Abstract
Background
The 21-gene Recurrence Score (RS) predicts outcome and benefit from adjuvant chemotherapy benefit in breast cancer patients treated with adjuvant endocrine therapy. In the NSABP B-28 study, we evaluated the 21-gene Recurrence Score (RS) for its prognostic impact and its ability to predict benefit from paclitaxel (P) in node-positive, estrogen receptor-positive (ER+) breast cancer patients treated with adjuvant chemotherapy plus tamoxifen.
Methods
The B-28 trial compared doxorubicin/cyclophosphamide (AC) with AC followed by P in 3060 patients. Tamoxifen for 5 years was also given to patients ≥50 years and those <50 years with ER+ and/or progesterone receptor-positive (PR+) tumors. The present study includes 1065 ER-positive, tamoxifen-treated patients with RS assessment. Median follow-up time was 11.2 years.
Results
In univariate analyses RS was a significant predictor of outcome. In multivariate analyses, RS remained a significant independent predictor of outcome beyond clinico-pathologic factors, age, and type of surgery (p<0.001). In the study population (n=1065), the disease-free survival (DFS) hazard ratio (HR) with adding P to AC was 0.87 (95% CI=0.72–1.05; P=0.14). RS was not a significant predictor of P benefit: for DFS, HRs for adding P to AC in RS low, intermediate, and high subgroups were 1.01 (95% CI=0.69–1.47; P=0.99), 0.84 (95% CI=0.62–1.14; P=0.26), and 0.81 (95% CI=0.60–1.10; P=0.21), respectively (interaction P=0.64). Similar findings were observed for the other study endpoints.
Conclusions
RS maintains significant prognostic impact in ER-positive, node-positive patients treated with adjuvant chemotherapy plus tamoxifen. However, RS did not significantly predict benefit from adding paclitaxel to AC chemotherapy.
Keywords: Breast Cancer, Node-Positive, Prognosis and Prediction, Recurrence Score
Introduction
Gene expression profiling is useful for assessing risk of distant recurrence in patients with early-stage breast cancer and provides additional information to that obtained from traditional histopathologic factors and biomarkers [1–6]. Several gene expression signatures predict risk of distant recurrence in untreated patients and those treated with hormonal therapy and/or chemotherapy [1–7]. The 21-gene Recurrence Score (RS) Assay (Oncotype DX®) quantifies the risk of distant recurrence in node-negative and node-positive, estrogen receptor (ER)-positive breast cancer patients treated with adjuvant endocrine therapy [5–8]. There are limited data on the prognostic impact of RS in node-positive patients treated with adjuvant chemo-endocrine therapy [9].
Besides its prognostic ability, the RS was also shown to significantly predict benefit from adding non-anthracycline-based chemotherapy to endocrine therapy in node-negative, ER-positive patients (NSABP B-20 trial) [10] and from adding anthracycline-based chemotherapy to endocrine therapy in postmenopausal, node-positive, ER-positive patients (SWOG 8814 trial) [10,11]. In both studies, patients with high RS significantly benefited from adding chemotherapy, whereas patients with intermediate or low RS did not. No study to date has formally evaluated the role of RS in predicting benefit from adding a taxane to an anthracycline-based regimen.
We sought to evaluate the prognostic impact of RS on residual risk of recurrence and death in node-positive, ER-positive patients treated with adjuvant chemo-endocrine therapy in the NSABP B-28 trial [12] and to also evaluate whether the RS predicts benefit from adding paclitaxel to AC chemotherapy.
Patients and Methods
The NSABP B-28 Trial
The NSABP B-28 trial evaluated whether the sequential addition of four cycles of paclitaxel (P: 225 mg/m2 every 3 weeks) to 4 cycles of doxorubicin/cyclophosphamide (AC every 3 weeks) would improve disease-free survival (DFS) and overall survival (OS) compared to 4 cycles of AC alone in patients with resected operable, node-positive breast cancer. Between August 1995 and May 1998, 3,060 patients were randomly assigned (AC: 1,529 patients and AC→P: 1,531 patients). Patients ≥50 years and those <50 with estrogen receptor (ER)-or progesterone receptor (PR)-positive tumors also received tamoxifen for 5 years starting with the first dose of AC. Post-lumpectomy radiotherapy was mandated. Post-mastectomy or regional-nodal radiotherapy was prohibited.
Aims, Eligibility, and Endpoints for the Present Study
The aims of the present study were to evaluate the prognostic impact of RS on DFS, distant recurrence-free interval (DRFI), OS, and breast cancer-specific survival (BCSS), and to examine the independent prognostic contribution of RS beyond traditional clinico-pathologic factors such as age, tumor size, grade, number of positive nodes, and adjuvant chemotherapy assignment. In addition, we sought to examine the association between RS and benefit from the sequential addition of P to AC chemotherapy.
Eligible patients had to be ER-positive by immunohistochemistry (IHC) on tissue microarray, tamoxifen-treated, and with successful 21-gene RS assay assessment. The primary pre-specified endpoint was DFS, defined as time from study entry to first loco-regional or distant recurrence, contralateral breast cancer, non-breast second primary cancer, or death from other causes. Other endpoints included DRFI, defined as time from study entry to first distant recurrence, with contralateral breast cancer or non-breast second primary cancers ignored, and deaths before distant recurrence considered as censoring events; OS; and BCSS, defined as time from study entry to death from breast cancer.
RNA Assessment Methodology for the Present Study
Available tumor specimens from B-28 that met the above eligibility criteria were centrally evaluated for histologic grade using the modified Bloom-Richardson score using 5-micron tissue sections stained by hematoxylin and eosin (H&E) [13]. All specimens were then analyzed for the Oncotype DX™ Recurrence Score as previously described [5,14,15]. Three 5-micron-thick sections were cut by the NSABP Division of Pathology laboratory. After sectioning, tumor-rich area in the tumor block was marked by the Genomic Health, Inc. (GHI) pathologist using H&E-stained sections as references. The tumor-rich area was manually micro-dissected with clean blades and RNA was extracted according to standard operating procedures for the Oncotype DX assay. The RNA was then assessed for quantity (Ribogreen assay™ Molecular Probes/Invitrogen Eugene, OR) and residual genomic DNA (using a DNA-specific PCR assay). RNA was subjected to reverse transcription (RT) with a universal RNA (Stratagene, Agilent, Santa Clara, CA) as a positive control and water as a negative control for each set of RT reactions followed by quantitative polymerase chain reaction analysis. The average reference gene expression served as a quality metric for each sample, and the limit of detection and limit of quantitation cutoffs and other quality metrics, as defined for the 21-gene assay, was applied as appropriate for the 21 genes in the RS [15]. In the end, the Oncotype DX assay was successfully performed in 1,065 patients with follow-up.
Statistical Methodology for the Present Study
Patients were grouped into low RS (<18), intermediate RS (18–30), and high RS (≥31). Kaplan-Meier estimates and log-rank tests were used to evaluate outcomes in different RS risk groups. Univariate Cox models were used to assess the strength of the association between RS and outcomes. Multivariate Cox proportional hazards models were used to examine whether RS provides independent prognostic information in addition to clinico-pathologic factors and whether the RS predicts benefit from the addition of adjuvant P to AC chemotherapy. Time-dependent Cox proportional hazards models were also used to explore whether or not the association between RS and risk of cancer recurrence varied over time. A P value <0.05 for the likelihood ratio test was used to determine the statistical significance of findings.
The study was approved by the Essex (NJ), Aultman Hospital (OH), and University of Pittsburgh IRBs (PA). Written, informed consent was obtained from all participants.
Results
Results of the Parent B-28 Trial
The results of the NSABP B-28 trial were first reported in 2005 [12] and demonstrated that the addition of P to AC significantly reduced the hazard for DFS event by 17% (Relative risk [RR]: 0.83, 95% CI: 0.72–0.95, P=0.006). Five-year DFS was 76%± 2% for patients randomly assigned to AC→P compared to 72%± 2% for those assigned to AC. Improvement in OS was small and not statistically significant (RR: 0.93, 95% CI: 0.78–1.12, P=0.46). Five-year OS was 85% ± 2% for both groups. Subset analysis of the effect of P according to hormone receptors or tamoxifen administration did not reveal statistically significant interaction (DFS: P=0.30; OS: P=0.44, respectively).
Patient Population for the Current Study
Of the 3060 patients participating in the B-28 trial, 1945 were excluded because they had either ER-negative tumors or tumor block was not available. An additional 32 patients were excluded for various reasons (8 were clinically ineligible, 17 did not receive tamoxifen, and 7 received post-mastectomy radiotherapy). Of the remaining 1083 tumors processed by GHI, 11 had insufficient RNA and 7 had poor qRT-PCR sample quality, leaving 1065 patients constituting the core group for the present study. Median follow up was 11.2 years.
Comparison of Included versus Excluded ER-Positive B-28 Patients
Among 1995 patients who were not included in this study, 999 had ER-positive tumors according to assessment from the participating institutions. When the 1065 B-28 ER-positive patients included in the present study were compared to the 999 ER-positive B-28 patients who were excluded, there were no significant differences in the distribution of age, number of positive nodes, or treatment group (AC or AC→P) (Supplementary Table 1). Compared to excluded patients, those who were included were significantly more likely to have undergone mastectomy (P=0.007), have larger tumors (P<0.001), and have higher grade tumors (P=0.004, Supplementary Table 1). When outcomes of the 1,065 patients who were included in the study were compared to those 999 ER-positive patients who were excluded, the former group of patients tended to have worse outcomes compared to the latter (DFS: 61.1 % vs. 66.5%, P=0.014; DRFI: 68% vs. 71.9%, P=0.04, respectively. Figure 1).
Fig. 1.
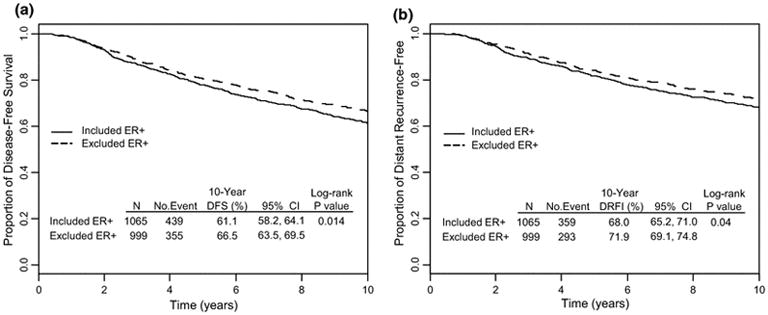
Comparison of (A) disease-free survival (DFS) and (B) distant relapse-free interval (DRFI) between 1,065 ER-positive, B-28 patients who were included in the present study and 999 ER-positive, B-28 patients who were excluded.
Distribution of the Recurrence Score in the Study Population
Among the 1065 patients included in the present study, 386 (36%) had a low RS (<18), 364 (34%) had an intermediate RS (18–30), and 315 (30%) had a high RS (≥31). The distribution of the RS was not statistically significantly different according to treatment, surgery type, or number of positive nodes. However, there were statistically significant differences in the distribution of RS according to age and tumor size, with older patients and those with small tumors being more likely to have low RS, although a wide distribution of RS values was seen in each age or size category (data not shown).
Univariate Analysis of Outcomes According to Recurrence Score Categories
In univariate analyses the RS was a statistically significant predictor of outcome for all four endpoints (DFS, DRFI, OS, and BCSS) (Figure 2A, 2B, 2C, and 2D, respectively). DFS at 10 years was 75.8% for patients with low RS compared to 57% for those with intermediate RS, and was 48% in those with high RS (log rank p<0.001); the percent distant relapse-free was 80.9%, 64.9%, and 55.8%, respectively (p<0.001); OS was 90%, 74.7%, and 63%, respectively (p<0.001); and breast cancer-specific survival was 95%, 78.9%, and 68.2%, respectively (p<0.001).
Fig. 2.
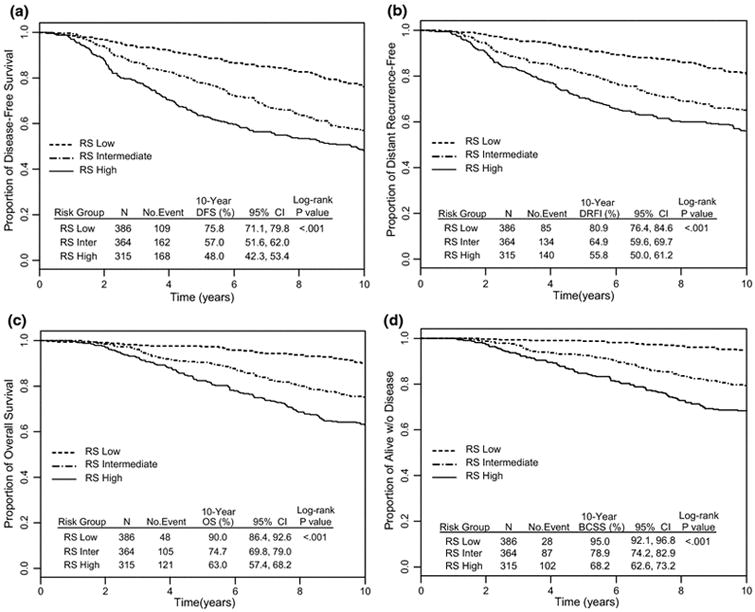
Kaplan-Meier curves for (A) disease-free survival (DFS), (B) distant relapse-free interval (DRFI), (C) overall survival (OS), and (D) breast cancer-specific survival (BCSS) according to Recurrence Score categories: NSABP B-28
Multivariate Analysis of Outcomes Adjusted for Clinico-pathologic Variables
The RS was an independent predictor of outcome on multivariate analysis (Table 1). Multivariate Cox proportional hazards models adjusting for treatment, age, number of positive nodes, type of surgery, tumor size, and tumor grade demonstrated a hazard ratio (HR) associated with a 50-unit increment in RS of 2.53 (95% CI=1.90, 3.38; p<0.001). (Table 1). Additional independent predictors on multivariate analysis included treatment (HR: 0.81; 95% CI=0.67, 0.99; P=0.037), number of positive nodes (HR: 1.82; 95% CI=1.49, 2.23), and tumor size (cm) (HR: 1.13 (95% CI=1.03, 1.23). The RS was also a significant independent predictor of outcome for the remaining three endpoints. The adjusted HRs corresponding to DRFI, OS, and BCSS were 2.42, 3.09, and 3.38, respectively. The likelihood ratio test p-values were all <0.001. The association of the RS with the risk of a DFS event was strongest in the first 5 years: up to 5 years, the adjusted HR associated with a 50-unit increment was 3.81 (95% CI=2.67, 5.43; P<0.001), whereas after 5 years the adjusted HR was 1.39 (95% CI=0.88, 2.19; P=0.16). Similar patterns were seen for the other endpoints.
Table 1.
Multivariate Cox proportional hazards model for disease-free survival excluding and including the Recurrence Score (RS): NSABP B-28
| Excluding RS | Including RS | |||
|---|---|---|---|---|
| HR (lower 95% CI, upper 95% CI) | P | HR (lower 95% CI upper 95% CI), | P | |
| Recurrence Score* | NA | NA | 2.53 (1.90,3.38) | <0.001 |
| Treatment | 0.83 (0.69,1.01) | 0.060 | 0.81 (0.67,0.99) | 0.037 |
| Age ≥ 50 | 0.96 (0.79,1.17) | 0.687 | 1.00 (0.82,1.22) | 0.967 |
| ≥4 positive nodes | 1.79 (1.47,2.19) | <0.001 | 1.82 (1.49,2.23) | <0.001 |
| Mastectomy | 1.23 (1.01,1.51) | 0.042 | 1.18 (0.97,1.45) | 0.105 |
| Tumor size (cm)* | 1.13 (1.04,1.23) | 0.005 | 1.13 (1.03,1.23) | 0.007 |
| Moderate grade | 1.57 (1.08,2.30) | <0.001 | 1.42 (0.97,2.08) | 0.133 |
| Poor grade | 1.95 (1.33,2.86) | 1.46 (0.98,2.16) | ||
Recurrence Score hazard ratio (HR) is associated with a 50 point difference. Tumor size HR is associated with a 1 cm difference.
Prognostic Impact of Recurrence Score across Various Patient Subgroups
The RS was a significant predictor of outcome (for all four endpoints) in various patient subgroups defined by treatment (AC or AC→P), type of surgery (lumpectomy or mastectomy), number of positive nodes (1–3 positive nodes or 4+ positive nodes), age at study entry (<50 or ≥ 50 years-old), and tumor size (0–2 cm or ≥2.1 cm) (Table 2). Notably among 268 patients with 1–3 positive nodes and low RS, only 20% had a DFS event (Figure 3A) and only 2% had died from breast cancer at 10 years (Figure 3C). Generally, outcomes of patients with 4 or more positive nodes and a low RS were as good as or better than those of patients with 1–3 positive nodes and intermediate or high RS. (Figure 3A, 3B, 3C, and 3D).
Table 2.
Prognostic discrimination of the Recurrence Score assessment within patient subsets defined by clinico-pathological factors: Kaplan-Meier estimates for the 10-year proportions of event-free for disease-free survival (DFS), distant recurrence-free interval (DRFI), overall survival (OS), and breast cancer-specific survival (BCSS): NSABP B-28
| 10-Year DFS (%) | P | 10-Year DRFI (%) | P | 10-Year OS (%) | P | 10-Year BCSS (%) | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Group | Risk Group | Risk Group | Risk Group | |||||||||||||
| Low | Inter | High | Low | Inter | High | Low | Inter | High | Low | Inter | High | |||||
| Treatment | ||||||||||||||||
| AC | 75.5 | 53.4 | 45.3 | <.001 | 80.8 | 62.5 | 53.2 | <.001 | 91.5 | 69.9 | 60.7 | <.001 | 94.2 | 75.3 | 67.9 | <.001 |
| AC-P | 76.1 | 60.4 | 50.5 | <.001 | 80.9 | 67.3 | 58.2 | <.001 | 88.5 | 79.3 | 65.3 | <.001 | 95.7 | 82.4 | 68.6 | <.001 |
| Surgery | ||||||||||||||||
| Lumpectomy | 80.0 | 63.3 | 53.5 | <.001 | 84.0 | 71.7 | 60.6 | <.001 | 92.3 | 81.0 | 71.3 | <.001 | 96.4 | 83.8 | 74.1 | <.001 |
| Mastectomy | 72.3 | 51.6 | 44.5 | <.001 | 78.2 | 59.0 | 52.7 | <.001 | 88.0 | 69.4 | 58.0 | <.001 | 93.8 | 74.8 | 64.6 | <.001 |
| # of Positive Nodes | ||||||||||||||||
| 1–3 | 79.8 | 64.8 | 57.0 | <.001 | 84.7 | 71.5 | 63.1 | <.001 | 93.3 | 79.2 | 70.7 | <.001 | 98.0 | 82.9 | 75.6 | <.001 |
| ≥4 | 66.7 | 40.3 | 31.1 | <.001 | 71.8 | 50.7 | 41.3 | <.001 | 82.4 | 65.2 | 48.5 | <.001 | 88.1 | 70.3 | 54.0 | <.001 |
| Age, y | ||||||||||||||||
| < 50 | 78.2 | 57.8 | 52.1 | <.001 | 83.4 | 65.5 | 57.9 | <.001 | 91.9 | 74.7 | 68.9 | <.001 | 95.2 | 75.7 | 72.7 | <.001 |
| ≥ 50 | 74.0 | 56.3 | 42.2 | <.001 | 79.0 | 64.4 | 52.8 | <.001 | 88.7 | 74.8 | 54.7 | <.001 | 94.8 | 82.0 | 61.8 | <.001 |
| Tumor size | ||||||||||||||||
| ≤ 2cm | 76.5 | 65.4 | 65.1 | 0.08 | 81.6 | 72.0 | 69.4 | 0.03 | 89.2 | 79.8 | 77.2 | 0.01 | 96.2 | 83.0 | 82.0 | <.001 |
| > 2cm | 74.7 | 49.2 | 38.3 | <.001 | 79.8 | 58.3 | 47.8 | <.001 | 90.6 | 70.1 | 55.1 | <.001 | 93.7 | 75.1 | 60.5 | <.001 |
Fig. 3.
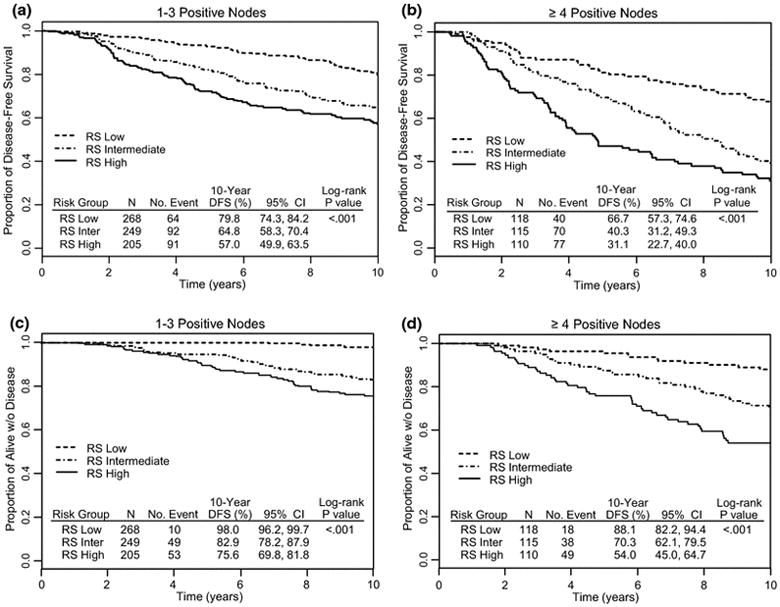
Kaplan-Meier curves for disease-free survival (DFS) according to Recurrence Score categories: NSABP B-28
Predictive Impact of Recurrence Score on Treatment Benefit from Addition of Paclitaxel to AC
Among 2007 B-28 patients with ER-positive tumors, as assessed by participating institutions, the addition of P to AC only improved risk of a DFS event marginally (HR=0.86; 95% CI=0.75, 0.99; P=0.04). Among the 1065 node-positive, ER-positive patients with RS information, a similar magnitude of treatment effect was observed but the difference was not statistically significant (HR=0.87; 95% CI=0.72, 1.05; P=0.14). For DFS, the HRs associated with the addition of P in RS low, intermediate, and high subsets were 1.01 (95% CI=0.69, 1.47; P=0.99), 0.84 (95% CI=0.62, 1.14; P=0.26), and 0.81 (95% CI=0.60, 1.10; P=0.21), respectively. The likelihood ratio test for interaction between P and RS risk groups did not suggest differential treatment effect across RS risk groups (P=0.65). Similar results were obtained for the other three endpoints (Figure 4) and when the subset of 937 patients who were assessed to be HER-2 negative or equivocal by RT-PCR was examined (data not shown).
Fig. 4.
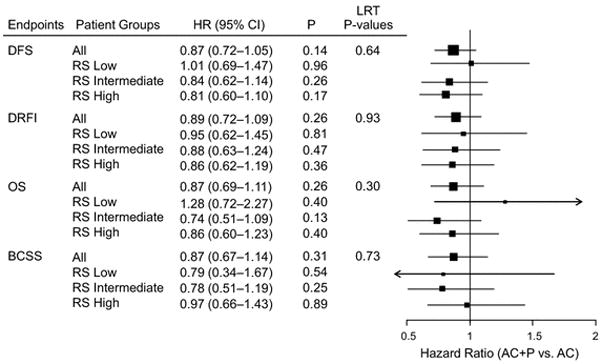
Forest Plots of the effect of paclitaxel (P) according to Recurrence Score (RS) category: NSABP B-28
Discussion
We have demonstrated that the 21-gene RS is a significant and independent predictor of outcome in node-positive, ER-positive breast cancer patients treated with adjuvant chemo-endocrine therapy. These results build on the previous experience on the prognostic impact of RS in node-negative patients treated with no adjuvant therapy, adjuvant endocrine therapy, or adjuvant chemo-endocrine therapy, as well as those in node-positive patients treated with adjuvant endocrine therapy alone [5,10,11]. Only one study (ECOG E2197) [9] has thus far evaluated the prognostic impact of the RS in node-positive breast cancer patients (≤3 positive nodes) treated with adjuvant chemo-endocrine therapy. In that study the RS was found to be independently prognostic in a cohort of 465 ER-positive patients with 0–3 positive nodes treated with AC or AT chemotherapy. Our study confirms those findings and expands the prognostic impact of RS to patients with ≥4 positive nodes. Our findings have potential clinical implications by refining residual risk of recurrence and death in node-positive, ER-positive patients and they can be useful in tailoring the extent of adjuvant chemotherapy and can help to identify patients with significant residual risk who could be candidates for clinical research protocols evaluating novel adjuvant therapies.
Our findings on the prognostic effect of RS according to the number of positive nodes suggest that the RS had a large, independent impact on prognosis within nodal subgroups. In fact, patients with ≥4 positive nodes and low RS had outcomes as good as or better than patients with 1–3 positive nodes and intermediate or high RS. This was true for all endpoints evaluated. These findings are in concordance with those reported from previous studies in node-positive patients treated with endocrine therapy alone [6,9,16] and underscore the complementary prognostic contribution of the RS in the context of the traditional clinico-pathologic prognostic factors.
The addition of taxanes to anthracycline-based chemotherapy regimens has resulted in a modest but statistically significant reduction in the odds of recurrence and death (approximately 15–20%) [12,17–19]. This reduction has translated to a small absolute improvement in DFS and OS (approximately 4–5%). Thus, the majority of patients who receive taxanes do not benefit from such treatment. Furthermore, the CALGB 9344 trial [19] demonstrated less benefit from the sequential addition of P to AC chemotherapy in patients with ER-positive tumors compared to those with ER-negative tumors. A similar benefit was observed in the parent NSABP B-28 trial in terms of recurrence-free survival, although no significant interaction between receptor status and treatment effect was observed.
Several investigators have attempted to identify predictors of taxane benefit with inconsistent results. Hayes et al. [19] examined the effect of ER, PR, and HER2 status in predicting benefit from adding P to AC in a subset of node-positive breast cancer patients who participated in the CALGB 9344 trial. Patients with a HER2-positive breast cancer benefited from P, regardless of ER status, but P did not benefit patients with HER2-negative, ER-positive cancers. Hugh et al. [20] investigated the predictive significance of tumor subtyping by IHC (for ER/PR/HER2 and Ki67) on the relative benefit from docetaxel in the BCIRG 001 trial, which compared TAC (docetaxel, doxorubicin, cyclophosphamide) versus FAC (5-fluorouracil, doxorubicin, cyclophosphamide) in patients with node-positive breast cancer. Patients were classified by tumor characteristics as (1) triple negative (ER/PR/HER2–negative, 14.5%), (2) HER2 (HER2-positive, ER/PR-negative, 8.5%), (3) luminal B (ER-positive and/or PR-positive and either HER2-positive and/or Ki67high, 61.1%), and (4) luminal A (ER-positive and/or PR-positive and not HER2-positive or Ki67high, 15.9%). Results demonstrated improved 3-year DFS with TAC versus FAC in the luminal B group (P=.025) and in a combined ER-positive/HER2-negative group treated with tamoxifen (P=.041), with a marginal trend in triple negative (P=.051) and HER2 (P=.068) subtypes. However, no DFS advantage was seen in the luminal A population.
The above data suggest that the benefit from adding taxanes to an anthracycline-containing regimen appears smaller for patients with ER-positive tumors. Even within that group, there is possibly another subset that may not benefit at all from the addition of the taxane. Although we did not observe significant interaction between treatment and RS for adding P to AC, our findings in the low RS group are not inconsistent with those from prior studies showing no benefit from the addition of adjuvant chemotherapy to endocrine therapy in patients with low RS. However, contrary to prior reports demonstrating significant benefit from adding chemotherapy to endocrine therapy in patients with high RS [10,11], our findings did not demonstrate such significant benefit from adding P to AC in patients with high RS. One possible explanation for the lack of a significant treatment by RS interaction is that the overall benefit from adding P to AC was modest not only in the B-28 ER-positive cohort (HR: 0.86) but also in the ER-positive cohort with RS information, which may have diminished the power to detect a significant treatment by RS interaction. In addition, the large benefit from adjuvant AC in patients with high RS may have minimized any additional benefit from the addition of P. Although inconclusive, the HRs of treatment effect from the addition of P to AC in patients with intermediate or high RS do not rule out a modest chemotherapy effect. The observation of possible treatment effect in patients with intermediate RS with the addition of a taxane to AC may have biologic implications, if confirmed in other datasets. To that end, the results of the TAILORx trial [21] on the effect of adjuvant chemotherapy in patients with RS of 11–25 are eagerly awaited, particularly because a considerable proportion of the patients randomly assigned to chemo-endocrine therapy in that trial have been treated with a taxane-containing regimen.
In conclusion, our results demonstrate a significant prognostic contribution of the Recurrence Score in node positive, ER-positive patients treated with chemo-endocrine therapy but no significant treatment by RS interaction. Our findings could help to identify subgroups of patients with low residual risk of recurrence, which could be adequately treated with shorter adjuvant chemotherapy regimens and may not necessarily need the addition of a taxane. Alternatively, they could also help to identify patients with considerable residual risk of recurrence who could be candidates for longer adjuvant chemotherapy regimens and for participation in adjuvant trials evaluating novel agents.
Supplementary Material
Comparison of distribution of age, number of positive nodes, treatment, tumor size, grade, and surgery type between ER-positive patients included in the present study and those who were excluded: NSABP B-28
Acknowledgments
Supported by: Public Health Service Grants U10CA-180868, U10CA-180822, UG1CA-189867, from the National Cancer Institute, Department of Health and Human Services, and from Bristol-Myers Squibb Pharmaceutical Research Institute.
The authors thank Barbara C. Good, Ph.D., for editorial assistance with this manuscript.
The work described in this manuscript is original research and has not been previously published. Related work has been published as follows and is provided upon submission:
Wolmark N, Mamounas EP, Baehner FL, et al. Prognostic impact of the combination of Recurrence Score and Quantitative Estrogen Receptor Expression (ESR1) on predicting late distant recurrence risk in estrogen receptor-positive breast cancer after 5 years of tamoxifen: Results from NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-28 and B-14. J Clin Oncol. 2016;34(20):2350–8.
Mamounas EP, Liu Q, Paik S, et al. 21-Gene Recurrence Score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. 2017; 109(4). pii: djw259.
Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive cancer: Results from NSABP B-28. J Clin Oncol 2005; 23(16):3686–96.
Yang SX, Costantino JP, Kim C, et al. Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer. J Clin Oncol 2010; 28(18):2974–81.
Mamounas EP, Bryant J, Lembersky BC, et al, NSABP investigators P. Paclitaxel (T) following doxorubicin/cyclophosphamide (AC) as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. Proc Am Soc Clin Oncol 2003; 22(ASCO 12). Abstr 12.
Mamounas EP. Evaluating the use of paclitaxel following doxorubicin/cyclophosphamide in patients with breast cancer and positive axillary nodes. Proc NIH Cons Devel Conf Adj Ther Br Cancer 2000; 17.
Yang SX, Costantino JP, Nguyen D, et al. Correlation of levels of Akt phosphorylation at Ser473 with benefit from paclitaxel chemotherapy in NSABP B-28 patients with node-positive breast cancer. J Clin Oncol (ASCO) 2009; 27(15S). Abstr 537.
Mamounas EP, Tang G, Paik S, et al. Prognostic impact of the 21-gene recurrence score (RS) on disease-free and overall survival of node-positive, ER-positive breast cancer patients (pts) treated with adjuvant chemotherapy: Results from NSABP B-28. ASCO Breast Cancer Symposium/J Clin Oncol 2012; 30(27S). Abstr 1.
Mamounas EP, Tang G, Paik S, et al. Association between the 21-Gene Recurrence Score and benefit from adjuvant paclitaxel in node-positive, ER-positive breast cancer patients: Results from NSABP B-28. San Antonio Breast Cancer Symposium/Cancer Res 2012; 72(24):Suppl 3. Abstr S1–10.
Mamounas EP, Tang G, Paik S, Baehner FL, et al. Recurrence score for prognosis and prediction of paclitaxel benefit in node(+)/ER(+) breast cancer. 13th St Gallen Intl BC Conf/The Breast 2013. Abstr P213.
The following authors declare the following potential conflict(s) of interest:
Eleftherios P. Mamounas, MD: GHI, Genentech: Consultant, Speakers’ Bureau; Biotheranostics,
GRAIL: Consultant; Roche, Celcuity, Macrogenics: Consultant
Gong Tang, PhD: Consulting from: ExxonMobil Biomedical Science Inc.
Soonmyung Paik, MD: None.
Frederick L. Baehner, MD: Employee, GHI: Salary and stock.
Qing Liu: None.
Jong-Hyeon Jeong, PhD: None.
Seong-Rim Kim, MD: None.
Steven M. Butler, PhD: Prior employee of GHI (Departed Dec, 2014), currently retired.
Farid Jamshidian, PhD: None.
Diana B. Cherbavaz, PhD: GHI Employment and equity.
Amy P. Sing, MD: Former employee of GHI, stock ownership.
Steven Shak, MD: GHI employee, shareholder.
Thomas B. Julian, MD: None.
Barry C. Lembersky, MD: None.
D. Lawrence Wickerham, MD: None.
Joseph P. Costantino, Dr PH: None.
Norman Wolmark, MD: None.
Footnotes
Trial Registration: PDQ: NSABP-B-28
References
- 1.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. PNAS. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Vijver MJ, He YD, van ‘t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 3.van ‘t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 4.Foekens JA, Atkins D, Zhang Y, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol. 2006;24:1665–1671. doi: 10.1200/JCO.2005.03.9115. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 7.Dowsett M, Cuzick J, Wales C, et al. Risk of distant recurrence using Oncotype DX in postmenopausal primary breast cancer patients treated with anastrozole or tamoxifen: A TransATAC study. Cancer Res. 2009;69(Suppl 2):75s. (Abstr 53) [Google Scholar]
- 8.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26:4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 11.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 13.Elston EW, Ellis IO. Method for grading breast cancer. J Clin Pathol. 1993;46:189–190. doi: 10.1136/jcp.46.2.189-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cronin M, Ghosh K, Sistare F, et al. Universal RNA reference materials for gene expression. Clin Chem. 2004;50:1464–1471. doi: 10.1373/clinchem.2004.035675. [DOI] [PubMed] [Google Scholar]
- 15.Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53:1084–1091. doi: 10.1373/clinchem.2006.076497. [DOI] [PubMed] [Google Scholar]
- 16.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 17.Martín M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 18.Martín M, Seguí MA, Antón A, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363:2200–2210. doi: 10.1056/NEJMoa0910320. [DOI] [PubMed] [Google Scholar]
- 19.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 20.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: Use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–76. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparano JA. TAILORx: Trial assigning individualized options for treatment (Rx) Clin Breast Cancer. 2006;7:347–350. doi: 10.3816/CBC.2006.n.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of distribution of age, number of positive nodes, treatment, tumor size, grade, and surgery type between ER-positive patients included in the present study and those who were excluded: NSABP B-28


