Abstract
We used a biobehavioral treatment consisting of melatonin and a standardized bed and wake time to decrease one girl’s head and mouth touches associated with sleep-related trichotillomania and trichophagia. We remotely coached the girl’s caregiver to implement all procedures and monitored response to treatment using a DropCam Pro video camera equipped with night-vision capabilities. Head and mouth touches decreased, and her sleep pattern improved with the combination of treatment strategies. We discuss our use of a novel mode of service delivery to treat sleep-related problem behavior.
Keywords: melatonin, remote treatment, sleep hygiene, trichophagia, trichotillomania
Trichotillomania is the recurrent pulling of one’s hair that results in hair loss and clinically significant distress (Criteria A and C of the Diagnostic and Statistical Manual of Mental Disorders, fifth edition [DSM-5], 2013) and may result in follicle damage, changes in appearance and regrowth of hair, and scalp irritation (Christenson & Mansueto, 1999). Individuals who engage in trichotillomania may later ingest the dislodged strands of hair (i.e., trichophagia), which can produce balls of hair in the gastrointestinal system and may cause anemia, abdominal pain, hematemesis, nausea, vomiting, bowel obstruction, and perforation in severe cases (American Psychiatric Association, 2013).
Treatment of trichotillomania in children typically involves a combination of behavioral (e.g., Azrin, Nunn, & Frantz, 1980) and pharmacological interventions (Franklin, Edson, & Freeman, 2010). However, trichotillomania that occurs prior to or during sleep (i.e., sleep-related trichotillomania; Murphy, Valerio, & Nath Zallek, 2006) may prove difficult to treat due to the inability to contrive the relevant stimulus conditions (e.g., alone in bed) in a clinical environment, thus requiring discrete monitoring of the child throughout the night.
An alternative to traditional in-situ monitoring is remote monitoring and telehealth (Lindgren et al., 2016), which involves delivering healthcare services via electronic modalities (Lustig, 2012). These modalities of service delivery allow for remote data collection (e.g., from a clinician’s office) at more practical times (e.g., during office hours), while ensuring the relevant stimulus conditions are present. Because such modalities of service delivery do not act directly upon problem behavior (as do more traditional approaches to treatment), it is critical to recruit and maintain the close participation of caregivers who will carry out the recommended treatment strategies.
The purpose of this study was to use a remote system of service delivery to coach a patient’s caregiver to implement a biobehavioral treatment consisting of melatonin (Ferracioli-Oda, Qawasmi, & Bloch, 2013) and sleep-hygiene recommendations under the relevant stimulus conditions in which sleep-related trichotillomania and trichophagia occurred.
Method
Subject and Setting
Kari was a 3-year-old girl diagnosed with autism spectrum disorder who was referred for the outpatient assessment and treatment of aggression, self-injury, and disruption. During her admission, Kari’s caregiver reported concerns of hair pulling and hair ingestion that occurred at night, which had begun to produce balding patches and strands of hair in Kari’s stool. We then began targeting these behaviors in addition to her primary referral concerns. The first author contacted Kari’s caregiver at least weekly via phone to obtain any important information not captured in the nightly videos (e.g., Kari becoming ill), provide relevant treatment information (e.g., changes to melatonin dosage), offer suggestions for improving compliance with those treatment procedures (e.g., ways to get Kari to bed on time), and provide feedback if needed.
We collected all data remotely via a networked DropCam Pro video camera equipped with night-vision and video-saving capabilities. The camera was mounted on a wall in Kari’s bedroom, permitting data collectors to see Kari’s entire bedroom, which measured 1.2 m by 2.1 m and contained a bed, pillows, blankets, and stuffed animals. Trained observers at a university-based program used encrypted computers to log onto the DropCam website to download and code videos of Kari from preceding nights. We screened all videos from each night of the previous week to evaluate their appropriateness. To score a video, we required (a) Kari to be in her own bedroom (e.g., not sleeping at grandparents) and remain there throughout the night (Kari was removed once because she became ill) and (b) the caregiver to follow the prescribed bed and wake times within 10 min. These videos were then randomized using the lists feature on www.random.org and distributed to available data collectors. Based on data-collector availability, we attempted to score three videos per week (range, 1-4).
Response Measurement and Interobserver Agreement
Data collection began when the caregiver shut off the light and left the room and ended when Kari was retrieved from her bed. Data collection paused if Kari’s head became invisible (e.g., under blankets) or if Kari’s caregiver re-entered her bedroom (this occurred once, and problem behavior ceased once the caregiver re-entered). We collected data on whether Kari’s head was visible using a 1-min whole-interval recording system. We excluded the entire video if the view of Kari’s head was obstructed for 50% or more of intervals that she was awake. This occurred only twice, once in baseline and once during treatment. For all other videos, we removed any intervals from data analysis in which Kari’s head was not visible. An average of 4.1% (range, 0%-39%) of intervals in which Kari was awake were removed from each video because Kari’s head was not visible.
The resolution of the night-vision camera enabled data collectors to detect discrete instances of Kari touching her head or mouth but prevented detection of whether Kari pulled out individual strands of her hair or ingested those strands. Therefore, the primary dependent variables were head and mouth touches. Head touches consisted of Kari touching any part of her head within her hairline with her hand. We did not score head touches if Kari rested her head on her hand or placed items (e.g., blankets) over her head. A mouth touch consisted of Kari placing one or both hands to her lips or in her mouth. We scored head and mouth touches only while Kari was awake. Sleeping was scored when Kari laid still without moving any part of her body more than 5 cm while having her eyes closed for 10 consecutive 1-min intervals. Sleep onset was defined as the first interval in which she met the definition of sleeping. We converted these measures to a percentage for each night by dividing the number of intervals with head or mouth touches by the total number of intervals (i.e., awake plus asleep intervals) and then multiplying by 100.
Data-collection procedures alternated throughout the night, depending on whether Kari was awake or asleep. We used 1-min partial-interval data collection to measure Kari’s head and mouth touches and whole-interval data collection to measure Kari’s sleep. Once Kari met the definition of sleeping, we transitioned to a 10-min, momentary-time-sampling (MTS) procedure to decrease the total observation time. When MTS began, data collectors noted the time at which Kari met the definition of sleeping and fast-forwarded the video 10 min, observed Kari for the first 5 s of the interval, and noted whether Kari remained asleep. Data collectors continued using MTS until Kari no longer met the definition for being asleep, at which point the data collectors transitioned back to whole-interval data collection.
We assessed interobserver agreement by having a second observer who was blind to the presence or absence of treatment collect data independently on 20% of scored videos. An agreement consisted of both observers recording the same behavior as occurring or not occurring within each interval. We divided the number of agreement intervals by the total number of intervals and then multiplied by 100. Coefficients averaged 91% (range, 64%-100%) for head touches, 97% (range, 88%-100%) for mouth touches, and 96% (range, 83%-100%) for sleeping.
Procedures
Baseline
Baseline consisted of sleep-hygiene recommendations similar to those described by Pipan and Blum (2011) and included going to bed at a standardized time, limiting distractions in the bedroom, and having unique discriminative stimuli (e.g., blanket, pillows) in the sleeping environment. Kari’s standardized bedtime was 7:30 p.m., which based on when she tended to wake, permitted the recommended 10 to 13 hours of sleep (Matricciani, Olds, Blunden, Rigney, & Williams, 2012). Within 10 min of the standardized bedtime, Kari’s caregiver entered the bedroom with Kari, laid her on the bed, wished her a goodnight, turned off the lights, and left the bedroom. Kari was able to move freely about her bedroom at night. These procedures remained in place across all conditions.
Melatonin
We collaborated with a pediatrician to determine an appropriate dosage of melatonin based on Kari’s age, weight, and height. We began titrating the dosage of melatonin Kari received each night starting with an initial dose of 1 mg. We then systematically increased the dosage of melatonin Kari received from 1 mg to 4 mg based on the results of her nightly and hourly analyses. Kari’s caregiver was instructed to deliver all doses of melatonin 45 min to 1 hr before bedtime. She then put Kari in bed at the standardized bedtime and left the room. She was instructed not to re-enter the room unless Kari became ill or needed immediate assistance. We later established a standardized wake time of 6:15 a.m. to address Kari’s head or mouth touches that persisted in the late morning hours. To ensure Kari received approximately the same amount of time for sleep as before the standardized wake time, we also extended her bedtime by 15 min.
Follow-up
We collected follow-up data 1 month following treatment to assess the maintenance of treatment effects.
Indirect Efficacy Assessment
We indirectly assessed the efficacy of the treatment by presenting photos of Kari’s head taken 5-months, 4-months, and 1-month pretreatment, as well as 1-month and 9-months posttreatment to five naïve adult caregivers whose children also were receiving services for the assessment and treatment of severe destructive behavior. Two randomly selected photos were presented side-by-side without any identifying information or indication as to when the photos were taken. Each permutation of photo pairs was presented once for each rater. We asked the raters using text displayed above each photo pair to select the photo in which they observed greater balding. Selection responses resulted in the removal of the photo pair and the presentation of a new photo pair until all photo pairs had been presented. For each rater, we divided the number of times each photo was selected by the total number of times that photo was presented, yielding a selection percentage. (See Supporting Information for the photos used.)
Results and Discussion
Figure 1 depicts the percentage of intervals in which Kari engaged in head or mouth touches and the percentage of intervals of the night she slept across nights. During baseline, Kari engaged in a relatively high percentage of head or mouth touches (M = 6.2%; range, 1.8%-9.7%) and slept a moderate amount of the night (M = 86.6%; range, 79.4%-96.4%). Introducing and then rapidly titrating the dosage of melatonin Kari received each night decreased the number of intervals with head or mouth touches, with the lowest levels occurring at the 3-mg dose (M = 1%; range, 0%-1.8%); however, the percentage of intervals that Kari slept each night did not change systematically with the use of melatonin. A return to baseline coincided with a sharp increase in the percentage of intervals with head or mouth touches (M = 6.7%; range, 5.6%-8.5%) and a decrease in the percentage of intervals each night that Kari slept (M = 74.9%; range, 68.5%-81.0%). We reinstated melatonin at the 3-mg dose and later instituted a standardized wake time (see below), which together decreased the percentage of intervals with head or mouth touches (M = 2.9%; range, 0%-7%) and increased the percentage of intervals each night she slept (M = 89.8%; range, 79.0%-98.1%). Finally, we increased Kari’s melatonin dosage from 3 mg to 4 mg. We later instructed the caregiver to remove Kari’s occasional naps, which occurred some days while riding in the car. The caregiver reported implementing antecedent- and consequent-based strategies to keep Kari awake, which included providing highly preferred tangible items, prompting Kari’s siblings to interact with her, and pulling over the car to wake her. With these final treatment modifications, the percentage of intervals Kari engaged in head or mouth touches decreased further (M = 2.3% range, 0%-15%), and she slept for a larger percentage of intervals each night (M = 92.9%; range, 76.7%-99.2%). Removing nights on which Kari was sick in this last phase (shaded area of Figure 1), we observed further decreases in the percentage of intervals with head or mouth touches (M = 1.5% range, 0%-8%) and increases in the percentage of intervals each night asleep (M = 93.9%; range, 83.6%-99.2%).
Figure 1.
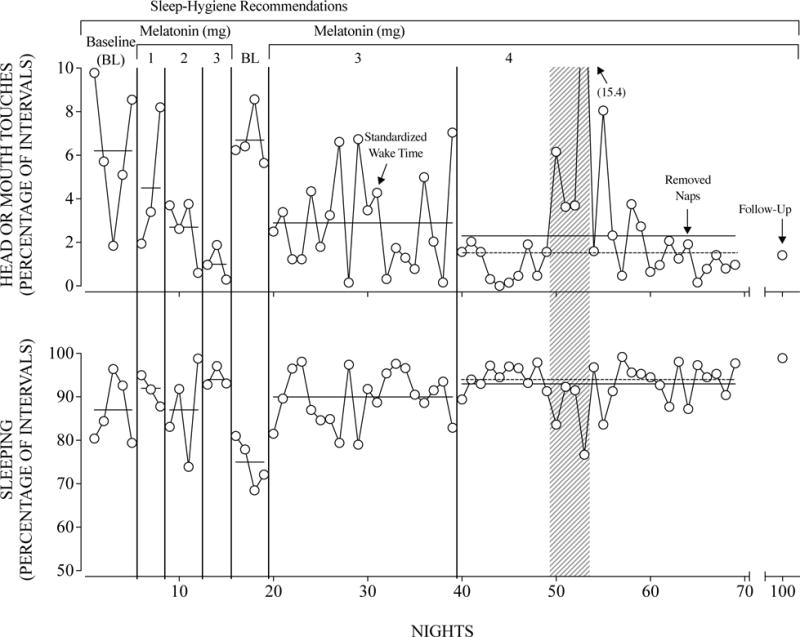
Percentage of intervals with head or mouth touches (top panel) and percentage of intervals of the night asleep (bottom panel) across nights. Nights on which Kari was sick are shaded gray. Solid horizontal lines indicate phase means. Dotted horizontal lines indicate the phase means without the nights on which Kari was sick.
Figure 2 displays the results of Kari’s hourly analysis. We used these data to supplement the nightly analysis and to detect patterns of behavior at each hour of the night. In constructing Figure 2, we plotted zero points (with no error bars) for hours in which Kari (a) had no head or mouth touches or (b) remained asleep throughout that hour across all nights from the corresponding phase of the evaluation. The results of Kari’s hourly analysis during baseline showed high levels of head or mouth touches, which were most likely to occur in the early or last hours of the night. Titrating the melatonin dosage produced reductions in head or mouth touches at both of these periods of the night. Returning to the 3-mg dose of melatonin (middle, rightmost panel) did not replicate prior hourly results, as Kari engaged in elevated levels of head or mouth touches in the late hours of the night. We then standardized her wake time and later increased her melatonin dosage and removed naps. Hourly results of the final treatment phase (bottom, rightmost panel) indicated that Kari engaged in low rates of head or mouth touches throughout the night.
Figure 2.
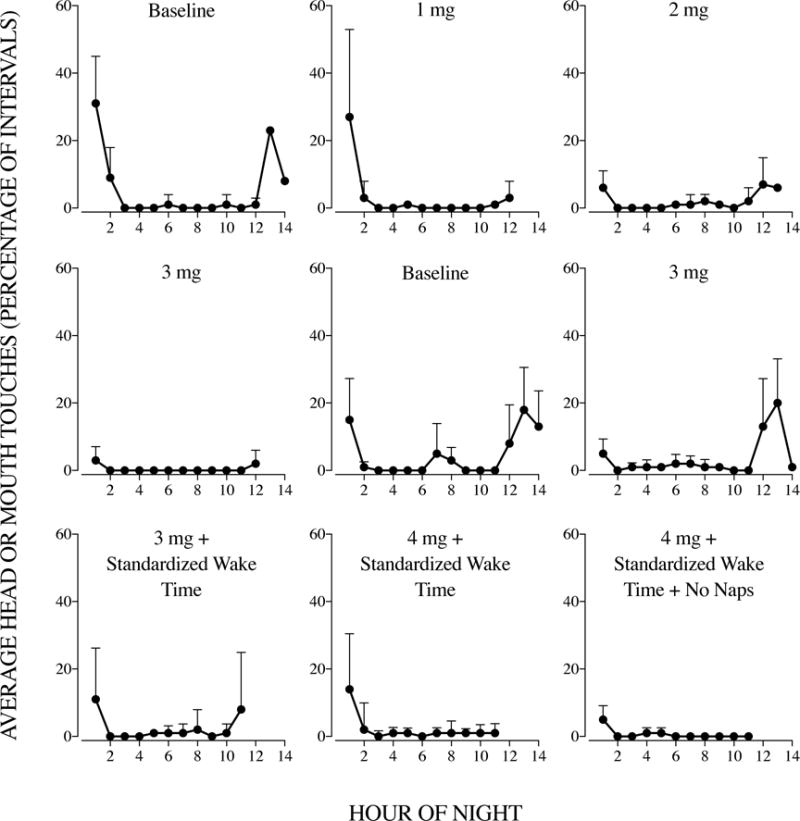
Average percentage of intervals with head or mouth touches throughout each hour of the night by each experimental manipulation. The temporal order of panels proceeds from left to right and then top to bottom (i.e., Baseline, 1 mg, 2 mg, 3 mg, etc.). Error bars indicate one standard deviation and appear only above the data points.
In the indirect efficacy assessment, raters selected pretreatment photos more often, indicating greater balding prior to treatment (5-month pretreatment photo selected on average 60%; range, 50%-75%; 4-month pretreatment photo selected on average 75%; range, 50%-100%; 1-month pretreatment photo selected on average 80%; range, 50%-100%). Posttreatment photos were selected less frequently, indicating less balding following treatment (1-month posttreatment photo selected on average 10%; range, 0%-25%; 9-month posttreatment photo was never selected). These results suggest that Kari’s trichotillomania decreased following treatment.
We evaluated the efficacy of a biobehavioral treatment delivered remotely to decrease head and mouth touches associated with trichotillomania and trichophagia. Unlike more traditional approaches to treatment, we had no direct contact with the patient related to treating either behavior. We showed a 66% decrease in the percentage of intervals in which Kari touched her head or mouth, even though our intervention did not act directly on either of these behaviors. It is likely that the combination of melatonin and sleep-hygiene recommendations indirectly decreased problem behavior by increasing the establishing operation for sleep, thereby decreasing the opportunity to engage in problem behavior. Although clear changes in Kari’s sleep were not always evident between baseline and treatment phases, Kari on average fell asleep 18 min sooner with the final treatment procedures (excluding the nights on which she was sick) when compared to the first baseline and 55 min sooner when compared to the second baseline. These improvements maintained at a 1-month follow-up.
A limitation of the current study is that although we demonstrated a functional relation between Kari’s problem behavior and the 3-mg dose of melatonin when compared to baseline (Phases 3 through 6 in Figure 1), we did not include a return to baseline after standardizing Kari’s wake time, increasing the dosage of melatonin to 4 mg, or removing naps. Thus, we did not demonstrate functional control over Kari’s problem behavior with the final treatment procedures, nor did we demonstrate functional control over Kari’s sleep with the final treatment procedures.
Another limitation was that we did not conduct a comprehensive functional analysis of Kari’s trichotillomania and trichophagia. Kari’s caregiver reported that Kari was most likely to pull and ingest her hair at night when alone in her bed. Considering this report in the context of the persistence of these behaviors in baseline suggests that her trichotillomania and trichophagia were, at least partly, automatically reinforced.
One of the obstacles of remote service delivery that we encountered involved assessing treatment integrity of procedures that the caregiver implemented in contexts outside of Kari’s bedroom. For example, we did not collect treatment-integrity data on the caregiver’s delivery of melatonin, nor could we monitor other activities which immediately preceded the caregiver putting Kari to bed, as these typically occurred in contexts other than the bedroom. Future researchers may consider instructing the individual implementing the treatment to conduct all procedures in the presence of the camera.
Studies addressing sleep-related problems have historically used time-consuming data-collection methods or ones that required adults to remain awake and conduct observations throughout the night (e.g., Piazza & Fisher, 1991). As technology advances, and opportunities to address unique problems (e.g., sleep-related trichotillomania and trichophagia) emerge, future research should continue examining ways to adapt technology to address unique concerns that positively affect a broader range of consumers and needs. This research provides one example of how traditional behavior-analytic methodology (e.g., in-situ observation and data collection) can be enhanced through technological advancements.
Supplementary Material
Acknowledgments
Grants #5R01HD079113 and #1R01HD083214 from The National Institute of Child Health and Human Development provided partial support for this research.
Footnotes
Action Editor, Iser DeLeon
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Azrin NH, Nunn RG, Frantz SE. Treatment of hairpulling (trichotillomania): a comparative study of habit reversal and negative practice training. Journal of Behavior Therapy and Experimental Psychiatry. 1980;11:13–20. doi: 10.1016/0005-7916(80)90045-2. [DOI] [Google Scholar]
- Christenson GA, Mansueto CS. Trichotillomania: Descriptive characteristics and phenomenology. Trichotillomania. 1999;1:42. [Google Scholar]
- Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8:1–6. doi: 10.1371/journal.pone.0063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin ME, Edson AL, Freeman JB. Research Behavior therapy for pediatric trichotillomania: Exploring the effects of age on treatment outcome. Child and Adolescent Psychiatry and Mental Health. 2010;4:18. doi: 10.1186/1753-2000-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig TA. The role of telehealth in an evolving health care environment: Workshop summary. Washington, DC: National Academy Press; 2012. [PubMed] [Google Scholar]
- Lindgren S, Wacker D, Suess A, Schieltz K, Pelzel K, Kopelman T, Lee J, Romani P, Waldren D. Telehealth and autism: Treating challenging behavior at lower cost. Pediatrics. 2016;137(S2):S167–S175. doi: 10.1542/peds.2015-28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: A brief history of sleep recommendations for children. Pediatrics. 2012;129:548–556. doi: 10.1542/peds.2011-2039. [DOI] [PubMed] [Google Scholar]
- Murphy C, Valerio T, Nath Zallek S. Trichotillomania: An NREM sleep parasomnia. Neurology. 2006;66:1276. doi: 10.1212/01.wnl.0000208516.19583.ad. [DOI] [PubMed] [Google Scholar]
- Piazza CC, Fisher W. A faded bedtime with response cost protocol for treatment of multiple sleep problems in children. Journal of Applied Behavior Analysis. 1991;24:129–140. doi: 10.1901/jaba.1991.24-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipan ME, Blum NJ. Basics of child behavior and primary care management of common behavioral problems. In: Voigt RG, Macias MM, Myers SM, editors. Developmental and behavioral pediatrics. American Academy of Pediatrics; 2011. pp. 37–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


