Abstract
Deletion in the 3p21 region, the chromosomal location of BAP1, has been reported in a subset of hepatocellular carcinoma (HCC), biliary and pancreatic cancers. This suggests that BAP1 could play a role in the pathogenesis of these tumors. We assessed the frequency of BAP1 loss by immunohistochemistry in 103 hepatic, biliary and pancreatic cancers. We also assessed chromosomal alterations in the BAP1 region in the same tumors by genotyping. We identified high frequency 4/8 (50%) of BAP1 loss in intrahepatic cholangicarcinoma (ICC). However the frequency was lower in HCC 9/51 (17.6%), pancreatic 1/42(2.4%) and extrahepatic biliary cancers (0/2). Loss of heterozygosity of at least one marker from the 3p21 region was observed in 75% of ICC, 52.9% of HCC and 45.2% of pancreatic cancers. Expression of hepatocytic (HepPar1) and bile duct (cytokeratin 7) markers were common (7/9, 77.8%) in the HCC tumors with loss or decrease of BAP1 compared with those with preserved BAP1 (18/42, 42.9%), (Fisher exact p=0.0751). Our results confirm the high frequency of BAP1 alterations in ICC and low frequency in pancreatic cancers. It also suggests that BAP1 is commonly altered in a subtype of HCC with both hepatocytic and biliary differentiation. Further studies of the therapeutic implications of our findings are warranted.
Keywords: BAP1, Loss of heterozygosity, 3p21 region, liver cancer, pancreatic cancer
Introduction
BAP1 (BRCA1 associated protein-1) is a tumor suppressor gene located in the 3p21 region[1]. BAP1 encodes a 729 amino acids long nuclear-localizing protein with an ubiquitin carboxy-terminal hydrolase (UCH) domain that gives BAP1 its deubiquitinase activity[1]. BAP1 functions as a component of the ubiquitin proteasome system. The tumor suppressor function of BAP1 is thought to be through its role in transcription regulation, cell cycle regulation, chromatin modification and DNA damage response [2-6].
Deletion of the 3p21 region, the chromosomal location of BAP1, is a frequent and early event in the formation of multiple tumors most significantly lung, breast and kidney cancers [7]. In addition, epigenetic inactivation of genes located in the region is a common and important event in the carcinogenesis of multiple tumors [8]. Multiple genes including, RASSF1, HYAL2, NPR2L, FUS1, BLU/ZMYND10, CYB561D2 (101F6), TMEM115 (PL6) and CACNA2D2 have been suggested as tumor suppressor driver genes in tumors with deletion in the 3p21 chromosomal region [9]. Given the important role of BAP1 in tumorigenesis of many cancers it is likely that it is a major driver for tumorigenesis in many tumors with deletion of this chromosomal region. Loss of heterozygosity (LOH) or deletion in the 3p21 region and its vicinity has been reported in a subset of HCC, biliary and pancreatic cancers suggesting that BAP1 could be an important tumor suppressor in the pathogenesis of these tumors [8, 10].
Germline mutation of BAP1 is associated with a tumor predisposition syndrome (BAP1-TPDS) with increased risk for four main cancers: uveal melanomas, cutaneous melanomas, malignant mesothelioma and lung adenocarcinoma[11-14]. However, several other cancers, including intrahepatic cholangiocarcinoma (ICC), hepatocellular carcinoma (HCC) and pancreatic adenocarcinoma (PDAC), have been reported in probands and families with germline mutation in BAP1[15, 16]. In addition, somatic mutations in BAP1 have been observed in the four main cancers associated with the BAP1-TPDS and in many other cancers in particularly ICC[15].
Hepatic, pancreatic and biliary carcinomas are aggressive tumors and represent three of the most lethal malignancies worldwide [17]. BAP1 is a potential target therapeutic candidate with multiple emerging studies targeting its downstream signals[18-20]. Also, in many cancers BAP1 loss is associated with tumor aggressiveness and prognosis[2, 21-23]. The goal of this study was to investigate the contribution of BAP1 to the tumorigenesis of the 3p21 deletion in hepatic, pancreatic and biliary tumors.
Materials and Methods
Tumor Samples
This study included tumor tissues from 51 HCC, eight ICC, two cases of lower common bile duct carcinoma (CBD) and 42 pancreatic tumors. The pancreatic tumors were five cases of ampullary carcinoma (AC) and 37 cases of PDAC. The eight cases of ICC included four peripheral and four hilar tumors. Corresponding non-tumor tissues were available in all cases. The clinicopathologic data for the 103 samples included in the study is summarized in Table 1. All specimens were retrospectively obtained from the archive of the pathology department National Liver Institute, Menoufia University or from the biorepository of the National Liver Institute Sustainable Sciences Institute Collaborative Research Center (NLISSICRC). The study was carried out according to an NLI institutional IRB approved protocol.
Table 1. Summary of the clinical and pathological features of samples included in the study.
| HCC | CC | AC | PDAC | |
|---|---|---|---|---|
| Total Number | No=51 | No=10 | No=5 | No= 37 |
| Age (Mean ±SD) | 57.1±6.8 | 52.5±3.5 | 57.40±7.5 | 54.92±12.91 |
| Gender | ||||
| Male | 42 (82.4%) | 6(60%) | 2(40%) | 25(67.6%) |
| Female | 9 (17.6%) | 4(40%) | 3(60%) | 12(32.4%) |
| Tumor size | ||||
| Mean ±SD | 7.3±13.4 | 7.3±13.4 | 2.5±0.3559 | 3.3±1.2 |
| Tumor grade | ||||
| Grade I | 0 | 0 | 2(40.0%) | 6(16.2%) |
| Grade II | 26(51%) | 7(70%) | 2(40.0%) | 23(62.2%) |
| Grade III | 25(49%) | 3(30%) | 1(20.0%) | 7(18.9%) |
| Grade IV | 0 | 0 | 0 | |
| NA | 1 (2.7%) | |||
| Vascular invasion | 24(47.1%) | 4(40%) | 0 | 10(27.0%) |
HCC: hepatocellular carcinoma, ICC: intrahepatic cholangiocarcinoma, DBD: distal bile duct carcinoma, AC: ampullary carcinoma, PDAC: pancreatic ductal adecarcinoma. NA: a case of signet ring carcinoma (not graded).SD: standard deviation from the mean.
Immunohistochemical (IHC) staining
IHC was carried out for all tumors except the ICC on 2mm diameter tissue microarrays (TMA) according to our previously published protocol[24]. At least two representative cores from each tumor and one core from matching non-tumor tissue were included. For the ICC cases tissue sections from the whole tumors were used. Tissue sections from the whole tumors were used for validation of the negative staining identified in the TMA.
After deparaffinization and rehydration, heat-induced antigen retrieval was performed using the DAKO high pH antigen retrieval solution in a vegetable steamer for 20 minutes at 97°C followed by incubation for an additional 20 minutes in the warm buffer. All antibodies were incubated overnight at 4 C°. Sections were incubated with a 1:50 dilution of a mouse monoclonal primary anti-BAP1 antibody (clone C-4, Santa Cruz Biotechnology, Dallas, Texas USA). Additionally, HCC samples were immunostained with HepPar1 (clone OCH1E5, DAKO A/S, Glostrup, Denmark, ready to use) and anti-cytokeratin 7 (clone OV-TL 12/30, DAKO A/S, Glostrup, Denmark, ready to use). Detection of the immunostaining was carried out utilizing the Envision™ FLEX/HRP detection system (DAKO A/S, Glostrup, Denmark) with the 3-diaminobenzidine (DAKO) as chromogen. For optimization of the immunohistochemistry protocol cell blocks were prepared from a melanoma cell line with strong expression of BAP1 (OCM3-positive control) and from the NCI-H226 a lung cancer cell line with biallelic loss of BAP1 (negative control), Figure 1. The expression of BAP1 in the cell lines was assessed by Western blot, Figure 1. In addition a negative control by omission of primary antibody was used for each run. After counterstaining with haematoxylin, the slides were independently assessed by two pathologists (MHA and DS) for detection of loss of nuclear staining of BAP1. Negative staining was defined as completely loss of nuclear staining[25-27], irrespective of cytoplasmic staining, in tumor cells with retained staining in non-tumor tissues (non-tumor liver or pancreas, blood vessels, lymphocyte and/or stromal cells) from the same section. Qualitative data were expressed as frequency and percent. Fisher exact test was used to measure association between BAP1 expression and hepPar1/CK7 expressions.
Figure 1. Optimization of BAP1 immunostaining and representative of tumors with negative and positive staining.
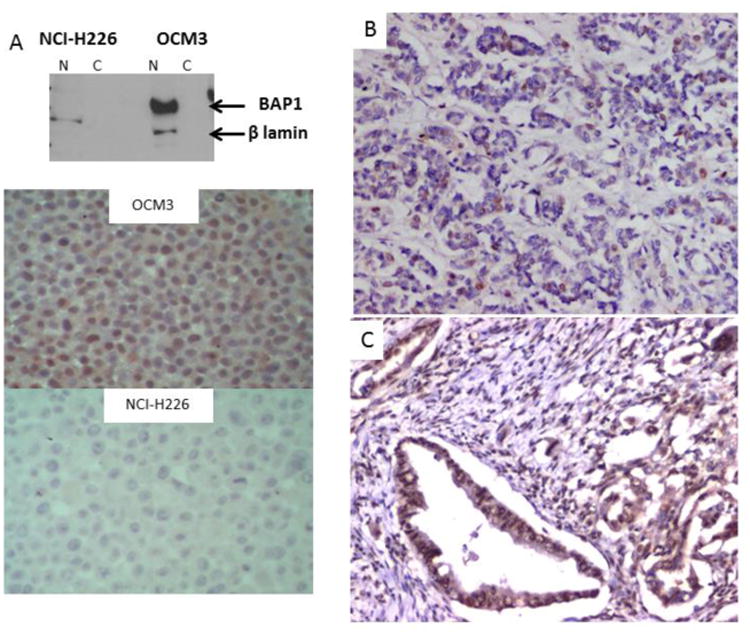
A) Two cell lines OCM3 (BAP1 positive) and NCI-H226 (BAP1 negative) were selected. Western blot confirmed BAP1 nuclear expression in OCM3. Immunostaining showed strong nuclear expression in OCM3 but no expression in the NCI-H226 cell line.
B) A cholangiocarcinoma case with loss of BAP1 expression occasional nuclear expression is seen in some ducts which are possibly non tumorous. Stroma and blood vessels endothelial cells are positive for nuclear BAP1 expression.
C) A cholangiocarcinoma with preserved nuclear expression of BAP1. Weak cytoplasmic expression is also observed.
Genotyping
Genomic DNA was extracted from both tumor and non-tumor samples using the Gentra PureGene extraction kit (Qiagen, Valencia, CA). Genomic DNA yield and quality were determined using a Nanodrop ND1000 spectrophotometer (ThermoScientific) and further visually inspected by agarose gel electrophoresis. LOH was assessed using microsatellite polymorphic markers flanking the BAP1 gene (D3S1578, D3S3561, D3S3026), as well, as two markers D3S3630 (3p26.3) and D3S3644 (3p14.1) in the p arm of chromosome 3.
The PCR products were analyzed using an ABI 377 sequencer and the GeneScan and Genotype software (Applied Biosystems, Foster City, CA, USA). The allelic imbalance factor (AIF) was determined by calculating the ratio of alleles for both the normal (N) and tumor (T) sample, and then the tumor ratio was divided by the normal ratio: T1:T2/N1:N2 as previously suggested[28, 29]. LOH was defined AIF of more than 1.5 or less than 0.67 for scoring regions with allelic imbalance. This ratio is equivalent to an allelic imbalance observed in at least 33% of the tumor cells[30].
Quantitative Reverse Transcription PCR (qRT-PCR)
RNA from frozen HCC tissue samples was isolated using the TRIZOL (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The extracted RNA was stored at -80°C until the time of experiments. Complementary DNA (cDNA) was synthesized using the SuperScript VILO cDNA Synthesis Kit according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). TaqMan 5′ nuclease quantitative real-time PCR assay was carried out using the pre-developed TaqMan assay according to the manufacturer's protocol (Applied Biosystems, Foster City, CA). The reactions were carried out in triplicates and performed on the ABI7500. The relative expression levels and fold changes of the target, normalized to GUSB levels, and relative to the matching non-tumors were determined by the comparative CT method using the formula 2-ΔΔCt.
Results
BAP1 protein expression
Table 2 summarizes the observed BAP1 IHC results. Forty two out of the 51 (82.4%) HCC tumors showed strong uniform nuclear expression of BAP1 in the tumor and non-tumor liver, Figure 1 supplement. In the remaining nine tumors, total loss of nuclear BAP1 protein expression was observed in three tumors, very weak expression in four tumors and heterogeneous expression with loss of expression in >50% of the tumor cells in two additional tumors, Figure 2. In total, no or markedly decrease expression of BAP1 was observed in 9/51 (17.6%) tumors, Table 2. The median age for the nine cases was 58 years and all were males.
Table 2. Summary of the BAP1 immunohistochemistry and genotyping results of the tumors included in the study.
| HCC No=51 | ICC No=8 | CBD No=2 | PDAC No=37 | AC No=5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Weak | Positive | Negative | Positive | Negative | Positive | Negative | weak | Positive | Positive | ||
| BAP1 IHC Number % | 3(5.9%) | 6(11.8%) | 42(82.41%) | 4(40%) | 4(40%) | 0 | 2(100%) | 1(2.4%) | 5(13.5%) | 30(81.08%) | 5(100%) | |
| LOH frequencies* | 2(66.6%) | 4(66.7%) | 21 (53.8%) | 4(100%) | 2(50%) | 0 | 2(100%) | 1(100%) | 4(10.8%) | 11(36.6%) | 1(20%) | |
| D3S3630 | 3p26.3 | 2(66.6)%) | 4(66.7%) | 13 (41.9%) | NA | NA | NA | NA | 0 | 2(5.4%) | 2(5.4%) | 0 |
| D3S1578 | 3p21.1 | 1(33.3)%) | 3 (33.3%) | 10(40.%) | NA | NA | NA | NA | 1(100%) | NA | 5(13.5%) | 0 |
| D3S3561 | 3p21.1 | 1(33.3%) | 4(80%) | 9(31%) | 4(100%) | 1 (25%) | 0 | 1(50%) | 0 | NA | 2(5.4%) | 0 |
| D3S3026 | 3p21 | 1(33.3%) | 2(40%) | 12(35.3%) | 2(50%) | 2(50%) | 0 | 0 | 1(100%) | 5(100%) | 0 | 1(20%) |
| D3S3644 | 3p14.1 | NA | 1(25%) | 12(42.9%) | 4(100%) | 2(50%) | 0 | 0 | 0 | NA | 2(5.4%) | 0 |
LOH: loss of heterozygosity, markers are sorted from telomeric to centromeric.
Note, Percentages represent samples with informative markers, non-informative markers were excluded.
Figure 2. Immunostaining of HCC showing association of BAP1 loss with positive staining with bile duct cell marker cytokeratin 7.
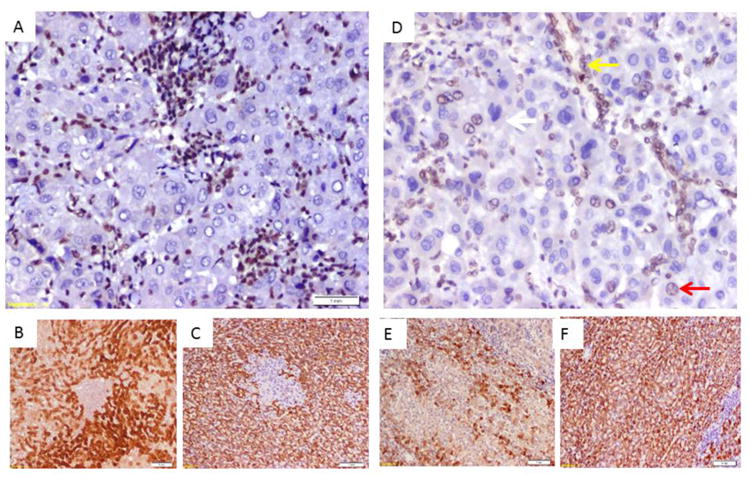
A) HCC case with homogenous loss of nuclear staining of BAP1, note positive staining in stromal and infiltrating inflammatory cells. B) CK7 immunostaining of the tumor in (A) showing positive expression of tumor cells to the bile duct epithelial marker. C) HepPar immunostaining of the tumor in (A) showing positive expression of tumor cells to the hepatocyte marker.
D) HCC case with heterogenous loss of nuclear staining of BAP1(white arrow loss of nuclear expression, red arrow show preserved expression, yellow arrow show preserved expression in a non-tumor bile duct). E) CK7 immunostaining of the tumor in (D) showing heterogenous expression of tumor cells to the bile duct epithelial marker. C) HepPar immunostaining of the tumor in (D) showing homogenous positive expression of tumor cells to the hepatocyte marker.
All 51 HCC tumors showed strong expression of HepPar1. While 25/51 (49%) tumors showed >10% expression of CK7. Seven out of the 9 (77.8%) HCC with loss or decrease in BAP1 expression showed strong combined CK7/HepPar1 expression, Figure 2. While showed loss or weak expression of BAP1, Figure 2. The difference in BAP1 expression between tumors with combined CK7 and HepPar1 expression (7/25 and those with only HepPar1 expression was not statistically significant (p=0.033). Identification of both CK7 and HepPar1 staining in the same tumor suggests a subtype of HCC with mixed hepatocellular and biliary differentiation of the tumor cells.
For the eight ICC, four tumors showed total loss of BAP1 (two peripheral and two hilar). All four ICC cases with loss of BAP1 were females, while the four cases with preserved BAP1 expression were males. The median age was 53 years. Perineural invasion was observed in all four cases with loss of BAP1. Lympho-vascular invasion was observed in three out of four cases with loss of BAP1 while it was observed in two out of the four in those with preserved BAP1 expression.
Out of the 37 PDAC tumors five tumors showed weak nuclear staining while only one showed total loss of BAP1 expression. The patient was 56 years old male and presented with grade III tumor with positive perineural and vascular invasions and lymph node metastasis. The two CBD and the five AC showed preserved BAP1 expression.
BAP1 RNA expression
Matching tumor and non-tumor liver tissue was available for assessment of RNA expression of BAP1 for the six HCC tumors (THCC-17, THCC-89, THCC-63, THCC-84, THCC71 and THCC82) with weak or markedly heterogeneous BAP1 expression. BAP1 RNA expression in each tumor was assessed relative to the matching non-tumor tissue, Table 3. In the tumor with strong expression of BAP1, a close to 5 folds increase in the expression was observed in the tumor compared to the matching control. While in the four tumors with weak expression 2.1-5 folds decrease in the BAP1 expression was observed and in the two tumors with heterogeneous expression mild increase to no change in expression of BAP1 was observed, Table 3.
Table 3. BAP1 RNA expression in HCC tumors with weak or heterogenous protein expression.
| Tumor | BAP1 RNA expression * (qRT-PCR) | BAP1 protein expression (IHC) |
|---|---|---|
| THCC-100 | 4.78 (± 0.96) | Strong |
| THCC-17 | 1.19 (± 0.36) | Heterogeneous |
| THCC-89 | 0.44 (± 0.35) | Weak |
| THCC-63 | 0.20 (±0.3) | Weak |
| THCC-84 | 0.47 (±0.26) | Weak |
| THCC-71 | 1.96 (±0.65) | Heterogeneous |
| THCC-82 | 0.45 (±0.40) | Weak |
Numbers represent fold changes of BAP1 expression in the tumors compared to its matching non-tumor tissues.
Genotyping
Table 2 summarizes the informative genotyping results for the five tested microsatellite markers. Loss of heterozygosity (LOH) in at least one marker was identified in 27 (52.9%), 6 (75%) and 21 (45.2%) of HCC, CC and pancreatic tumors respectively. BAP1 biallelic inactivation as evident by loss of protein expression was observed in only a small subset of HCC, PDAC, AC and CBD. However, for ICC out of the four tumors with loss of BAP1 expression three showed LOH suggesting that BAP1 is a major tumor suppressor gene in the region in ICC but not in the other tumors.
Discussion
Hepatic, pancreatic and biliary cancers have been reported in patients with germline mutations in BAP1 suggesting it as potential driver in these tumors. The role of BAP1 in tumorigenesis of a subset of ICC is well documented, however its role in HCC and PDAC is still not clear[31-33]. The most significant finding in this study is the loss of BAP1 in a subtype of HCC tumors with coexpression of hepatic and biliary markers. Although BAP1 loss was observed in a small, 17.6%, subset of HCC tumors, all of the BAP1 negative cases and 4/6 of low BAP1 expressing cases showed coexpression of hepatocytic cellular marker (HepPar 1) and a biliary marker CK7. The weak or heterogenous expression of BAP1 protein in HCC tumors was further validated by qRT-PCR, which showed significance decrease in the tumors compared to the matching non-tumor tissues. Positive combined HepPar1 and CK7 expressions in this subset of tumors could be explained by either arrest of hepatic progenitor cells (HPCs) maturation during the process of hepatocarcinogenesis or dedifferentiation of mature hepatocytes to a progenitor cell/biliary phenotype. HPCs are bipotential cells expressing both CK7 and HepPar1 markers. This observation suggests that BAP1 negative HCC is a unique molecular subtype of the tumor. After submission of our manuscript another publication came out showing higher frequency of BAP1 mutation in an aggressive subset of HCC expressing stemness genes[34]., Utilizing multi-omics integration of the copy number variation, DNA methylation and messenger RNA expression the authors of that study showed three subclasses of HCC with BAP1 more frequently mutated in the more aggressive subclass[34]. In addition, BAP1 knock down was associated with significant over expression of stemness genes (CA9, EPCAM, KRT19 and PROM1). Taken together with our findings, BAP1 loss is associated with a subtype of HCC that is characterized by dedifferentiation, expression of stemness genes and likely more aggressive phenotype. Further studies on a larger cohort will be needed to delineate the molecular biology of this unique tumor subtype.
Although we didn't have survival information for patients included in the study, an association between BAP1 loss in cases with biliary differentiation, high tumor grade (66.7%) and perineural invasion (100%) indicates the aggressive nature of these tumors. Loss of nuclear BAP1 expression correlates with poor prognosis in other tumors such as uveal melanoma[25, 26]. Further studies of the outcome of HCC and ICC patients and their response to therapy are needed. The strong correlation between BAP1 protein expression as detected by IHC and RNA expression assessed by qRT-PCR indicates that IHC could be considered as a good representative for BAP1 expression status in HCC tumors.
The incidence rates of CC vary among different areas of the world, and this is mostly due to genetic differences and variation in geographical risk factors [35]. In the West, primary sclerosing cholangitis is the most common cause while in Southeast Asia[36], liver fluke infection is the most common etiology[37]. In Egypt, the etiology of ICC is not clear. Liver fluke is not a known etiology in Egyptian patients and we didn’t observe evidence of sclerosing cholangitis in the eight patients with ICC included in the study. Contamination of drinking water with many pollutants such as chemical carcinogens, heavy metals, pesticides, and polychlorinated biphenyls as a result of contamination of drinking water with industrial wastes and agricultural irrigation wastewater [38] could be a potential etiology. In addition, Hepatitis C viral infection, which has been observed commonly in our patients, has been implicated in the carcinogenesis of ICC [39, 40], however, the information on the status of the infection was not available for most of our patients.
Our study confirmed the important role of BAP1 in the tumorigenesis of ICC including both peripheral and hilar subtypes. We observed a high frequency (50%) of loss of BAP1 expression in the tumors. In addition, BAP1 protein expression was strongly linked to LOH in all ICC cases, which indicates biallelic inactivation of BAP1 gene in these tumors. Our result is consistent with the high frequency (25%) of somatic biallelic inactivating of BAP1 reported in ICC [33]. In ICC, loss of BAP1 expression occurred in large tumors with lymph node metastasis 75%. This is consistent with previous reports suggesting association between BAP1 loss and poor prognosis in ICC [31]. Metastatic liver adenocarcinoma is very difficult to distinguish from ICC either by morphology or IHC. The high frequency of BAP1 loss in ICC may be of diagnostic importance for surgical pathologists.
For pancreatic tumors (PDAC and AC) the loss of BAP1 protein expression was found in one out of 42 (2.4%). The low frequency of BAP1 loss in pancreatic tumors is consistent with other reports suggesting that it is not a major tumor suppressor driver. The relative high frequency of LOH in these tumors suggests that genes other than BAP1 are main drivers for the pathogenesis of the 3p21 chromosomal loss in these tumors. Frequent allelic loss and large homozygous deletions of human chromosome 3p.21 region are specially one of the earliest molecular changes of many cancers suggesting that it contains many tumor suppressor gene candidates, with varying role, in different types of tumors[10].
Given the high frequency of BAP1 alterations in many tumors there is an increasing interest in its utilization as potential target for therapy. However, targeting a tumor suppressor is quite challenging. There is some evidence that targeting downstream signaling of BAP1 could have potential therapeutic utility. It has been suggested that histone deactylase inhibitors could have a therapeutic effect in uveal melanoma, lung cancer and mesothelioma in particular those with BAP1 loss[19, 20].EZH2 and PARP inhibitors have been suggested as other potential therapeutic targets[18, 41]. Currently several clinical trials are ongoing utilizing these potential therapeutic targets (clinicaltrials.gov: NCT02860286, NCT03207347 and NCT00121225). Knowledge of BAP1 expression status will be important for proper selection of patients for individualization of patients care.
In conclusion, BAP1 loss is common in ICC and a subtype of HCC with bile cell differentiation of tumor cells and it is a major driver for cancer in these tumors. Our result also suggests that BAP1 loss is rare in pancreatic cancers including both PDAC and AC. It also suggests that BAP1 expression could be used as a biomarker to identify a subset of hepatic tumors with biliary differentiation. Based on the recent progress in molecular based therapies targeting BAP1 our results support the utility of these agents in ICC and a subset of HCC tumors.
Supplementary Material
Highlights.
Loss of BAP1 is common in cholangiocarcinoma and a subtype of hepatocellular carcinoma but rare in pancreatic cancer.
Hepatocellular carcinomas with BAP1 loss show co-expression of biliary and hepatic epithelial markers suggesting that they are a unique subtype of the tumor.
Loss of heterozygosity of the 3p21 region is commonly detected in the hepatobiliary and pancreatic tumors.
Acknowledgments
This project was supported by a grant from the Sustainable Sciences Institute, San Francisco California and the R21CA191943 grant from the National Cancer Institute (PI: Abdel-Rahman, MH).
Footnotes
Conflict of interest: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz DC, Wilkinson KD, Maul GG, Barlev N, Berger SL, Prendergast GC, Rauscher FJ., 3rd BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 2.Hakimi AA, Chen YB, Wren J, Gonen M, Abdel-Wahab O, Heguy A, Liu H, Takeda S, Tickoo SK, Reuter VE, Voss MH, Motzer RJ, Coleman JA, Cheng EH, Russo P, Hsieh JJ. Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. European urology. 2013;63:848–54. doi: 10.1016/j.eururo.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu H, Mashtalir N, Daou S, Hammond-Martel I, Ross J, Sui G, Hart GW, Rauscher FJ, 3rd, Drobetsky E, Milot E, Shi Y, Affar el B. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Molecular and cellular biology. 2010;30:5071–85. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, Wilkinson KD. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–62. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen DE, Rauscher FJ., 3rd Defining biochemical functions for the BRCA1 tumor suppressor protein: analysis of the BRCA1 binding protein BAP1. Cancer Lett. 1999;143(1):S13–7. doi: 10.1016/s0304-3835(99)90004-6. [DOI] [PubMed] [Google Scholar]
- 6.White AE, Harper JW. Cancer. Emerging anatomy of the BAP1 tumor suppressor system. Science. 2012;337:1463–4. doi: 10.1126/science.1228463. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Zabarovska VI, Braga E, Alimov A, Klein G, Zabarovsky ER. Loss of heterozygosity in tumor cells requires re-evaluation: the data are biased by the size-dependent differential sensitivity of allele detection. FEBS letters. 1999;462:121–8. doi: 10.1016/s0014-5793(99)01523-9. [DOI] [PubMed] [Google Scholar]
- 8.Tischoff I, Markwarth A, Witzigmann H, Uhlmann D, Hauss J, Mirmohammadsadegh A, Wittekind C, Hengge UR, Tannapfel A. Allele loss and epigenetic inactivation of 3p21.3 in malignant liver tumors. Int J Cancer. 2005;115:684–9. doi: 10.1002/ijc.20944. [DOI] [PubMed] [Google Scholar]
- 9.da Costa Prando É, Cavalli LR, Rainho C. Evidence of epigenetic regulation of the tumor suppressor gene cluster flanking RASSF1 in breast cancer cell lines. Epigenetics. 2011;6:1413–24. doi: 10.4161/epi.6.12.18271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikiforova MN, Nikiforov YE, Biddinger P, Gnepp DR, Grosembacher LA, Wajchenberg BL, Fagin JA, Cohen RM. Frequent loss of heterozygosity at chromosome 3p14.2-3p21 in human pancreatic islet cell tumours. Clin Endocrinol (Oxf) 1999;51:27–33. doi: 10.1046/j.1365-2265.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Rahman MH, Pilarski R, Cebulla CM, Massengill JB, Christopher BN, Boru G, Hovland P, Davidorf FH. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48:856–9. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, Hesdorffer M, Nasu M, Powers A, Rivera Z, Comertpay S, Tanji M, Gaudino G, Yang H, Carbone M. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I, Viale A, Lash AE, Pirun M, Socci ND, Rutten A, Palmedo G, Abramson D, Offit K, Ott A, Becker JC, Cerroni L, Kutzner H, Bastian BC, Speicher MR. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–21. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popova T, Hebert L, Jacquemin V, Gad S, Caux-Moncoutier V, Dubois-d'Enghien C, Richaudeau B, Renaudin X, Sellers J, Nicolas A, Sastre-Garau X, Desjardins L, Gyapay G, Raynal V, Sinilnikova OM, Andrieu N, Manie E, de Pauw A, Gesta P, Bonadona V, Maugard CM, Penet C, Avril MF, Barillot E, Cabaret O, Delattre O, Richard S, Caron O, Benfodda M, Hu HH, Soufir N, Bressac-de Paillerets B, Stoppa-Lyonnet D, Stern MH. Germline BAP1 mutations predispose to renal cell carcinomas. American journal of human genetics. 2013;92:974–80. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet. 2016;89:285–94. doi: 10.1111/cge.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilarski R, Rai K, Cebulla C, Abdel-Rahman M. BAP1 Tumor Predisposition Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [PubMed] [Google Scholar]
- 17.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 18.Parasramka M, Yan IK, Wang X, Nguyen P, Matsuda A, Maji S, Foye C, Asmann Y, Patel T. BAP1 dependent expression of long non-coding RNA NEAT-1 contributes to sensitivity to gemcitabine in cholangiocarcinoma. Mol Cancer. 2017;16:22. doi: 10.1186/s12943-017-0587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacco JJ, Kenyani J, Butt Z, Carter R, Chew HY, Cheeseman LP, Darling S, Denny M, Urbe S, Clague MJ, Coulson JM. Loss of the deubiquitylase BAP1 alters class I histone deacetylase expression and sensitivity of mesothelioma cells to HDAC inhibitors. Oncotarget. 2015;6:13757–71. doi: 10.18632/oncotarget.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, Bowcock AM, Harbour JW. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res. 2012;18:408–16. doi: 10.1158/1078-0432.CCR-11-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farzin M, Toon CW, Clarkson A, Sioson L, Watson N, Andrici J, Gill AJ. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology. 2015;47:302–7. doi: 10.1097/PAT.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 22.Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, Leng N, Pavia-Jimenez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, Kinch L, Hambuch T, Jain S, Lotan Y, Margulis V, Sagalowsky AI, Summerour PB, Kabbani W, Wong SW, Grishin N, Laurent M, Xie XJ, Haudenschild CD, Ross MT, Bentley DR, Kapur P, Brugarolas J. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–9. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–3. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Rahman MH, Agour AA, El-Azab DS. Tissue microarray as a research tool to study non-neoplastic liver diseases. Egyptian Liver Journal. 2014;4:69–74. 10.1097/01.ELX.0000451425.36015.99. [Google Scholar]
- 25.Koopmans AE, Verdijk RM, Brouwer RW, van den Bosch TP, van den Berg MM, Vaarwater J, Kockx CE, Paridaens D, Naus NC, Nellist M, van IWF, Kilic E, de Klein A. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol. 2014;27:1321–30. doi: 10.1038/modpathol.2014.43. [DOI] [PubMed] [Google Scholar]
- 26.Kalirai H, Dodson A, Faqir S, Damato BE, Coupland SE. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer. 2014;111:1373–80. doi: 10.1038/bjc.2014.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah AA, Bourne TD, Murali R. BAP1 protein loss by immunohistochemistry: a potentially useful tool for prognostic prediction in patients with uveal melanoma. Pathology. 2013;45:651–6. doi: 10.1097/PAT.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 28.Skotheim RI, Diep CB, Kraggerud SM, Jakobsen KS, Lothe RA. Evaluation of loss of heterozygosity/allelic imbalance scoring in tumor DNA. Cancer Genet Cytogenet. 2001;127:64–70. doi: 10.1016/s0165-4608(00)00433-7. [DOI] [PubMed] [Google Scholar]
- 29.Cawkwell L, Bell SM, Lewis FA, Dixon MF, Taylor GR, Quirke P. Rapid detection of allele loss in colorectal tumours using microsatellites and fluorescent DNA technology. Br J Cancer. 1993;67:1262–7. doi: 10.1038/bjc.1993.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devilee P, Cleton-Jansen AM, Cornelisse CJ. Ever since Knudson. Trends Genet. 2001;17:569–73. doi: 10.1016/s0168-9525(01)02416-7. [DOI] [PubMed] [Google Scholar]
- 31.Andrici J, Goeppert B, Sioson L, Clarkson A, Renner M, Stenzinger A, Tayao M, Watson N, Farzin M, Toon CW, Smith RC, Mittal A, Samra JS, Hugh TJ, Chou A, Lawlor RT, Weichert W, Schirmacher P, Sperandio N, Ruzzenente A, Scarpa A, Gill AJ. Loss of BAP1 Expression Occurs Frequently in Intrahepatic Cholangiocarcinoma. Medicine (Baltimore) 2016;95:e2491. doi: 10.1097/MD.0000000000002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putra J, de Abreu FB, Peterson JD, Pipas JM, Mody K, Amos CI, Tsongalis GJ, Suriawinata AA. Molecular profiling of intrahepatic and extrahepatic cholangiocarcinoma using next generation sequencing. Exp Mol Pathol. 2015;99:240–4. doi: 10.1016/j.yexmp.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, Offerhaus GJ, Roa JC, Roberts LR, Gores GJ, Popescu I, Alexandrescu ST, Dima S, Fassan M, Simbolo M, Mafficini A, Capelli P, Lawlor RT, Ruzzenente A, Guglielmi A, Tortora G, de Braud F, Scarpa A, Jarnagin W, Klimstra D, Karchin R, Velculescu VE, Hruban RH, Vogelstein B, Kinzler KW, Papadopoulos N, Wood LD. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–3. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo HG, Choi JH, Yoon S, Jee BA, Cho EJ, Lee JH, Yu SJ, Yoon JH, Yi NJ, Lee KW, Suh KS, Kim YJ. Integrative analysis of genomic and epigenomic regulation of the transcriptome in liver cancer. Nat Commun. 2017;8:839. doi: 10.1038/s41467-017-00991-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maroni L, Pierantonelli I, Banales JM, Benedetti A, Marzioni M. The significance of genetics for cholangiocarcinoma development. Annals of translational medicine. 2013:1. doi: 10.3978/j.issn.2305-5839.2012.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. The American journal of gastroenterology. 2004;99:523–6. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 37.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dekov V, Komy Z, Araujo F, Van Put A, Van Grieken R. Chemical composition of sediments, suspended matter, river water and ground water of the Nile (Aswan-Sohag traverse) Science of the Total Environment. 1997;201:195–210. doi: 10.1016/s0048-9697(97)84057-0. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer science. 2004;95:592–5. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donato F, Gelatti U, Tagger A, Favret M, Ribero M, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case–control study in Italy. Cancer Causes and Control. 2001;12:959–64. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 41.Schoumacher M, Le Corre S, Houy A, Mulugeta E, Stern MH, Roman-Roman S, Margueron R. Uveal melanoma cells are resistant to EZH2 inhibition regardless of BAP1 status. Nat Med. 2016;22:577–8. doi: 10.1038/nm.4098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


