Abstract
Introduction
Understanding the extent to which murine models of TBI replicate clinically relevant neurological outcomes is critical for mechanistic and therapeutic studies. We determined sensorimotor outcomes in mouse model of TBI and validated the use of a standardized neurological examination scoring system to quantify the extent of injury.
Materials and Methods
We utilized a lateral fluid percussion injury model of TBI, and compared TBI animals to those that underwent sham surgery. We measured neurobehavioral deficits using a standardized 12-point neurological examination, magnetic resonance imaging, a rotating rod test, and longitudinal acoustic startle testing.
Results
TBI animals had a significantly decreased ability to balance on a rotating rod and a marked reduction in the amplitude of acoustic startle response. The neurological exam had a high inter-rater reliability (87% agreement) and correlated with latency to fall on a rotating rod (R2 = 0.72).
Conclusions
TBI impairs sensorimotor function in mice, and the extent of impairment can be predicted by a standardized neurological examination.
Keywords: Traumatic brain injury, neurological assessment, injury severity score, sensorimotor function, acoustic startle response
INTRODUCTION
Each year, over 1.7 million Americans sustain a traumatic brain injury (TBI), which is known to be a contributing factor to over 30 % of all injury related deaths in the United States1, 2. Although improvements in emergency medicine and neurosurgical care have increased the survivability of TBI, survivors frequently endure a range of long-term cognitive and sensorimotor sequelae, or secondary injuries, including headaches, loss of concentration, difficulty recalling information, impaired coordination, decreased reaction time, aggressive behavior, frustration, anxiety, and even seizures3–6. These aforementioned symptoms of clinical outcomes after TBI can vary greatly among individuals and persist for long periods of time, due to variables which are poorly understood, and few currently available therapeutic options7.
Abnormalities in sensorimotor function that occur after TBI are of special interest to certain populations including professional athletes and military troops for whom even subtle changes in such function can be devastating. The acoustic startle response (ASR) paradigm has been used in both human and rodent models of TBI to screen for abnormalities in sensorimotor pathways. In several studies a decreased ASR was found after TBI in humans and rodents8–12. Several models of TBI have been established in rodents such as controlled cortical impact (CCI)13, controlled concussion14, the weight-drop method15, blast, and fluid percussion injury (FPI). In the FPI method, injury severity can be titrated to deliver a consistent focal force that produces long-term secondary injuries with significant neurological deficits16–18. It is important to understand the extent to which murine models of TBI replicate clinical outcomes, so that investigators can conduct studies into pathophysiological mechanisms and perform pre-clinical trials of potential therapeutic interventions.
Our study aimed to determine the natural history of sensorimotor outcomes in a mouse model of TBI. We used magnetic resonance imaging (MRI) techniques to visualize the brain after the insult and a standardized neurological and behavioral assessment scoring system to quantify the neurological deficit after surgery. We measured sensorimotor function both by determining latency on a rotating rod, and by measuring acoustic startle response amplitude. We also validate the utility of a rapid neurological scoring system to predict sensorimotor dysfunction following a TBI in a mouse model.
METHODS
Animals
Adult male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME), of 8–12 weeks of age, were allowed to acclimate to their cages and the facility for at least one week before experimentation. Mic were kept on a 12 hour light/dark cycle, with ad libitum access to food and water and were housed in groups of 5. All studies were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals (NIH), Guidelines for Survival Rodent Surgery (NIH), and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Vermont.
Fluid Percussion Injury and Survival Studies
A fluid percussion injury (FPI) method was used to induce a TBI, similar to that previously described by Larson et al19. Briefly, mice were anesthetized with 2–5 % isoflurane and the animal’s head was placed into a restraint. A craniotomy was performed at a point midway between lambda and bregma, approximately 2 mm lateral to the sagittal suture. A 3 mm diameter hole is drilled through the skull into the underlying extradural space using a variable speed power drill (Dremel, Racine, WI). A custom engineered stainless steel, hollow intracranial screw with a 2 mm internal diameter was fitted tightly into the burr hole. Once secured, the intracranial screw was filled with 0.9% normal saline and attached with tubing to the fluid percussion device. A pendulum was released to impact the fluid percussion device and induce TBI. Sham operated animals received anesthesia and a scalp incision only. During surgery the intracranial pressure and its instantaneous elevation by the fluid percussion wave were recorded using an in-line pressure transducer connected to a high-speed data acquisition unit (Instrumentation and Modeling Facility, University of Vermont, Burlington VT). The peak level of fluid percussion injury, equivalent to the measured intracranial pressure during percussion, was measured. Changing the height of the pendulum allowed titration of the level of injury in a stepwise fashion across groups of mice. We sought to determine a level of injury which was survivable in the majority of animals, but with demonstrable neurologic deficits. For each set of three mice, the level of injury was incrementally increased. FPI was started at 20 PSI for the first set and survivability was measured 48 hours after the injury. The FPI was then increased by 10 PSI for each set of mice, survivability was measured 48 hours after injury, and the process was repeated to a PSI of 50 which yielded 0 % survival. Mortality increased along with the incremental increases in PSI, and the level of injury used for all subsequent trials was 37 PSI which yielded 66 % survival. This intensity was chosen as it was between 40 and 35 PSI which yielded 100 % and 33 % survival, respectfully. All animals received 0.02mg/kg buprenorphine for analgesia while under anesthesia and 5mg/kg of meloxicam was used to treat post-operative pain, to reduce interference with behavioral studies. Animals were recovered from surgery and allowed access to food and water ad libitum.
Neurological Examinations and Assessment
Once the survivable level of injury was established, additional animals were utilized to develop and validate a neurological scoring system sensitive to TBI. To develop the scoring system, we performed serial neurological examinations on cohorts of TBI and sham animals in a pilot period. We chose to study the majority of animals which received an FPI at 37 PSI as this level of injury was survival (66 %) and generated demonstrable neurological deficits. In addition, a second subset of animals in the TBI group received a lower level of injury (20 PSI) to establish a correlation between the level of injury and the neurological assessment score. Using these two levels of injury allowed us to further validate the neurological scoring system at different degrees of FPI. To develop our scoring system, we selected those neurological examination features based on previously published literature for rodent brain injury, which could be performed “at the bedside”, without conditioning the animals or requiring special equipment20, 21. During the pilot phase, neurological examinations were performed in TBI and control animals prior to surgery and at longitudinal time points after surgery. The examination findings that were observed by multiple examiners in multiple TBI animals remained in the standardized examination (Table 1). Based on pilot survival studies and preliminary neurological scoring results, we chose 37 PSI as the level of injury for all further TBI experiments. This level of injury resulted in a consistent insult, with the majority of animals surviving, and clearly observable changes in neurological functions as quantified by the neuroscore.
Table 1. Neurobehavioral assessment of sham and TBI mice one day after surgery.
Neurological assessment scores between groups were broken into two sections which quantified motor and sensory deficits and behavioral findings. The inter-rater reliability (IRR) was calculated (agreement 87%) between examiners. The table describes the specific motor/sensory deficits and behavioral findings under normal and abnormal conditions. The total scores for the sham and TBI groups are shown in the right columns (n=12 for sham and TBI groups).
| Sensory/Motor Deficits | Normal | Abnormal | TBI | Sham |
|---|---|---|---|---|
| Grasps | 0) Strong / Strength unchanged | 1) Weak and decreased strength | 6 | 0 |
| Gait | 0) Balanced / Coordinated | 1) Unbalanced / Uncoordinated | 4 | 0 |
| Postural Reflex | 0) Present and attempted | 1) Absent / Not observed | 3 | 0 |
| Abnormal Position | 0) Absent / Not observed | 1) Paretic head tilt / contorted body | 2 | 0 |
| Edge Perception | 0) Present in both eyes | 1) present unilaterally or Absent bilaterally | 1 | 0 |
| Vision | 0) Reacts / Blinks / Turns away | 1) Unable to detect stimuli / Absent | 1 | 0 |
| Circular Motion | 0) Absent / Not observed | 1) Consistently moves counterclockwise or clockwise | 0 | 0 |
| Totals by group | 17 | 0 |
| Behavioral Findings | Normal | Abnormal | TBI | Sham |
|---|---|---|---|---|
| Posture (Pain) | 0) No pain observed | 1) Hunched posture, piloerect fur, squinted eyes, nose angled down & decreased locomotion | 9 | 4 |
| Apathy | 0) Normal response and exploration | 1) No Movement or response | 6 | 0 |
| Anxiety-like Behavior | 0) Freely moving about cage | 1) Avoidant and crouched in the corner | 6 | 1 |
| Aggressive Behavior | 0) Passive interaction with handler | 1) Attempt to bite handler | 1 | 3 |
| Grooming | 0) Grooming present | 1) Crust at eyes, oily, unkempt fur | 1 | 0 |
| Totals by group | 23 | 8 |
The first part of the examination assessed sensorimotor deficits. The first three evaluations were completed as passive observations of the mouse outside of the cage. Contortions of the body with or without a tilt of the head qualified as an abnormal position, which was marked as a positive finding. A gait deficit was considered present if the mouse was unable to maintain coordination while ambulating. If the mouse was observed to consistently ambulate around a fixed point in a clockwise or counter-clockwise fashion this was considered a positive finding for circular motion. The final four assessments required the evaluator to handle the mouse. To test the animal’s vision, the mouse was suspended by its tail and the investigator advanced a cotton-swab toward the left eye to test for corneal or menace reflex. A reaction or series of blinks to the cotton swab was considered normal, while no reflex to the cotton swab was considered a positive finding for visual deficit. In addition, while the mouse was suspended it was moved near the edge of a laboratory table to assess edge perception. A normal response is for the mouse to attempt to reach back to the table and a lack of this movement was considered a positive finding of edge perception deficit. While the mouse was suspended, the animal was tested for a postural reflex, which under normal conditions, consists of stretched fore and rear limbs as if the animal is preparing for a fall and lack of this posture was considered a positive mark for the postural reflex test. Grasp was tested as a surrogate measure of strength. This was completed by placing the mouse on the underside of the cage top from their home cage. The underside of the cage top has wire mesh, interlaced at 1 mm intervals, providing an optimal surface for the mouse to grasp. The cage top and the mouse were then inverted 10 to 12 inches above the home cage. Falling into the cage within 15 seconds of suspension indicated poor grasp strength and resulted in a positive mark for grasp deficit. Mice that maintained grasp and did not fall into the cage within 15 seconds were considered within normal limits for grasp strength.
The second part of the neurological exam assessed pain and abnormal behavior. Signs indicating pain included: hunched posture, piloerect fur, squinted eyes and decreased locomotion22–24. Presence of one or more of these findings was considered positive for the pain/posture portion of the neurological score. The apathy part of the neurological score was assessed through passive observation of the mouse. After the mouse was removed from its cage it was placed on a disposable drape for observation. Free exploration of the new environment was considered normal. Mice that did not freely explore were manually stimulated to ambulate by gentle pressure on the hind legs. The animals that did not explore or respond to stimulation received a positive mark for presence of apathy. Anxiety-like behavior was scored for those mice that actively and frantically avoided external stimulation. Mice that made several attempts to bite the examiner yielded a positive mark for aggressive behavior. The behavioral portion of the neurological score concluded with observations of the animal’s hygiene. Mice that appeared to have dusty, oily or unkempt fur indicating a lack of grooming received a positive mark for grooming deficit.
To establish the inter-rater reliability of this scoring system, two technicians, blinded to the treatment group and to other examiner’s findings, were asked to independently examine a cohort of TBI and sham animals 24 hours after surgery. The standardized neurological examination system was explained to the examiners, but they did not receive any other special training other than basic animal husbandry and handling experience. The examiners assessed each mouse using the 12-point neurological scoring system, and each component of the neurological score was categorized as present, marked as +1, or absent, marked as 0. A positive mark on the neurological score indicated an abnormality or impairment. The total neurological score for an individual mouse was determined by adding the score from each of the 12 tests. A total score of 12 indicated the highest morbidity, whereas a score of 0 indicated a recovered/healthy animal without observable deficits. The two sets of scores were later compared to determine the inter-rater reliability (IRR), which we report as percent agreement between the two reviewers.
Bodyweights
Bodyweights of all animals were measured during the course of the study. Each animal was weighed prior to surgery and then daily thereafter until euthanasia. Animals that lost > 2.5 g in 24 hours were injected with 3 ml of sterile 0.9 % saline subcutaneously in the tissue overlying the dorsal lumbar fascia to help prevent dehydration.
Magnetic Resonance Imaging
In a subset of mice, in vivo magnetic resonance imaging (MRI) was conducted with an Achieva 3.0 Tesla TX magnet (Philips Medical Systems International B.V., Best, Netherlands) under isoflurane anesthesia (2–5 %) 36 to 48 hours after surgery to demonstrate the extent of injury. A 16 channel Torso-XL receive coil (InVivo Corp., Gainesville, FL) was used in combination with a small single-channel e-Coil (Medtronic Inc., Minneapolis, MN) for image acquisition. Two axially-acquired pulse sequences were used to assess brain injury: T2-weighted (T2W) gradient spin echo (GRaSE) and fluid attenuated inversion recovery (FLAIR). The GRaSE acquisition was performed with TR=4266 ms, TE=80 ms, acquired matrix=200×190, field-of-view (FOV)=60 mm × 49 mm, slice thickness=0.8 mm, signal averages (NEX)=3. For the FLAIR sequence, images were acquired with TR=8000 ms, TE=125 ms, acquired matrix=200×171, FOV=60 mm × 49 mm, slice thickness=1 mm, NEX=2.
Sensorimotor Coordination
Sensorimotor coordination was assessed in mice using a Rota-Rod (Med-Associates, Georgia, VT) – a device that measures the length of time a mouse is able to stay on a rotating rod as previously described25. Animals were pre-acclimated to the Rota-Rod on the day prior to sham or TBI surgery. Twenty-four hours after surgery, animals were tested in five consecutive trials, for a maximum of 300 seconds each. The initial velocity of the rod was 4 RPM, and was accelerated every 10 seconds by 4 RPM until reaching 40 RPM. The amount of time spent on the rod before falling was recorded for each animal.
Acoustic Startle Response
Acoustic startle response (ASR) measurements were obtained using a stabilimeter chamber as previously described in mice26 and rats27. Briefly, the chamber was suspended between compression springs in a sound-attenuating cubicle, such that any movement in the chamber caused displacement of an accelerometer fixed to the bottom of the cage. Startle amplitude was defined as the maximal peak-to-trough voltage measured by the accelerometer during the first 200 ms after the stimulus onset. The startle response was evoked using computer generated 50 ms white-noise bursts (5 ms rise-decay). Data collection and the control and sequencing of all stimuli were controlled by Med-Associates startle reflex hardware and software (Georgia, VT).
A baseline startle test was administered 2 days prior to surgery in which mice were placed in the startle chambers and allowed a 5 min acclimation period. During this acclimation period mice were exposed to a continuous 60 dB background noise. Following the acclimation period the animal was then exposed to 30 noise bursts (as described above) which varied in acoustic intensity (95 dB, 100 dB, or 105 dB) with a 30 s inter-trial interval. Mice were tested for changes in baseline startle amplitude following sham and TBI surgery. Pre-sham startle baselines at 105 dB were used to divide the entire cohort into sham and TBI sub-groups. Animals were ordered by their average startle amplitude from largest to lowest amplitude. Grouping was then determined by alternating assignment in descending order and placing every-other animal into a group. The two groups were randomly designated to receive either a TBI or sham surgery. On days 3, 11, and 15 post-surgery, mice were returned to the startle chambers and tested as described above. The percent change in startle was calculated by normalizing every animal’s startle response after treatment (sham or TBI) with its pre-treatment startle response.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Differences between experimental groups in experiments were determined by a two-tailed non-parametric t-test (Mann-Whitney), a two-tailed paired t-test (Wilcoxon Rank Sum), ordinary one- or two-way analysis of variance (ANOVA) with a Bonferroni correction for multiple comparisons, or a Kruskal-Wallis test (one-way ANOVA on ranks) as needed. For correlation between the neurological assessment and Rota-Rod time, the Spearman correlation coefficient was calculated using non-parametric correlation analysis. Inter-rater reliability was determined by comparing the two independent evaluators’ exam scores in a separate “agreement” column and marking +1 when the two ratings for the same category matched and 0 when the evaluators differed in scoring. The agreement column was summed together to get a total agreement score, which was divided by 12, the total number of scoring categories. This fraction, the inter-rater reliability or agreement, was expressed as a percent. Any percent over 81% was considered to be of almost perfect or very good reliability28. All plots were generated in GraphPad Prism 6.0 (GraphPad Software, Lo Jolla, CA). In all analyses, P<0.05 was considered to indicate statistical significance.
RESULTS
Survival and Injury Titration
We sought to determine a level of FPI that resulted in a moderate TBI with demonstrable neurological deficits, but was survivable in the majority of mice at 48 hours post-surgery. All the animals subject to FPI at 20 or 30 PSI survived for more than 48 hours (Figure 1). For mice receiving an FPI of 40 PSI survival was 33 % and no mice recovered from surgery at 50 PSI. Thirty-five (35) PSI yielded 100 % survival. Thirty-seven (37) PSI was determined to be the ideal level of injury producing between 100 % and 33 % survival.
Figure 1. Fluid percussion injury (FPI) titration survival curve 48 hours after injury.
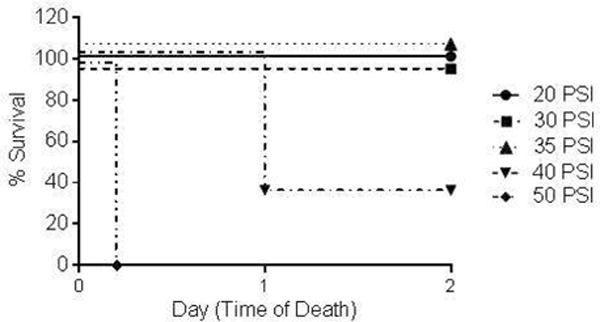
The legend shows the peak instantaneous intracranial pressure induced by the fluid wave (20 to 50 PSI). Survival in the 20, 30, and 35 PSI groups was 100%. In the 40 PSI group, survival was 33%, and 0% of the mice in the 50 PSI group survived (n=3 for all groups).
Neurological and Behavioral Examinations
We developed a standardized neurological exam to quantify the extent of neurological deficits after TBI. In the pilot period, we established 12 neurological findings, grouped as “sensory/motor deficits” or “behavioral findings”, more likely to be present in animals after TBI than in animals with a sham operation (scalp incision) alone (Table 1). The standardized examination was developed based on testing for these 12 findings and each finding received a score of +1 (present) or 0 (absent). The neurological score evaluation was completed by two independent examiners and inter-rater reliability (IRR) was determined to be at 87 % agreement. Increasing neuroscores correlated with increasing injury severity. The average total neurological scores 1 day after surgery for the three groups were: 0.7 ± 0.2 (sham, n=12), 1.4 ± 0.5 (moderate TBI, 20 PSI, n=13), and 3.3 ± 0.5 (severe TBI, 37 PSI, n=12), and the difference between the groups was statistically significant (P<0.05; Kruskal-Wallis test) (Figure 2A). These findings confirm that the neurological score is a rapid and reliable means for determination of the extent of the TBI. When we looked at specific features of the neuroscore, TBI mice at 37 PSI displayed significantly more abnormal findings in both the behavioral (1.9 ± 0.4) and sensory/motor (1.4 ± 0.3) subsets of the neurological assessment 1 day following surgery when compared to sham mice (0.7 ± 0.2; 0 ± 0, respectively) (Figure 2B, n=12 for both groups, P<0.05). TBI mice in the 20 PSI group (n=13) again produced higher neuroscores than the sham mice and lower scores than the 37 PSI TBI mice in both behavioral and sensory/motor tests (0.9 ± 0.1; 0.5 ± 0.1, respectively), and differences within the sub groups again were significantly different (P<0.05; Kruskal-Wallis test). The 37 PSI TBI group had a total score of 23 for behavioral and 17 for sensory/motor deficits compared to 12 behavioral and 6 sensory/motor deficits in the 20 PSI TBI group and 8 behavioral and 0 neurological deficits in the sham group. Both TBI and sham mice appeared to have a posture indicating pain, although TBI animals displayed this finding more than twice as often as sham animals. The most common sensory/motor deficit among TBI mice grasp. All of the remaining abnormal behavioral findings, aside from aggressive behavior, were more common in TBI than in sham mice. Gait and postural reflex were also common findings in TBI mice while the remaining abnormalities were not as common. These less frequent abnormalities were seen only in mice with more severe injuries (reflected by higher total neurological scores) and likely indicate a greater neurological insult. Based on the neuroscores and survival of the three treatment groups, a moderate level of TBI (37 PSI) was chosen for all subsequent experimental series.
Figure 2. Summary data for neurological assessments of sham and TBI groups 1 day post-surgery.
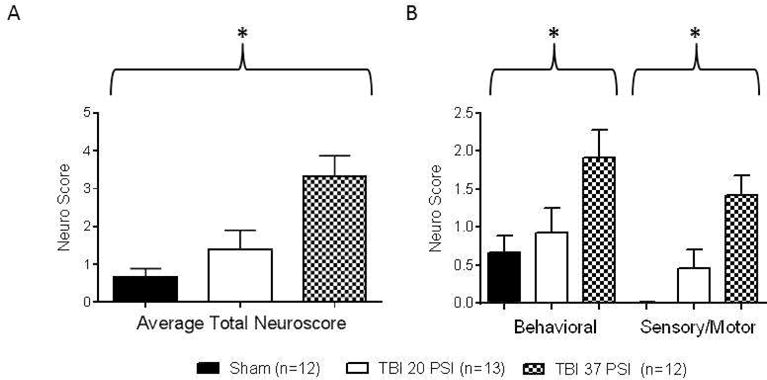
(A) The average total neurological assessment scores of behavioral findings and sensory/motor deficits one day after surgery increased along with increasing injury severity (sham, moderate TBI (20PSI) or severe TBI (37 PSI); group differences were significant (P<0.05, Kruskal-Wallis test). (B) The specific components (behavioral and sensory/motor) of the neurological outcome assessments were also significantly different between groups (P<0.05, Kruskal-Wallis test).
Bodyweights
The body weights of the sham and TBI treatment groups were not significantly different before surgery on “Day 0” (29 ± 0.5 g sham and 29 ± 0.7 g TBI, n=12 for both groups) (Figure 3). All subsequent bodyweight measurements were compared to the corresponding animal’s Day 0 baseline. One day after surgery TBI mice had lost significantly more weight from the previous day than the sham operated group (25.1 ± 0.5 g and 28.2 ± 0.4 g respectively, n=12 for both groups, P<0.05). Two days after surgery TBI mice were still significantly underweight when compared to the sham operated group (25 ± 0.8 g and 28.4 ± 0.4 g respectively, n=12 and n=11, P<0.05). Fifteen days after surgery, a recovery in body weight was observed in the TBI group to near baseline levels and was not significantly different when compared to the sham operated group (27.7 ± 0.8 g TBI and 29 ± 0.3 g sham respectively, n=12 and n=10, n.s.). Two animals from the TBI group died during the course of this study.
Figure 3. Summary data for average body weights of sham and TBI mice at 0; 1; 2; and 15 days after surgery.
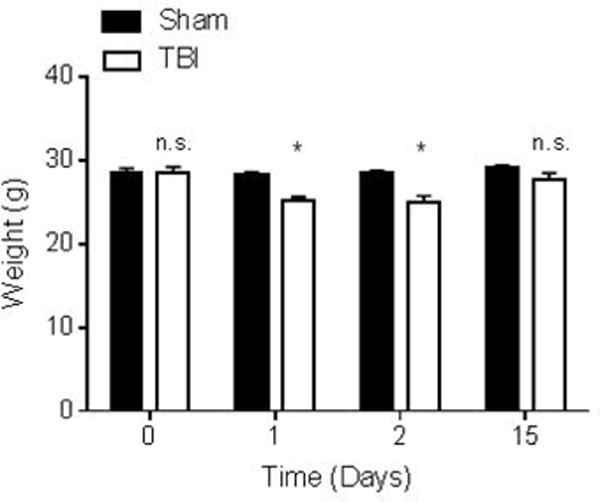
Average body weights at day 0 for sham (28.6 ± 0.5 g) and TBI (28.6 ± 0.7 g) (n=12 for both groups) are not significantly different. Average body weights at day 1 for sham (28.2 ± 0.4 g) and TBI (25.1 ± 0.5 g) (n=12 for both groups) are significantly different (P<0.05). Average body weights at day 2 for sham (28.4 ± 0.4 g) and TBI (25 ± 0.8 g) (n=12 sham and n=11 TBI) are significantly different (P<0.05). Average body weights at day 15 for sham (29 ± 0.3 g) and TBI (27.7 ± 0.8 g) (n=12 sham n=10 TBI) are not significantly different. Day 0 corresponds to 8 hours after surgery. The data are expressed as mean ± SEM in grams and ordinary two-way ANOVA with Bonferroni correction for multiple comparisons was used to compare groups. Bodyweights on days 1, 2, and 15 were compared to the corresponding animal’s baseline weight on day 0.
Magnetic Resonance Imaging
MRI of sham and TBI mice under isoflurane anesthesia shows the extent of cortical damage after FPI (Figure 4). T2 FLAIR images show intra-parenchymal hemorrhage extending from the site of the craniotomy (Figure 4A). In addition there is increased signal intensity on GRaSE sequences (Figure 4B and C) in the same hemisphere, consistent with cerebral edema without midline shift.
Figure 4. Magnetic Resonance Imaging (MRI) with Achieva 3.0T Tx magnet after TBI or sham surgery.
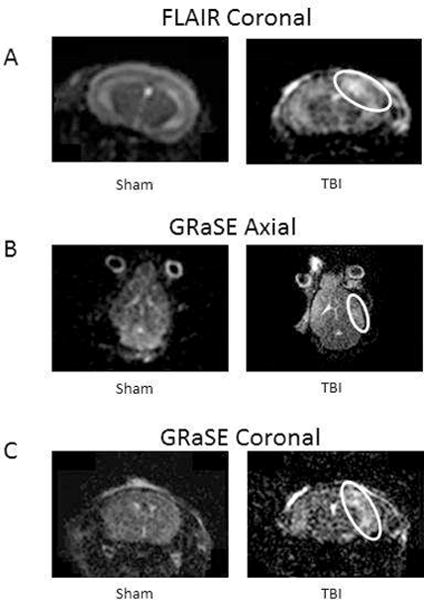
Imaging obtained from anesthetized mice 36–48 h after surgery. Representative sequences are shown with (white circles) indicating structural injury and cerebral edema (T2-weighted gradient and spin echo [GRaSE]).
Acoustic Startle Responses
On day 3 post-surgery, at 95 dB the sham group had an 11 ± 24 % (n=11) increase in ASR from baseline compared to a 65 ± 7 % (n=21) decrease in ASR from baseline in the TBI group. At 100 dB both groups decreased from baseline 29 ± 16 % (n=10) in shams compared to 61 ± 8 % (n=21) in TBIs, and at 105 dB the sham group increased 1 ± 34 % (n=11) compared to a 65 ± 7 % (n=21) decrease in the TBIs. On day 11 post-surgery, at 95 dB the sham group had a 22 ± 20 % (n=11) decrease in ASR from baseline compared to a 63 ± 6 % (n=21) decrease in ASR from baseline in the TBI group. At 100 dB both groups decreased as well from baseline 32 ± 14 % (n=10) in shams compared to 60 ± 7 % (n=21) in TBIs, and at 105 dB the sham group decreased 54 ± 8 % (n=10) compared to a 67 ± 6 % (n=21) decrease in the TBIs. On day 15 post-surgery, at 95 dB the sham group had a 41 ± 16 % (n=10) decrease in ASR from baseline compared to a 56 ± 8 % (n=21) decrease in the TBI group. At 100 dB both groups decreased 51 ± 15 % (n=10) in the shams compared to 59 ± 9 % (n=21) in the TBIs, and at 105 dB both groups decreased 45 ± 16 % (n=11) in the shams and 59 ± 10 % (n=21) in the TBIs.
Overall, TBI mice displayed a significantly diminished acoustic startle response at all acoustic intensities (95, 100, and 105 dB) compared to sham animals 3 and 11 days post-surgery (Figure 5A and 5B, P<0.05). However, on day 15 there was no significant difference in ASR between sham and TBI groups at any of the three acoustic intensities (Figure 5C) (Ordinary two-way ANOVA, P<0.05).
Figure 5. Acoustic startle response (ASR) in sham and TBI mice at 3, 11, and 15 days after surgery.
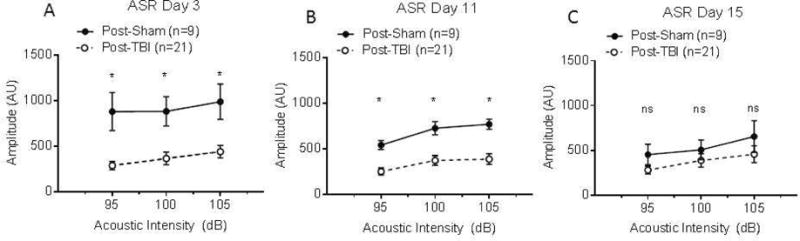
Sham (n=10–11) and TBI (n=21) mice were tested at 3, 11, and 15 days post-injury using three acoustic intensities per day (95, 100, and 105 dB) (Figure 5A–C). All intensities were significantly different between treatment groups on days 3 and 11 post-surgery (P<0.05). The ASR at all intensities for sham mice was not significantly different from TBI mice on day 15 post-surgery. The amplitude of the ASR was measured in arbitrary units (AU) and ordinary two-way ANOVA was used to compare groups.
Sensorimotor Coordination
As a result of neuronal damage confirmed by MRI, sensorimotor coordination was tested using the Rota-Rod and was found to be impaired in TBI mice 1 day after surgery. TBI mice exhibited a significantly impaired performance on this task (206 ± 41 s; n=9) when compared to sham mice (290 ± 5 s; n=13; Mann-Whitney P<0.05) (Figure 6C). Sham mice consistently ran the entire duration of each trial (300 s) with no significant difference being found between trials before and after sham surgery (Figure 6A; n=13; Wilcoxon Rank Sum, n.s.). Rota-Rod performance was significantly decreased between trials in TBI mice following injury (Figure 6B; n=9; Wilcoxon Rank Sum, P<0.05). Performance on this task by TBI mice was also correlated with poor neurological score outcomes; mice with higher neurological scores generally performed worse on the Rota-Rod task (Figure 7; n=9 mice; P<0.05; R = −0.809 Spearman correlation coefficient).
Figure 6. Sensorimotor coordination is impaired following TBI one day after injury.
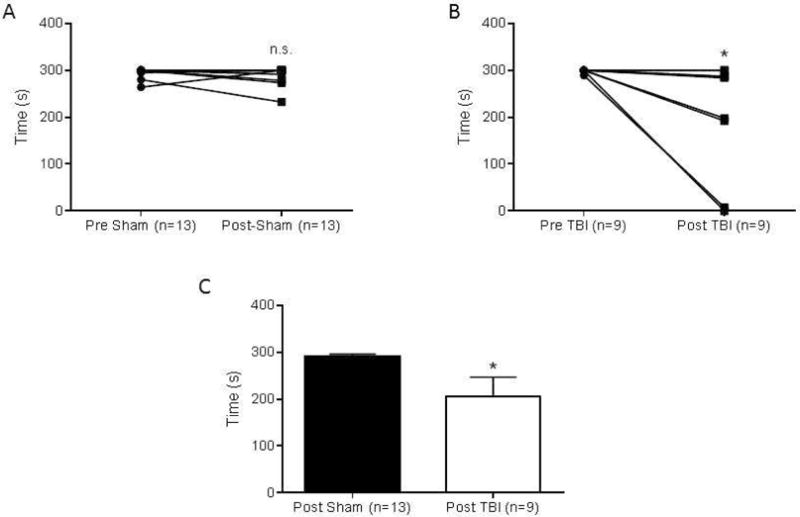
Each point represents the average of five trials per mouse. (A) No significant difference in latency to fall was observed in mice before and after sham surgery (n=13). (B) TBI mice exhibited significantly lower latency to fall times on the Rota-Rod after injury (n=9, P<0.05). (C) Rota-Rod times were 290 ± 5 s in the sham injury group (n=13) and 206 ± 41 s in the TBI group (n=9) which were significantly decreased. Numbers are expressed as mean ± SEM and the Mann-Whitney test was used to compare groups (P<0.05).
Figure 7. A higher neurological assessment score is correlated with impaired sensorimotor function.
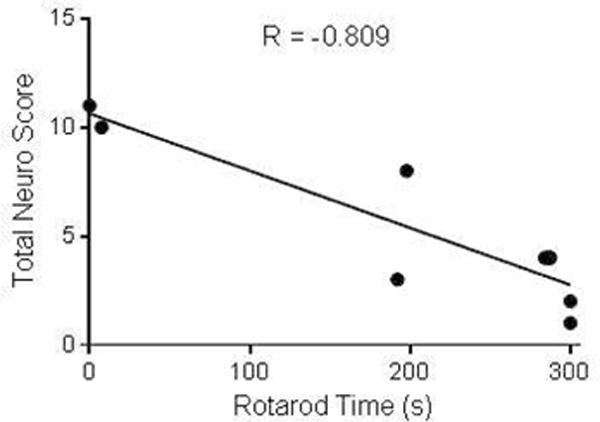
Correlation plot between total neuro score for TBI mice (n=9) and time on the Rota-Rod 1 day after injury. A higher neurological assessment score corresponded to a lower latency to fall on the Rota-Rod. The Spearman correlation coefficient was R = −0.809, P<0.05.
DISCUSSION
We have demonstrated that the fluid percussion method of injury with a PSI of 37 produces a survivable model of TBI in mice, which reproduced the effects of mild-to-moderate TBI on behavior and sensorimotor functions. The neurological score was successful in detecting these outcomes after surgery and helped provide a method for determining the severity and specific outcomes of the injury. MRI imaging confirmed neurologic insult, brain swelling, and edema in TBI mice providing a basis for reduced sensorimotor reactivity following injury.
Establishing the level of TBI induced by fluid percussion injury which is survivable in mice is an important contribution to the literature. Since survival was 33 % and lower at PSI levels higher than 37, we focused on this level of severity. Survivability of 33 % or lower was not acceptable due to concerns for animal welfare, experimental throughput, and costs. In addition, at PSI levels over 37, the mice that did survive had seizures, apnea, and were comatose making neurological assessments nearly impossible as there was nothing to measure. Thirty-seven (37) PSI was the ideal injury severity which yielded mice with measurable neurological deficits at an appropriate survival rate. We compared TBI at 37 PSI to sham operated mice in all our experiments except for the neurological examination, which we validated using an intermediate severity of 20 PSI. Using this intermediate injury severity also reaffirmed the correlation between injury severity and observable neurological deficits as demonstrated using our neurological scoring system. Our standardized neurological examination scoring system is rapid and easy to perform, and we demonstrated that it has an excellent inter-rater reliability and can discriminate between TBI and sham surgery. Other scoring systems have been previously published, such as a 100-point scoring system to determine neurological outcomes following subarachnoid hemorrhage in rats20. This scoring system requires more time and specialized equipment, and it has not been validated for the mouse. Another neurological scoring system is the Neurobehavioral Severity Score for Rodents (NSS-R) which does not report an IRR in a mouse model of TBI12, 21. We have improved upon these previous exams by focusing on highly relevant neurobehavioral outcomes present in mouse TBI, and furthermore we determined the IRR for our scoring system. Our rapid, 12-point scoring system can be conducted “at the bedside” in an efficient manner, and it does not require prior training or baseline testing of the mice. We show that this standardized examination detects neurobehavioral deficits caused by TBI, has a high IRR between examiners (87 % agreement) and correlates with the extent of sensorimotor impairment. This is useful as it allows the investigator a quick and consistent method to quantify injury severity before continuing on with the planned experimental series or treatment. Because there is inherent heterogeneity in every disease model, including TBI, having a rapid means to evaluate each animal post-injury can provide investigators with a tool to generate a consistent cohort for further testing. Behavioral deficits were occasionally observed in sham mice after surgery, but there were no motor or sensory deficits present. The behavioral findings in the sham animals were likely manifestations of stress from the scalp incision procedure or side effects of the analgesia. Implementation of this standardized examination will allow investigators to quantify injury in individual experimental animals after surgery, to compare the degree of injury across experimental groups and laboratories, and better assess the outcomes of experimental interventions.
Recent studies have shown that sensorimotor coordination measured as the ASR is altered in a rodent models of TBI but only one has utilized a mouse model 8, 11, 12. To take advantage of the powerful tools provided by genetic engineering, many investigators in the field of neurotrauma have shifted from rat to mouse models. These researchers are challenged by the limited published data available showing clinically relevant, neurological outcomes in murine survival models of TBI, which we have successfully demonstrated in this study. We show that TBI caused observable neurobehavioral deficits, decreased latency to fall on a rotating rod, and an impaired acoustic startle response after injury. The outcomes observed in the murine model are clinically relevant to human patients, as several studies have reported poor sensorimotor function and diminished ASR in human concussion and TBI patients9, 10. This is an important finding, as establishing an animal model which mirrors a human disease is the first step in translational research.
Our results suggest that the acoustic startle paradigm is an effective tool for detecting decreased sensorimotor reactivity following traumatic brain injury, as proposed by Wiley et al8. One advantage of using the startle paradigm is that the neural circuits underlying this behavior have been clearly delineated. Following the presentation of the startle stimulus, sound energy is transduced and relayed to the dorsal cochlear nucleus, the cochlear root nucleus, the ventral cochlear nucleus and the lateral superior olive. The projections of each structure converge on the caudal pontine reticular nucleus (PnC) and then to spinal motor neurons generating a reflexive response29, 30. Wiley et al found decreased sensorimotor reactivity after experimental TBI in rats and postulated a traumatic disruption in this pathway8. TBI is known to produce inflammation and subsequent glutamate excitotoxicity in the brain31–35 and Krase et al reported a role for both subtypes of ionotropic glutamate receptors in the PnC in the acoustic startle response in rats36. We hypothesize that the diminished ASR after TBI is due to a glutamate induced excitotoxicity in the PnC of the mouse.
Startle responses to the incremental increases in acoustic stimulus intensity in the TBI group were severely attenuated at 3, 11, and 15 days post-surgery, indicating chronic sensorimotor impairment following an injury. Although significantly elevated when compared to the TBI group on day 3 and 11, the sham-operated mice did show decreased ASR on day 11 and 15 when compared to the initial responses on day 3. On day 15 post-surgery there was no significant difference in the startle responses between sham and TBI groups; however, the TBI group remained severely diminished compared to the pre-injury baseline responses. The attenuation of the startle response in the sham group on day 15 post-surgery, and the overall decrease over the course of the study, could be attributed to long-term habituation to the startle stimulus37, 38 On day 15, TBI mice no longer showed a difference in bodyweight when compared to sham mice. It is recognized that the weight loss in the TBI group on days 1 and 2 post surgery was likely a combination of dehydration and/or loss of appetite. On day 15, TBI mice had regained bodyweight and showed no significant difference when compared to the shams; however, signs of sensorimotor impairment were still evident using the ASR paradigm. The TBI groups diminished startle amplitudes cannot be attributed to weight loss of the mice, since bodyweights had restored to baseline levels on day 15.
A limitation to our study is that although our neurological assessment has been validated at varying degrees of FPI, the system has not been compared to other models of TBI such as CCI, blast, or acceleration/deceleration. In this regard our exam is not generalizable to TBI as a whole as it would have to be validated across these other methods of injury. However, for FPI we maintain that it is an appropriate tool to quantify the extent of a fluid percussion injury.
In conclusion, these results will fill a knowledge gap in understanding the natural history of neurological dysfunction in mouse models of TBI, providing critical information for any investigators seeking to perform mechanistic or therapeutic research in this important area of surgical research.
Acknowledgments
We thank the University of Vermont Instrumentation and Model Facility (IMF) for their technical prowess and support with the fluid percussion apparatus.
FUNDING SOURCES
This work was supported by the Totman Medical Research Trust and the National Institute of Health (K08-GM-098795 and UM1-HL-120877).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
AMS, DS, NV, and LH drafted the article. AMS and DS performed animal surgery and collected data with assistance from CLS and SR. AMS and DS analyzed the data and generated the figures. SEH, and KF oversaw the project and provided conceptual guidance.
DISCLOSURES
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Faul MXL, Wald MM, Coronado VG. Traumatic brain injury in the united states: Emergency department visits, hospitalizations and deaths 2002–2006. 2010 [Google Scholar]
- 2.Marin JR, Weaver MD, Yealy DM, Mannix RC. Trends in visits for traumatic brain injury to emergency departments in the united states. JAMA. 2014;311:1917–1919. doi: 10.1001/jama.2014.3979. [DOI] [PubMed] [Google Scholar]
- 3.Alexander MP. Mild traumatic brain injury: Pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 4.Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. International review of psychiatry. 2003;15:341–349. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- 5.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM, Jr, Ricker JH. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001;16:343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: A neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatric disease and treatment. 2005;1:311–327. [PMC free article] [PubMed] [Google Scholar]
- 8.Wiley JL, Compton AD, Pike BR, Temple MD, McElderry JW, Hamm RJ. Reduced sensorimotor reactivity following traumatic brain injury in rats. Brain Res. 1996;716:47–52. doi: 10.1016/0006-8993(96)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Saunders JC, McDonald S, Richardson R. Loss of emotional experience after traumatic brain injury: Findings with the startle probe procedure. Neuropsychology. 2006;20:224–231. doi: 10.1037/0894-4105.20.2.224. [DOI] [PubMed] [Google Scholar]
- 10.Williams C, Wood RL. Affective modulation of the startle reflex following traumatic brain injury. J Clin Exp Neuropsychol. 2012;34:948–961. doi: 10.1080/13803395.2012.703641. [DOI] [PubMed] [Google Scholar]
- 11.Pang KC, Sinha S, Avcu P, Roland JJ, Nadpara N, Pfister B, Long M, Santhakumar V, Servatius RJ. Long-lasting suppression of acoustic startle response after mild traumatic brain injury. J Neurotrauma. 2015;32:801–810. doi: 10.1089/neu.2014.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A, Chandran R, Barry ES, Bhomia M, Hutchison MA, Balakathiresan NS, Grunberg NE, Maheshwari RK. Identification of serum microrna signatures for diagnosis of mild traumatic brain injury in a closed head injury model. PLoS One. 2014;9:e112019. doi: 10.1371/journal.pone.0112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: Cognitive and histopathologic effects. Journal of neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 14.Goldman H, Hodgson V, Morehead M, Hazlett J, Murphy S. Cerebrovascular changes in a rat model of moderate closed-head injury. Journal of neurotrauma. 1991;8:129–144. doi: 10.1089/neu.1991.8.129. [DOI] [PubMed] [Google Scholar]
- 15.Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part i: Pathophysiology and biomechanics. Journal of neurosurgery. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: Characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 17.Cernak I. Animal models of head trauma. NeuroRx: the journal of the American Society for Experimental NeuroTherapeutics. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabadi SV, Hilton GD, Stoica BA, Zapple DN, Faden AI. Fluid-percussion-induced traumatic brain injury model in rats. Nat Protoc. 2010;5:1552–1563. doi: 10.1038/nprot.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson BE, Stockwell DW, Boas S, Andrews T, Wellman GC, Lockette W, Freeman K. Cardiac reactive oxygen species after traumatic brain injury. J Surg Res. 2012;173:e73–81. doi: 10.1016/j.jss.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thal SC, Mebmer K, Schmid-Elsaesser R, Zausinger S. Neurological impairment in rats after subarachnoid hemorrhage–a comparison of functional tests. J Neurol Sci. 2008;268:150–159. doi: 10.1016/j.jns.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Yarnell AM, Barry ES, Mountney A, Shear D, Tortella F, Grunberg NE. The revised neurobehavioral severity scale (nss-r) for rodents. Curr Protoc Neurosci. 2016;75:9 52 51–59 52 16. doi: 10.1002/cpns.10. [DOI] [PubMed] [Google Scholar]
- 22.Roughan JV, Flecknell PA. Evaluation of a short duration behaviour-based post-operative pain scoring system in rats. Eur J Pain. 2003;7:397–406. doi: 10.1016/S1090-3801(02)00140-4. [DOI] [PubMed] [Google Scholar]
- 23.Peterson NC. Assessment of pain scoring. Contemporary topics in laboratory animal science / American Association for Laboratory Animal Science. 2004;43:74–76. [PubMed] [Google Scholar]
- 24.Hawkins P. Recognizing and assessing pain, suffering and distress in laboratory animals: A survey of current practice in the uk with recommendations. Lab Anim. 2002;36:378–395. doi: 10.1258/002367702320389044. [DOI] [PubMed] [Google Scholar]
- 25.Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The rotarod test: An evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. Journal of neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 26.Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mcpp on acoustic startle. Behav Neurosci. 2008;122:943–948. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- 27.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (pacap) and brain-derived neurotrophic factor (bdnf) mrna expression in the bed nucleus of the stria terminalis (bnst): Roles for pacap in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wongpakaran N, Wongpakaran T, Wedding D, Gwet KL. A comparison of cohen ‘s kappa and gwet ‘s ac1 when calculating inter-rater reliability coefficients: A study conducted with personality disorder samples. BMC Med Res Methodol. 2013;13:61. doi: 10.1186/1471-2288-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebert U, Koch M. Glutamate receptors mediate acoustic input to the reticular brain stem. Neuroreport. 1992;3:429–432. doi: 10.1097/00001756-199205000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Hinzman JM, Wilson JA, Mazzeo AT, Bullock MR, Hartings JA. Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J Neurotrauma. 2016 doi: 10.1089/neu.2015.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prow NA, Irani DN. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J Neurochem. 2008;105:1276–1286. doi: 10.1111/j.1471-4159.2008.05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntosh TK, Smith DH, Meaney DF, Kotapka MJ, Gennarelli TA, Graham DI. Neuropathological sequelae of traumatic brain injury: Relationship to neurochemical and biomechanical mechanisms. Lab Invest. 1996;74:315–342. [PubMed] [Google Scholar]
- 35.Dorsett CR, McGuire JL, DePasquale EA, Gardner AE, Floyd CL, McCullumsmith RE. Glutamate neurotransmission in rodent models of traumatic brain injury. J Neurotrauma. 2016 doi: 10.1089/neu.2015.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krase W, Koch M, Schnitzler HU. Glutamate antagonists in the reticular formation reduce the acoustic startle response. Neuroreport. 1993;4:13–16. doi: 10.1097/00001756-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Davis M, Wagner AR. Habituation of startle response under incremental sequence of stimulus intensities. J Comp Physiol Psychol. 1969;67:486–492. doi: 10.1037/h0027308. [DOI] [PubMed] [Google Scholar]
- 38.Lane ST, Franklin JC, Curran PJ. Clarifying the nature of startle habituation using latent curve modeling. Int J Psychophysiol. 2013;88:55–63. doi: 10.1016/j.ijpsycho.2013.01.010. [DOI] [PubMed] [Google Scholar]


