 | 0.34 | 263.50 | 38. 88 |
- | - | - | - | - |
| Predial ysis |
Std. Error |
0.45 | 1.86 | 0.07 | 0.23 | 0.03 | 0.02 | 21.83 | 0.1 0 |
- | - | - | - | - |
|
| ||||||||||||||
| 2 | Ave. (n=8) |
7.49 | 23.82 | 0.99 | 1.73 | 0.34 | 0.37 | 280.33 | 38. 88 |
- | - | - | - | - |
| mCSF | Std. Error |
0.55 | 2.45 | 0.13 | 0.26 | 0.02 | 0.01 | 33.64 | 0.1 0 |
- | - | - | - | - |
|
| ||||||||||||||
| 3 | Ave. (n=8) |
6.76 | 23.17 | 0.84 | 1.86 | 0.30 | 0.36 | 231.42 | 38. 88 |
- | - | - | - | - |
| 50μM DAMG O |
Std. Error |
0.50 | 2.10 | 0.07 | 0.18 | 0.02 | 0.02 | 24.12 | 0.1 0 |
- | - | - | - | - |
|
| ||||||||||||||
| 4 | Ave.(n =8) |
7.19 | 24.05 | 0.64 | 1.60 | 0.28 | 0.38 | 220.38 | 38. 88 |
- | - | - | - | - |
| mCSF | Std. Error |
1.07 | 3.85 | 0.08 | 0.32 | 0.03 | 0.06 | 38.58 | 0.1 0 |
- | - | - | - | - |
| Study D |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/m in) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mm Hg) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=9) |
8.05 | 29.19 | 0.85 | 1.41 | 0.29 | 0.35 | 284.19 | 39. 52 |
110.9 3 |
70.36 | 41.64 | 96.70 | 7.4 6 |
| Predial ysis |
Std. Error |
0.57 | 3.09 | 0.08 | 0.14 | 0.01 | 0.03 | 30.48 | 0.2 3 |
8.98 | 2.79 | 1.13 | 5.60 | 0.0 1 |
|
| ||||||||||||||
| 2 | Ave. (n=9) |
7.75 | 27.19 | 0.90 | 1.58 | 0.31 | 0.36 | 278.85 | 39. 40 |
87.20 | 71.52 | 41.42 | 101.33 | 7.4 5 |
| mCSF | Std. Error |
0.64 | 3.01 | 0.08 | 0.23 | 0.02 | 0.02 | 28.61 | 0.2 0 |
9.22 | 3.97 | 0.99 | 5.38 | 0.0 1 |
|
| ||||||||||||||
| 3 | Ave. (n=9) |
7.38 | 26.96 | 0.79 | 1.67 | 0.29 | 0.38 | 263.00 | 39. 25 |
86.55 | 71.25 | 41.30 | 101.03 | 7.4 6 |
| 100μM DAMG O |
Std. Error |
0.68 | 3.12 | 0.07 | 0.25 | 0.02 | 0.03 | 33.68 | 0.1 7 |
7.90 | 4.22 | 1.59 | 3.57 | 0.0 1 |
|
| ||||||||||||||
| 4 | Ave.(n =9) |
7.47 | 28.19 | 0.74 | 1.63 | 0.28 | 0.40 | 244.26 | 39. 21 |
85.67 | 71.08 | 40.12 | 100.92 | 7.4 6 |
| mCSF | Std. Error |
0.60 | 3.14 | 0.74 | 0.23 | 0.02 | 0.02 | 27.63 | 0.1 8 |
6.39 | 3.43 | 1.07 | 5.18 | 0.0 1 |
Figure 3.
Because of the trend of a transient reduction in V̇I over the last 30 minutes of DAMGO dialysis (Figure 2), a moving time average was computed using 3 successive 5 minute intervals and the lowest value for each goat over the last 30 minutes of DAMGO dialysis and mCSF are plotted in this figure (filled circles). Average values for each dose of DAMGO are plotted using mean symbols, data are means ± SE. One-way ANOVA indicated V̇I as a percent of control differed significantly (P < 0.042) over the 4 conditions shown. The post hoc test indicated the responses to 0 and 50 μM differed significantly (P < 0.05).
Figure 4.
Visual inspection of ventilatory tracings indicates variability of breathing increased during and after unilateral dialysis of 100μM DAMGO. The four tracings were from a single goat for a portion of the last 15 minutes of the predialysis control period (panel A) and the last 15 minutes of each of three hours of mCSF dialysis (panels B-D) with DAMGO increased during the second of the three hours (Panel C).
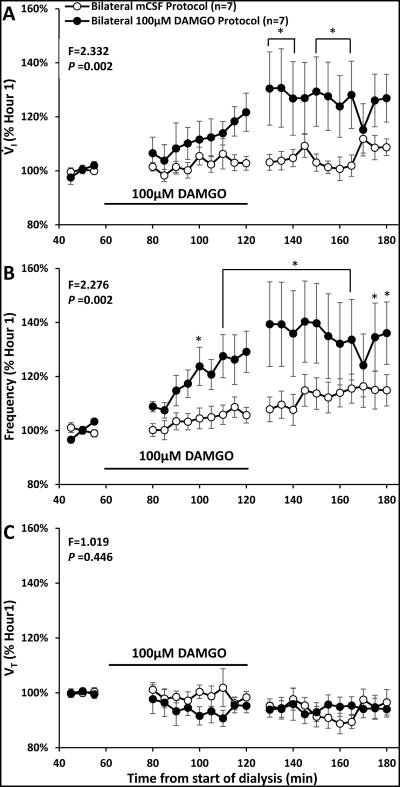
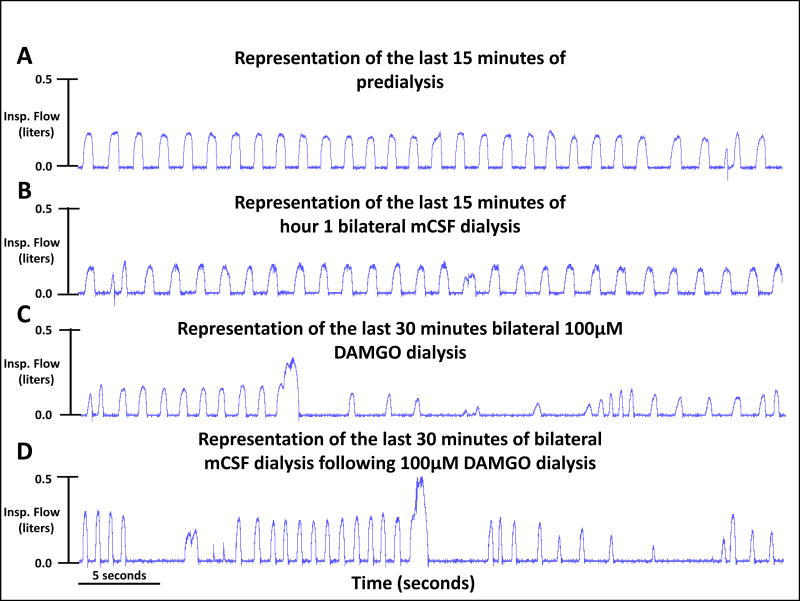
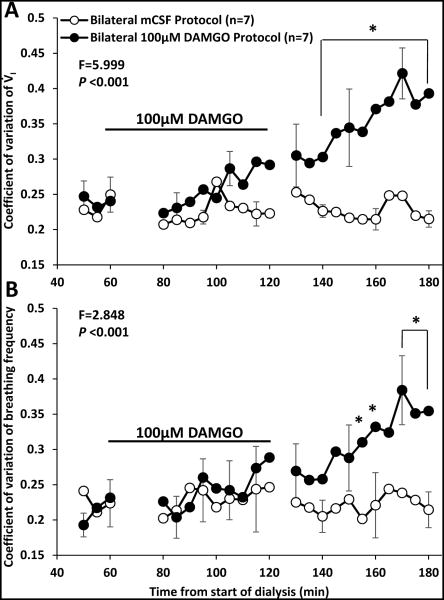
There were significant (P <0.05) effects of time and drug for V̇I, f, TI TE, and VT/TI when comparing bilateral dialysis of mCSF with bilateral dialysis of 100 μM DAMGO during the day. Expressing the data as absolute values over the entire study protocol (Table 3), or when expressed as percent of control values immediately prior to DAMGO dialysis (Figures 5 and 6), we found that bilateral dialysis of 100 μM DAMGO during the day significantly (P <0.05) increased V̇I, f, TE, and VT /TI and decreased TI. Bilateral dialysis of DAMGO also destabilized breathing (Figure 7), where the coefficient of variation of V̇I and f (Figure 8) were significantly (P <0.001) greater during the hour after bilateral DAMGO dialysis compared to the hour after mCSF dialysis. There were no significant (P ≥0.516) changes in any other measured variable during bilateral dialysis of DAMGO during the day (Table 3).
Table 3.
Averages of raw values for physiologic variables obtained during studies testing the effect of bilateral dialysis of DAMGO into the ventral respiratory column of awake goats. Presented are data for four, fifteen minute time periods for each of two studies (A and B). The first period of each study was predialysis control. During the subsequent three periods of both A and B studies, mock cerebral spinal fluid (mCSF) was dialyzed. The only difference between A and B was in study B when DAMGO was added to the mCSF in period 3 to obtain a concentration of 100 μM. Abbreviation of physiologic variables are defined in the text. Results of statistical analyses using two-way repeated measures ANOVA (time and study as factors) indicated there was a significant interaction term for V̇I, (P=0.025), f (P=0.019), and TI (P=0.007).
| Study A |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/mi n) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mm Hg) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=7) |
8.14 | 30.49 | 0.90 | 1.26 | 0.28 | 0.38 | 264.62 | 39. 13 |
- | - | - | - | - |
| Predial ysis |
Std. Error |
0.74 | 2.76 | 0.10 | 0.10 | 0.01 | 0.04 | 21.93 | 0.1 4 |
- | - | - | - | - |
|
| ||||||||||||||
| 2 | Ave. (n=7) |
8.15 | 33.50 | 0.80 | 1.17 | 0.25 | 0.34 | 275.29 | 39. 15 |
- | - | - | - | - |
| mCSF | Std. Error |
0.79 | 2.93 | 0.08 | 0.11 | 0.02 | 0.03 | 25.65 | 0.1 2 |
- | - | - | - | - |
|
| ||||||||||||||
| 3 | Ave. (n=7) |
8.49 | 35.79 | 0.75 | 1.12 | 0.25 | 0.36 | 288.43 | 39. 19 |
- | - | - | - | - |
| mCSF | Std. Error |
0.90 | 3.50 | 0.07 | 0.10 | 0.02 | 0.04 | 23.92 | 0.1 1 |
- | - | - | - | - |
|
| ||||||||||||||
| 4 | Ave.(n =7) |
8.93 | 38.63* | 0.70 * |
1.04 | 0.24 | 0.37 | 308.33 | 39. 21 |
- | - | - | - | - |
| mCSF | Std. Error |
0.92 | 4.08 | 0.08 | 0.10 | 0.02 | 0.04 | 32.00 | 0.1 0 |
- | - | - | - | - |
| Study B |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/m in) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mm Hg) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=7) |
7.24*** | 32.74** | 0.80** | 1.20** | 0.23*** | 0.29*** | 290.14 | 38. 88 |
72.51 | 79.72 | 41.75 | 88.75 | 7.4 5 |
| Predial ysis |
Std. Error |
0.53 | 2.29 | 0.07 | 0.08 | 0.02 | 0.02 | 24.20 | 0.1 2 |
3.44 | 1.64 | 0.95 | 2.45 | 0.0 0 |
|
| ||||||||||||||
| 2 | Ave. (n=7) |
7.72 | 36.28 | 0.77 | 1.12 | 0.22 | 0.33 | 302.29 | 38. 91 |
73.23 | 80.49 | 41.95 | 91.25 | 7.4 5 |
| mCSF | Std. Error |
0.95 | 4.08 | 0.12 | 0.12 | 0.02 | 0.04 | 40.71 | 0.1 1 |
2.61 | 3.92 | 0.15 | 13.15 | 0.0 1 |
|
| ||||||||||||||
| 3 | Ave. (n=7) |
8.84 | 45.95 | 0.51 | 1.09 | 0.21 | 0.41 | 282.62 | 39. 05 |
70.07 | 76.95 | 43.40 | 89.70 | 7.4 3 |
| 100μM DAMG O |
Std. Error |
0.82 | 5.16 | 0.06 | 0.16 | 0.02 | 0.05 | 32.99 | 0.1 0 |
1.92 | 3.05 | 1.30 | 13.10 | 0.0 2 |
|
| ||||||||||||||
| 4 | Ave.(n =7) |
9.09 | 47.34 | 0.47 | 1.34 | 0.21 | 0.45 | 272.43 | 39. 12 |
68.94 | 82.78 | 41.60 | 83.65 | 7.4 3 |
| mCSF | Std. Error |
0.88 | 6.73 | 0.07 | 0.18 | 0.02 | 0.06 | 37.04 | 0.1 1 |
1.47 | 2.16 | 0.90 | 7.55 | 0.0 3 |
There were significant (P<0.05) effects of time indicated as follows:
4 differed from 1,
4 differed from 1 and 2,
1 differed from 2, 3, & 4.
Figure 5.
Bilateral dialysis of 100 μM DAMGO during the day (filled circles, n=7) significantly (P<0.002) increased ventilation (V̇I, panel A) and breathing frequency (f, panel B) but did not alter (P<0.446) tidal volume (VT, panel C) compared to dialysis of mCSF alone (open circles, n=7). Data are means ± SE. Dialysis of 100μM DAMGO occurred between 60 and 120 minutes (indicated by solid line). The values shown during and after DAMGO dialyses are the percent of the last 15 minutes prior to DAMGO dialyses. The F and P values are interaction terms from two-way repeated measures ANOVA using dose and time as factors. Asterisks denote values different between open and closed symbols ((P < 0.05), post hoc test).
Figure 6.
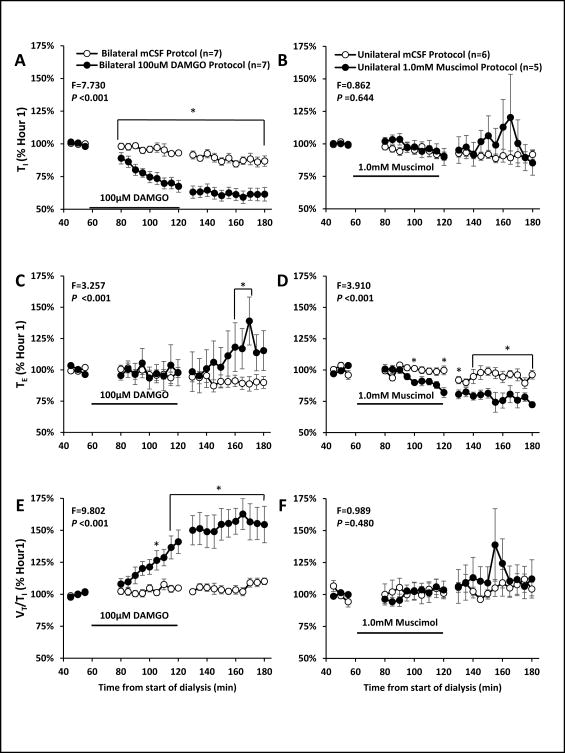
Inspiratory time (TI), expiratory time (TE) and tidal volume/ inspiratory time changed differently between bilateral dialysis of DAMGO (left panels) and unilateral dialysis of muscimol (right panel). Data are means ± SE. Bilateral dialysis of 100 μM DAMGO (n=7) during the day significantly (P < 0.001) decreased TI, increased TE during the post DAMGO dialyses period, and increased VT/TI during and after DAMGO dialysis. TI and VT /TI increased after muscimol dialysis while TE was significantly (P < 0.001) decreased during and after muscimol dialysis. Filled circles indicate studies during which 100 μM DAMGO was dialyzed during 60 to 120 minutes (solid line indicates period of DAMGO dialysis). Two-way repeated measures ANOVA was used to obtain F and P values and values shown are the interaction term of the ANOVA. Asterisks denote values different between open and closed symbols (P < 0.05, post hoc test).
Figure 7.
Visual inspection of ventilatory tracings indicates variability of breathing increased and inspiratory time decreased during and after bilateral dialysis of 100μM DAMGO. The four tracings were from a single goat for a portion of the last 15 minutes of the predialysis control period (panel A) and the last 15 minutes of each of three hours of mCSF dialysis (panels B-D) with DAMGO increased during the second of the three hours (Panel C).
Figure 8.
The coefficient of variation of ventilation (V̇I, panel A) and breathing frequency (f, panel B) within 5 minute periods was significantly (P < 0.001) greater during the period after bilateral 100 μM DAMGO dialysis than after bilateral mCSF dialysis. Data are means ± SE. The x Axis is time from start of 180 minutes of dialysis. As indicated by the black line, DAMGO was dialyzed between 60 and 120 minutes. The F and P values are interaction terms from two-way repeated measures ANOVA using dose and time as factors. Asterisks denote values different between open and closed symbols (P < 0.05, post hoc test).
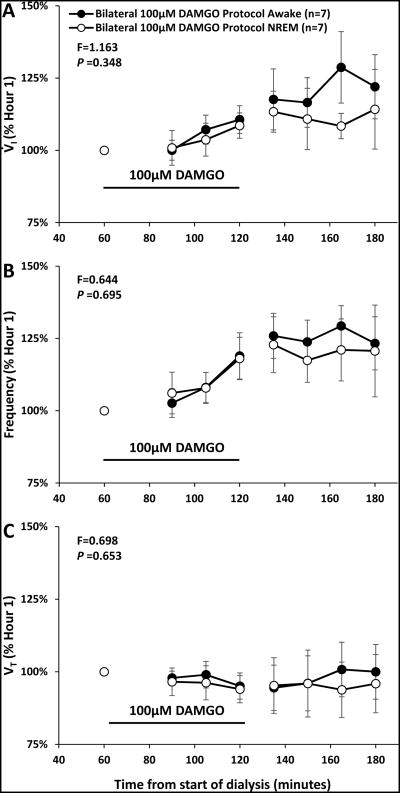
At night, for unilateral and bilateral (Figure 9) dialysis of 100 μM DAMGO, there were no significant interaction terms in two-way ANOVA analysis (time and state as factors) when comparing responses during awake and NREM sleep (Tables 4 and 5).
Figure 9.
During bilateral dialysis of 100 μM DAMGO (n=7) at night, there were no significant (P ≥ 0.348) interaction terms for any ventilatory variable of the two-way ANOVA comparing awake and NREM sleep (state and time). Data are means ± SE. Data from each state were averaged in 15 minute bins, the filled circles indicate the awake state and open circles indicate the NREM sleep state. Dialysis of 100 μM DAMGO occurred during minutes 60-120 of the study and is indicated by the solid line.
Table 4.
Average of raw values for physiologic variables obtained during studies testing the effect of unilateral dialysis of DAMGO into the ventral respiratory column of goats during awake and NREM sleep states. Presented are data for the last fifteen minutes of three hours of mCSF dialysis. During the second hour, DAMGO was added to the mCSF to provide a concentration of 100 μM. Abbreviation of physiologic variables are defined in the text. Ventilatory data were obtained on seven goats, but HR, BP, blood gases, and pH were obtained on only four goats. For all variables, there was no statistically significant (P>0.05) interaction value from the two-way repeated measures ANOVA (time and study as factors) comparing awake and NREM sleep.
| Period | Data | V̇I
(L/min) |
f (br/min) |
TI
(sec) |
TE
(sec) |
VT
(L/br) |
HR (bpm) |
BP (mmHg) |
PaCO2
(mmHg) |
PaO2
(mmHg) |
pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (awake) | Average | 7.46 | 24.65 | 1.14 | 1.84 | 0.32 | 81.57 | 74.49 | 37.50 | 87.54 | 7.47 |
| mCSF | Std. Error |
0.77 | 2.73 | 0.09 | 0.19 | 0.02 | 10.21 | 7.90 | 1.88 | 4.86 | 0.01 |
| 2 (awake) | Average | 7.99 | 27.03 | 0.95 | 1.64 | 0.31 | 83.98 | 73.37 | 35.64 | 85.66 | 7.46 |
| 100μM DAMGO |
Std. Error |
0.78 | 2.88 | 0.10 | 0.17 | 0.02 | 10.45 | 7.45 | 1.11 | 4.01 | 0.01 |
| 3 (awake) | Average | 7.67 | 27.39 | 0.95 | 1.71 | 0.29 | 83.47 | 71.56 | 36.73 | 82.78 | 7.46 |
| mCSF | Std. Error |
0.89 | 3.19 | 0.11 | 0.17 | 0.02 | 11.54 | 5.81 | 0.74 | 4.60 | 0.00 |
| Period | Data |
V̇I
(L/min) |
f
(br/min) |
TI
(sec) |
TE
(sec) |
VT
(L/br) |
HR
(bpm) |
BP
(mmHg) |
PaCO2
(mmHg) |
PaO2
(mmHg) |
pH |
| 1 (NREM) | Average | 6.79 | 23.09 | 1.14 | 1.81 | 0.30 | 77.90 | 80.06 | - | - | - |
| mCSF | Std. Error |
0.56 | 1.97 | 0.08 | 0.14 | 0.02 | 11.27 | 5.54 | - | - | - |
| 2 (NREM) | Average | 7.54 | 25.87 | 0.97 | 1.61 | 0.29 | 78.95 | 72.97 | - | - | - |
| 100μM DAMGO |
Std. Error | 0.68 | 2.34 | 0.11 | 0.15 | 0.02 | 10.14 | 8.35 | - | - | - |
| 3 (NREM) | Average | 6.17 | 24.82 | 1.01 | 1.79 | 0.26 | 90.82 | 65.95 | - | - | - |
| mCSF | Std. Error |
0.45 | 2.25 | 0.11 | 0.13 | 0.02 | 13.57 | 6.47 | - | - | - |
Table 5.
Average of raw values for physiologic variables obtained during studies testing the effect of bilateral dialysis of DAMGO into the ventral respiratory column of goats during awake and NREM sleep states. Presented are data for the last fifteen minutes of three hours of mCSF dialysis. During the second hour, DAMGO was added to the mCSF to provide a concentration of 100 μM. Abbreviation of physiologic variables are defined in the text. For all variables, there was no statistically significant (P>0.05) interaction value from the two-way repeated measures ANOVA (time and study as factors) comparisons of all 4 studies. Ventilatory data were obtained on seven goats, but HR, BP, blood gases, and pH were only obtained on four goats.
| Period | Data | V̇I
(L/min ) |
f (br/min ) |
TI
(sec ) |
TE
(sec ) |
VT
(L/br ) |
HR (bpm ) |
BP (mmHg ) |
PaCO2
(mmHg) |
PaO2
(mmHg) |
pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (awake) | Averag e |
9.20 | 35.33 | 0.85 | 1.16 | 0.27 | 91.55 | 61.83 | 42.95 | 90.58 | 7.4 5 |
| mCSF | Std. Error |
1.30 | 3.78 | 0.14 | 0.16 | 0.02 | 15.69 | 3.95 | 1.80 | 4.72 | 0.0 2 |
| 2 (awake) | Averag e |
10.16 | 42.82 | 0.67 | 1.20 | 0.25 | 89.38 | 62.52 | 42.30 | 93.63 | 7.4 5 |
| 100μM DAMGO |
Std. Error |
1.45 | 6.01 | 0.12 | 0.26 | 0.02 | 14.61 | 4.56 | 1.19 | 5.71 | 0.0 2 |
| 3 (awake) | Ave. (n=7) |
10.87 | 44.59 | 0.60 | 1.29 | 0.26 | 92.03 | 63.70 | 42.80 | 89.58 | 7.4 4 |
| mCSF | Std. Error |
1.50 | 6.62 | 0.10 | 0.25 | 0.02 | 13.01 | 5.25 | 1.62 | 4.55 | 0.0 1 |
| 1 (NREM) | Averag e |
7.80 | 33.05 | 0.89 | 1.16 | 0.24 | 88.97 | 61.30 | - | - | - |
| mCSF | Std. Error |
1.00 | 3.57 | 0.16 | 0.12 | 0.02 | 9.72 | 2.22 | - | - | - |
| 2 (NREM) | Averag e |
8.42 | 39.38 | 0.67 | 1.22 | 0.23 | 90.36 | 63.71 | - | - | - |
| 100μM DAMGO |
Std. Error |
1.05 | 5.08 | 0.10 | 0.25 | 0.02 | 8.89 | 4.17 | - | - | - |
| 3 (NREM) | Averag e |
8.57 | 40.89 | 0.60 | 1.46 | 0.23 | 89.55 | 64.14 | - | - | - |
| mCSF | Std. Error |
1.24 | 7.44 | 0.09 | 0.30 | 0.03 | 9.20 | 2.21 | - | - | - |
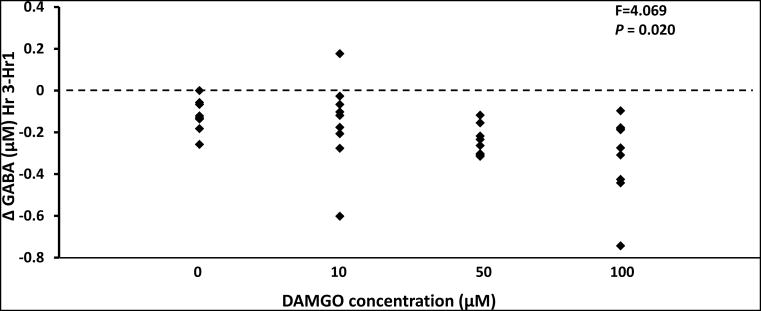
During daytime unilateral DAMGO dialysis, a one-way ANOVA analysis of the change in GABA between pre and post dialysis of DAMGO indicated there was a significant (P < 0.020) decrease in GABA during dialysis of 50 μM and 100 μM DAMGO (Figure 10). In addition, during both unilateral and bilateral dialysis of 100 μM DAMGO at night, there were significant (P <0.001) decreases in GABA in the effluent mCSF. However, bilateral dialysis of 100 μM DAMGO during the day did not significantly (P > 0.05) alter GABA. Lastly, there were no significant changes (P > 0.05) in 5-HT, SP, glutamine, glycine, dopamine, norepinephrine and the metabolites DOPAC, HIAA, and HVA in the effluent dialysate during DAMGO dialyses studies (data not shown).
Figure 10.
Unilateral dialysis of 50 μM and 100 μM DAMGO during the day significantly (P < 0.02) decreased GABA in the effluent dialyzed mCSF. Data are means ± SE. On the x-axis are the DAMGO doses and y axis indicates the change in GABA between hours 1 and 3 of dialysis. Statistical analysis was by one-way ANOVA (drug as factor).
Muscimol Microdialysis
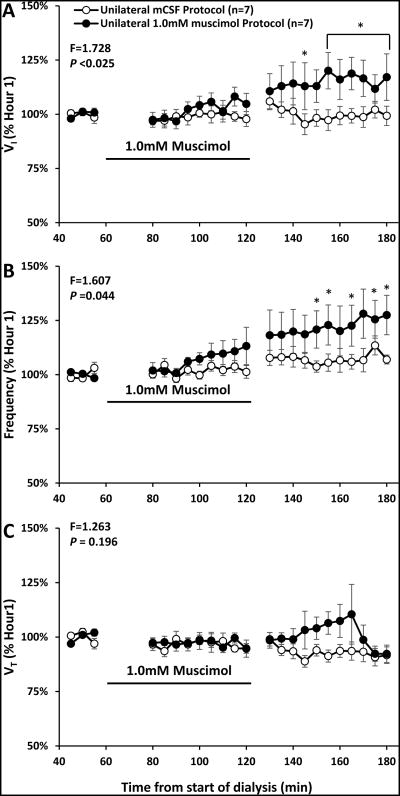
A two-way ANOVA (time and dose as factors) indicated there were no significant (P > 0.05) differences in absolute values of physiologic variables between mCSF dialysis and dialysis of three muscimol concentrations (0.5, 1.0, or 10 mM) (Table 6). However, when variables during and after muscimol dialysis were expressed as a percent of the periods just prior to muscimol dialysis, there were significant effects of time and dose for V̇I (P < 0.025) and f (P < 0.044) when all muscimol doses were compared and when each elevated muscimol concentration was individually compared to the mCSF study (Figure 11, 1.0 mM shown). Expressed as percent change, only 0.5 mM muscimol significantly (P < 0.005) altered VT. All doses of muscimol significantly (P < 0.023) decreased TE but did not significantly (P ≥ 0.577) alter TI or VT /TI (Figure 6).
Table 6.
Average of raw values for physiologic variables obtained during studies testing the effect of unilateral dialysis of muscimol into the ventral respiratory column of awake goats. Presented are data for four, fifteen minute time periods for each of four studies (A, B, C, D). The first period of each study was predialysis control. During the subsequent periods of all studies, mock cerebral spinal fluid (mCSF) was dialyzed. During period 3 of studies B-D, muscimol was added to the mCSF to provide concentrations of 0.5 μM, 1.0 μM or 10 μM respectively. Abbreviation of physiologic variables are defined in the text. For all variables, there was no statistically significant (P>0.05) interaction value from the two-way repeated measures ANOVA (time and study as factors) comparisons of all 4 studies.
| Study A |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/mi n) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mmH g) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=7) |
7.73 | 25.03 | 0.94 | 1.47 | 0.32 | 0.33 | 287.71 | 39. 04 |
- | - | - | - | - |
| Predialy sis |
Std. Error |
0.57 | 1.86 | 0.06 | 0.09 | 0.04 | 0.03 | 36.34 | 0.2 1 |
- | - | - | - | - |
|
| ||||||||||||||
| 2 | Ave. (n=7) |
7.39 | 26.84 | 0.88 | 1.44 | 0.29 | 0.34 | 273.81 | 39. 07 |
- | - | - | - | - |
| mCSF | Std. Error |
0.71 | 1.89 | 0.07 | 0.11 | 0.03 | 0.04 | 28.49 | 0.1 6 |
- | - | - | - | - |
|
| ||||||||||||||
| 3 | Ave. (n=7) |
7.25 | 27.14 | 0.81 | 1.41 | 0.28 | 0.34 | 265.38 | 39. 12 |
- | - | - | - | - |
| mCSF | Std. Error |
0.59 | 2.48 | 0.06 | 0.10 | 0.03 | 0.04 | 26.72 | 0.1 6 |
- | - | - | - | - |
|
| ||||||||||||||
| 4 | Ave.(n =7) |
7.65 | 29.47 | 0.79 | 1.28 | 0.28 | 0.35 | 256.14 | 39. 25 |
- | - | - | - | - |
| mCSF | Std. Error |
0.61 | 3.03 | 0.07 | 0.10 | 0.02 | 0.04 | 28.52 | 0.2 1 |
- | - | - | - | - |
| Study B |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/m in) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mm Hg) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=7) |
8.24 | 27.90 | 0.80 | 1.21 | 0.31 | 0.37 | 301.96 | 29. 23 |
- | - | 40.54 | 97.99 | 7.4 5 |
| Predial ysis |
Std. Error |
0.57 | 3.32 | 0.04 | 0.13 | 0.02 | 0.01 | 25.00 | 6.3 8 |
- | - | 0.70 | 4.82 | 0.0 1 |
|
| ||||||||||||||
| 2 | Ave. (n=7) |
8.22 | 29.03 | 0.77 | 1.13 | 0.30 | 0.40 | 285.33 | 32. 52 |
68.68 | 60.16 | 40.58 | 98.87 | 7.4 4 |
| mCSF | Std. Error |
0.85 | 4.07 | 0.05 | 0.11 | 0.02 | 0.01 | 28.32 | 4.9 0 |
12.76 | 7.37 | 1.14 | 5.22 | 0.0 1 |
|
| ||||||||||||||
| 3 | Ave. (n=7) |
9.36 | 31.62 | 0.72 | 1.03 | 0.31 | 0.44 | 304.95 | 33. 40 |
86.53 | 71.96 | 40.38 | 102.74 | 7.4 7 |
| 0.5mM Muscim ol |
Std. Error |
1.06 | 4.47 | 0.05 | 0.10 | 0.02 | 0.02 | 29.36 | 4.8 8 |
9.92 | 4.04 | 1.56 | 5.49 | 0.0 1 |
|
| ||||||||||||||
| 4 | Ave.(n =7) |
9.06 | 30.22 | 0.75 | 1.08 | 0.30 | 0.41 | 276.67 | 33. 41 |
82.00 | 62.76 | 40.75 | 96.80 | 7.4 3 |
| mCSF | Std. Error |
1.01 | 4.26 | 0.05 | 0.12 | 0.02 | 0.02 | 27.21 | 4.8 9 |
11.34 | 3.75 | 0.75 | 4.38 | 0.0 1 |
| Study C |
| Perio d |
Data | V̇I
(L/mi n) |
f (br/m in) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/m in) |
TR (°C ) |
HR (bp m) |
BP (mm Hg) |
PaCO2
(mm Hg) |
PaO2
(mm Hg) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=7) |
7.83 | 27.80 | 1.02 | 1.24 | 0.31 | 0.32 | 289.75 | 39. 22 |
89.63 | 69.36 | 42.09 | 88.17 | 7.4 4 |
| Predial ysis |
Std. Error |
0.65 | 3.30 | 0.11 | 0.15 | 0.02 | 0.03 | 35.94 | 0.1 3 |
7.59 | 5.63 | 1.17 | 4.76 | 0.0 1 |
|
| ||||||||||||||
| 2 | Ave. (n=7) |
7.75 | 30.63 | 0.80 | 1.18 | 0.28 | 0.32 | 292.92 | 39. 19 |
88.96 | 66.42 | 41.67 | 88.98 | 7.4 4 |
| mCSF | Std. Error |
0.47 | 4.03 | 0.08 | 0.17 | 0.02 | 0.01 | 31.42 | 0.1 0 |
6.37 | 5.23 | 0.80 | 4.61 | 0.0 1 |
|
| ||||||||||||||
| 3 | Ave. (n=7) |
8.21 | 34.54 | 0.77 | 1.06 | 0.27 | 0.32 | 288.63 | 39. 24 |
91.47 | 63.67 | 42.20 | 87.42 | 7.4 2 |
| 1.0mM Muscim ol |
Std. Error |
0.69 | 5.50 | 0.08 | 0.16 | 0.02 | 0.01 | 30.31 | 0.1 2 |
6.72 | 3.07 | 1.15 | 4.00 | 0.0 1 |
|
| ||||||||||||||
| 4 | Ave.(n =7) |
8.90 | 39.98 | 0.68 | 0.87 | 0.25 | 0.34 | 311.67 | 39. 29 |
90.66 | 68.59 | 42.53 | 87.80 | 7.4 3 |
| mCSF | Std. Error |
0.97 | 6.28 | 0.08 | 0.10 | 0.02 | 0.03 | 36.91 | 0.1 4 |
9.40 | 3.58 | 1.07 | 4.24 | 0.0 1 |
| Study D |
| Perio d |
Dat a |
V̇I
(L/mi n) |
f (br/mi n) |
TI
(se c) |
TE
(se c) |
VT
(L/b r) |
VT/ TI |
VO2
(ml/mi n) |
TR (°C ) |
HR (bp m) |
BP (mmH g) |
PaCO2
(mmH g) |
PaO2
(mmH g) |
p H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ave. (n=6 ) |
8.58 | 30.46 | 0.53 * |
0.72 | 0.29 | 0.39 | 326.78 | 39.2 2 |
101.3 9 |
77.11 | 42.93 | 95.20 | 7.4 2 |
| Predialy sis |
Std. Erro r |
0.98 | 3.96 | 0.18 | 0.24 | 0.01 | 0.04 | 47.34 | 0.29 | 1.38 | 5.93 | 1.12 | 6.70 | 0.0 2 |
| 2 | Ave. (n=6 ) |
8.27 | 30.03 | 0.48 | 0.76 | 0.29 | 0.40 | 298.33 | 39.1 1 |
88.75 | 77.92 | 43.68 | 100.15 | 7.4 3 |
| mCSF | Std. Erro r |
0.84 | 4.16 | 0.16 | 0.26 | 0.01 | 0.03 | 45.33 | 0.22 | 0.97 | 6.86 | 1.18 | 3.20 | 0.0 1 |
| 3 | Ave. (n=6 ) |
9.40 | 32.66 | 0.44 | 0.74 | 0.30 | 0.49 | 306.00 | 39.2 3 |
88.52 | 73.82 | 42.35 | 98.65 | 7.4 3 |
| 10.0m M Muscim ol |
Std. Erro r |
1.27 | 5.54 | 0.15 | 0.25 | 0.01 | 0.06 | 49.11 | 0.22 | 1.33 | 4.18 | 1.75 | 2.29 | 0.0 1 |
| 4 | Ave. (n=6 ) |
10.61 | 36.17 | 0.45 | 0.61 | 0.30 | 0.51 | 303.56 | 39.1 8 |
92.75 | 74.36 | 44.13 | 93.18 | 7.4 1 |
| mCSF | Std. Erro r |
2.16 | 7.10 | 0.16 | 0.21 | 0.05 | 0.13 | 59.56 | 6.05 | 21.88 | 17.69 | 8.24 | 17.33 | 1.3 5 |
Figure 11.
Unilateral dialysis of 1.0 mM (n=7) muscimol (filled circles) during the day significantly increased ventilation (V̇I, panel A, P =0.025) and breathing frequency (f panel B, P =0.044) but did not alter tidal volume (VT, panel C, P =0.196) compared to dialysis of mCSF alone (open circles) (n=7). Data are means ± SE. The solid lines indicate the period during which muscimol was dialyzed (60-120 minutes). Two-way repeated measures ANOVA was used (dose and time as factors) to obtain F and P values. Asterisks denote values significantly different between open and closed symbols (P < 0.05, post hoc test).
The highest concentration of muscimol (10.0 mM) induced varied degrees of behavioral excitability, nystagmus, spastic limb movements, and postural instability lasting up to 4 hours following the completion of studies. In 3 of 9 studies, these behavioral effects were too severe to warrant continuation of the study beyond the second hour. Two of the three animals had a dramatic increase in f accompanied by severe behavioral effects, while the third animal’s breathing frequency decreased eventually leading to terminal apnea. Despite these major behavioral changes, there were no significant changes during or after muscimol dialysis in all neurochemicals analyzed in the effluent mCSF, although norepinephrine increased in 4 of 6 goats during dialysis of 10.0 mM muscimol (data not shown). For all doses of muscimol, there were no significant (P ≥ 0.094) changes in paO2, paCO2, BP, HR, TR, or V̇O2 (Table 6).
Discussion
Microdialysis of antagonists to excitatory, neuromodulatory, G protein-coupled receptors into the VRC of awake and sleeping goats (Langer et al., 2016; Muere et al., 2013, 2015a; Muere et al., 2015b; Muere et al., 2015c) support the concept of neuromodulator compensation, whereby decreases in excitatory receptor activity are compensated by increases in other (predominantly excitatory) neuromodulators to prevent a decrease in ventilation and breathing frequency (Doi and Ramirez, 2008, 2010). In other words, there is “compensation” defined as no change in ventilation with dialysis of excitatory antagonists and that this may be due to the observed concomitant increase in excitatory (SP) and decrease in inhibitory neurotransmitters (GABA).
The objective herein was to determine whether compensation would also occur during VRC dialyses of agonists to inhibitory, G protein-coupled (μ-opioid) or inhibitory, ionotropic (GABAA) receptors in awake and sleeping goats. Ventilatory data indicated that what appears to be compensation occurred during dialysis of both types of inhibitory receptor agonists. Furthermore, it appears the compensation in neuromodulators differed between agonists to inhibitory G protein-coupled (μ-opioid) receptors and agonists of inhibitory ionotropic (muscimol) receptors.
Ventilatory and neurochemical effects of opioids dialyzed into the VRC
μ-opioid receptors are widely and abundantly expressed throughout respiratory control regions in the brainstem, including the preBötC region of the VRC (Gray et al., 1999; Haji et al., 2003; Krause et al., 2009a; Pattinson, 2008; Stornetta et al., 2003). These inhibitory, neuromodulatory receptors may contribute to the regulation of normal eupneic ventilation through the activity of endogenous opioids. The activation of these receptors also contributes to unwanted depression of ventilation with opiate use clinically, or cardiorespiratory failure in cases of opiate abuse/overdose. Previous studies have addressed the effects of exogenously applied opioid receptor agonists within the neural networks that control breathing, but these studies show inconsistent effects on ventilatory variables (Krause et al., 2009a; Lalley, 2008; Montandon et al., 2011; Mustapic et al., 2010; Pattinson, 2008). Montandon et al. dialyzed DAMGO into the preBötC of awake and anesthetized rats and found that breathing frequency was depressed at low concentrations, and found complete apnea at higher concentrations (Montandon et al., 2011). In contrast, Mustapic et al. reported that the injection of DAMGO into the preBötC of decerebrate (unanesthetized) dogs increased breathing frequency and phrenic nerve output (Mustapic et al., 2010). Different still were studies by Krause et al., in which DAMGO bilaterally injected into the preBötC of awake goats did not affect respiratory rhythm or pattern while breathing room air (Krause et al., 2009a). Our current results differ from each of the above as unilateral DAMGO dialysis decreased ventilation, but bilateral dialysis increased ventilation, breathing frequency and ventilatory drive while it decreased inspiratory time. In addition, both unilateral and bilateral dialysis of DAMGO destabilized breathing frequency, which is similar to previous reports that opioids can cause increased variability or destabilization of breathing (Mitsis et al., 2009; Pattinson, 2008; van den Aardweg, 2009; Walker et al., 2007). Collectively, these data suggest that the effect of opioids on ventilation might differ between species, state of wakefulness, and the nature of the experimental preparation.
During unilateral DAMGO dialysis, a local decrease in GABA (as indicated in the collected effluent mCSF, Figure 10) may have provided neuromodulatory compensation for a presumed DAMGO-induced depression of ventilation (Figure 3). In other words, an initial direct depression of ventilation (Figure 3) by DAMGO may have been offset within minutes by a secondary/compensatory decrease in GABA or by a change in another neurochemical to return ventilation to normal. This secondary/compensatory response may have been relatively greater with 100 μM compared to 50 μM which is why a significant depression in ventilation was found with 50 μM but not 100 μM DAMGO. A secondary/compensatory response is sufficiently rapid to prevent a detectable decrease in ventilation during dialysis of antagonists to excitatory neuromodulator receptors (Muere et al., 2013, 2015a; Muere et al., 2015b; Muere et al., 2015c). Compensation during DAMGO dialysis may also have been within the contralateral VRC. However, findings herein (Figure 5) do not support this possibility as bilateral DAMGO dialysis increased ventilation. This finding is similar to bilateral dialysis of antagonists to excitatory receptors which also increased ventilation even though ventilation did not change during unilateral dialysis of these antagonists (Langer et al., 2016). Accordingly, during unilateral and bilateral dialysis, there likely are multiple factors affecting ventilation, which makes it difficult to measure a steady-state, maximal effect, or recovery effect. The cause of the increased ventilation with bilateral DAMGO was not associated with decreased GABA; thus, we speculate it is due to other mechanisms such as increases in excitatory neuromodulators not yet measured and/or changes in second messengers downstream of G protein-coupled neuromodulator receptors. We have no explanation for why the increased ventilation with bilateral DAMGO dialysis did not result in arterial hypocapnia even though metabolic rate did not change. Nevertheless, whatever the exact mechanism of the neurochemical response to perturbation of neuromodulator receptors, it does not appear unique to awake and NREM sleep states as Langer et al found increases in 5-HT and SP equal in awake and anesthetized goats during dialysis of 50 mM atropine (Langer et al., 2016).
Opioids administered clinically in humans for pain management clearly cause respiratory depression, and when given intravenously likely simultaneously depress multiple respiratory nuclei (Pattinson, 2008). Why does neuromodulator compensation not prevent this respiratory depression? It is not known whether neuromodulator compensation is widespread throughout the brain. Thus, the specific neural sites providing a tonic drive to breathe (chemoreceptors) and/or sites of respiratory-associated motor neurons (such as the hypoglossal nuclei) might not display neuromodulator compensation during opiate-induced depression, and thus could be the major substrate for respiratory depression during clinical intravenous opioid administration. Indeed opioid respiratory depression has been linked to increased incidence of obstructive sleep apnea and sleep disordered breathing (Randerath and George, 2012; Zutler and Holty, 2011), which as reported by Skulsky et al. (Skulsky et al., 2007) and Hajiha et al. (Hajiha et al., 2009) could be due to opioid depression of hypoglossal muscle activity.
In reduced preparations opioids added to a perfusate of the medulla induced a quantal or step-wise, rather than progressive, slowing of respiratory frequency (Mellen et al., 2003). In awake goats quantal slowing does not occur during opioid administration to the VRC (which includes the preBötC). These findings suggest a fundamental difference in the effects of opioids between reduced and intact preparations. Indeed, an index of the drive to breathe (VT/TI) was increased during bilateral μ-opioid receptor stimulation, which likely represents the effects of compensation (or overcompensation) by neuromodulators. Moreover, the present findings demonstrate a fundamental difference between the effects of DAMGO and muscimol on respiratory drive and timing mechanisms.
Ventilatory and Neurochemical Effects of unilateral Muscimol Dialysis in the VRC
The current and past dialysis studies in unanesthetized goats found neuromodulator compensation during perturbation of G protein-coupled receptors (Muere et al., 2013, 2015a; Muere et al., 2015b; Muere et al., 2015c). Herein we found what could be interpreted as overcompensation for increases in inhibitory ionotropic GABA receptor activity during muscimol dialysis. Several previous studies (Akilesh et al., 1997; Curran et al., 2000; Curran et al., 2001; Darnall et al., 2001; Gatti et al., 1987; Yamada et al., 1981; Yamada et al., 1982) found that administering muscimol to various brainstem regions depressed breathing and/or arousal from sleep. On the other hand, other studies (Nattie and LI 2008, and Taylor et al.2006) found that administration of muscimol increased breathing and/or increased the ventilatory response to CO2 and hypoxia (Nattie and Li, 2000; Taylor et al., 2006). Our findings are similar to these latter studies as we found that muscimol dialysis increased ventilation and breathing frequency.
We found no significant change in the levels of any measured neurochemicals in the effluent mCSF that could counter a depressant effect of muscimol on neuronal activity. However, 10.0 mM muscimol dialysis increased norepinephrine in effluent dialysate in 4 of 6 goats, which could have contributed to the increased V̇I in these goats. A mechanism of GABA receptor-mediated increase in norepinephrine release has been proposed by Scatton et al.,1981 who found that administration of a GABA receptor agonist (Progabide) in the rat hypothalamus activated noradrenergic neurons and increased norepinephrine turnover (Scatton and Bartholini, 1981). Another possible explanation is that muscimol acts on presynaptic GABA receptors to increase glutamate release and postsynaptic neuronal activity (Jang et al., 2006; Koga et al., 2005); thus, presynaptic, GABA-mediated glutamate responses may contribute to the increased V̇I and f for all doses of muscimol. The behavioral effects observed with muscimol dialysis could also be due to increased glutamate release, as these effects were similar to those observed by Wenninger et al. after injection of ibotenic acid (an excitatory neurotoxin which acts glutamate receptors) into the preBötC of goats (Wenninger et al., 2004).
Irrespective of the mechanism and/or pathway, the ventilatory responses to muscimol indicate that some type of compensation and/or secondary effect exists to prevent respiratory depression during perturbations of inhibitory ionotropic receptors, which instead increased ventilation in the behaving goat.
μ-opioid and GABA receptor-mediated breathing instabilities
There was significant ventilatory instability with dialyses of the μ-opioid receptor agonist (Figures 4,7,8) and to some degree during muscimol dialysis. This instability contrasts to studies (Langer et al., 2016; Muere et al., 2013, 2015a; Muere et al., 2015b; Muere et al., 2015c) in which dialyses of antagonists to multiple excitatory receptors in the VRC did not destabilize breathing. The destabilization with DAMGO could be due to time-dependent changes in the direct effect of opioid-induced depression and the indirect effect of potential compensatory mechanisms. Or a compensatory increase in an unknown excitatory neuromodulator may have created a “high-gain controller”, which destabilizes breathing (Asyali et al., 2002; Khoo, 2000). Finally, destabilization in the muscimol studies could be due to increased norepinephrine which during pathological states induced increased variability in breathing (Viemari et al., 2013; Zanella et al., 2014).
Caveats and Limitations
We previously summarized (Muere et al., 2013, 2015a; Muere et al., 2015b; Muere et al., 2015c) the limitations and caveats of dialyses studies in the medulla of awake and sleeping goats. One limitation is that the microtubules were not all implanted at the same site (Figure 1). The variation in placement was due primarily to the need for avoiding blood vessels on the dorsal surface during surgical implantation. A second limitation is the uncertainty of the exact boundaries of the targeted preBötC. To assess the proximity of the microtubule to the preBötC, we utilize the ventilatory response to the glutamate receptor agonist NMDA. Herein, there was a positive response to NMDA even in some goats (see # 11 and 15) whose post mortem histologic measurements indicated the microtubule was outside the estimated boundaries of the preBötC; thus, it is possible our anatomic estimate of the preBötC may be too restrictive. A third limitation is the study may have been under powered which could be why we did not detect a significant depression of ventilation with 100 μM unilateral dialysis of DAMGO even though there was a significant depression with 50 μM unilateral dialysis of DAMGO. A fourth limitation is uncertainty regarding the diffusion of the drugs dialyzed. A final limitation is that we currently only have the capability of measuring the selected neurochemicals in the effluent mCSF, and as a result we likely missed potential changes in other local neuromodulatory molecules.
Summary and Conclusions
We found that: 1) V̇I decreased during unilateral but increased during bilateral dialysis of DAMGO, 2) dialyses of DAMGO destabilized breathing, 3) unilateral dialysis of muscimol increased breathing and 4) dialysis of DAMGO decreased GABA in the effluent mCSF. We conclude: 1) neuromodulatory compensation can occur during altered inhibitory neuromodulator receptor activity, and 2) the mechanism of compensation differs between G protein-coupled excitatory and inhibitory receptors and between G protein-coupled and ionotropic inhibitory receptors, and 3) the effects of presumed perturbations of inhibitory receptor activity within the respiratory control network shown herein likely have general applicability to neuronal networks throughout the brain.
Highlights.
Increased inhibitory receptor activity in the VRC alters & destabilizes breathing.
Changes occur in neuromodulators when inhibitory receptor activity is increased.
Neuromodulator compensation depends on receptor type altered.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
T.L. performed surgeries and experiments, analyzed data and wrote the MS, S.E.N. performed surgeries and experiments and histological analyses, contributed to data analysis and figures, and MS editing, N.J.B. performed experiments and contributed to intellectual discussions, S.T. performed experiments and contributed to data analysis and histological analyses, L.P. performed all surgeries, M.R.H. contributed to intellectual discussions, MS writing and editing, H.V.F. contributed to intellectual discussions and MS writing and editing. Funding for this study was provided by NIH HL 112996 and HL-007852 and the Veterans Administration. All experiments were performed at Medical College of Wisconsin, Milwaukee, Wisconsin, U.S.A.
Disclosures:
There are no conflicts of interest to disclose.
References
- Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J.Appl.Physiol. 1997;82:469–479. doi: 10.1152/jappl.1997.82.2.469. [DOI] [PubMed] [Google Scholar]
- Asyali MH, Berry RB, Khoo MC. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Trans Biomed Eng. 2002;49:206–216. doi: 10.1109/10.983454. [DOI] [PubMed] [Google Scholar]
- Curran AK, Chen G, Darnall RA, Filiano JJ, Li A, Nattie EE. Lesion or muscimol in the rostral ventral medulla reduces ventilatory output and the CO(2) response in decerebrate piglets. Respir Physiol. 2000;123:23–37. doi: 10.1016/s0034-5687(00)00143-2. [DOI] [PubMed] [Google Scholar]
- Curran AK, Darnall RA, Filiano JJ, Li A, Nattie EE. Muscimol dialysis in the rostral ventral medulla reduced the CO(2) response in awake and sleeping piglets. J Appl Physiol. 2001;90:971–980. doi: 10.1152/jappl.2001.90.3.971. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Curran AK, Filiano JJ, Li A, Nattie EE. The effects of a GABA(A) agonist in the rostral ventral medulla on sleep and breathing in newborn piglets. Sleep. 2001;24:514–527. doi: 10.1093/sleep/24.5.514. [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir.Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti PJ, DaSilva AM, Gillis RA. Cardiorespiratory effects produced by injecting drugs that affect GABA receptors into nuclei associated with the ventral surface of the medulla. Neuropharmacology. 1987;26:423–431. doi: 10.1016/0028-3908(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Okazaki M, Ohi Y, Yamazaki H, Takeda R. Biphasic effects of morphine on bulbar respiratory neuronal activities in decerebrate cats. Neuropharmacology. 2003;45:368–379. doi: 10.1016/s0028-3908(03)00154-0. [DOI] [PubMed] [Google Scholar]
- Hajiha M, DuBord MA, Liu H, Horner RL. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol. 2009;587:2677–2692. doi: 10.1113/jphysiol.2009.171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J.Appl.Physiol. 2004;96:1815–1824. doi: 10.1152/japplphysiol.00992.2003. [DOI] [PubMed] [Google Scholar]
- Jang IS, Nakamura M, Ito Y, Akaike N. Presynaptic GABAA receptors facilitate spontaneous glutamate release from presynaptic terminals on mechanically dissociated rat CA3 pyramidal neurons. Neuroscience. 2006;138:25–35. doi: 10.1016/j.neuroscience.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- Koga H, Ishibashi H, Shimada H, Jang IS, Nakamura TY, Nabekura J. Activation of presynaptic GABAA receptors increases spontaneous glutamate release onto noradrenergic neurons of the rat locus coeruleus. Brain Res. 2005;1046:24–31. doi: 10.1016/j.brainres.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Krause KL, Neumueller SE, Marshall BD, Kiner T, Bonis JM, Pan LG, Qian B, Forster HV. Micro-opioid receptor agonist injections into the presumed pre-Botzinger complex and the surrounding region of awake goats do not alter eupneic breathing. J Appl Physiol. 2009a;107:1591–1599. doi: 10.1152/japplphysiol.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KL, Neumueller SE, Marshall BD, Kiner T, Bonis JM, Pan LG, Qian B, Forster HV. Micro-opioid receptor agonist injections into the presumed pre-Botzinger complex and the surrounding region of awake goats do not alter eupneic breathing. J Appl Physiol (1985) 2009b;107:1591–1599. doi: 10.1152/japplphysiol.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1287–1304. doi: 10.1152/ajpregu.00199.2003. [DOI] [PubMed] [Google Scholar]
- Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol. 2008;164:160–167. doi: 10.1016/j.resp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer TM, 3rd, Neumueller SE, Crumley E, Burgraff NJ, Talwar S, Hodges MR, Pan LG, Forster HV. State-dependent and -independent effects of dialyzing excitatory neuromodulator receptor antagonists into the ventral respiratory column. J Appl Physiol. 2016;(1985) doi: 10.1152/japplphysiol.00619.2016. jap 00619 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol. 2000;27:126–131. doi: 10.1046/j.1440-1681.2000.03193.x. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsis GD, Governo RJ, Rogers R, Pattinson KT. The effect of remifentanil on respiratory variability, evaluated with dynamic modeling. J Appl Physiol (1985) 2009;106:1038–1049. doi: 10.1152/japplphysiol.90769.2008. [DOI] [PubMed] [Google Scholar]
- Montandon G, Horner R. Rebuttal from Gaspard Montandon and Richard Horner. J Physiol. 2014;592:1167. doi: 10.1113/jphysiol.2013.268300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–1301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Atropine microdialysis within or near the pre-Botzinger Complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol. 2013;114:694–704. doi: 10.1152/japplphysiol.00634.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Evidence for respiratory neuromodulator interdependence after cholinergic disruption in the ventral respiratory column. Respir Physiol Neurobiol. 2015a;205:7–15. doi: 10.1016/j.resp.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muere C, Neumueller S, Olesiak S, Miller J, Langer T, Hodges MR, Pan L, Forster HV. Combined unilateral blockade of cholinergic, peptidergic, and serotonergic receptors in the ventral respiratory column does not affect breathing in awake or sleeping goats. J Appl Physiol (1985) 2015b;119:308–320. doi: 10.1152/japplphysiol.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muere C, Neumueller SE, Olesiak SJ, Miller JR, Hodges MR, Pan LG, Forster HV. Blockade of neurokinin-1 receptors in the ventral respiratory column does not affect breathing but alters neurochemical release. J Appl Physiol. 2015c;(1985) doi: 10.1152/japplphysiol.00884.2014. jap 00884 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Botzinger complex region. J Neurophysiol. 2010;103:409–418. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis into the caudal aspect of the Nucleus tractus solitarii of conscious rats inhibits chemoreception. Respir Physiol Neurobiol. 2008;164:394–400. doi: 10.1016/j.resp.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100:747–758. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- Randerath WJ, George S. Opioid-induced sleep apnea: is it a real problem? J Clin Sleep Med. 2012;8:577–578. doi: 10.5664/jcsm.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatton B, Bartholini G. Drug-induced changes of epinephrine turnover in the rat hypothalamus. Life Sci. 1981;29:1161–1170. doi: 10.1016/0024-3205(81)90205-8. [DOI] [PubMed] [Google Scholar]
- Skulsky EM, Osman NI, Baghdoyan HA, Lydic R. Microdialysis delivery of morphine to the hypoglossal nucleus of Wistar rat increases hypoglossal acetylcholine release. Sleep. 2007;30:566–573. doi: 10.1093/sleep/30.5.566. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Botzinger complex in vivo. J Neurophysiol. 1999;81:1150–1161. doi: 10.1152/jn.1999.81.3.1150. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Inspiratory augmenting bulbospinal neurons express both glutamatergic and enkephalinergic phenotypes. J Comp Neurol. 2003;455:113–124. doi: 10.1002/cne.10486. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol. 2006;153:203–216. doi: 10.1016/j.resp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, Medina-Kauwe L, Gibson KM. gamma-Aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol. 1995;112:247–263. doi: 10.1016/0300-9629(95)00099-2. [DOI] [PubMed] [Google Scholar]
- van den Aardweg JG. Respiratory variability after opioids: see it happen. J Appl Physiol (1985) 2009;106:1029–1030. doi: 10.1152/japplphysiol.00155.2009. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Garcia AJ, 3rd, Doi A, Elsen G, Ramirez JM. beta-Noradrenergic receptor activation specifically modulates the generation of sighs in vivo and in vitro. Front Neural Circuits. 2013;7:179. doi: 10.3389/fncir.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, Shilling KC. Chronic Opioid Use is a Risk Factor for the Development of Central Sleep Apnea and Ataxic Breathing. Journal of Clinical Sleep Medicine. 2007;3:455. + [PMC free article] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Botzinger complex area eliminate eupneic respiratory rhythm in awake goats. J.Appl.Physiol. 2004;97:1629–1636. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Hamosh P, Gillis RA. Respiratory depression produced by activation of GABA receptors in hindbrain of cat. J Appl Physiol Respir Environ Exerc Physiol. 1981;51:1278–1286. doi: 10.1152/jappl.1981.51.5.1278. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Norman WP, Hamosh P, Gillis RA. Medullary ventral surface GABA receptors affect respiratory and cardiovascular function. Brain Res. 1982;248:71–78. doi: 10.1016/0006-8993(82)91148-9. [DOI] [PubMed] [Google Scholar]
- Zanella S, Doi A, Garcia AJ, 3rd, Elsen F, Kirsch S, Wei AD, Ramirez JM. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J Neurosci. 2014;34:36–50. doi: 10.1523/JNEUROSCI.3644-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutler M, Holty JE. Opioids, sleep, and sleep-disordered breathing. Curr Pharm Des. 2011;17:1443–1449. doi: 10.2174/138161211796197070. [DOI] [PubMed] [Google Scholar]