Abstract
Recent evidence has suggested that dietary polyunsaturated fatty acids (PUFAs) modulate inflammation; however, few studies have focused on the pathobiology of PUFA using isocaloric and isolipidic diets and it is unclear if the associated pathologies are due to dietary PUFA composition, lipid metabolism or obesity, as most studies compare diets fed ad libitum. Our studies used isocaloric and isolipidic liquid diets (35% of calories from fat), with differing compositions of omega (ω)-6 or long chain (Lc) ω-3 PUFA that were pair-fed and assessed hepatic pathology, inflammation and lipid metabolism. Consistent with an isocaloric, pair-fed model we observed no significant difference in diet consumption between the groups. In contrast, the body and liver weight, total lipid level and abdominal fat deposits were significantly higher in mice fed an ω-6 diet. An analysis of the fatty acid profile in plasma and liver showed that mice on the ω-6 diet had significantly more arachidonic acid (AA) in the plasma and liver, whereas, in these mice ω-3 fatty acids such as eicosapentaenoic acid (EPA) were not detected and docosahexaenoic acid (DHA) was significantly lower. Histopathologic analyses documented that mice on the ω-6 diet had a significant increase in macrovesicular steatosis, extramedullary myelopoiesis (EMM), apoptotic hepatocytes and decreased glycogen storage in lobular hepatocytes, and hepatocyte proliferation relative to mice fed the Lc ω-3 diet. Together, these results support PUFA dietary regulation of hepatic pathology and inflammation with implications for enteral feeding regulation of steatosis and other hepatic lesions.
Keywords: Diet, PUFA, Omega-3, Hepatic Steatosis, Fish Oil, Fatty Liver
1 Introduction
Omega-6 (ω-6) and omega-3 (ω-3) polyunsaturated fatty acids (PUFA) are essential fatty acids (FA), which cannot be interconverted in humans due to lack of ω-3 desaturase.[1] Besides their role in energy storage and production, PUFA are important constituents of biological membranes and are precursors to prostaglandin and pro-resolving lipid mediator pathways.[2],[3] Many lipid mediators derived from ω-6 PUFA have pro-inflammatory functions,[4] whereas; those synthesized from ω-3 PUFAs have anti-inflammatory properties.[5, 6] Both ω-3 and ω-6 PUFA are metabolized by the same enzymes resulting in signaling molecules with opposing bioactivities. Studies have suggested that humans evolved on a diet containing approximately a 1:1 ratio of ω-6:ω-3 PUFA[7]; however, current western diets has a high ω-6:ω-3 ratio.[8] A diet high in ω-6 PUFAs, such as linoleic acid (LA), results in decreased tissue levels of ω-3 long-chain (Lc) PUFAs, including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)[9], and a heightened risk of chronic inflammatory disease processes.[10] In contrast, a diet containing a low ω-6:ω-3 PUFA ratio, or one that is supplemented with Lc ω-3 PUFAs, reduces risk factors for chronic inflammatory diseases, including cardiovascular disease,[11, 12] cancer,[13, 14] and obesity.[15, 16] Dietary Lc ω-3 PUFA can also reduce hepatic inflammation, fibrosis, and steatosis in non-alcoholic fatty liver diseases (NAFLD), and non-alcoholic steatohepatitis (NASH).[17, 18]
Although hepatic lipid storage is normal, excessive intrahepatic lipid accumulation (>5.6% of liver weight) [19] is associated with steatosis, inflammation and cardiometabolic syndromes. Several mechanisms are involved in the accumulation of intrahepatic lipids, including increased accumulation of triglycerides in the liver, increased de novo lipogenesis, and/or reduced clearance and obesity.[20] Approximately 30% of Americans have a fatty liver, [19] and this is increased up to 75% amongst obese individuals.[21] Further, hepatic steatosis occurs rapidly with excess calorie consumption, independent of dietary composition.[22] In contrast, in humans a hypocaloric diet reduces steatosis,[23, 24] suggesting that it is crucial to differentiate between the effects mediated by total caloric intake versus obesity to understand the role of dietary composition in the modulation of the hepatic microenvironment. The majority of studies on hepatic steatosis have used high-fat diets (60% of calories from saturated fatty acids (SFAs)) and overfeeding to provide an animal model of NAFLD.[25, 26] To date, little is known about the effects of ω-6 and ω-3 PUFA dietary composition, independent of caloric intake on the hepatic microenvironment.
Mature myeloid cells are terminally differentiated and continuously renewed by the proliferation of committed hematopoietic precursors, such that myelopoiesis is critical to expand and replenish the myeloid cell pool. Numerous pathologic conditions stimulate myelopoiesis including, infections, autoimmune and inflammatory conditions, neoplasia and obesity in association with neutrophilia, splenomegaly and multifocal, hepatic extramedullary myelopoiesis (EMM), i.e. the formation of myeloid tissue outside of the bone marrow.[27] Hepatic EMM is normal during fetal and early development;[28, 29] however, EMM in adult tissues is associated with pathological conditions. Hepatocyte apoptosis indicates liver injury and found significantly increased in patients with NASH.[30] High fat diets have also been reported to increase the osmotic fragility of red blood cell (RBC) membranes resulting in decreased RBC counts and hemoglobin concentration.[31]
In the present study, we used an isocaloric, isolipidic liquid diet combined with pair feeding that allows for controlled dietary caloric intake and limited weight changes between groups. Using this model, we examined the effects of dietary ω-6 and ω-3 PUFA composition on hepatic pathobiology and report that dietary PUFA composition regulates hepatic steatosis, proliferation, apoptosis and EMM using a dietary model containing comparatively lower fat calories as a percent of total relative to previously reported studies.[25, 26]
2. Materials and Methods
2.1. Animals and Pair-fed Model
Female BALB/c mice (6 weeks old) purchased from Charles River Laboratories were housed in micro-isolators in groups of five mice per cage, under standard conditions of temperature and humidity, with a 12 h light-dark cycle. The micro-isolators were attached to high efficiency particulate air (HEPA)-filtered ventilation blowers for clean air. The animal housing facility was maintained as a specific pathogen free (SPF) area. The Institutional Animal Care and Use Committee at the University of Nebraska Medical Center approved the animal protocol for the study.
After 2 weeks of acclimation (Fig. 1A) to each diet, mice were divided at random into two dietary groups (n=20) on the basis of the experimental liquid diet (ω-6 or ω-3 diet). Our diets were isocaloric and isolipidic and had identical protein, fiber, and micronutrient contents. The ω-6 diet was the Lieber-DeCarli control diet (Dyets # 710027) containing 28.4 gm/L olive oil, while the ω-3 diet was customized by using the base diet (Dyets # 710166) and adding 8.4 gm/L olive oil and 20 gm/L of encapsulated fish oil (NutriGold Triglyceride Omega-3 Gold capsules; Lot# 0081-3180-2) (Table 1). Both liquid diets provided 1.0 Kcal energy per ml of diet and equal calories from macronutrients (35% derived from fat, 47% from carbohydrate, and 18% derived from protein). The ω-6 and ω-3 diets differed primarily by the absence or presence of Lc ω-3 PUFA from fish oil and ω-6 PUFA from olive oil respectively. Omega-3 capsules and Lieber-DeCarli powder diets were stored at 4°C. Diets were prepared and delivered every 24-26 hours and daily intake was monitored. Ad libitum access to the liquid diets was provided for the first 5 days to acclimatize the mice to the diets. From day 6, the ω-6 diet group mice were pair-fed based on mean consumption of the ω-3 diet group from the previous day and this was continued throughout the study for 10 or 20 weeks. Body weights were recorded twice a week. At the end of the experiment, blood was collected without fasting prior to the mice being euthanized and hematological parameters, such as total white blood cells count (WBC) count, red blood cells (RBC) count, hemoglobin level, hematocrit and mean corpuscular volume (MCV) were determined by vet ABC animal blood counter (Scil animal care company, Grayslake, IL). Livers and abdominal adipose tissue were removed and weighed and the livers processed as described below. In addition, a limited number of control studies were undertaken with age matched, chow fed (#7912, Teklad) mice as a baseline control.
Figure 1. Isocaloric and isolipidic diets, pair-fed model and their impact on body weight.
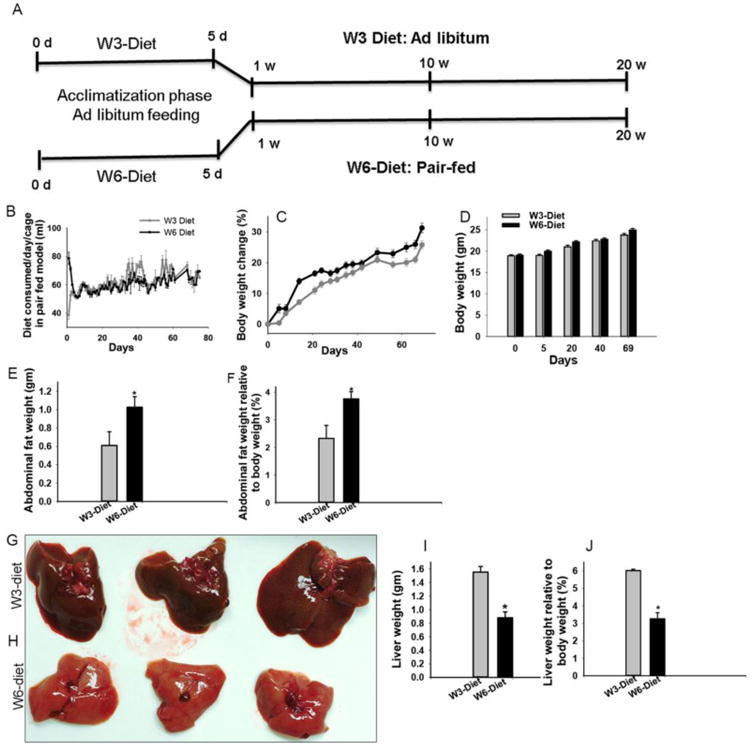
Mice were pair-fed omega- (ω-6) and omega-3 (ω-3) PUFA diets for 10 or 20 weeks. Experimental design for a pair-fed model (A). Mice were acclimatized to liquid diets by feeding ad libitum amounts for the first five days. Thereafter, the ω-6 diet group mice were pair-fed based on the diet consumed by the ω-3 mice on a cage basis on the preceding day. The average amount of diet consumed/day (B). Differences in percent changes in body weights between the pair-fed groups [n= 20] compared by a repeated measure test (C). Body weights before the start of the liquid diet [day 0], at the start of pair-feeding [day 5], on day 20, and day 40, of pair feeding and before autopsy on [day 69] (n=20) (D). Comparison of abdominal adipose tissues between the groups (E and F) (n=3), based on abdominal fat weight (E), as well as, fat weight relative to body weight (%) at autopsy (F). Photographs of representative livers document differences in color and size from mice fed ω-3 (G) or ω-6 (H) diets. Comparison of liver weight (I) and liver weight relative to body weight (J) (n=3). * = p ≤ 0.05
Table 1. Composition of the experimental diets.
The omega-6 diet (ω-6 diet) is the Lieber-Decarli control diet, which contains 28.4 gm/L of olive oil. Omega-3 diet (ω-3 diet) was customized by replacing 20 gm/L olive oil with the ω-3 oil (NutriGold omega 3 capsule). All the other components were the same in both of the diets to maintain iso-composition and isocaloric properties.
| Ingredient in Diet | ω-6 Diet Grams/L | ω-3-Diet Grams/L |
|---|---|---|
| Casein | 41.4 | 41.4 |
| L-Cystine | 0.5 | 0.5 |
| DL-Methionine | 0.3 | 0.3 |
| Corn Oil | 8.5 | 8.5 |
| Olive Oil | 28.4 | 8.4 |
| Safflower Oil | 2.7 | 2.7 |
| Maltose Dextrin | 115.2 | 115.2 |
| Cellulose | 10 | 10 |
| Mineral Mix | 8.75 | 8.75 |
| Vitamin Mix | 2.5 | 2.5 |
| Choline Bitartrate | 0.53 | 0.53 |
| Xanthum gum | 3 | 3 |
| Encapsulated omega-3 oil | 0 | 20 |
|
| ||
| Total | 221.8 | 221.8 |
2.2. Fatty Acid Analysis
Portions of liver tissues and plasma samples from the diet fed mice for 10 weeks were snap frozen and stored at -80°C for FA analysis. Portions of ready-to-use ω-3 and ω-6 diets were also frozen until needed for lipid extraction. Omega-3 capsules were stored at 4°C and used directly for FA analysis. Total lipid extraction from tissues and diets were undertaken using antioxidant β-hydroxy-toluene (0.05%, wt/vol) as previously described.[32, 33] Heptadecanoic and nonadecanoic acid (100 μg each) were added to all samples to estimate the recovery of FA. Complex lipids were hydrolyzed by adding 0.5 ml of methanol containing 1% (wt/vol) sulfuric acid and 0.25 ml of toluene, and then were incubated at 60°C overnight. Following incubation, 1.25 ml of water containing sodium chloride (5%, wt/vol) was added and esters were extracted using hexane (2 × 1.25 ml per sample). The hexane layer was washed using 1 ml of water containing potassium bicarbonate (2%, wt/vol), and dried over anhydrous sodium sulfate. The solvent was removed under a stream of nitrogen and the FA methyl esters were analyzed by gas chromatography-mass spectrometry (GC-MS) using an Agilent 6890 series gas chromatograph equipped with a 5873 mass-selective detector. For assessment of sterols, 100 μg of 5α-cholestane was added to samples as an internal standard. Sterols were separated and detected by GC-MS using a DB-17ms column. FA concentration was analyzed as μg/μl or μg/mg of the samples, analyzed, and compared between the diets and tissues from ω-3 and ω-6 diet fed mice
2.3. Histology and immunohistochemistry (IHC)
Liver tissues were fixed in zinc fixative for 24-48 hours, and transferred to 70% ethanol, prior to paraffin embedding, and sectioned at 4-6 μm. For Oil Red O (ORO) staining, fresh liver tissues were embedded in optimal cutting temperature (O.C.T) compound medium (Tissue-Tek #4583, Sakura Finetek, CA U.S.A) and stored at -80°C until sectioned and stained. Sections were stained with hematoxylin and eosin (H & E), examined under light microscopy using a Zeiss Axioplan-2 microscope with a HRc camera, and analyzed using Zeiss AxioVision Rel 4.8 software. Two persons examined the sections as “blinded” group assignments and an experienced pathologist validated the results. Steatosis was examined in H & E stained sections, then further confirmed and quantified by counting ORO stained lipid droplets in hepatocytes. Lipid droplets the size of a hepatic nucleus or larger were enumerated to analyze macro-vesicular steatosis, whereas smaller lipid droplets were considered for micro-vesicular steatosis analysis. To quantify hepatic steatosis, the number of lipid droplets per 1000× magnification field were counted for 10 random fields for each mouse liver. EMM was assessed based on foci of immature inflammatory cells and their nuclear morphology in H & E stained sections. The number of foci per field and the number of inflammatory cells per focus were counted in 10 fields at 200× magnification. Biliary duct size and the area of biliary epithelium were measured in the biliary ducts present in 10 random fields at 200× magnification using Image-J software. Hepatic glycogen deposition was measured using Periodic Acid-Schiff (PAS) staining on paraffin embedded liver tissue sections. Magenta color PAS positive hepatocytes were confirmed as glycogen deposited hepatocytes by comparing glycogen degradation in a serial section treated with PAS-diastase (PAS-digest). The average number of PAS-positive hepatocytes were counted in 10 fields of lobular regions (200×).
Rabbit polyclonal antibody to CD45 (ab10558 Abcam Inc, Cambridge, MA) and rabbit monoclonal antibody to Ki67 (ab16667, Abcam Inc.) were used to detect leukocytes and proliferation markers respectively. For IHC staining, deparaffinized and rehydrated liver sections were steamed in preheated sodium citrate antigen retrieval buffer (pH 6.0) for 20 minutes using a steamer (HS1000, Black and Decker, Miramar, Florida), slides were cooled, washed 3 times in TRIS-buffered saline, pH 7.6 (TBS) containing tween-20 (TBST), endogenous peroxidase blocked by hydrogen peroxide, washed in TBST and blocked with 5% goat serum in TBST (ab7481; Abcam Inc.) for 1 hour at room temperature (RT). Primary antibodies were diluted in antibody diluent (BD559148; BD), and incubated at 4°C overnight. For negative controls, serial sections were incubated in the diluent without the primary antibody and all other staining aspects undertaken. Sections were washed 3 times with TBST and incubated in Signal Stain Boost IHC detection reagent (HRP, Rabbit 8114S Cell Signaling Technology; Danvers, MA.) for 30 minutes at RT in a humid chamber. The washed sections were incubated with DAB chromogen (BD550880; Becton Dickinson, New Jersey) until a mild brown color was detected. Sections were briefly dipped in a hematoxylin solution (MHS32; Sigma-Aldrich, St. Louis, MO) followed by 0.1% sodium bicarbonate for counter staining. Images were captured on a Zeiss Axioplan-2 microscope as described above. The number of CD45+ cells in each cluster, and the number of clusters of CD45+ cells per 100× field were counted in 10 random fields per sample (n=5).
2.4. TUNEL Labeling
The terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay was performed on deparaffinized liver sections according to the manufacturer's instructions (Roche, Indianapolis, IN). The label solution without TdT was used as a negative control and liver sections with known tumor metastases were used as positive controls. Slides were cured overnight, in the dark, with ProLong® Diamond Antifade Mountant with DAPI (P36971; Thermo-Fisher, Grand Island, NY). Fluorescent images were captured on a Zeiss LSM-710 confocal microscope at 630× magnification, and the images were processed using the Zeiss Zen 2012 stitching software and merged into a single image. The number of TUNEL positive nuclei and DAPI positive hepatic nuclei per field at 630× magnification were counted in 10 random fields to calculate the number of apoptotic nuclei and relative percentage of apoptotic hepatocytes.
2.5. Protein extraction of hepatic lysate and western blotting
Freshly isolated liver sections were collected in ice-cold Tissue Protein Extraction Reagent (T-PER™; #77510; Thermo-Scientific, Grand Island, NY) containing complete™ ULTRA Tablets, EDTA-free, Protease Inhibitor Cocktail (#5892953001, Roche, Indianapolis, IN) and PhosSTOP™ phosphatase inhibitor cocktail (#04906837001, Roche, Indianapolis, IN), and then homogenized using tissue homogenizer with disposable tips (Omni TH; Omni International, Kennesaw, GA). The homogenized tissues were centrifuged at 10,000g for 10 minutes at 4°C. Protein concentrations of the whole tissue extracts were determined according to the manufactur's protocol (Pierce™ BCA Protein Assay Kit; #23227, Thermo-Fisher, Grand Island, NY). For Western blotting, whole cell protein samples were separated by SDS PAGE using a 4-15% Mini-PROTEAN TGX Precast Gel (456-1084, Bio-Rad), and blotted using the Trans-Blot® Turbo™ Mini PVDF Transfer Packs (1704156, Bio-Rad) Trans-Blot® Turbo™ Transfer System (Bio-Rad, Hercules, California). The blotted membranes were blocked using 5% BSA in TBST for 1 hour at RT, followed by incubation in diluted primary antibodies anti-NF-kB p65 [E379] (ab32536), anti-beta Actin (ab8227) overnight at 4°C. After washing 3 times, blots were incubated with the secondary antibody [goat anti-rabbit IgG H&L chain, HRP] (ab6721, Abcam Inc.) for 1 hr at RT. All the antibodies were purchased from Abcam Inc. The protein bands were examined by using SuperSignal™ West Pico Chemiluminescent Substrate (34078, Thermo-Fisher, Grand Island, NY). Blots were exposed and digital images were acquired using a myECL™ Imager, and relative protein quantities were determined by using myImageAnalysis™ Software (62237, Thermo-Fisher, Grand Island, NY).
2.6 Flow cytometry and colony forming units-granulocyte, macrophage (CFU-GM)
Freshly isolated livers were minced into small pieces, treated with collagenase-IV (17104-019, Gibco) and DNAse I (D5025, Sigma), and filtered through cell dissociation sieve to prepare a single cell suspension. Hepatic leukocytes (WBCs) were isolated using mouse cell separation media, Lympholyte M (CL5035, Cedarlane). Then the total hepatic WBC count was analyzed using vet ABC animal blood counter and data presented as WBC count/gram of the liver. For flow cytometry, isolated leukocytes were stained with the indicated antibodies for 30 minutes at 4°C. The antibodies used were anti-CD45-V450 (clone 30-F11, BD Pharmingen) anti-CD201-APC (clone ebio1560, ebioscience) and anti-CD27-BV650 (clone LG3A10, BD Pharmingen). Flow cytometry was performed on BD LSRFortessa ×50 platforms and results were analysed using FlowJo software version 9.9.5 (TreeStar). For CFU-GM assay, 105 cells were cultured in 1.1 ml of methylcellulose-based medium for myeloid progenitor cells (MethoCult™ GF M3534, STEM cell technologies) as per company protocol. Colonies of at least 50 cells were scored for analysis of CFU-GM.
2.7. Statistical Analysis
Results were expressed as mean +/- standard error of mean (SEM) for animal weights, liver weight, liver weight/body weight, abdominal fat weight, abdominal fat weight/body weight, fatty acid levels, histology and IHC quantification values. Data from the two groups was compared by Student's t-test for independent samples. Plots for change in body weights were compared by repeated measures test. All graphs were plotted using Sigma Plot software. All data was analyzed using SPSS. Data differing by p≤0.05 were considered to be significant.
3. Results
3.1. Pair-feeding, food consumption and body weight
During the initial 5-day ad libitum diet acclimation period (Fig. 1A), mice on the ω-6 diet consumed more feed than those in the ω-3-diet group (p<0.05). Therefore, when pair-feeding was initiated on day 6, the average amount of ω-3 diet consumed was used as the baseline for the ω-6 diet (Fig. 1B). Studies of changes in body weight over time with the isocaloric and isolipidic diets using a repeated measures test revealed significant increases in weight in the mice (n=20/group) given the ω-6 diets (Fig. 1C). In part due to the increased consumption of the ω-6 diet during a brief acclimation period a significant difference in body weight was observed between the groups on day 5 (Fig. 1D). Similarly, at the pair-feeding mid points (day 20, and day 40) and at autopsy on day 69 (Fig. 1D) significant differences in body weights were also observed. Consistent with the higher body weights at autopsy (20 weeks) mice fed the ω-6 diet had significantly more abdominal fat (Fig. 1E) and abdominal fat weight relative to the body weight (Fig. 1F) as compared to ω-3 diet fed mice. On gross examination of the livers from the ω-3 (Fig. 1G) and ω-6 diet fed mice (Fig. 1H), livers from ω-6 diet fed mice were lighter in color (pink) and significantly smaller than those from the ω-3 fed mice, which were red-brown in color (Fig. 1H). In both groups liver weights (Fig. 1I) and liver:body weight ratios (Fig. 1J) were significantly lower in mice fed the ω-6 diet.
3.2. Effect of PUFA diet composition on the lipid profile of plasma and liver
The lipid composition of the plasma and livers were analyzed following autopsy at 10 weeks post dietary initiation (Table-2). These studies revealed an 8-fold higher level of AA (0.64 μg/μl) in the plasma of ω-6 fed mice. However, as the ω-6 diet contained no AA (C20:4); the AA is of a metabolic origin from the ω-6 PUFA precursor, linoleic acid (C18:2), which is contained in both diets. Both of the diets and the plasma of mice on these diets had a comparable level of C18:2, but AA product:precursor ratio (C20:4/C18:2) was 5.8 fold higher in the plasma of ω-6 diet fed mice. Further, there was a 2.8-fold higher level of total ω-6 FAs in the plasma of ω-6 diet fed mice compared to ω-3 diet fed mice (p<0.05); even though both of the diets had comparable amounts of total ω-6 PUFAs. In contrast to ω-6 FA levels, the ω-3 PUFA, EPA was not detected in the plasma or livers of mice consuming the ω-6-diet. Similarly, levels of the ω-3 PUFA, DHA were 2.8-fold lower (0.05 vs. 0.14 μg/μl) in the plasma of mice fed the ω-6 diet (p<0.05). There were also 1.9-fold and 2.4-fold increase in the level of linoleic acid and AA respectively in the liver, resulting in a significantly higher level of total ω-6 PUFA in livers of ω-6 diet fed mice as compared to ω-3 diet fed mice (p<0.05). However, the C20:4:C18:2 FA ratio was not significantly different between the livers of ω-6 vs. ω-3 diet fed mice. Consistent with the plasma EPA levels, EPA was not detected in the livers of mice fed the ω-6 diet and the DHA content was significantly (11-fold) lower (1.5 vs. 16.5 μg/mg), compared to mice fed the ω-3 diet (p<0.05). We note that the mono unsaturated fatty acid (MUFA) content was significantly higher in the liver, but not in plasma of mice fed the ω-6 diet. Table 2 also has an analysis of the FA composition of the fish oil used in the diet, which contained 83.2% of total FAs in the form of ω-3 FA (from C20:5 and C22:6) resulting in a ω-6:ω-3 FA ratio of 0.7:1 in the ω-3 diet compared to 21:1 in the ω-6 diet. The ω-6: ω-3 FA ratios of the plasma was similar to the dietary ratio.
Table 2. Fatty acid profiles of diets, liver, and plasma measured by gas chromatographic mass spectrophotometry (GC-MS) analysis.
ω-6 vs. ω-3 diets were freshly prepared daily using ready to feed liquid diets. The fish oil used was from pharmaceutical grade ω-3 capsules. Liver and plasma were collected from the mice fed respective diets for 10 weeks. (n=5) The average liver weights for the two groups were significantly different (ω-3> ω-6) and are shown in figure 1G and 1H
| Carbon chain length and double bonds | Fatty Acid (FA) Name | Concentration | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Diet (μg/μl) | Plasma (μg/μl ± SEM) | Liver (μg/mg ± SEM) | Fish Oil (μg/μl) | |||||
|
| ||||||||
| ω3 | ω6 | ω3 | ω6 | ω3 | ω6 | |||
| 14:0 | Tetradecanoate | 0.00 | 0.20 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| 16:0 | Hexadecanoic | 1.53 | 3.02 | 0.79 ± 0.08 | 0.79 ± 0.10 | 12.32 ± 4.12 | 15.83 ± 1.30 | 9.65 |
| 16:1 | 9-Hexadecenoic | 0.00 | 0.20 | 0.00 ± 0.00 | 0.01 ± 0.01 | 1.01 ± 0.29 | 1.42 ± 0.18 | 0.00 |
| 18:0 | Octadecanoic | 0.28 | 0.76 | 0.57 ± 0.14 | 0.38 ± 0.01 | 5.18 ± 1.42 | 5.19 ± 0.21 | 0.00 |
| 18:1 | 9-Octadecenoic | 3.77 | 15.62 | 0.20 ± 0.01 | 0.35 ± 0.02 * | 8.35 ± 0.85 | 42.87 ± 3.11 * | 27.44 |
| 18:2 | cis-8,11-Octadecadienoic (Linoleic) (ω-6) | 6.67 | 5.03 | 0.31 ± 0.01 | 0.44 ± 0.03 | 5.78 ± 0.62 | 11.22 ± 0.83 * | 4.73 |
| 18:3 | cis-9,12,15-Octadecatrienoic acid (Linolenic) (ω-3) | 0.14 | 0.24 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 |
| 20:1 | cis-13-Eicosenoic | 0.70 | 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.10 ± 0.10 | 0.86 ± 0.05 * | 26.99 |
| 20:3 | cis-7,10,13-Eicosatrienoic | 0.00 | 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.69 ± 0.21 | 0.49 ± 0.04 | 0.00 |
| 20:4 | cis-5,8,11,14-Eicosatetraenoic (AA) (ω-6) | 0.51 | 0.00 | 0.08 ± 0.01 | 0.64 ± 0.03 * | 2.13 ± 0.38 | 5.14 ± 0.20 * | 37.60 |
| 20:5 | cis-5,8,11,14,17-Eicosapentaenoic (EPA) (ω-3) | 7.52 | 0.00 | 0.19 ± 0.01 | 0.00 ± 0.00 * | 5.58 ± 1.07 | 0.00 ± 0.00 * | 409.70 |
| 22:1 | 13-Docosenoic acid | 0.25 | 0.00 | 0.02 ± 0.02 | 0.00 ± 0.00 | 0.09 ± 0.09 | 0.00 ± 0.00 * | 12.54 |
| 22:5 | cis-4,7,10,13,16-Docosapentaenoic acid (ω-3) | 0.26 | 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.22 ± 0.33 | 0.68 ± 0.08 * | 17.97 |
| 22:6 | cis-4,7,10,13,16,19-Docosahexaenoic (DHA) (ω-3) | 2.64 | 0.00 | 0.14 ± 0.01 | 0.05 ± 0.01 * | 16.55 ± 2.92 | 1.50 ± 0.06 * | 159.78 |
|
| ||||||||
| Total omega 6 FA level | 7.18 | 5.03 | 0.39 ± 0.01 | 1.08 ± 0.04 * | 7.91 ± 0.73 | 16.36 ± 0.85 * | 42.33 | |
| Total omega 3 FA level | 10.56 | 0.24 | 0.33 ± 0.01 | 0.05 ± 0.01 * | 24.35 ± 3.13 | 2.18 ± 0.10 * | 587.45 | |
| Total MUFA level | 4.72 | 15.82 | 0.23 ± 0.02 | 0.36 ± 0.02 * | 9.55 ± 0.91 | 45.15 ± 3.12 * | 66.97 | |
| Total SA level | 1.81 | 3.98 | 1.37 ± 0.16 | 1.17 ± 0.10 | 17.50 ± 4.35 | 21.02 ± 1.32 | 9.65 | |
|
| ||||||||
| Total Fatty Acid Concentration (μg/mg or μg/uL) | 24.27 | 25.07 | 2.32 ± 0.16 | 2.66 ± 0.11 | 60.00 ± 5.49 | 85.20 ± 3.49 | 706.40 | |
|
| ||||||||
| Ratio of total ω-6 FA to ω-3 FA (ω-6:ω-3) | 0.7:1 | 21:01 | 1.2:1 | 21.6:1 * | 0.32:1 | 7.5:1 * | ||
=p<0.05.
3.3. Effect of dietary ω-3:ω-6 PUFA hepatic histopathology and steatosis
Histopathologic analysis of H & E stained liver sections from mice fed the different diets for 10 weeks (Fig. 2A) revealed significant effects on hepatocyte steatosis. The livers from mice fed the ω-3 diet had occasional hepatocyte lipid microvacuoles; whereas mice on the ω-6 diet had an increase in macro- and micro-vesicular steatosis in their hepatocytes (Fig. 2A). Steatosis was quantified by counting the ORO positive fat droplets that were about the size of a hepatic nucleus. There was a 7-fold increase in macrovesicular steatosis in mice fed the ω-6 diet, compared to the ω-3 diet fed mice (p<0.05) (Fig. 2B and 2C). Moreover, an increase in microvesicular steatosis was observed in the livers of ω-6 diet fed mice (Fig. 2C). This suggested that the increase in the dietary hepatic ω-6:ω-3 ratio in mice receiving the ω-6 diet (Table 2) enhanced hepatic macro-steatosis, independent of total caloric consumption.
Figure 2. Differential effects of dietary PUFA on hepatic steatosis.
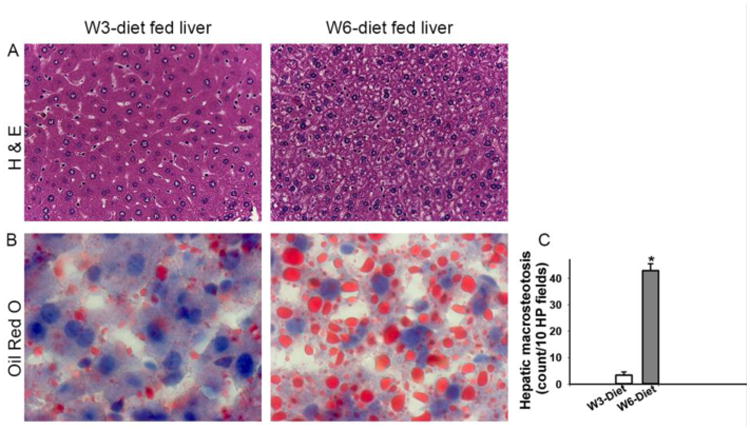
Hepatic steatosis analysis of livers by H & E staining (A). Oil Red O-stained liver sections analyzed for hepatic steatosis (B and C). Images were taken with magnification of 400× (A) and 1000× (B) (n=5) [*=p<0.05].
Hepatic glycogen storage was assessed by the histological analysis of PAS (+/- diastase digestion) stained liver sections of mice fed the diets for 20 weeks (Fig. 3). The result showed that livers from mice fed the ω-3 diet had significant glycogen storage in hepatocytes around portal triad and central vein of hepatic lobules, which was absent in mice fed the ω-6 diet (Fig. 3A-3C). The presence of glycogen deposits in PAS positive cells was confirmed by a comparison to a serial section that underwent glycogen digestion (Fig. 3C). Thus, the results indicate that the dietary ω-6:ω-3 FA ratio might modulate energy storage and metabolism pathways such that increasing consumption of a diet with a high ω-6:ω-3 FA ratio enhances hepatic fat storage whereas consumption of Lc ω-3 FA reduces fat storage but enhances energy storage in the form of glycogen. Collectively, the increase in the dietary ω-6:ω-3 FA ratio results in macrosteatosis, and decreased glycogen deposition in lobular hepatocytes. We also undertook a histological analysis of liver tissue from mice fed a chow control diet using H & E and ORO stained sections (Fig. S1 (A-C)). The histological features of livers from chow controls were distinct from the mice receiving liquid diets and had intermediate level of steatosis (more than the ω-3 group but less than the ω-6 group. Also, there were a few microgranulomas in the liver from chow fed mice but the size, number and features of those immune cells were distinct than what we reported as EMM in this study (Fig. S1B). Thus, mice in a chow control group were not used in further analysis as their dietary composition had multiple differences from our experimental liquid diets including lipid types, percentages, protein and carbohydrate levels and calories consumption.
Figure 3. Differential regulation of hepatic glycogen storage by dietary PUFA.
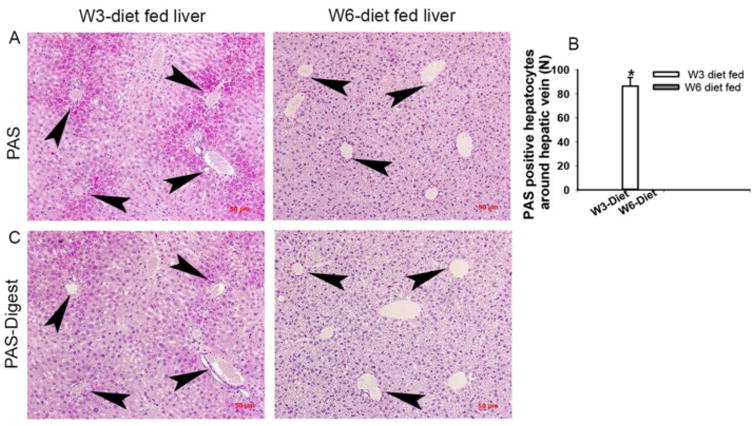
Liver sections stained with Periodic Acid-Schiff (PAS) showed glycogen-containing hepatocytes around the regions of central vein and portal area of hepatic lobules in mice fed ω-3 diet but absent in ω-6 diet group (A and B). The hepatic glycogen was digested using PAS-diastase staining (PAS-Digest) (C). Images were taken with magnification of 200× (A and C) and (n=5) [*=p<0.05].
The total WBC count of blood and liver was determined. There was no significant difference in the peripheral blood WBC count between the dietary groups, i.e.; 11.3 × 106 +/- 1.1 × 106 and 9.3 × 106 +/- 1.0 ×106 WBC cells per ml of blood in mice from ω-3 and ω-6 diet groups respectively (n=20). In contrast, mice fed ω-6 diet had a significant (10-fold) increase in the number of WBC cells per gram of liver (7.9 × 106 +/- 1.3 × 106) compared to ω-3 diet fed mice (0.8 × 106 +/- 0.5 × 106). We also evaluated hepatic inflammatory cell infiltration by H & E and anti-CD45 staining from mice receiving the diets for 10 and 20 weeks (Fig 4A-4G). The differentiated inflammatory cells were observed in cellular clusters composed of immature myeloid cells with variable size and nuclear morphology (Fig. 4A), supporting hepatic EMM. Mice given the ω-6 diet for 10 weeks had a significant (9-fold) increase in EMM foci (0.9+/-0.2 per 100× magnified field), compared to ω-3 diet fed mice (Fig. 4A and 4B). Similarly, mice given the ω-6 diet for 20 weeks had an inflammatory appearance, with significantly higher number of EMM foci (2-fold) compared to ω-3 diet fed mice (0.2+/-0.06 per 100× magnified field) (Fig. 4B). However, there was no significant difference in the number of EMM foci between the 10- or 20-week diet fed groups (Fig. 4B). Further, the EMM foci in ω-6 fed mice were larger and located adjacent to the central veins, whereas the EMM foci in ω-3 diet fed animals were smaller and located randomly in the hepatic sinusoids (Fig. 4A). The presence of inflammatory cells (individually distributed or in clusters) was confirmed by staining the liver sections with the anti-CD45 antibody (Fig. 4C - 4G). The ω-6 diet fed mice had significantly higher numbers of individual CD45+ cells compared to the ω-3 diet fed groups, in both the 10 and 20 weeks' diet fed studies (Fig. 4C & 4D). Morphologically, these CD45+ cells included both myeloid and lymphoid cells. In addition, ω-6 diet fed mice had a significantly higher number of CD45+ inflammatory cell foci (6.8+/0.4) compared to ω-3 diet fed mice (0.4+/-0.3 per 100× magnified field). The foci were approximately 3 times larger (23.2+/-2.4 cells/cluster) in the livers of ω-6 diet fed mice compared to the ω-3 diet fed mice (8+/-5.3 cells/cluster) (Fig. 4E-4G). Similar results were observed in the livers of mice maintained on the diets for 20 weeks. Additionally, the number of inflammatory cells per EMM foci were significantly increased in the 20-week diet fed mice compared to the 10-week mice when studied using mice from both dietary groups. (Fig. 4F and 4G). Confirmation of the increase in hepatic hematopoietic progenitor (CD201+CD27+) cells [34] was obtained by flow analysis of isolated hepatic non-parenchymal cells and found as a 24.4+/-4.9% of CD45+ cells in ω-6 diet fed mice versus 3.7+/-1 % of CD45+ cells in ω-3 diet fed mice were of progenitor phenotype respectively. The myeloid phenotypes of progenitor cells were further confirmed by CFU-GM analysis, which showed that ω-6 and ω-3 dietary groups had 14.9+/-1.1 and 9.4+/-2.1 CFU-GM colonies/100,000 cells plated respectively.
Figure 4. Differential regulation of hepatic extramedullary myleopoeisis (EMM) by PUFA.
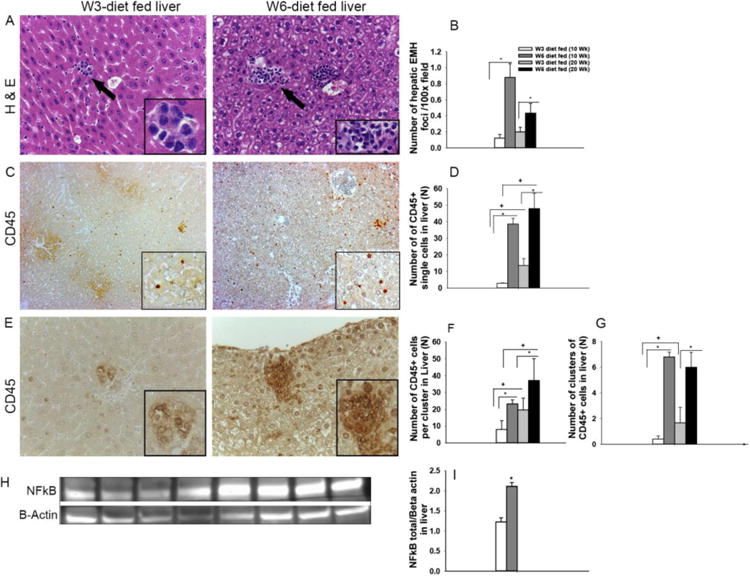
Livers of mice were compared for hepatic EMM by counting the number and size of EMM foci in 10 microscopic fields/sample by H & E staining (A) (n= 3 to10). Arrows indicate a focus of EMM. Comparison of EMM foci between 10 weeks and 20-week diet fed mice of the dietary groups (B). Analysis of 100× magnified fields of CD45 stained liver sections for CD45+ single cells (C and D). Analysis of number and size of CD45+ cell clusters in dietary groups (E, F, and G). Images were taken at a magnification of 400× (A) and 100× (C and E). Hepatic NF kB protein expression was significantly higher in the ω-6 as assessed based on Western blots (H and I) [*=p<0.05].
Since NFκB expression is associated with myelopoiesis, we examined the expression of NFκB protein in the livers of mice fed the diets for 10 weeks and found that livers from ω-6 diet fed mice had a significantly higher level of NFκB expression (Fig. 4H and Fig. 4I) (p<0.05, n=5), indicating inflammatory cell activation. Collectively, the higher ω6:ω3 FA ratio in the diets appeared to be associated with inflammation and EMM in the hepatic microenvironment.
3.4. Dietary ω3:ω6 PUFA regulation of hepatocyte proliferation, degeneration, and apoptosis
We examined hepatocyte proliferation (Ki67) and apoptosis (TUNEL) by IHC using mice fed the experimental diets for 10 or 20 weeks. Mice fed the diet for ten weeks did not have a difference in hepatocyte proliferation (data not shown). However, mice fed the ω-6 diet for 20-weeks had a significantly lower number of proliferating hepatic nuclei (1.5-fold) as compared to ω-3 diet fed animals (Fig. 5A and 5B). In contrast to hepatocyte proliferation, a 4-fold increase in the number of TUNEL positive hepatocyte nuclei was observed in the livers of mice fed the ω-6 diet for 20 weeks (Fig. 5C and 5D). When the number of TUNEL positive nuclei relative to total hepatocyte nuclei were compared, a significantly higher frequency of hepatocytes in the livers of ω-6 diet fed mice were TUNEL positive (41%) as compared to 15% of the hepatocytes in the livers of ω-3 diet fed mice (Fig. 5E). Collectively, these results suggest a potential mechanism for the decrease in liver weight in the ω-6 diet fed animals.
Figure 5. Dietary PUFA regulation of hepatocyte proliferation, and apoptosis.
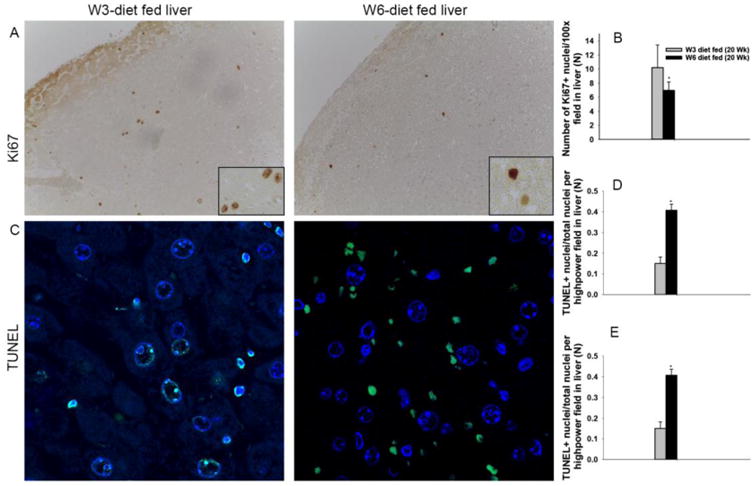
The livers of mice fed ω-3 and ω-6 diets for 20 weeks were compared based on proliferation (Ki67) and apoptosis (TUNEL) by counting the number of positive cells per 10 microscopic fields per sample (n=3). The number of proliferating hepatocytes observed are shown in A and B. The number of TUNEL+ (apoptotic) nuclei (C and D) and apoptotic nuclei relative to total nuclei (C and E) observed in are shown. Images were taken at a magnification of 100× (A) and 630× (C) respectively [*=p<0.05].
Additionally, morphological differences were observed in the bile ducts of ω-3 versus ω-6 diet fed mice (Fig. S2).
4. Discussion
In the present study, we compared the biological effects of feeding ω-3 versus ω-6 PUFA using Lieber-DeCarli, isocaloric liquid diets and a pair-fed model. During acclimatization, the dietary preference was for the ω-6 diet, which was consistent with our earlier observations. Thus, the ω-3 diet consumption was used as the base-line for pair feeding. In contrast to prior reports of pair-fed mice given isocaloric diets,[35, 36] in our studies significant differences in body weight were observed between mice fed an isolipidic ω-3 versus ω-6 diet. It is noted that in the referenced prior studies, diets differed in components other than PUFA and so are not directly comparable. This is relevant as in the one published study, in which mice were pair fed, isocaloric and isolipidic diets differing in PUFA composition (safflower oil (ω-6) versus fish oil (ω-3)) mice on the safflower oil diet for nine months had a 31% increase in weight versus mice on the fish oil diet.[37] These observations support a need for pair-feeding to achieve an isocaloric diet due to dietary preferences by laboratory rodents, and a need for isolipidic diets to study PUFA regulation of body weight. Further, despite providing isocaloric and isolipidic diets, we observed a significant difference in fat mass measured as abdominal fat in ω-6 diet fed mice, suggesting differential metabolism between the two groups. This is consistent with prior reports of PUFA regulation of dietary fat content and body fat deposition and distribution.[38, 39] In one clinical study, consumption of PUFA diets was reported to decrease visceral fat and increase lean muscle mass as compared to individuals receiving high saturated fat diets.[38] Among the subtypes of PUFA, ω-3 can limit hypertrophy of abdominal fat deposits[40] and reduce weight gain in pre-obese animals and humans by a reduction in visceral fat[41-44]. Potential mechanisms for the divergent effects of ω-3 and ω-6 FAs in adipose tissue biology include the regulation of adipogenesis,[45] lipid homeostasis,[46] brain-gut-adipose tissue axis,[47] and systemic inflammation,[48] suggesting that lowering the dietary ω-6:ω-3 ratio might help control obesity.[49] The level of Lc ω-3 PUFA used in this study was higher than the dose used in clinical study [50], because the current study was primarily designed to analyze the effects of a ω-6: ω-3 ratio of 1:1, which has been reviewed in literatures as the ω-6: ω-3 ratio upon which humans evolved. [7]
The FA composition of diets can modulate the composition of stored and structural lipids;[51] including the FA profile of plasma and tissues.[52] [53] In the present study, we observed a significant increase in total ω-6 PUFA, AA, and a decrease in DHA, and an absence of EPA in the plasma of mice fed a ω-6-diet. These observations are in agreement with a previous report, in which rats were fed a diet with a 1:1 ratio of ω-6:ω-3 FA resulting in a significantly higher levels of plasma EPA, DHA, and a lower level of AA compared to the rats fed a diet with a 30:1 ratio of ω-6:ω-3 PUFA.[52] The results are also consistent with a clinical study in which the plasma FAs in humans were associated with their dietary FA composition.[54]
Our results suggest that the plasma level of Lc ω-3 PUFA, such as EPA and DHA, reflect dietary intake. In contrast, plasma AA (C20:4) levels could only be explained by the elongation and desaturation of the ω-6 linoleic acid (C18:2) in mice given the ω-6 diet. Further, the ω-6 and ω-3 PUFAs compete for incorporation into phospholipids and as substrates contributing to these differences.[55] In this study, the concentration of total ω-6 PUFAs and linoleic acid (precursor to AA) did not differ between the experimental diets, although the AA: precursor linoleic acid was 5.8-fold higher in the plasma of mice fed ω-6 diets. Thus, the decreased level of plasma AA in the ω-3 diet fed mice is apparently regulated by dietary ω-3 Lc PUFA, as a modulator of AA biosynthesis. This observation is supported by reports that both EPA and DHA can reduce proinflammatory cytokines[56] and hepatic steatosis.[57] The observation of significantly higher MUFA level in mice fed ω-6 reflected the dietary composition of the ω-6 diet. Previous studies into the bioactivity of olive oil showed that an iso-energetic MUFA diet can reduce liver fat in diabetic patients [58], higher oxidation of MUFA in the liver of rats [59] and protects against experimental inflammation [60]. Thus, we posit that our observations of inflammatory signals in ω-6 diet fed mice is primarily due to changes in dietary ω-6: ω-3 or the absence of Lc- ω-3-PUFA, rather than an increase in MUFA with the ω-6 diet. In our studies we measured the ω-3 PUFAs DHA and EPA in the livers of mice fed ω-3 and ω-6 diets. In a previous report, a decrease in total hepatic ω-6 PUFA and AA levels were observed in a mouse model of NAFLD, which was associated with the metabolic utilization of AA during chronic inflammation.[61] The majority of dietary NAFLD studies have been based on obesity such as the use of a model of over-consumption versus a control diet;[62] ad-libitum feeding of a high saturated fat diet (>60% calories from fat)[26, 63] or a high fat Lieber-DeCarli diet (71% of energy from fat) compared to the original Lieber-DeCarli control diet (35% fat, that we used herein).[64] Thus, the role of PUFA composition, as opposed to obesity, in the pathogenesis of “fatty liver” has to date been poorly evaluated. In these studies, we report an increase in macro-vesicular steatosis in ω-6 diet fed mice, an observation consistent with previous clinical and animal model studies, which emphasized that a western diet-induced hepatic steatosis.[63, 65] However, unlike previous animal studies, we observed lower relative liver weights and higher steatosis in the ω-6 diet fed mice,[63] supporting a role for dietary PUFA composition on the hepatic microenvironment, independent of total caloric intake.[66] Most studies used high fat/high caloric /high saturated fat diets to induce hepatic steatosis and compared the results with liver from control mice fed a standard laboratory chow diet, which has ω-6:ω-3 ratio of around 10:1 with variable dietary composition.[67, 68] The present study compared the results between two liquid diets differed in FA composition only. Further, an increase in liver weights in diet induced NAFLD/NASH studies might be a consequences of severe steatosis along with induction of fibrosis in liver. Thus, it is possible that feeding the moderately fat ω-6 diet for 20 weeks may be insufficient for the development of other NASH symptoms such as fibrosis, which might be responsible for increasing liver weight in steatosis studies using NAFLD/NASH model. In this study, the lobular hepatocytes from ω-6 diet mice lacked glycogen storage (Fig.3) which is supported by the significant increase in macrosteatosis in lobular and portal regions of the ω-6 mice (Fig. 2I). Hepatic steatosis and glycogen storage levels vary depending on the strain of mice and the percent of glycogen positive cells decrease with an increase in steatosis in high fat diet fed BALB/c mice.[69] Similarly, increased macrosteatosis in the livers of mice fed a high carbohydrate diet was associated with a decrease in glycogen storage, which recoverd by inclusion of ω-3 FA in the diet. [70] The difference in hepatic glycogen storage might be due to specific regulatory roles of ω-6 and ω-3 FA in metabolic pathways. [71] However, a comparison of hepatic glycogen content between moderately fat, isocaloric, isolipidic and pair-fed model has not been reported previously, suggesting a need for further studies to understand the metabolic regulation of the diets which might have modulated mechanisms of energy storage in the form of fat or glycogen. In summary, the decreased glycogen content (Fig. 3), together with increased steatosis (Fig. 2), decreased hepatocyte proliferation and increased hepatocyte apoptosis (Fig. 5) might contribute to the lower hepatic weight of the ω-6 diet fed mice observed in the current study.
In these studies, we observed a significant increase in hepatic inflammatory cells in an absence of leukophilia in the ω-6 diet group suggesting that the outcome of higher number of inflammatory cells in the liver was independent of a systemic inflammatory response. Further, histological analysis documented a significant increase in the number and size of inflammatory cell foci, containing immature myeloid cells in the livers of mice fed a high ω-6:ω-3 diet. The foci closely resembles hepatic EMH as defined by National Toxicological program [72] however, as majority of cells in the foci were early myeloid cells, likely myeloid progenitor cells with a lack of erythroid progenitor cells [as defined in EMH], we addressed the foci as EMM. We further confirmed the increase in hematopoietic progenitors in livers of the ω-6 diet fed mice by flow analysis and CFU-GM counts. Unlike humans, mice have a smaller medullary space resulting in EMH during early development; however, our observation of a significant increase in number and size foci of EMM in 30 weeks old mice fed an ω-6 diet compared to age matched ω-3 diet fed mice cannot be considered as a normal developmental phenomenon and needs further evaluation. An increase in EMM foci in association with hepatic steatosis has not been reported previously; however, prior hepatic steatosis studies were based on a NASH model, which might have an infiltration of mature inflammatory cells, such that enhanced EMM was obscured by regional inflammation. Modulation of the hepatic microenvironment and the production of growth factors such as granulocyte-monocyte colony stimulating factor (GM-CSF) by inflammatory cells may result in EMM.[72] Further, our observation of degenerating hepatocyte morphology with large lipid inclusions, higher numbers of apoptotic hepatocytes, and an increase in NFκB levels support an inflammatory cell role in the modulation of the hepatic microenvironment resulting in the mobilization of hematopoietic precursors leading to hepatic EMM in mice fed a high ω-6:ω-3 PUFA diet.
Hepatocyte proliferation [73] maintains hepatic mass and in our studies we observed decreased hepatocyte proliferation in the ω-6 diet fed mice. However, this differs from a previous report of an increased hepatocyte proliferation in NAFLD hepatic steatosis.[74] The basis of this difference needs further evaluation, but likely contributes to the decreased liver size in the ω-6 diet-consuming mice. Further, hepatocyte apoptosis is a prominent clinical feature of NASH and positively correlates with hepatic inflammation.[30] Thus, the observation of increased apoptotic hepatocytes in livers of mice fed ω-6 diet, steatosis and EMM supports a role of for dietary PUFA in the initiation of liver inflammation.
The significant increase in bile duct diameters at comparable levels of the biliary tree and morphological alterations on the biliary epithelium in mice fed ω-3 versus ω-6 diet may be secondary to the changes in liver mass between the dietary groups (Fig. S2). However, additional mechanistic studies are needed to analyze a potential role for dietary PUFA on the biliary system.
In summary, employing a pair fed, liquid diet with a lipid composition of 35% of dietary calories, the present study demonstrates that consuming a high ω-6:ω-3 PUFA diet regulates hepatic steatosis, EMM, glycogen deposition, and hepatocyte apoptosis. These results indicate that dietary Lc PUFA ω-3 suppresses steatosis and prevents the “first hit” mechanism of fatty liver development, and potentially lowers the risk of NAFLD and liver injury associated hepatic disorders. This may support a role for a ω-6:ω-3 FA ratio, but not the average calories consumed from fat, (∼35%) in the initiation of the formation of a “fatty liver”. However, other attributes of metabolic changes due to hypercaloric consumption and obesity might promote progression to NAFLD and NASH.
Supplementary Material
Acknowledgments
Support: We gratefully acknowledge funding from the Fred & Pamela Buffett Cancer Center's NIH Cancer Center Support Grant (P30CA036727) for this project.
Abbreviations
- AA
Arachidonic acid
- CFU-GM
Colony forming unit-granulocyte-macrophage
- DHA
Docosahexaenoic acid
- EMH
Extramedullary hematopoiesis
- EMM
Extramedullary myelopoiesis
- EPA
Eicosapentaenoic acid
- FA
Fatty acid
- Lc
Long chain
- MCV
Mean corpuscular volume
- MUFA
Monounsaturated fatty acid
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- ORO
Oil Red O
- PAS
Periodic Acid-Schiff
- PAS-Digest
Periodic Acid-Schiff-Diastase
- PUFA
Polyunsaturated fatty acid
- RBC
Red blood cell
- SFA
Saturated Fatty Acid
Footnotes
Conflict of Interest: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spychalla JP, Kinney AJ, Browse J. Identification of an animal omega-3 fatty acid desaturase by heterologous expression in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1142–7. doi: 10.1073/pnas.94.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. European journal of pharmacology. 2011;668(Suppl 1):S50–8. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 4.Kuehl F, Egan R. Prostaglandins, arachidonic acid, and inflammation. Science. 1980;210:978–84. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment that Counter Proinflammation Signals. Resolvins. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulsen RC, Gotlinger KH, Serhan CN, Kruger MC. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and omega-3 intake. Am J Hematol. 2008;83:437–45. doi: 10.1002/ajh.21170. [DOI] [PubMed] [Google Scholar]
- 7.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–7. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 8.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American journal of clinical nutrition. 2011;93:950–62. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz AA, Cruz-Hernandez C, Plouffe LJ, Casey V, Xiao C, Nimal Ratnayake WM. Increasing Dietary alpha-linolenic acid enhances tissue levels of long-chain n-3 PUFA when linoleic acid intake is low in hamsters. Annals of nutrition & metabolism. 2010;57:50–8. doi: 10.1159/000317345. [DOI] [PubMed] [Google Scholar]
- 10.Harris WS, Poston WC, Haddock CK. Tissue n – 3 and n – 6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Song J, Kwon N, Lee MH, Ko YG, Lee JH, Kim OY. Association of serum phospholipid PUFAs with cardiometabolic risk: beneficial effect of DHA on the suppression of vascular proliferation/inflammation. Clinical biochemistry. 2014;47:361–8. doi: 10.1016/j.clinbiochem.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Abeywardena MY, Patten GS. Role of ω3 long-chain polyunsaturated fatty acids in reducing cardio-metabolic risk factors. Endocr Metab Immune Disord Drug Targets. 2011;11:232–46. doi: 10.2174/187153011796429817. [DOI] [PubMed] [Google Scholar]
- 13.Kimura Y, Kono S, Toyomura K, Nagano J, Mizoue T, Moore MA, et al. Meat, fish and fat intake in relation to subsite-specific risk of colorectal cancer: The Fukuoka Colorectal Cancer Study. Cancer Sci. 2007;98:590–7. doi: 10.1111/j.1349-7006.2007.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–77. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luzio GA, Parnaik VK, Mayer RM. A D-glucosylated form of dextransucrase: demonstration of partial reactions. Carbohydr Res. 1983;121:269–78. doi: 10.1016/0008-6215(83)84023-3. [DOI] [PubMed] [Google Scholar]
- 16.Mejia-Barradas CM, Del-Rio-Navarro BE, Dominguez-Lopez A, Campos-Rodriguez R, Martinez-Godinez M, Rojas-Hernandez S, et al. The consumption of n-3 polyunsaturated fatty acids differentially modulates gene expression of peroxisome proliferator-activated receptor alpha and gamma and hypoxia-inducible factor 1 alpha in subcutaneous adipose tissue of obese adolescents. Endocrine. 2014;45:98–105. doi: 10.1007/s12020-013-9941-y. [DOI] [PubMed] [Google Scholar]
- 17.Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Alimentary pharmacology & therapeutics. 2006;23:1143–51. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka N, Sano K, Horiuchi A, Tanaka E, Kiyosawa K, Aoyama T. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. Journal of clinical gastroenterology. 2008;42:413–8. doi: 10.1097/MCG.0b013e31815591aa. [DOI] [PubMed] [Google Scholar]
- 19.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. Journal of Gastroenterology. 2013;48:434–41. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Annals of internal medicine. 2000;132:112–7. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer RW, Hammer S, Lamb HJ, Frolich M, Diamant M, Rijzewijk LJ, et al. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab. 2008;93:2702–8. doi: 10.1210/jc.2007-2524. [DOI] [PubMed] [Google Scholar]
- 23.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–8. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitola BE, Deivanayagam S, Stein RI, Mohammed BS, Magkos F, Kirk EP, et al. Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity (Silver Spring) 2009;17:1744–8. doi: 10.1038/oby.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825–34. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Depner CM, Philbrick KA, Jump DB. Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr(-/-) mouse model of western diet-induced nonalcoholic steatohepatitis. J Nutr. 2013;143:315–23. doi: 10.3945/jn.112.171322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Malley DP. Benign extramedullary myeloid proliferations. Mod Pathol. 2007;20:405–15. doi: 10.1038/modpathol.3800768. [DOI] [PubMed] [Google Scholar]
- 29.Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. Journal of blood medicine. 2010;1:13–9. doi: 10.2147/JBM.S7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–43. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 31.Abdelhalim MA, Moussa SA. Biochemical changes of hemoglobin and osmotic fragility of red blood cells in high fat diet rabbits. Pak J Biol Sci. 2010;13:73–7. [PubMed] [Google Scholar]
- 32.Sealls W, Gonzalez M, Brosnan MJ, Black PN, DiRusso CC. Dietary polyunsaturated fatty acids (C18:2 omega6 and C18:3 omega3) do not suppress hepatic lipogenesis. Biochimica et biophysica acta. 2008;1781:406–14. doi: 10.1016/j.bbalip.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez M, Sealls W, Jesch ED, Brosnan MJ, Ladunga I, Ding X, et al. Defining a relationship between dietary fatty acids and the cytochrome P450 system in a mouse model of fatty liver disease. Physiol Genomics. 2011;43:121–35. doi: 10.1152/physiolgenomics.00209.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez SE, Inlay MA, Serwold T. CD201 and CD27 identify hematopoietic stem and progenitor cells across multiple murine strains independently of Kit and Sca-1. Exp Hematol. 2015;43:578–85. doi: 10.1016/j.exphem.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Lian F, Liu C, Hu KQ, Wang XD. Isocaloric Pair-Fed High-Carbohydrate Diet Induced More Hepatic Steatosis and Inflammation than High-Fat Diet Mediated by miR-34a/SIRT1 Axis in Mice. Sci Rep. 2015;5:16774. doi: 10.1038/srep16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnet N, Somm E, Rosen CJ. Diet and gene interactions influence the skeletal response to polyunsaturated fatty acids. Bone. 2014;68:100–7. doi: 10.1016/j.bone.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–68. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 39.Boozer CN, Schoenbach G, Atkinson RL. Dietary fat and adiposity: a dose-response relationship in adult male rats fed isocalorically. Am J Physiol. 1995;268:E546–50. doi: 10.1152/ajpendo.1995.268.4.E546. [DOI] [PubMed] [Google Scholar]
- 40.Belzung F, Raclot T, Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am J Physiol. 1993;264:R1111–8. doi: 10.1152/ajpregu.1993.264.6.R1111. [DOI] [PubMed] [Google Scholar]
- 41.Buckley JD, Howe PR. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes Rev. 2009;10:648–59. doi: 10.1111/j.1467-789X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 42.Baillie RA, Takada R, Nakamura M, Clarke SD. Coordinate induction of peroxisomal acyl-CoA oxidase and UCP-3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot Essent Fatty Acids. 1999;60:351–6. doi: 10.1016/s0952-3278(99)80011-8. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Matute P, Perez-Echarri N, Martinez JA, Marti A, Moreno-Aliaga MJ. Eicosapentaenoic acid actions on adiposity and insulin resistance in control and high-fat-fed rats: role of apoptosis, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2007;97:389–98. doi: 10.1017/S0007114507207627. [DOI] [PubMed] [Google Scholar]
- 44.Hassanali Z, Ametaj BN, Field CJ, Proctor SD, Vine DF. Dietary supplementation of n-3 PUFA reduces weight gain and improves postprandial lipaemia and the associated inflammatory response in the obese JCR:LA-cp rat. Diabetes Obes Metab. 2010;12:139–47. doi: 10.1111/j.1463-1326.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- 45.Amri EZ, Ailhaud G, Grimaldi PA. Fatty acids as signal transducing molecules: involvement in the differentiation of preadipose to adipose cells. J Lipid Res. 1994;35:930–7. [PubMed] [Google Scholar]
- 46.Clarke SD, Jump D. Polyunsaturated fatty acids regulate lipogenic and peroxisomal gene expression by independent mechanisms. Prostaglandins Leukot Essent Fatty Acids. 1997;57:65–9. doi: 10.1016/s0952-3278(97)90494-4. [DOI] [PubMed] [Google Scholar]
- 47.Schwinkendorf DR, Tsatsos NG, Gosnell BA, Mashek DG. Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int J Obes (Lond) 2011;35:336–44. doi: 10.1038/ijo.2010.159. [DOI] [PubMed] [Google Scholar]
- 48.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–8S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 49.Simopoulos A. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodson L, Bhatia L, Scorletti E, Smith DE, Jackson NC, Shojaee-Moradie F, et al. Docosahexaenoic acid enrichment in NAFLD is associated with improvements in hepatic metabolism and hepatic insulin sensitivity: a pilot study. Eur J Clin Nutr. 2017 doi: 10.1038/ejcn.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohamed AI, Hussein AS, Bhathena SJ, Hafez YS. The effect of dietary menhaden, olive, and coconut oil fed with three levels of vitamin E on plasma and liver lipids and plasma fatty acid composition in rats. J Nutr Biochem. 2002;13:435–41. doi: 10.1016/s0955-2863(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 52.Kassem AA, Abu Bakar MZ, Yong Meng G, Mustapha NM. Dietary (n-6 : n-3) fatty acids alter plasma and tissue fatty acid composition in pregnant Sprague Dawley rats. ScientificWorldJournal. 2012;2012:851437. doi: 10.1100/2012/851437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruxton CH, Calder PC, Reed SC, Simpson MJ. The impact of long-chain n-3 polyunsaturated fatty acids on human health. Nutr Res Rev. 2005;18:113–29. doi: 10.1079/NRR200497. [DOI] [PubMed] [Google Scholar]
- 54.Astorg P, Bertrais S, Laporte F, Arnault N, Estaquio C, Galan P, et al. Plasma n-6 and n-3 polyunsaturated fatty acids as biomarkers of their dietary intakes: a cross-sectional study within a cohort of middle-aged French men and women. Eur J Clin Nutr. 2008;62:1155–61. doi: 10.1038/sj.ejcn.1602836. [DOI] [PubMed] [Google Scholar]
- 55.Abayasekara DR, Wathes DC. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukot Essent Fatty Acids. 1999;61:275–87. doi: 10.1054/plef.1999.0101. [DOI] [PubMed] [Google Scholar]
- 56.Zivkovic AM, Telis N, German JB, Hammock BD. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif Agric (Berkeley) 2011;65:106–11. doi: 10.3733/ca.v065n03p106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–85. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 58.Bozzetto L, Prinster A, Annuzzi G, Costagliola L, Mangione A, Vitelli A, et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. 2012;35:1429–35. doi: 10.2337/dc12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bessesen DH, Vensor SH, Jackman MR. Trafficking of dietary oleic, linolenic, and stearic acids in fasted or fed lean rats. Am J Physiol Endocrinol Metab. 2000;278:E1124–32. doi: 10.1152/ajpendo.2000.278.6.E1124. [DOI] [PubMed] [Google Scholar]
- 60.Liehr M, Mereu A, Pastor JJ, Quintela JC, Staats S, Rimbach G, et al. Olive oil bioactives protect pigs against experimentally-induced chronic inflammation independently of alterations in gut microbiota. PLoS One. 2017;12:e0174239. doi: 10.1371/journal.pone.0174239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Cao Y, Fu Y, Guo G, Zhang X. Liver fatty acid composition in mice with or without nonalcoholic fatty liver disease. Lipids Health Dis. 2011;10:234. doi: 10.1186/1476-511X-10-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, et al. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–14. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 63.Jump DB, Depner CM, Tripathy S, Lytle KA. Impact of dietary fat on the development of nonalcoholic fatty liver disease in Ldlr(–/–) mice. The Proceedings of the Nutrition Society. 2016;75:1–9. doi: 10.1017/S002966511500244X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, et al. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–9. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 65.Nobili V, Bedogni G, Alisi A, Pietrobattista A, Rise P, Galli C, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–3. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 66.Bialostosky K, Wright JD, Kennedy-Stephenson J, McDowell M, Johnson CL. Dietary intake of macronutrients, micronutrients, and other dietary constituents: United States 1988-94. Vital Health Stat. 2002;11:1–158. [PubMed] [Google Scholar]
- 67.Porras D, Nistal E, Martinez-Florez S, Pisonero-Vaquero S, Olcoz JL, Jover R, et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 68.Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295:G987–95. doi: 10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jovicic N, Jeftic I, Jovanovic I, Radosavljevic G, Arsenijevic N, Lukic ML, et al. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS One. 2015;10:e0134089. doi: 10.1371/journal.pone.0134089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alwayn IP, Gura K, Nose V, Zausche B, Javid P, Garza J, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res. 2005;57:445–52. doi: 10.1203/01.PDR.0000153672.43030.75. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed AA, Balogun KA, Bykova NV, Cheema SK. Novel regulatory roles of omega-3 fatty acids in metabolic pathways: a proteomics approach. Nutr Metab (Lond) 2014;11:6. doi: 10.1186/1743-7075-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Craig CE, Quaglia A, Dhillon AP. Extramedullary haematopoiesis in massive hepatic necrosis. Histopathology. 2004;45:518–25. doi: 10.1111/j.1365-2559.2004.01970.x. [DOI] [PubMed] [Google Scholar]
- 73.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–94. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 74.Vansaun MN, Mendonsa AM, Lee Gorden D. Hepatocellular proliferation correlates with inflammatory cell and cytokine changes in a murine model of nonalchoholic fatty liver disease. PLoS One. 2013;8:e73054. doi: 10.1371/journal.pone.0073054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


