Pain behaviors, verbal and non-verbal [34] expressions of pain [i.e., resting, guarding, facial/vocal expressions, asking for assistance; [9; 38]] are important indicators of functioning in children with chronic pain. Pain behaviors communicate a child’s pain experience, and are a useful metric for understanding patient wellbeing. While potentially adaptive during acute pain (i.e., signaling danger), greater pain behaviors are associated with significant impairment in chronic pain [5; 13; 24; 25; 29; 33] including higher rates of depressive symptoms [13; 24; 25], pain severity levels [13; 24; 29] and pain-related disability [5; 10; 13; 24; 29; 33] in adults with chronic pain, and higher levels of pain catastrophizing, depressive symptoms and disability [27; 28] in children. Adequate assessment of pain behaviors in youth is important for both caregivers and providers because it can identify youth with chronic pain at greatest risk for adverse outcomes and guide interventions that target adaptive ways to communicate about pain.
In addition to being associated with poorer psychosocial functioning, children’s pain behaviors have been shown to impact medical interventions received. For example, preschoolers with the most facial distress after a minor injury were most likely to receive medical intervention from staff, regardless of the severity of the injury [41]. Additionally, in an experimental study of medical decision making, pediatric nurses reported a correlation between the likelihood of administering analgesics with the level of vocal expression of pain [11]. Little is currently known about how pediatric pain behaviors impact providers and caregiver’s responses.
Traditional methods of assessing pain behaviors typically involve lengthy and complex observational systems that [22] are impractical for use in medical clinic settings [34]. A more practical approach for use in medical settings is a self-reported measure of pain behaviors. A parent-reported measure of pain behavior exists for use with adolescents [27]; however, discrepancies between parent and child report of child health functioning are common and patient reported information is generally considered more sensitive indicators of child health and wellbeing [45].
The Patient Reported Outcomes Measurement Information System (PROMIS) is the product of a cooperative research program initiated by the National Institutes of Health to apply advancements in measurement science to support development and validation of precise instruments that assess self-reported health domains uniformly across medical conditions. As part of the PROMIS initiative [5; 34], a self-report measure of pain behavior was developed. Psychometric evidence is available for adults [5; 34] that supports the notion that self-reports of pain behaviors can be reliable and valid when compared to other methods of assessing these behaviors [5]. However, there is currently no validated self-report measure of pain behaviors for children and adolescents. The purpose of this study was to create such a measure. Our research group has published the rigorous qualitative methods used to develop the pediatric pain behavior item bank [19]. This report presents the results of calibration testing and evaluation of psychometric properties of the pediatric PROMIS pain behavior item bank and development of a fixed short form.
Methods
Development of the pain behavior item pool
Candidate items for the pediatric pain behavior measure were developed following the rigorous, methodological standards of PROMIS [3; 8]. Items were selected based on a comprehensive literature review, expert feedback, and focus groups and semi-structured qualitative interviews conducted with youth with chronic pain [19]. Item bank development was also informed by the PROMIS item banks for self-report of pain behaviors in adults to facilitate future linkage studies for measurement across the lifespan. A total of 47 items were included in the final pediatric pain behavior item bank.
The pediatric pain behavior item bank encompassed a variety of self-reported external manifestations of pain: behaviors that typically communicate to others that an individual is experiencing pain. These actions or reactions can be verbal or nonverbal, and involuntary or deliberate. They include observable displays of pain (i.e., sighing, crying, resting, guarding, facial expressions), and verbal reports of pain (i.e., talking about it, yelling, asking for help). Items assess pain behavior over a response period of the past seven days. Items are in the format, “In the past 7 days, when I was in pain I [representative behavior]…” with response options for each item of “had no pain,” “never,” “almost never,” “sometimes,” “often,” and “almost always”. The “had no pain” option was to indicate if the respondent was free of pain, whereas the frequency options were to indicate whether or not a particular pain behavior was endorsed by those respondents who did experience pain in the past 7 days.
Participants
Participants in the study were N = 450 pediatric patients between the ages 8 to 17 years with chronic pain associated with juvenile fibromyalgia (JFM), juvenile idiopathic arthritis (JIA) or sickle cell disease (SCD). During recruitment, candidates were screened for whether they had experienced pain in the past 7 days to ensure that those with recurrent pain conditions (JIA and SCD) were currently or within a few days of experiencing an episode of pain. Participants were recruited through outpatient clinics at 3 pediatric medical centers in three regions of the United States (Midwest, Northeast and Southeast). This study was approved by the Institutional Review Boards at each institution and patients and parents provided written informed consent/assent for participation in the study.
Procedure
Caregivers provided data on socio-demographic information and health history. Children completed 47 candidate pain behavior self-report items that were previously developed as part of a larger pool of PROMIS items pertaining to their pain experience [19]. In addition, self-report measures of pain interference, depressive symptoms, fatigue, average pain intensity, and pain catastrophizing were collected.
Measures
Pediatric PROMIS Pain Measures
Participants completed psychometrically validated [39] measures of 1) pain interference (8 item short form) [40] i.e., difficulties in completing daily activities, socioemotional problems, and impairment in physical functioning due to pain, 2) depressive symptoms (8 item short form) [15], and 3) fatigue (10 item short form) [26]. All PROMIS measures assessed presence of symptoms in the past 7 days via a 5-point Likert-type scale. Response options were - never, almost never, sometimes, often, and almost always. The summed scores on each scale were converted to a standardized T-score metric. Higher scores indicate a greater symptoms or a higher level of impairment. Pediatric short forms were used as these measures have been found to be more precise as compared to adaptive PROMIS measures in children [39].
Average pain intensity
Average pain intensity in the past week was collected via patient self-report using a 0–10 numeric rating scale (NRS) [2; 43]. Score level classifications were based on the following: 0 = no pain, 1–3 = “mild pain”, 4–6 = “moderate pain”, and 7–10 = “severe pain”.
Pain Catastrophizing
The Pain Catastrophizing Scale, Child Version (PCS-C), contains 13 items related to the child’s thoughts and feelings about pain when the child is in pain [6]. Response options include - not at all (0), mildly (1), moderately (2), severely (3), and extremely (4). Total scores range from 0 to 52, with higher scores reflecting greater pain catastrophizing.
Data Analysis
Confirmatory factor analyses (CFA) were conducted (Mplus Version 7.1) [31] with a mean-and-variance adjusted weighted least squares estimator and delta parameterization to assess unidimensionality of responses to the 47 pediatric pain behavior items (16). Model fit was examined using empirically validated fit indices and levels [14]. Specifically, a root mean square error of approximation (RMSEA) < .05, and a comparative fit index (CFI) and Tucker-Lewis Index (TLI) > 0.90 would suggest that the hypothesized model structure fits the data well. IRTFIT [37] was used to compute the item parameters (i.e., slope and thresholds) based on the graded response model [35]. The slopes indicate the strength of the relationship between an item and the target construct (i.e., pain behaviors). Threshold parameters provide information on item severity and location along the measured construct. IRTFIT computes the S-X2 (a Pearson X2 statistic) and S-G2 (a likelihood ratio G2 statistic) fit statistics that use the sum score of all items and compare the predicted and observed response frequencies for each level of the scale sum score. Statistically significant differences indicate poor fit of that particular item.
Differential item functioning (DIF) was examined by age and diagnoses. DIF exists when an item functions differently for respondents with similar trait levels, but from different subgroups or at different time points. DIF can be consistent across the range of the construct being measured (uniform DIF), or its impact can vary depending on the level of the construct (non-uniform DIF). Logistic regression as described by Zumbo [46] was used to for detecting DIF.
Finally, reliability was assessed using Cronbach’s alpha and test information curves based on scores from the full item bank and the short form. Convergent validity was assessed with bivariate correlations between scores on pain behavior short form and scores on three other pediatric PROMIS short form measures (pain interference, depressive symptoms, fatigue ), PCSC and average pain intensity scores from the past week on the 0–10 NRS scale, categorized into “no pain” (0), “mild” (1–3), “moderate” (4–6), and “severe” (7–10) pain groups. We conducted a known groups analyses by pain intensity category with a one-way ANOVA.
Results
Sample Characteristics
A total of 450 children (Mean age = 13.54, SD = 2.8) participated in the study (see Table 1 for demographics and diagnoses). The majority of patients with JIA and JFM were Caucasian and those diagnosed with SCD were predominantly African-American. Overall, there was a larger proportion of female participants (71.6%) which is consistent with the higher prevalence of chronic pain in girls, particularly adolescents [23], and female predominance in JIA. The subgroup of SCD patients was gender balanced. There were other statistically significant differences in group characteristics, including higher mean age of JFM patients and higher pain intensity (NRS) scores compared to JIA and SCD. Additionally, SCD patients had higher mean pain behavior scores overall, and higher mean pain interference scores compared to JIA patients.
Table 1.
Demographic and clinical characteristics by diagnosis groups
| Juvenile Fibromyalgia | Juvenile Idiopathic Arthritis | Sickle Cell Disease | |
|---|---|---|---|
| N | 151 | 175 | 115 |
| Mean Age (SD) | 14.83 (1.96) | 12.83 (2.94) | 12.97 (2.89) |
| % Female | 89.4% | 69.1% | 52.2% |
| % Caucasian | 84.1% | 89.1% | 0% |
| % African American | 6.6% | 4.6% | 97.4% |
| % Hispanic | 5.3% | 3.4% | 3.5% |
| Mean Pain NRS Score | 5.13 (2.61) | 2.91 (2.11) | 4.38 (2.55) |
| Mean PROMIS Pain Interference Score (SD) | 53.13 (9.43) | 44.50 (9.09) | 54.09 (8.18) |
| Mean PROMIS Pain Behavior Score (SD) | 50.62 (9.24) | 44.94 (9.29) | 56.63 (7.62) |
Note. The Mean Pain Behavior Score was significantly different between each of the diagnosis groups (p < .001). In addition, mean age was different by diagnosis (p < .001).
Item Bank Results
The CFA of item responses for the total sample indicated that all 47 items identified during qualitative work (16) fit a unidimensional model well, χ2 (1034) = 4227.12, p < .001, RMSEA = .08, CFI = .95, TLI = .95, indicating that all the items could be accounted for by a single factor, pain behaviors. Standardized factor loadings ranged from 0.63 to 0.86 and were all statistically significant at p < .001. Therefore, we proceeded to model the responses using a graded response IRT model.
After calibration to the graded response model, item fit statistics were inspected to evaluate misfit. None had p-values less than 0.001. Thus all were included in the final item bank. The item parameters of the final 47 items are shown in Table 2. The slope parameters ranged from 1.81 (“I rubbed my body where it hurt”) to 4.58 (“I had to stop what I was doing”). The threshold values ranged from −2.02 to 1.95, reflecting adequate coverage of the range of the pain behavior construct.
Table 2.
Item parameters for pediatric PROMIS pain behavior full bank.
| Item Stem | Slope | Location Threshold 1 | Location Threshold 2 | Location Threshold 3 | Location Threshold 4 | Location Threshold 5 |
|---|---|---|---|---|---|---|
| I tried not to move. | 3.66 | −1.39 | −0.13 | 0.18 | 0.67 | 1.05 |
| I asked for help with doing things. | 3.66 | −1.51 | −0.17 | 0.2 | 0.73 | 1.12 |
| I asked people to let me be by myself. | 2.84 | −1.56 | 0.13 | 0.41 | 0.95 | 1.41 |
| I moved stiffly. | 2.74 | −1.82 | −0.49 | −0.09 | 0.48 | 0.99 |
| I yelled for someone to help me. | 3.76 | −1.36 | 0.35 | 0.67 | 1.04 | 1.25 |
| I cried. | 3.55 | −1.42 | 0.03 | 0.32 | 0.76 | 1.07 |
| I used something for support (cane, crutches, wheelchair) to move from place to place. | 2.85 | −1.44 | 0.76 | 0.99 | 1.38 | 1.55 |
| I limped. | 2.70 | −1.6 | −0.12 | 0.18 | 0.69 | 1.07 |
| I became quiet. | 2.65 | −1.55 | −0.22 | 0.09 | 0.68 | 1.16 |
| I asked for help getting around. | 4.01 | −1.19 | 0.25 | 0.55 | 0.89 | 1.15 |
| I groaned. | 3.49 | −1.5 | −0.12 | 0.27 | 0.82 | 1.2 |
| I stayed away from other people. | 3.40 | −1.71 | 0.08 | 0.39 | 0.89 | 1.26 |
| I asked for medicine. | 2.65 | −1.75 | −0.27 | 0.01 | 0.65 | 1.13 |
| I screamed. | 3.65 | −1.37 | 0.42 | 0.77 | 1.12 | 1.35 |
| I protected the part of my body that hurt. | 3.55 | −1.46 | −0.32 | −0.03 | 0.47 | 0.83 |
| I tightened my jaw. | 2.42 | −1.5 | 0.12 | 0.46 | 0.98 | 1.49 |
| I talked about my pain. | 2.22 | −1.93 | −0.47 | 0.06 | 0.8 | 1.29 |
| I didn’t let anyone touch me. | 2.89 | −1.45 | −0.02 | 0.33 | 0.8 | 1.15 |
| I rubbed my body where it hurt. | 1.81 | −2.02 | −0.73 | −0.33 | 0.39 | 1.06 |
| I moved slower. | 4.39 | −1.41 | −0.46 | −0.17 | 0.37 | 0.74 |
| I got angry at people. | 2.88 | −1.54 | 0.05 | 0.38 | 0.91 | 1.27 |
| I didn’t want anyone to touch me. | 3.27 | −1.54 | −0.07 | 0.17 | 0.58 | 0.96 |
| I avoided standing. | 3.55 | −1.81 | −0.19 | 0.14 | 0.65 | 1.01 |
| I asked people to bring me things (food, games). | 3.51 | −1.47 | −0.09 | 0.21 | 0.71 | 1.03 |
| I couldn’t stay still. | 2.67 | −1.66 | −0.21 | 0.11 | 0.71 | 1.27 |
| I was restless. | 3.00 | −1.6 | −0.26 | 0.02 | 0.54 | 1.02 |
| I tried to think of something nice/fun. | 2.17 | −1.79 | −0.46 | −0.05 | 0.72 | 1.28 |
| I went to sleep. | 2.38 | −1.68 | −0.36 | 0.05 | 0.6 | 1.14 |
| I had to stop what I was doing. | 4.58 | −1.26 | −0.32 | 0.03 | 0.62 | 0.99 |
| I avoided lifting or carrying heavy things. | 3.40 | −1.57 | −0.22 | 0.04 | 0.45 | 0.87 |
| I stayed near to someone who cares about me. | 2.52 | −1.82 | −0.59 | −0.23 | 0.34 | 0.85 |
| I tried to rest or relax. | 2.65 | −1.79 | −0.91 | −0.62 | 0.22 | 0.85 |
| I complained. | 3.09 | −1.67 | −0.48 | −0.06 | 0.51 | 0.92 |
| I asked to see a doctor. | 2.49 | −1.64 | 0.27 | 0.54 | 1.16 | 1.56 |
| I pulled away if someone touched me where it hurt. | 3.31 | −1.53 | −0.19 | 0.09 | 0.51 | 0.9 |
| I argued with people. | 2.56 | −1.61 | 0.07 | 0.38 | 0.96 | 1.54 |
| I avoided using the part of my body that hurt. | 3.51 | −1.67 | −0.39 | −0.06 | 0.41 | 0.86 |
| I said mean words to people. | 2.36 | −1.8 | 0.31 | 0.64 | 1.23 | 1.76 |
| I got mad and threw or hit something. | 2.19 | −1.72 | 0.58 | 1.02 | 1.59 | 1.95 |
| I sighed. | 2.86 | −1.81 | −0.22 | 0.09 | 0.66 | 1.12 |
| I took breaks from what I was doing. | 3.91 | −1.59 | −0.4 | −0.16 | 0.49 | 0.97 |
| I told people I couldn’t do my usual chores. | 3.81 | −1.28 | 0.09 | 0.41 | 0.82 | 1.22 |
| It showed on my face. | 3.44 | −1.44 | −0.29 | 0 | 0.52 | 0.89 |
| I told people I couldn’t do things with them. | 3.58 | −1.33 | −0.07 | 0.25 | 0.75 | 1.12 |
| I asked for someone to help me. | 3.65 | −1.41 | −0.14 | 0.23 | 0.73 | 1.04 |
| I lay down. | 3.26 | −1.41 | −0.6 | −0.25 | 0.33 | 0.89 |
| I felt my body get tense. | 3.26 | −1.47 | −0.2 | 0.08 | 0.6 | 1.06 |
Differential Item Functioning
Ordinal logistic regression [46] was used to assess items for DIF. DIF was assessed for age (8–12 versus 13 and older), consistent with age cut points in prior PROMIS pediatric studies [16; 18], and diagnosis (JIA versus JFM and SCD). The IRT theta score was used as the conditioning variable. DIF was determined to be significant when the p-value for the χ2 was less than 0.001.
No items had non-uniform DIF due to age (data not shown). Three items had uniform DIF due to age. After reviewing the content of these items, two of these three items (“I asked for someone to help me”, “I stayed near someone who cares about me”) were associated with increased interaction with a caregiver, and potentially more “easily” endorsed by younger children. The other item that showed uniform DIF due to age was “I felt my body get tense”, which, after matching on pain behavior levels, was more likely to be endorsed by older children.
No items had non-uniform DIF due to diagnosis (data not shown). Six items had uniform DIF for the JIA versus JFM comparison (“I limped”, “I asked for medicine”, “I talked about my pain”, “I felt my body get tense”, “I didn’t want anyone to touch me”, “I was restless”), such that these items were more likely to be endorsed by patients with JIA than JFM, with similar levels of pain behaviors. In addition, six items had uniform DIF for the JIA versus SCD comparison (“I moved stiffly”, “I got angry at people”, “I lay down”, “I went to sleep”, “I argued with people”, “I said mean words to people”), where patients with JIA were more likely to endorse these items than patients with SCD with similar levels of pain behaviors.
Next, we examined whether the item-level bias identified in the DIF analyses would influence mean-based conclusions [30]. We compared model-based estimates from the partially invariant model to estimates from a model ignoring DIF [1]. Specifically, JIA patients were assigned a mean of zero on the “pain behaviors” factor for purposes of model identification. We examined whether JFM and SCD patient’s factor means were significantly different from zero and found that they were not. In addition, the factor mean for ages 8 to 12 was set to zero and we found that the older age group (13 to 18) did not have a factor mean significantly different from zero. Thus, we concluded that the observed DIF between the age groups and among disease diagnoses did not substantively influence mean-based conclusions.
Short Form Results
Selection of items for inclusion in a pediatric pain behaviors short form was an iterative process that included a blend of qualitative and quantitative methods. First, content of the 47 items in the bank was assessed by a panel of 12 experts published in the area of pediatric pain, including pediatric pain physicians (total n=5, specialties included an anesthesiologist, a hematologist, and three rheumatologists), pediatric pain psychologists (n=3), quantitative psychologists (n=3), and an anthropologist with an expertise in qualitative data analysis (n=1). The selected items encompassed the following pain behavior dimensions: verbal complaints, resting, restricting activity, emotional displays (verbal or non-verbal), guarding, withdrawal, involuntary vocalization, altered gait/movement, and seeking help. Of note, individual pain behavior items may have been categorized by one or more of the aforementioned domains. Items were ranked by the expert panel based on how informative they were (using the IRT parameters) and whether they encompassed dimension(s) of pain behavior. After the ranking of items, votes were tallied, and inconsistencies were addressed via discussion and a group consensus process.
In addition, those items that overlapped with the adult pain behavior bank were identified and panelists were asked to weigh these items more highly in consideration for the short form to facilitate potential future linking of the adult and pediatric measures’ metrics. Fifteen candidate items were identified for possible inclusion in the short form, distributed across the pain behavior dimensions specified above. Four (out of the 15) items overlapped with the PROMIS adult pain behavior item bank. The list of 15 candidate short form items was further narrowed by the experts to eight items (the typical number of items included in PROMIS short forms) after a selection process similar to that described above, including reviewing a list of items, ranking the top 8 items, tallying votes, and working with the group to reconcile inconsistencies. Note that the resulting 8 items included two overlapping items with adult short form. Current versions of PROMIS measures are available at http://www.healthmeasures.net/search-view-measures.
CFA was conducted on responses to the 8 items chosen for inclusion in the short form measure (Table 3). Fit statistics indicated good fit to a unidimensional model (χ2 (20) = 60.76, p < .001, CFI = .995, TLI = .993, RMSEA = .068). Standardized factor loadings ranged from .71 to .87 and were all statistically significant. Table 4 includes information on converting raw scores obtained on the short form into T-scores.
Table 3.
PROMIS pain behavior 8 item short form
| 1 | In the past 7 days, when I was in pain… | it showed on my face.* |
| 2 | In the past 7 days, when I was in pain | I moved slower.# |
| 3 | In the past 7 days, when I was in pain | I protected the part of my body that hurt.* |
| 4 | In the past 7 days, when I was in pain | I had to stop what I was doing. |
| 5 | In the past 7 days, when I was in pain | I asked for someone to help me.# |
| 6 | In the past 7 days, when I was in pain | I lay down*. |
| 7 | In the past 7 days, when I was in pain | I asked for medicine. |
| 8 | In the past 7 days, when I was in pain | I talked about my pain. |
Item overlaps or similarly worded as item in adult PROMIS pain behavior item bank. #Similarly worded to item in adult pain behavior short form. Adult PROMIS Pain Behavior items available at: http://www.healthmeasures.net/search-view-measures
Table 4.
Summed Score to Scale Score Conversion Table for PROMIS Pediatric Pain Behavior 8-item Short Form
| Summed Score | Scale Score |
|---|---|
| 0 | 20.0 |
| 1 | 28.4 |
| 2 | 30.7 |
| 3 | 32.6 |
| 4 | 34.2 |
| 5 | 35.7 |
| 6 | 37.1 |
| 7 | 38.5 |
| 8 | 39.8 |
| 9 | 40.9 |
| 10 | 41.9 |
| 11 | 42.9 |
| 12 | 43.9 |
| 13 | 44.8 |
| 14 | 45.7 |
| 15 | 46.5 |
| 16 | 47.3 |
| 17 | 48.1 |
| 18 | 48.8 |
| 19 | 49.6 |
| 20 | 50.3 |
| 21 | 51.0 |
| 22 | 51.8 |
| 23 | 52.5 |
| 24 | 53.2 |
| 25 | 53.9 |
| 26 | 54.7 |
| 27 | 55.4 |
| 28 | 56.2 |
| 29 | 57.0 |
| 30 | 57.7 |
| 31 | 58.5 |
| 32 | 59.4 |
| 33 | 60.3 |
| 34 | 61.2 |
| 35 | 62.2 |
| 36 | 63.3 |
| 37 | 64.6 |
| 38 | 66.2 |
| 39 | 67.9 |
| 40 | 80.0 |
Reliability and Validity
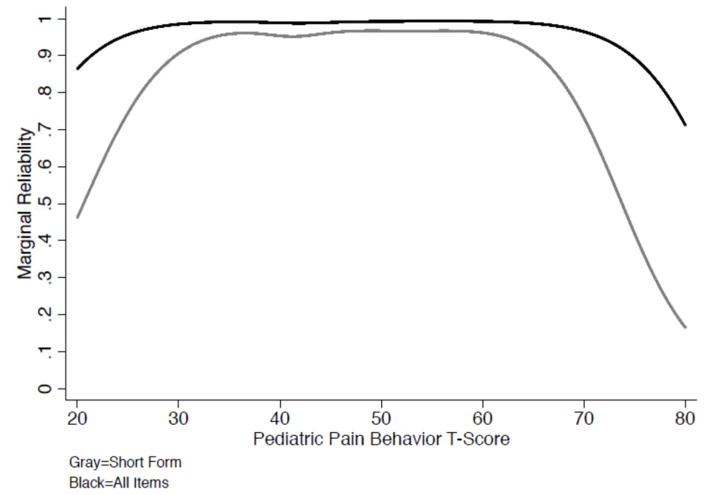
Cronbach’s alpha for the full item bank was .98 and .92 for the short form pain behavior scale. Figure 1 shows the marginal reliability of the short form and full item bank across the range of pain behavior T-scores. For the short form, the level of reliability approximates 0.90 between T-Scores of 35 to 65, and the item bank achieves higher reliability across almost the full range of the latent trait (T-Scores of 22 – 75). Table 5 summarizes the bivariate correlations among the PROMIS short form measures, pain NRS and the PCS-C. Scores on the PROMIS Pain Behavior short form were significantly positively correlated with scores on PROMIS fatigue, depressive symptoms, and pain interference measures. In addition, there were significant positive correlations between PROMIS pain behavior scores and scores on both the PCS-C and the NRS of pain intensity. Finally, we evaluated differences in mean pain behavior short form scores by pain intensity categories (no pain = 0, mild = 1–3; moderate pain = 4–6; severe pain = 7–10). An ANOVA revealed that pain behavior means increased stepwise with increases in pain intensity, F (3, 439) = 72.33, p < .001, such that the “no pain” group had the lowest mean pain behavior T-scores (M = 37.72, SD = 12.00), “mild” pain intensity group had significantly higher pain than the no pain group (M = 46.86, SD = 8.74, p < .001), “moderate” pain intensity had significantly higher mean pain behavior scores than “mild” (M = 53.11, SD = 6.93, p < .001) and “severe” pain intensity had the highest mean pain behavior T-scores (M = 57.18, SD = 6.57, p < .01).
Figure 1.
Marginal Reliability for PROMIS Pediatric Pain Behavior Items
Table 5.
Bivariate correlations among measures, N = 450.
| PROMIS Pain Interference | PROMIS Depressive Symptoms | PROMIS Fatigue | Pain Intensity NRS | PCS-C | PROMIS Pain Behavior Full Item Bank | |
|---|---|---|---|---|---|---|
| PROMIS Depressive Symptoms | .59** | -- | -- | -- | -- | |
| PROMIS Fatigue | .82** | .65** | -- | -- | -- | |
| Pain Intensity NRS | .65** | .41** | .59** | -- | ||
| PCS-C | .73** | .68** | .69** | .55** | -- | |
| PROMIS Pain Behavior Short Form | .78** | .48** | .67** | .58** | .62** | .95** |
p < .001
Discussion
This paper describes the rigorous psychometric development and validation process of a new, brief measure of self-reported pain behaviors for children and adolescents with chronic pain as part of the PROMIS initiative. The pediatric PROMIS pain behavior short form and calibrated item bank will complement other validated measures in the suite of PROMIS measures for pain interference, fatigue, depressive symptoms, anxiety and mobility that have strong psychometric properties in pediatric pain populations [39]. Moreover, the measure has the potential to be linked with the adult PROMIS pain behavior measure for use in studies that include adult and pediatric pain populations or longitudinal research of youth with pain. In line with established PROMIS methodology, we followed a systematic process of first developing a large item pool using literature review of existing measures and stakeholder input (patients, parents and pain experts) to ensure adequate coverage of the construct from varying perspectives[19] . In this study, we used a graded IRT model and found that all 47 pain behavior items that were initially identified demonstrated excellent model fit and, therefore were all considered appropriate items for inclusion in the final pediatric pain behavior item bank. Next, a rigorous psychometric examination of the items using modern test theory methods was performed. Results were combined with further expert input and alignment with PROMIS adult measures, to arrive at a highly precise 8-item pediatric pain behavior patient-report short form measure.
For this study, we included a large sample of 450 children and adolescents suffering from widespread musculoskeletal pain (JFM group), rheumatic disease (JIA group) and hematologic disease (SCD group). By targeting these specific disorders, we were able to include a range of diagnoses and demographically diverse participants (which would aid generalizability), but we also had sufficient numbers of patients in each condition to test whether the measure performed differently based on age, gender and diagnosis. Overall, the SCD group had the highest mean scores on pain behavior, whereas the JIA group had the lowest scores. This is consistent with prior observations in these clinical populations [7], in which children with SCD reporting experience of pain in the prior week (similarly to our study population) had significantly higher reports of pain interference, as well as fatigue, depressive symptoms, compared to children with JIA, and children with SCD not experiencing pain in the prior week. Children with JFM generally experience more constant daily pain compared to those with SCD or JIA and their pain behavior scores fell somewhere between the JIA and SCD groups.
Though we did find that some items were more easily endorsed by one group or another, there were not a large number of items with DIF, nor did DIF meaningfully impact scores. This finding validates the use of the measure across pediatric pain conditions, particularly for musculoskeletal pain conditions.
Findings from the current investigation demonstrated model fit and reliability of an 8item short form. This short form measure could be readily integrated into busy clinical practice or research studies, imposing minimal burden on respondents and clinical staff. This PROMIS pediatric short form of pain behaviors has distinct advantages over burdensome coding systems completed by observer ratings [22; 42] ) and caregiver reports of child pain behaviors [27] that may be subject to informant bias when the caregiver is the only respondent. It will be beneficial for future studies to utilize ecological momentary assessment of pain behaviors via electronic diaries or actigraphy, given the tendency of children to rely on global perceptions or halo effects when using retrospective reports [17]. It may also be beneficial to explore effects of different types of pain behaviors. Those related to gait and motor functioning, such as guarding, may be particularly important indicators of functioning in musculoskeletal pain conditions [21], for example. Further, patients who present with multiple forms of pain behavior (guarding, rubbing, and bracing) may evidence greater levels of disability [20].
Limitations of this study included potential confounding variables for assessment of pain behaviors (i.e., anxiety), sample composition, and sample sizes for the psychometric analyses. First, patient characteristics (i.e., anxiety) may bias pain behavior reporting. However, as noted above, the self-report tool for pain behavior should ideally be used in conjunction with observer report to provide a more comprehensive picture. Although both the initial item pool and final 8item scale best fit a unidimensional model of pain behavior, the identification of pediatric pain-behavior subdomains (or specific pain behaviors that may be adaptive/maladaptive) may be helpful in future research. For example, studies of pain behavior in adults have shown that some pain behaviors (such as “guarding”) may more strongly predict disability compared to others [33]. The measure developed in this study does not provide subscale scores for different kinds of pain behaviors. Also, because, this study was limited to United States participants, it would be informative to study whether child reports of pain behaviors and implications for pain-related disability differ across cultures. Additionally, although the sample size was comparable to other studies of patient-reported outcomes in pediatric research, the sample sizes for proper evaluation of DIF were relatively small. Future research needs to further examine DIF by gender, diagnosis, race/ethnic group, and psychosocial functioning (i.e., do participant responses differ based on high or low levels of depression/anxiety?). Although item order effects are often small [12; 36], future studies may wish to examine this issue.
The development of a psychometrically sound child-reported item bank of pain behaviors is important given that understanding the child’s report of pain behaviors may serve to better understand their level of pain-related disability. Increased child pain behaviors may be associated with increased medical intervention and healthcare utilization, which can be risky to patients and costly to families. Having a measure of child pain behaviors can facilitate identification of maladaptive pain behaviors expressed which is especially relevant in the context of recurrent and chronic pain conditions, where parents and caregivers’ responses have been shown to be influential in the child’s ability to function when in pain [4; 32; 44]. Greater awareness of the meaning of children and adolescents’ pain behaviors can be used in the treatment planning process to guide a child and family towards developing more constructive approaches for dealing with pain. For example, if a child reported engaging in guarding due to fear of experiencing real or perceived pain symptoms, a clinician could help facilitate exposure to feared activities in a safe and structured environment, thereby increasing engagement in daily activities and potentially reducing pain-related fears and behaviors.
In conclusion, the newly developed brief PROMIS pediatric pain behavior short form is an important step that sets the foundation for further research into pain behavior in children and adolescents with chronic pain. Further validation of this measure examining how this measure performs in comparison to existing observational coding systems of pain behavior will be helpful in further supporting its construct validity. A validated self-reported measure of pain behavior offers a convenient alternative or complementary tool to existing measures which would be a useful advance for the field of pediatric pain research.
Acknowledgments
We are indebted to C. Jeffrey Jacobson, PhD for his extensive contributions to the qualitative work that underlay development of the Pain Behavior item bank. This project would not have been possible without the excellent research assistance from Jennifer Farrell, Kimberly Barnett, Jenna Tress, Leann Schilling, and Caravella McCuistian for assistance with patient recruiting, administration of questionnaires and regulatory compliance. We are grateful to the patients and parent participants in this research study.
Funding: The Patient-Reported Outcomes Measurement Information System (PROMIS) is an NIH Roadmap initiative to develop a computerized system measuring PROs in respondents with a wide range of chronic diseases and demographic characteristics. PROMIS II was funded by cooperative agreements with a Statistical Center (Northwestern University, PI: David Cella, PhD, 1U54AR057951), a Technology Center (Northwestern University, PI: Richard C. Gershon, PhD, 1U54AR057943), a Network Center (American Institutes for Research, PI: Susan (San) D. Keller, PhD, 1U54AR057926) and thirteen Primary Research Sites which may include more than one institution (State University of New York, Stony Brook, PIs: Joan E. Broderick, PhD and Arthur A. Stone, PhD, 1U01AR057948; University of Washington, Seattle, PIs: Heidi M. Crane, MD, MPH, Paul K. Crane, MD, MPH, and Donald L. Patrick, PhD, 1U01AR057954; University of Washington, Seattle, PIs: Dagmar Amtmann, PhD and Karon Cook, PhD, 1U01AR052171; University of North Carolina, Chapel Hill, PI: Darren A. DeWalt, MD, MPH, 2U01AR052181; Children’s Hospital of Philadelphia, PI: Christopher B. Forrest, MD, PhD, 1U01AR057956; Stanford University, PI: James F. Fries, MD, 2U01AR052158; Boston University, PIs: Stephen M. Haley, PhD and David Scott Tulsky, PhD (University of Michigan, Ann Arbor), 1U01AR057929; University of California, Los Angeles, PIs: Dinesh Khanna, MD and Brennan Spiegel, MD, MSHS, 1U01AR057936; University of Pittsburgh, PI: Paul A. Pilkonis, PhD, 2U01AR052155; Georgetown University, PIs: Carol. M. Moinpour, PhD (Fred Hutchinson Cancer Research Center, Seattle) and Arnold L. Potosky, PhD, U01AR057971; Children’s Hospital Medical Center, Cincinnati, PI: Esi M. Morgan DeWitt, MD, MSCE, 17 1U01AR057940; University of Maryland, Baltimore, PI: Lisa M. Shulman, MD, 1U01AR057967; and Duke University, PI: Kevin P. Weinfurt, PhD, 2U01AR052186). NIH Science Officers on this project have included Deborah Ader, PhD, Vanessa Ameen, MD, Susan Czajkowski, PhD, Basil Eldadah, MD, PhD, Lawrence Fine, MD, DrPH, Lawrence Fox, MD, PhD, Lynne Haverkos, MD, MPH, Thomas Hilton, PhD, Laura Lee Johnson, PhD, Michael Kozak, PhD, Peter Lyster, PhD, Donald Mattison, MD, Claudia Moy, PhD, Louis Quatrano, PhD, Bryce Reeve, PhD, William Riley, PhD, Ashley Wilder Smith, PhD, MPH, Susana Serrate-Sztein, MD, Ellen Werner, PhD and James Witter, MD, PhD.
Footnotes
There are no conflicts of interest for the study authors.
References
- 1.Carle AC, Weech-Maldonado R, Ngo-Metzger Q, Hays RD. Evaluating Measurement Equivalence across Race and Ethnicity on the CAHPS® Cultural Competence Survey. Medical care. 2012;50(9 Suppl 2):S32. doi: 10.1097/MLR.0b013e3182631189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castarlenas E, Jensen MP, von Baeyer CL, Miró J. Psychometric Properties of the Numerical Rating Scale to Assess Self-Reported Pain Intensity in Children and Adolescents: A Systematic Review. The Clinical journal of pain. 2016 doi: 10.1097/AJP.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 3.Cella D, Chang C-H. Response to Hays et al and McHorney and Cohen: A discussion of item response theory and its applications in health status assessment. Medical Care. 2000;38(9):II-66–II-72. doi: 10.1097/00005650-200009002-00010. [DOI] [PubMed] [Google Scholar]
- 4.Chambers CT, Craig KD, Bennett SM. The impact of maternal behavior on children’s pain experiences: An experimental analysis. Journal of pediatric psychology. 2002;27(3):293–301. doi: 10.1093/jpepsy/27.3.293. [DOI] [PubMed] [Google Scholar]
- 5.Cook KF, Keefe F, Jensen MP, Roddey TS, Callahan LF, Revicki D, Bamer AM, Kim J, Chung H, Salem R. Development and validation of a new self-report measure of pain behaviors. PAIN®. 2013;154(12):2867–2876. doi: 10.1016/j.pain.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104(3):639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 7.DeWalt DA, Gross HE, Gipson DS, Selewski DT, DeWitt EM, Dampier CD, Hinds PS, Huang I-C, Thissen D, Varni JW. PROMIS® pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Quality of Life Research. 2015;24(9):2195–2208. doi: 10.1007/s11136-015-0953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeWalt DA, Rothrock N, Yount S, Stone AA. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45(5 Suppl 1):S12–21. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fordyce W. Behavioral methods for chronic pain and illness. Pain. 1977;3(3):291–292. [Google Scholar]
- 10.Fordyce WE, Brockway JA, Bergman JA, Spengler D. Acute back pain: a control-group comparison of behavioral vs traditional management methods. Journal of Behavioral Medicine. 1986;9(2):127–140. doi: 10.1007/BF00848473. [DOI] [PubMed] [Google Scholar]
- 11.Hamers JP, Abu-Saad HH, van den Hout MA, Halfens RJ, Kester AD. The influence of children’s vocal expressions, age, medical diagnosis and information obtained from parents on nurses’ pain assessments and decisions regarding interventions. Pain. 1996;65(1):53–61. doi: 10.1016/0304-3959(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 12.Hays RD, Kim S, Spritzer KL, Kaplan RM, Tally S, Feeny D, Liu H, Fryback DG. Effects of mode and order of administration on generic health-related quality of life scores. Value in Health. 2009;12(6):1035–1039. doi: 10.1111/j.1524-4733.2009.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haythornthwaite JA, Sieber WJ, Kerns RD. Depression and the chronic pain experience. Pain. 1991;46(2):177–184. doi: 10.1016/0304-3959(91)90073-7. [DOI] [PubMed] [Google Scholar]
- 14.Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal. 1999;6(1):1–55. [Google Scholar]
- 15.Irwin DE, Stucky B, Langer MM, Thissen D, DeWitt EM, Lai J-S, Varni JW, Yeatts K, DeWalt DA. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2010;19(4):595–607. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin DE, Stucky BD, Thissen D, DeWitt EM, Lai JS, Yeatts K, Varni JW, DeWalt DA. Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Quality of Life Research. 2010;19(4):585–594. doi: 10.1007/s11136-010-9618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaaniste T, Noel M, von Baeyer CL. Young children’s ability to report on past, future, and hypothetical pain states: a cognitive-developmental perspective. Pain. 2016;157(11):2399–2409. doi: 10.1097/j.pain.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson CJ, Farrell JE, Kashikar-Zuck S, Seid M, Verkamp E, DeWitt EM. Disclosure and self-report of emotional, social, and physical health in children and adolescents with chronic pain—a qualitative study of PROMIS pediatric measures. Journal of pediatric psychology. 2013;38(1):82–93. doi: 10.1093/jpepsy/jss099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson CJ, Kashikar-Zuck S, Farrell J, Barnett K, Goldschneider K, Dampier C, Cunningham N, Crosby L, DeWitt EM. Qualitative Evaluation of Pediatric Pain Behavior, Quality, and Intensity Item Candidates and the PROMIS Pain Domain Framework in Children With Chronic Pain. The Journal of Pain. 2015;16(12):1243–1255. doi: 10.1016/j.jpain.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keefe FJ, Bradley LA, Crisson JE. Behavioral assessment of low back pain: identification of pain behavior subgroups. Pain. 1990;40(2):153–160. doi: 10.1016/0304-3959(90)90066-M. [DOI] [PubMed] [Google Scholar]
- 21.Keefe FJ, Hill RW. An objective approach to quantifying pain behavior and gait patterns in low back pain patients. Pain. 1985;21(2):153–161. doi: 10.1016/0304-3959(85)90285-4. [DOI] [PubMed] [Google Scholar]
- 22.Keefe FJ, Smith S. The assessment of pain behavior: implications for applied psychophysiology and future research directions. Applied psychophysiology and biofeedback. 2002;27(2):117–127. doi: 10.1023/a:1016240126437. [DOI] [PubMed] [Google Scholar]
- 23.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Koho P, Aho S, Watson P, Hurri H. Assessment of chronic pain behaviour: reliability of the method and its relationship with perceived disability, physical impairment and function. Journal of rehabilitation medicine. 2001;33(3):128–132. doi: 10.1080/165019701750165970. [DOI] [PubMed] [Google Scholar]
- 25.Krause SJ, Wiener RL, Tait RC. Depression and pain behavior in patients with chronic pain. The Clinical journal of pain. 1994;10(2):122–127. doi: 10.1097/00002508-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Lai J-S, Stucky BD, Thissen D, Varni JW, DeWitt EM, Irwin DE, Yeatts KB, DeWalt DA. Development and psychometric properties of the PROMIS® pediatric fatigue item banks. Quality of Life Research. 2013;22(9):2417–2427. doi: 10.1007/s11136-013-0357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch-Jordan AM, Kashikar-Zuck S, Goldschneider KR. Parent perceptions of adolescent pain expression: The adolescent pain behavior questionnaire. PAIN®. 2010;151(3):834–842. doi: 10.1016/j.pain.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch-Jordan AM, Kashikar-Zuck S, Szabova A, Goldschneider KR. The interplay of parent and adolescent catastrophizing and its impact on adolescents’ pain, functioning, and pain behavior. The Clinical journal of pain. 2013;29(8):681. doi: 10.1097/AJP.0b013e3182757720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCahon S, Strong J, Sharry R, Cramond T. Self-report and pain behavior among patients with chronic pain. The Clinical journal of pain. 2005;21(3):223–231. doi: 10.1097/00002508-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Millsap RE, Yun-Tein J. Assessing factorial invariance in ordered-categorical measures. Multivariate Behavioral Research. 2004;39(3):479–515. [Google Scholar]
- 31.Muthén LK, Muthén BO. Mplus: Statistical analysis with latent variables: User’s guide, seventh edition. Muthén & Muthén; Los Angeles: 1998–2016. [Google Scholar]
- 32.Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: an integrative approach. Pain. 2005;119(1–3):1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 33.Prkachin KM, Schultz IZ, Hughes E. Pain behavior and the development of pain-related disability: the importance of guarding. The Clinical journal of pain. 2007;23(3):270–277. doi: 10.1097/AJP.0b013e3180308d28. [DOI] [PubMed] [Google Scholar]
- 34.Revicki DA, Chen W-H, Harnam N, Cook KF, Amtmann D, Callahan LF, Jensen MP, Keefe FJ. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146(1):158–169. doi: 10.1016/j.pain.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samejima F. Handbook of modern item response theory. Springer; 1997. Graded response model; pp. 85–100. [Google Scholar]
- 36.Schell KL, Oswald FL. Item grouping and item randomization in personality measurement. Personality and Individual Differences. 2013;55(3):317–321. [Google Scholar]
- 37.Terry R, Lee S, Milburn N. IRT-FIT: Fitting IRT models in SAS®. Technical Manual for Users. 2005 [Google Scholar]
- 38.Turk DC, Wack JT, Kerns RD. An empirical examination of the “pain-behavior” construct. Journal of Behavioral Medicine. 1985;8(2):119–130. doi: 10.1007/BF00845516. [DOI] [PubMed] [Google Scholar]
- 39.Varni JW, Magnus B, Stucky BD, Liu Y, Quinn H, Thissen D, Gross HE, Huang IC, DeWalt DA. Psychometric Properties of the PROMIS® Pediatric Scales: Precision, Stability, and Comparison of Different Scoring and Administration Options. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014;23(4):1233–1243. doi: 10.1007/s11136-013-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varni JW, Stucky BD, Thissen D, DeWitt EM, Irwin DE, Lai J-S, Yeatts K, DeWalt DA. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. The Journal of Pain. 2010;11(11):1109–1119. doi: 10.1016/j.jpain.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Baeyer C, Baskerville S, McGrath P. Everyday pain in three-to five-year-old children in day care. Pain Research & Management. 1998;3(2):111–116. [Google Scholar]
- 42.von Baeyer CL, Spagrud LJ. Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 years. Pain. 2007;127(1):140–150. doi: 10.1016/j.pain.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 43.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. PAIN®. 2009;143(3):223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani TA. Parent attention versus distraction: impact on symptom complaints by children with and without chronic functional abdominal pain. Pain. 2006;122(1):43–52. doi: 10.1016/j.pain.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters E, Stewart-Brown S, Fitzpatrick R. Agreement between adolescent self-report and parent reports of health and well-being: results of an epidemiological study. Child: care, health and development. 2003;29(6):501–509. doi: 10.1046/j.1365-2214.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- 46.Zumbo BD. A handbook on the theory and methods of differential item functioning (DIF): Logistic Regression Modeling as a Unitary Framework for Binary and Likert-Type (Ordinal) Item Scores. Ottawa: National Defense Headquarters; 1999. [Google Scholar]