Abstract
Background
Acute rest SPECT-MPI has high predictive value for acute coronary syndrome (ACS) in emergency department patients. Prior studies have shown excellent agreement between rest/stress CT perfusion (CTP) and SPECT-MPI, but the value of rCTP in acute chest pain triage remains unclear. We sought to determine the diagnostic accuracy of early rCTP, incremental value beyond obstructive CAD (≥50% stenosis), and compared early rCTP to late stress SPECT-MPI in patients with CAD presenting with suspicion of ACS to the emergency department.
Methods and Results
In this pre-specified sub-analysis of 183 patients (58.1±10.2years, 33%female) we included patients with any CAD by CCTA from ROMICAT I. rCTP was assessed semi-quantitatively, blinded to CAD interpretation. Overall, 31 had ACS and 48 had abnormal rCTP. Sensitivity and specificity of rCTP for ACS were 48% (95%CI: 30-67) and 78% (95%CI: 71-85), respectively. rCTP predicted ACS [adjusted OR 3.40 (95%CI: 1.37-8.42), p=0.008] independently of obstructive CAD, and sensitivity for ACS increased from 77% (95%CI: 59%-90%) for obstructive CAD to 90% (95%CI: 74%-98%) with addition of rCTP (p=0.05). In a subgroup undergoing late rest/stress SPECT-MPI (n=81), CCTA/rCTP had non-inferior discriminatory value to CCTA/SPECT-MPI [AUC: 0.88 vs. 0.90; p=0.64] using a non-inferiority margin of 10%.
Conclusions
Early rCTP provides incremental value beyond obstructive CAD to detect ACS. CCTA/rCTP is non-inferior to CCTA/SPECT-MPI to discriminate ACS, and presents an attractive alternative to triage patients presenting with acute chest pain.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00990262.
Keywords: perfusion, emergency department, acute chest pain, computed tomography
Coronary CT angiography (CCTA) has emerged in recent years as an effective, efficient, and safe tool in the emergency department (ED) to evaluate low-intermediate risk patients with acute chest pain. 1, 2 Although a completely normal CCTA result provides excellent negative predictive value to exclude acute coronary syndrome (ACS), the sole reliance on the presence of obstructive CAD to identify ACS is less robust.3, 4 This can be due to pathophysiologic explanations such as luminal thrombosis followed by re-canalization, endothelial dysfunction with decreased coronary flow reserve, and vasospasm.3, 5-11 It can also be secondary to technical factors such as image degradation during arrhythmia, and inadequate resolution with heavily calcified plaque and branch vessel disease.12 In any of these situations, myocardial perfusion assessment can provide complementary physiologic data to improve the sensitivity for detecting ACS.
Earlier studies have presented convincing evidence for the use of myocardial perfusion defects on early resting SPECT-MPI performed during symptoms for prediction of prognosis in patients with acute chest pain.13, 14 Despite these promising results, most SPECT-MPI in the ED setting is performed after the clinical exclusion of MI/ACS via serial biomarkers and electrocardiography during a 12 hour stay in a chest pain observation unit, in the form of stress and rest SPECT on the following day.2, 15-17 This allows for the evaluation of reversible myocardial ischemia, which offers incremental diagnostic value, albeit at the expense of increased time and technical complexity. Moreover, the presence of stress-induced ischemia following serial negative troponins does not completely link the underlying CAD to the presenting symptoms as an ACS. However, resting myocardial ischemia or infarct can also be evaluated on routine clinical CCTA datasets by rCTP, and such studies have shown good diagnostic accuracy in identifying myocardial ischemia and infarct when compared to SPECT-MPI in both the acute 18-20 and stable outpatient settings.10, 11, 21, 22 For example, Gupta et al. found that single-phase rCTP analysis had a sensitivity of 91% and specificity of 94% for detection of an irreversible perfusion defect (myocardial infarction) on SPECT-MPI.20 Similarly, in a cohort of 76 patients presenting with acute chest pain to the ED, Feuchtner et al. found that rCTP assessment had a sensitivity of 92%, specificity of 95%, PPV of 80%, and NPV of 95% on a per-patient level versus late resting SPECT-MPI. 18 In the workup of stable outpatients, stress and rest CTP has been shown to have incremental diagnostic value to identify coronary disease amenable to therapy.23
Based on the results of early resting SPECT-MPI studies in the ED and the excellent agreement of CCTA with SPECT-MPI to identify perfusion deficits under rest/stress, we hypothesized that evaluation of resting myocardial perfusion on early CCTA in patients presenting with acute chest pain to the ED would improve prediction of ACS beyond obstructive CAD assessment. In addition, we hypothesized that early combined assessment of CAD and myocardial perfusion on CT would render excellent agreement with late rest/stress SPECT-MPI performed after clinical rule out of myocardial infarction.
Methods
Study population
Details of the ROMICAT I (Rule Out Myocardial Infarction Using Computer Assisted Tomography) observational trial of using CCTA in adults at low-to-intermediate likelihood of ACS presenting to the emergency department (ED) with acute chest pain but without objective signs of myocardial ischemia on initial electrocardiogram (ECG) or necrosis on initial biomarkers have been previously reported. Exclusion criteria included atrial fibrillation, serum creatinine > 1.3 mg/dl, and history of coronary artery disease (CAD). 3
Of the initial ROMICAT I cohort of 368 patients, we included 183 patients with presence of any CAD by CCTA; flow-chart of exclusion criteria is provided in Figure 1. Therefore, for this secondary analysis, we excluded all patients found to have no plaque on CCTA (i.e., those with completely normal coronary arteries), since none of those had ACS.3 Moreover, we excluded two subjects who had incomplete CCTA datasets to permit evaluation of resting CT perfusion. The institutional review board approved the study protocol, and all patients provided written informed consent.
Figure 1. Study Flow Diagram.
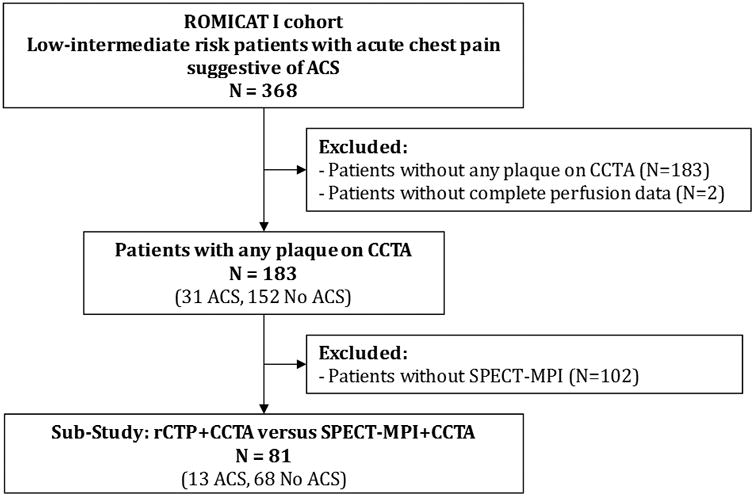
The original ROMICAT I (Rule out Myocardial Infarction with Computer-Assisted Tomography) consisted of 368 patients presenting with symptoms suggestive of acute coronary syndrome (ACS) to the ED. This secondary analysis evaluates the 183 patients with identifiable coronary artery disease (CAD) on CCTA, who had complete data for rest perfusion (rCTP) analysis. Sub-study analysis (n=81) was undertaken evaluating combined performance of rCTP/CCTA compared to combined performance of SPECT-MPI/CCTA.
Coronary CT Angiography (CCTA)
The 64-detector-row single-source CCTA protocol in ROMICAT I has previously been described. 24 The presence and extent of coronary atherosclerotic plaque for each coronary segment was determined using a modified 17-segment model of the coronary artery tree. Subjects were classified by blinded expert readers based on the presence and severity of CAD into the following strata: no CAD (no plaque), non-obstructive CAD, and obstructive CAD, and reported previously.3 Obstructive CAD was defined either as any epicardialcoronary artery stenosis with ≥50% luminal diameter obstruction or if coronary stenosis could not be confidently excluded (i.e. intention-to-diagnose paradigm), whereas non-obstructive CAD was defined as the presence of a coronary artery stenosis <50%.
Resting CT Myocardial Perfusion (rCTP)
Special care was employed to ensure that readers for rCTP were blinded to coronary artery evaluation images. This was achieved by displaying preformatted 8-mm-thick MPR images on the dedicated workstation, which preclude accurate assessment for coronary artery plaque and stenosis but are optimal for assessment of myocardial perfusion. 25 Readers were not allowed to display thin slice images in dedicated planes for coronary assessment. Image review was performed using anonymized DICOM datasets on a dedicated multiplanar workstation (TeraRecon, Aquarius Intuition Version 4.4.8, San Mateo, CA). Images were reconstructed as 8-mm thick average intensity projection images in short and long-axis planes, using a narrow starting window width of approximately 200 Hounsfield Units (HU) with a window level of 100 HU (i.e. 2:1 ratio).10, 25 Areas of myocardial hypo-attenuation were scored by myocardial territory26, 27 on a scale of 0-3 (0 = normal, with 1-3 representing increasing likelihood an area of hypo-attenuation represents a true perfusion defect as opposed to artifact, by consensus judgment of an experienced readers with at least 4 years experience as well as dedicated fellowship training in cardiac imaging (BG and AP). Specifically, score of 1= hypo-enhanced region more likely due to beam-hardening artifact, and not following a myocardial territory of a coronary artery, 2= hypo-enhanced region but does not clearly satisfy perfusion defect criteria described in 3, possibly due to degraded image quality, 3= hypo-enhanced region contained within a vascular region, extending from the subendocardial toward the epicardium. For analytic purposes, the rCTP results of 1, 2, and 3 were collapsed into one group representing abnormal rCTP. To help exclude artifact, multiple phases were available and evaluated after initial review of the default mid-diastolic phase reconstructed for coronary assessment.
SPECT-MPI
The SPECT-MPI protocol and analysis has been previously described by our group.28 Briefly, SPECT-MPI was performed using Tc-99m-MIBI according to American College of Cardiology/American Heart Association image acquisition guidelines using a standard one-day rest-stress protocol.29 Patients performed a treadmill exercise protocol or underwent pharmacological stress with intravenous adenosine (Adenoscan, Astellas Pharma US) at 140 mcg/kg/min for at least 3 minutes prior to radiotracer injection) if unable to exercise. SPECT-MPI was performed after complete serial biomarker and ECG evaluation. The nuclear MPR images were analyzed individually by 2 independent readers who were blinded to clinical history, CCTA results, and patient outcome, and discrepancies adjudicated by a third reader, as published previously. Analysis was performed in a semi-quantitative fashion using a standard AHA 17-segment model and a 5-point scoring system using a commercially available SPECT-MPI image analysis program (4DM SPECT, Ann Arbor, Michigan).27 Global summed scores were computed for the stress images (summed stress score [SSS], reflecting the combined extent and severity of ischemia plus scar) and rest images (summed rest score [SRS], reflecting the extent and severity of myocardial scar), as well as their difference (summed difference score [SDS], reflecting the combined extent and magnitude of myocardial ischemia). A SPECT-MPI result was considered to be abnormal if the SSS was ≥4 and/or the SDS ≥1. 30
Definitions of Test Results of Combined CCTA/rCTP and CCTA/SPECT-MPI
When both CCTA and rCTP were combined as a predictor (CCTA/rCTP) for ACS, a positive result was defined as there being either abnormal rest CT perfusion or the presence of obstructive CAD. CCTA/rCTP was negative if rCTP was normal, and obstructive CAD was not present on CCTA. This decision rule was proposed to optimize sensitivity and negative predictive value (NPV) for the purpose of more efficient triage of low-intermediate risk acute chest pain patients presenting to the Emergency Department. However, for completeness, we also assessed the diagnostic test characteristics using an alternate decision rule in which both abnormal rest CT perfusion and obstructive CAD were required to define a positive result: CCTA (+) and rCTP (+).
Similarly, when both CCTA and SPECT-MPI were combined as a predictor (CCTA/SPECT-MPI) for ACS, a positive result was defined as there being either abnormal SPECT-MPI (SSS≥4 or SDS≥1), or the presence of obstructive CAD. CCTA/SPECT-MPI was negative if SPECT-MPI was normal, and obstructive CAD was not present on CCTA.
Definition of ACS
The definition of ACS during the index hospitalization in the ROMICAT I cohort has been published previously.3 Briefly, ACS was defined as either an acute myocardial infarction or unstable angina pectoris. Establishment of this diagnosis was based on an outcome panel of two experienced physicians each with more than 10 years experience who reviewed prospectively collected patient clinical information as well as medical records during the index hospitalization. The outcome panel was blinded to the findings of CCTA, and disagreement was resolved by the adjudication of an additional cardiologist.
Statistical Analysis
Baseline demographics of patients with and without ACS were compared with use of independent sample t-test for continuous variables, Fisher's exact test for categorical variables, and the Wilcoxon rank-sum test for ordinal variables. Continuous variables were expressed as mean ± standard deviation; categorical variables were described by frequency. All analyses were performed using Stata (Version 13.1, StatCorp, College Station, Texas). A multivariable logistic regression analysis was performed to assess whether rCTP predicted ACS independent of patient characteristics (TIMI score) and obstructive CAD, and an area under the curve (AUC) analysis was employed to determine whether rCTP is incremental to the presence of obstructive CAD. 31
We compared the combined strategies of CCTA/SPECT-MPI and CCTA/rCTP for the detection of ACS in a non-inferiority analysis32 in the subset of patients that later underwent SPECT-MPI (n=81). (Figure 1) Non-inferiority was pre-specified as a maximal difference of the AUC between a CCTA/SPECT-MPI and CCTA/rCTP strategy of 10%.
Results
Baseline characteristics of our analytic study cohort (n=183) are shown in Table 1, stratified by outcome of ACS. There were 31 patients with ACS (MI: n=8 had MI, unstable angina: n=23), and 152 patients without ACS during the index hospitalization. The TIMI risk score was significantly higher in the subgroup of patients with ACS during the index hospitalization compared to those without ACS. All 183 patients had CAD by CCTA, among them 67 patients with obstructive CAD (≥50% stenosis). As reported previously, presence of obstructive CAD by CCTA had a sensitivity of 77% (24/31) for ACS. 3
Table 1. Patient Demographics and Risk Factors in Patients with and without ACS.
| ACS (N = 31) | No ACS (N = 152) | P-Value* | |
|---|---|---|---|
| Age (yrs) | 62.1 ± 12.6 | 57.3 ± 11.2 | 0.05 |
| Male sex (%) | 23 (74.2) | 100 (65.8) | 0.41 |
| BMI (kg/m²) | 28.8 ± 4.4 | 29.5 ± 6.2 | 0.47 |
| Cardiovascular risk factors (%) | |||
| Hypertension | 20 (64.5) | 77 (49.3) | 0.17 |
| Diabetes mellitus | 5 (16.1) | 22 (14.5) | 0.78 |
| Hyperlipidemia | 18 (58.1) | 75 (49.3) | 0.43 |
| Smoking | 17 (54.8) | 86 (56.6) | 1.00 |
| No. of CV risk factors (%) | 0.49 | ||
| 0 or 1 | 35.5 | 45.4 | |
| 2 or 3 | 64.5 | 50.0 | |
| 4 | 0.0 | 4.6 | |
| TIMI Score (%) | 0.004 | ||
| 0 | 12.9 | 38.8 | |
| 1 | 35.5 | 34.2 | |
| 2 | 22.6 | 21.1 | |
| 3 | 29.0 | 5.9 |
Values are expressed as mean (SD) or as percentages as indicated.
Comparisons between groups were performed with the use of an independent sample t-test for continuous variables, Fisher's exact test for categorical variables, and the Wilcoxon rank-sum test for ordinal variables.
ACS= Acute Coronary Syndrome; BMI= Body Mass Index; CV= cardiovascular; TIMI=Thrombolysis In Myocardial Infarction.
Resting CT Myocardial Perfusion
For the purpose of this analysis, we dichotomized the rCTP findings into either normal or abnormal (score ≥1). CCTA based findings of CAD and resting myocardial perfusion in those with and without ACS are depicted in Table 2. rCTP defects were identified in 48/183 (26%) patients, with sensitivity for detection of ACS of 48% (15/31) and specificity of 78%. The sensitivity of abnormal rCTP was 38% (3/8) for MI and 52% (12/23) for unstable angina pectoris The majority of rCTP defects identified were in the LAD coronary territory (66%). Among 549 total coronary territories examined (183 patients × 3 coronary territories each), there was agreement between abnormal rCTP findings and CCTA-detected obstructive CAD in 75% (410/549) of coronary territories (results not shown). Representative case examples are depicted in Figures 2-4.
Table 2. CCTA and Early Rest CT Perfusion Findings.
| ACS (N = 31) | No ACS (N = 152) | P-value* | |
|---|---|---|---|
| CCTA findings, N (%) | <0.001 | ||
| Non-obstructive CAD | 7 (22.6) | 109 (71.7) | |
| Obstructive CAD | 24 (77.4) | 43 (28.3) | |
| rCTP findings, N (%) | 0.003 | ||
| No defect | 16 (51.6) | 119 (78.3) | |
| Any defect | 15 (48.4) | 33 (21.7) | |
| rCTP score, N (%) | 0.001 | ||
| Score=0 | 16 (51.6) | 119 (78.3) | |
| Score=1 | 7 (22.6) | 21 (13.8) | |
| Score=2 | 3 (9.7) | 6 (4.0) | |
| Score=3 | 5 (16.1) | 6 (4.0) |
Percentages in each cohort are represented in parentheses.
Comparisons between groups were performed with the use of Fisher's exact test or Wilcoxon Rank Sum test.
ACS= Acute Coronary Syndrome; CCTA= Coronary CT Angiography; rCTP= resting CT perfusion analysis; CAD= coronary artery disease
Figure 2. Agreement between coronary stenosis severity and resting CT perfusion.
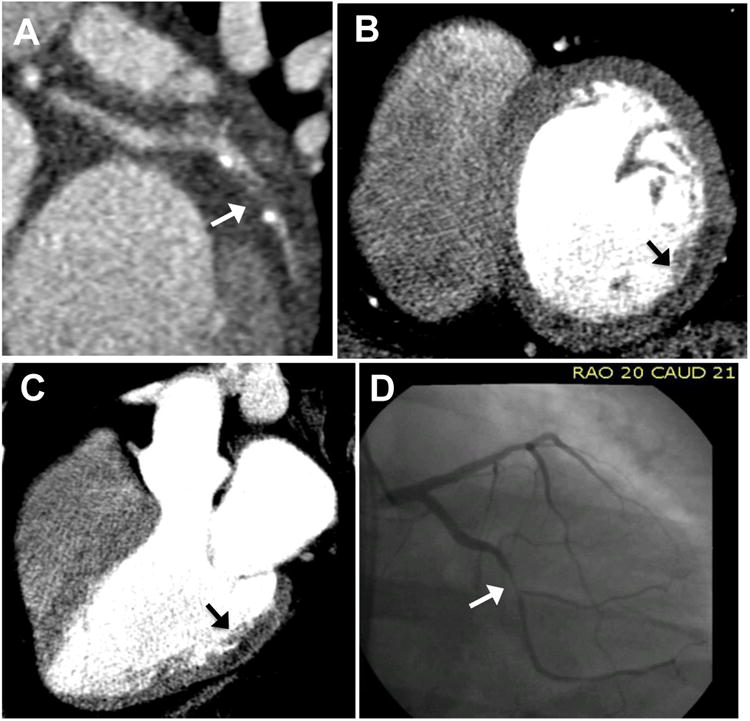
Coronary CT angiography reveals obstructive CAD (arrow) with severe stenosis of the left circumflex artery (A). Resting CT perfusion 8-mm thick MPR short-axis reconstruction demonstrates a subendocardial to mid-myocardial mid-basal inferolateral rest perfusion defect (arrow) (B) which is confirmed in the 3-chamber view (C). This 39-year-old man subsequently underwent SPECT-MPI which revealed an inferolateral fixed stress and rest defect with peri-infarct ischemia (not shown). Invasive coronary angiography (D) revealed a 95% left circumflex stenosis.
Figure 4. Discordance between coronary anatomy and resting CT perfusion.
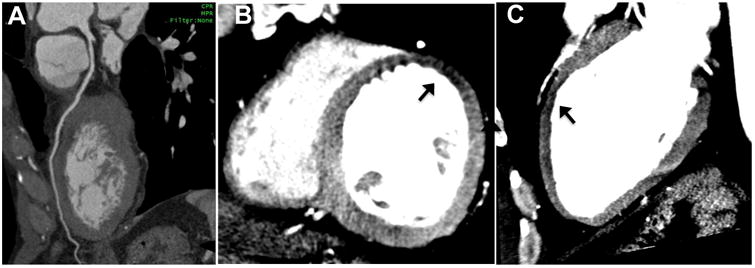
Curved multiplanar reconstruction CTA image reveals no CAD along LAD (A), but resting CT perfusion analysis (B and C) using 8-mm thick MPR demonstrates a mid-anterior rest perfusion defect (arrows) in short-axis and 2-chamber views. In this 52-year-old man presenting with chest pain, serial troponin was mildly elevated leading to urgent invasive coronary angiography (not shown) which revealed mild narrowing of the LAD that resolved with intra-coronary nitroglycerin, consistent with coronary vasospasm.
The overall relationship between abnormal resting CT perfusion, obstructive CAD on CCTA, and presence of ACS is displayed in Figure 5. Of note, there were only three subjects that would have been “missed” even with incorporating analysis of rCTP, including two with unstable angina and one with NSTEMI. (Supplemental Table).
Figure 5. Distribution of Abnormal rCTP and Obstructive CAD in relation to ACS in 183 subjects with CAD in the ROMICAT I trial.
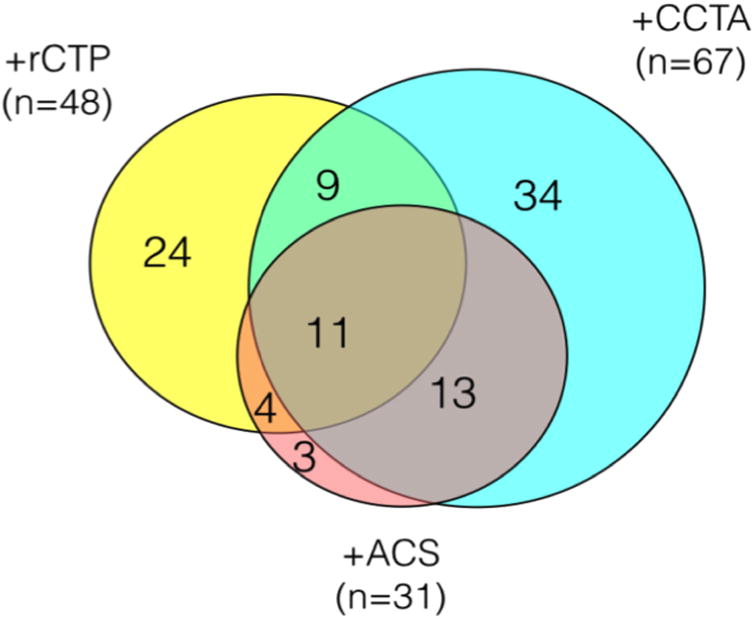
Key: +rCTP: Abnormal rCTP with possible perfusion defect (score≥1); +CCTA: Obstructive (≥50%) plaque or indeterminate stenosis; +ACS: Adjudicated endpoint of MI or unstable angina
In multivariable logistic regression abnormal rCTP independently predicted ACS [adjusted OR 3.4 (95%CI:1.4-8.4), p=0.008] after adjusting for obstructive CAD and TIMI risk score (Table 3). Moreover, combined assessment of CAD and perfusion increased sensitivity of CCTA from 77% (95%CI: 59%-90%) for obstructive CAD alone to 90% (95%CI: 74%-98%) with addition of rCTP (p=0.05), using the pre-specified decision rule for a positive result being either obstructive CAD or abnormal rCTP.). Furthermore, an AUC comparison revealed that abnormal rCTP is incremental to the CCTA presence of obstructive CAD [CCTA: 0.75 (95%CI: 0.66-0.83); CCTA/rCTP : 0.80 (95%CI: 0.72-0.88), p=0.01] in discriminating the presence from the absence of an ACS. However, this occurred at the expense of decrease in specificity from 72% (for CCTA alone) to 56% (combined)(Table 4).
Table 3. Multivariable Logistic Regression Analysis for Prediction of ACS.
| Odd Ratio (95%CI) | P-value | |
|---|---|---|
| CCTA + | 6.8 (2.5-18.5) | <0.001 |
| rCTP + | 3.4 (1.4-8.4) | 0.008 |
| TIMI Score | 1.5 (0.9-2.3) | 0.11 |
Multivariable Logistic Regression Model adjusting for CCTA(+) defined as presence of stenosis≥50%, and TIMI score. ACS=Acute Coronary Syndrome; CCTA= Coronary CT Angiography; rCTP= resting CT perfusion analysis; TIMI=Thrombolysis In Myocardial Infarction score
Table 4. Diagnostic Test Characteristics of Combined CCTA and Rest CT Perfusion Analysis for Prediction of ACS.
| Sensitivity (95%CI) | Specificity (95%CI) | PPV (95% CI) | NPV (95% CI) | Balanced Accuracy* | |
|---|---|---|---|---|---|
| CCTA (+) | 77.4 (58.9-90.4) | 71.7 (63.8-78.7) | 35.8 (24.5-48.5) | 94.0 (88.0-97.5) | 74.6 (66.3-82.9) |
| rCTP (+) | 48.4 (30.2-66.9) | 78.3 (70.9-84.6) | 31.3 (18.7-46.3) | 88.1 (81.5-93.1) | 63.3 (53.8-72.9) |
| CCTA (+) or rCTP (+) | 90.3 (74.2-98.0) | 55.9 (47.6-64.0) | 29.5 (20.6-39.7) | 96.6 (90.4-99.3) | 73.1 (66.5-79.7) |
| CCTA (+) and rCTP (+) | 35.5 (19.2-54.6) | 94.1 (89.1-97.3) | 55.0 (31.5-76.9) | 87.7 (81.7-92.3) | 64.8 (56.0-73.5) |
ACS= Acute Coronary Syndrome; CCTA= Coronary CT Angiography; CCTA(+) refers to obstructive (≥50%) or indeterminate; rCTP (+) possible perfusion defect (score≥1); rCTP=resting CT perfusion analysis;
Balanced Accuracy = 0.5 * (SE + SP) = AUC
Of note, by using the alternate decision of a positive result being both obstructive CAD and abnormal rCTP, specificity was increased from 72% (95%CI: 64-79) to 94% (95%CI: 89-97), at the expense of significantly reduced sensitivity of only 36% (95%CI: 19-55).
Sub-study Comparison of rCTP to SPECT-MPI
Among the subset of patients that underwent SPECT-MPI (n=81), 13 subjects had ACS. Diagnostic test results stratified by ACS outcome and test accuracy are shown in Tables 5 and 6. CCTA/rCTP had a sensitivity of 100%, specificity of 50%, NPV of 100%, and PPV of 28% for the detection of ACS in this subgroup. CCTA/SPECT-MPI had a sensitivity of 92%, specificity of 62%, NPV of 98%, and PPV of 32% for the detection of ACS in this subgroup.
Table 5. CCTA and Rest CT Perfusion Findings in the 81 Subjects Undergoing Later SPECT-MPI.
| ACS (N = 13) | No ACS (N = 68) | P-Value* | |
|---|---|---|---|
| CCTA, N (%) | <0.001 | ||
| Non-obstructive CAD | 1 (7.7) | 48 (70.6) | |
| Obstructive CAD | 12 (92.3) | 20 (29.4) | |
| rCTP, N (%) | 0.050 | ||
| No defect | 6 (46.2) | 51 (75.0) | |
| Any Defect | 7 (53.8) | 17 (25.0) | |
| rCTP score, N (%) | 0.032 | ||
| Score = 0 | 6 (46.2) | 51 (75.0) | |
| Score = 1 | 4 (30.8) | 12 (17.7) | |
| Score = 2 | 2 (15.4) | 2 (2.9) | |
| Score = 3 | 1 (7.7) | 3 (4.4) | |
| SPECT-MPI: SSS, N (%) | <0.001 | ||
| 0-3 | 4 (30.8) | 61 (89.7) | |
| 4-8 | 2 (15.4) | 3 (4.4) | |
| 9-13 | 3 (23.1) | 3 (4.4) | |
| >13 | 4 (30.8) | 1 (1.5) | |
| SPECT-MPI: SDS, N (%) | <0.001 | ||
| 0 | 7 (53.9) | 65 (95.6) | |
| 1-3 | 2 (15.4) | 2 (2.9) | |
| 4-7 | 1 (7.7) | 0 (0.0) | |
| >8 | 3 (23.1) | 1 (1.5) |
rCTP perfusion defect were scored as normal or abnormal (1-3) with scores of 1 to 3 representing increasing reader confidence that an area of hypo-attenuation represented a true perfusion defect.
Comparisons between groups were performed with the use of Fisher's exact test or Wilcoxon Rank Sum test.
ACS= Acute Coronary Syndrome; CCTA=Coronary CT Angiography; rCTP=resting CT perfusion analysis; SPECT-MPI=Single-photon emission computed tomography-myocardial perfusion imaging; SSS=Summed Stress Score; SDS=Summed Difference Score.
Table 6. Diagnostic Accuracy of CCTA, rCTP, and combined predictors for ACS in the 81 Subjects Undergoing Later SPECT-MPI.
| Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | Balanced Accuracy | |
|---|---|---|---|---|---|
| CCTA (+) | 92.3 (64.0-99.8) | 70.6 (58.3-81.0) | 37.5 (21.1-56.3) | 98.0 (89.1-99.9) | 81.4 (72.1-90.8) |
| rCTP (+) | 53.8 (25.1-80.8) | 75.0 (63.0-84.7) | 29.2 (12.6-51.1) | 89.5 (78.5-96.0) | 64.4 (49.4-79.4) |
| SPECT (+) | 69.2 (38.6-90.9) | 88.2 (78.1-94.8) | 52.9 (27.8-77.0) | 93.8 (84.8-98.3) | 78.7 (65.1-92.3) |
| CCTA (+) or rCTP (+) | 100.0 (75.3-100.0) | 50.0 (37.6-62.4) | 27.7 (15.6-42.6) | 100.0 (89.7-100.0) | 75.0 (69.0-81.0) |
| CCTA (+) or SPECT (+) | 92.3 (64.0-99.8) | 61.8 (49.2-73.3) | 31.6 (17.5-48.7) | 97.7 (87.7-99.9) | 77.0 (67.5-86.6) |
Key: CCTA(+) obstructive (≥50%) or indeterminate; rCTP (+) possible perfusion defect (score≥1); SPECT (+) if SSS ≥ 4 or SDS ≥1.
To test whether CCTA/rCTP is non-inferior to CCTA/SPECT-MPI for the detection of ACS, we compared the AUC for both strategies. The AUC for CCTA/SPECT-MPI was 0.88 (95%CI: 0.81-0.96), and the AUC for CCTA/rCTP was 0.90 (95%CI: 0.79-1.00). Therefore, the AUC analysis met the pre-specified margin of non-inferiority between the strategies of 10% (i.e. the lower bound of the CI of CCTA/rCTP, 0.79, exceeded the non-inferiority threshold of 0.78 = 0.88 – 0.10).
Discussion
Our findings demonstrate that early resting CT myocardial perfusion analysis provides incremental value for prediction of ACS to obstructive CAD assessment by CCTA in low-intermediate risk patients presenting to the ED. In addition, a combined early CCTA and rCTP analysis is non-inferior to CCTA and late (after exclusion of MI) SPECT-MPI to predict ACS. Hence, comprehensive early CCTA including rest perfusion assessment has the potential to improve efficiency of CCTA in a safe manner without additional cost, infrastructure, radiation exposure, or contrast administration.
Our findings are consistent with previous trials of SPECT-MPI in the ACS exclusion setting demonstrating safe triage and high accuracy for ACS diagnosis, when testing was performed as early as possible after ED presentation. Our results support the concept that analysis of CT resting first pass perfusion during initial chest pain evaluation provides incremental value to anatomic CTA data to include or exclude ACS.13 The current study also extends previous work by our group and others in evaluating resting perfusion defects in smaller cohorts of higher-risk acute chest pain patients.33-35 In comparison to these studies in which the majority had ACS and underwent cardiac catheterization, our study cohort was at low-intermediate risk for ACS. On the other hand, a recent study by Branch et al represents a similar patient population (9 ACS events in 105 patients). 36 However, they had only 3 patients with abnormal resting perfusion and showed improved specificity but reduced sensitivity when added to stenosis assessment. In contrast, we had a more granular assessment of myocardial perfusion abnormality (reporting on a 1-3 scale of confidence in there being a true perfusion defect) and therefore had more patients with abnormal perfusion, and our focus was on improving triage of patients with CAD in the ED setting, or optimizing sensitivity and NPV rather than specificity.
Overall, our results suggest that combined CCTA/rCTP is non-inferior to CCTA/SPECT-MPI to discriminate ACS, and hence presents an attractive alternative to accurately triage and manage patients presenting with acute chest pain to the ED found to have any plaque by CCTA. (Those without any plaque were shown to have a 0% ACS and hence were not part of this study cohort.) The improved sensitivity/NPV of CCTA/rCTP would result in less “missed ACS” cases being discharged to home. In addition, the non-inferiority of CCTA/rCTP to CCTA/SPECT-MPI for detection of ACS (based upon AUC) suggests that assessment of rCTP in our cohort would be an efficient use of resources without the additional cost and radiation exposure of sequential diagnostic testing.
However, our study is not without limitations. Our cohort is modest in size, with a relatively small number of events, particularly in our subgroup analysis. Nevertheless, our outcomes were adjudicated events that were independent of any findings on CCTA, and we succeeded to show non-inferiority of combined CCTA/rCTP compared to CCTA/SPECT-MPI. In addition, the ROMICAT I participants were recruited between 2005 and 2007, and CCTA technical parameters have significantly improved over the last several years. For example, arrhythmia, calcium burden, and even renal insufficiency play a decreasing role in CCTA contraindications in the era of dual-source computed tomography, arrhythmia rejection algorithms, single-heartbeat imaging, and iterative reconstruction algorithms. However, the ROMICAT I cohort was performed with 64-detector row CCTA, which is still considered an acceptable minimum standard for clinical CCTA.
Several adjunctive techniques have emerged in recent times that promise to offer improvements in the diagnostic profile of traditional CCTA. Each of these is not without limitations, but merit discussion and evaluation, particularly with an eye toward a practical implementation in the acute chest pain population. Perhaps the most widely publicized development is fractional flow reserve with CT (FFR-CT) utilizing computational fluid dynamic modeling to predict the hemodynamic consequence of anatomic lesions. While initial results were only somewhat comforting, additional generations of the proprietary algorithm have demonstrated improved results. 37-39 However, this method demands pristine datasets, and requires off-site processing by experts and high-level processing workstations, thus eliminating the possibility of timely disposition decisions based on results in the ED setting. Stress and rest perfusion CT is a second promising technique, but with it come all the practical limitations of stress and rest SPECT MPI in the ED setting: for safety reasons, most hospitals require more than one set of cardiac enzymes and ECG before allowing a patient to proceed to a stress exam, given concerns for ischemia even with pharmacologic stress (in the past years pharmacologic stress agents have even been issued “black box” warnings by the Food and Drug Administration due to concerns for causing ischemia in rare cases). Dual energy has been proposed as a potential answer to resting (and stress) perfusion; while this technique may alleviate some issues by allowing a more quantitative evaluation of myocardial enhancement, it is neither widely available on all platforms, nor is it validated to independently improve diagnostic perfusion evaluation beyond single-energy CTP. Additional methodologies such as wide-area-detectors, advanced iterative reconstructions and beam-hardening corrections, and even spectral CT are available or on the horizon, but none are yet validated across multiple vendor platforms. Thus, though our cohort was scanned on relatively common and decidedly aging CT technology, we feel that our cohort represents a reasonable minimum standard for modern ED CCTA practice.
In the era of radiation dose reduction, cost containment, and increasing use of prospective ECG-triggering (thus forgoing the potential for wall motion analysis) our findings are of particular interest. However, further larger scale studies including randomized controlled trials are necessary to confirm our findings and apply them to other populations of patients.
Conclusions
Early rCTP provides incremental value beyond obstructive CAD to detect ACS. Combined CCTA/rCTP is non-inferior to CCTA/SPECT-MPI to discriminate ACS, and hence presents an attractive alternative to accurately triage and manage patients presenting with acute chest pain to the ED.
Supplementary Material
Figure 3. Incremental value of resting CT Perfusion.
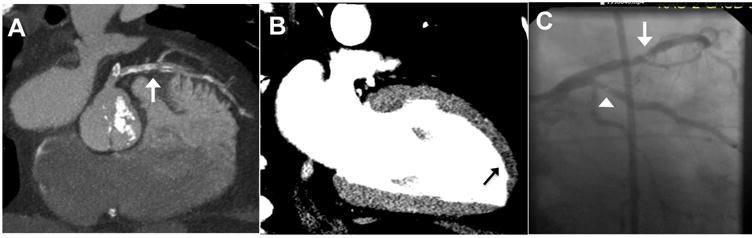
CTA images (A) yielded indeterminate coronary stenosis severity secondary to heavy calcification and slab artifact (arrow). Resting CT perfusion reconstruction via 2-chamber view 8-mm thick MPR (B) demonstrate an apical anterior rest perfusion defect (arrow). In this 64-year-old man, the standard care evaluation included a stress test which revealed inferior ischemia and transient ischemic dilatation, and subsequent invasive coronary angiography (C) revealed a 70% LAD stenosis (arrowhead) and a 90% ramus intermedius stenosis (arrow).
Acknowledgments
Sources of Funding: This work was supported by grants from the National Heart, Lung, and Blood Institute (R01 HL080053 and 5K24HL113128). Dr. Pursnani, Dr. Ahmed, and Dr. Uthamalingam received funding from NIHT32 HL076136. Dr. Ferencik was supported by a grant from the American Heart Association 13FTF1645000
Footnotes
Disclosures: None.
References
- 1.Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O'Neil BJ, Shaw LJ, Shen MY, Valeti US, Raff GL. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414–22. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–50. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863–71. doi: 10.1016/j.jacc.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 5.Nageh T, Sherwood RA, Wainwright RJ, Shah AM, Thomas MR. The clinical relevance of raised cardiac troponin I in the absence of significant angiographic coronary artery disease. Int J Cardiol. 2005;100:325–30. doi: 10.1016/j.ijcard.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–11. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 8.Min JK, Berman DS, Budoff MJ, Jaffer FA, Leipsic J, Leon MB, Mancini GB, Mauri L, Schwartz RS, Shaw LJ. Rationale and design of the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy) study. J Cardiovasc Comput Tomogr. 2011;5:301–9. doi: 10.1016/j.jcct.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 10.Blankstein R, Shturman LD, Rogers IS, Rocha-Filho JA, Okada DR, Sarwar A, Soni AV, Bezerra H, Ghoshhajra BB, Petranovic M, Loureiro R, Feuchtner G, Gewirtz H, Hoffmann U, Mamuya WS, Brady TJ, Cury RC. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol. 2009;54:1072–84. doi: 10.1016/j.jacc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 11.George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang HJ, Bluemke DA, Becker L, Yousuf O, Texter J, Lardo AC, Lima JA. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009;2:174–82. doi: 10.1161/CIRCIMAGING.108.813766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC., Jr ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Udelson JE, Beshansky JR, Ballin DS, Feldman JA, Griffith JL, Handler J, Heller GV, Hendel RC, Pope JH, Ruthazer R, Spiegler EJ, Woolard RH, Selker HP. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. JAMA. 2002;288:2693–700. doi: 10.1001/jama.288.21.2693. [DOI] [PubMed] [Google Scholar]
- 14.Heller GV, Stowers SA, Hendel RC, Herman SD, Daher E, Ahlberg AW, Baron JM, Mendes de Leon CF, Rizzo JA, Wackers FJ. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and nondiagnostic electrocardiograms. J Am Coll Cardiol. 1998;31:1011–7. doi: 10.1016/s0735-1097(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 15.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53:2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Candell-Riera J, Oller-Martinez G, de Leon G, Castell-Conesa J, Aguade-Bruix S. Yield of early rest and stress myocardial perfusion single-photon emission computed tomography and electrocardiographic exercise test in patients with atypical chest pain, nondiagnostic electrocardiogram, and negative biochemical markers in the emergency department. Am J Cardiol. 2007;99:1662–6. doi: 10.1016/j.amjcard.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 17.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, O'Gara PT, Carabello BA, Russell RO, Jr, Cerqueira MD, St John Sutton MG, DeMaria AN, Udelson JE, Kennedy JW, Verani MS, Williams KA, Antman EM, Smith SC, Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42:1318–33. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Feuchtner GM, Plank F, Pena C, Battle J, Min J, Leipsic J, Labounty T, Janowitz W, Katzen B, Ziffer J, Cury RC. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart. 2012;98:1510–7. doi: 10.1136/heartjnl-2012-302531. [DOI] [PubMed] [Google Scholar]
- 19.Schepis T, Achenbach S, Marwan M, Muschiol G, Ropers D, Daniel WG, Pflederer T. Prevalence of first-pass myocardial perfusion defects detected by contrast-enhanced dual-source CT in patients with non-ST segment elevation acute coronary syndromes. Eur Radiol. 2010;20:1607–14. doi: 10.1007/s00330-010-1725-7. [DOI] [PubMed] [Google Scholar]
- 20.Gupta M, Kadakia J, Jug B, Mao SS, Budoff MJ. Detection and quantification of myocardial perfusion defects by resting single-phase 64-slice cardiac computed tomography angiography compared with SPECT myocardial perfusion imaging. Coron Artery Dis. 2013;24:290–7. doi: 10.1097/MCA.0b013e32835f2fe5. [DOI] [PubMed] [Google Scholar]
- 21.Ghoshhajra BB, Maurovich-Horvat P, Techasith T, Medina HM, Verdini D, Sidhu MS, Blankstein R, Brady TJ, Cury RC. Infarct detection with a comprehensive cardiac CT protocol. J Cardiovasc Comput Tomogr. 2012;6:14–23. doi: 10.1016/j.jcct.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cury RC, Nieman K, Shapiro MD, Butler J, Nomura CH, Ferencik M, Hoffmann U, Abbara S, Jassal DS, Yasuda T, Gold HK, Jang IK, Brady TJ. Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology. 2008;248:466–75. doi: 10.1148/radiol.2482071478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha-Filho JA, Blankstein R, Shturman LD, Bezerra HG, Okada DR, Rogers IS, Ghoshhajra B, Hoffmann U, Feuchtner G, Mamuya WS, Brady TJ, Cury RC. Incremental value of adenosine-induced stress myocardial perfusion imaging with dual-source CT at cardiac CT angiography. Radiology. 2010;254:410–9. doi: 10.1148/radiol.09091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann U, Bamberg F. Is computed tomography coronary angiography the most accurate and effective noninvasive imaging tool to evaluate patients with acute chest pain in the emergency department?: CT coronary angiography is the most accurate and effective noninvasive imaging tool for evaluating patients presenting with chest pain to the emergency department. Circ Cardiovasc Imaging. 2009;2:251–63. doi: 10.1161/CIRCIMAGING.109.850347. discussion 263. [DOI] [PubMed] [Google Scholar]
- 25.Ghoshhajra BB, Rogers IS, Maurovich-Horvat P, Techasith T, Verdini D, Sidhu MS, Drzezga NK, Medina HM, Blankstein R, Brady TJ, Cury RC. A comparison of reconstruction and viewing parameters on image quality and accuracy of stress myocardial CT perfusion. J Cardiovasc Comput Tomogr. 2011;5:459–66. doi: 10.1016/j.jcct.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerci RJ, Arbab-Zadeh A, George RT, Miller JM, Vavere AL, Mehra V, Yoneyama K, Texter J, Foster C, Guo W, Cox C, Brinker J, Di Carli M, Lima JA. Aligning coronary anatomy and myocardial perfusion territories: an algorithm for the CORE320 multicenter study. Circ Cardiovasc Imaging. 2012;5:587–95. doi: 10.1161/CIRCIMAGING.111.970608. [DOI] [PubMed] [Google Scholar]
- 27.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol. 2002;9:240–5. doi: 10.1067/mnc.2002.123122. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed W, Schlett CL, Uthamalingam S, Truong QA, Koenig W, Rogers IS, Blankstein R, Nagurney JT, Tawakol A, Januzzi JL, Hoffmann U. Single resting hsTnT level predicts abnormal myocardial stress test in acute chest pain patients with normal initial standard troponin. JACC Cardiovasc Imaging. 2013;6:72–82. doi: 10.1016/j.jcmg.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen CL, Goldstein RA, Akinboboye OO, Berman DS, Botvinick EH, Churchwell KB, Cooke CD, Corbett JR, Cullom SJ, Dahlberg ST, Druz RS, Ficaro EP, Galt JR, Garg RK, Germano G, Heller GV, Henzlova MJ, Hyun MC, Johnson LL, Mann A, McCallister BD, Jr, Quaife RA, Ruddy TD, Sundaram SN, Taillefer R, Ward RP, Mahmarian JJ. Myocardial perfusion and function: single photon emission computed tomography. J Nucl Cardiol. 2007;14:e39–60. doi: 10.1016/j.nuclcard.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–43. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 32.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 33.Bezerra HG, Loureiro R, Irlbeck T, Bamberg F, Schlett CL, Rogers I, Blankstein R, Truong QA, Brady TJ, Cury RC, Hoffmann U. Incremental value of myocardial perfusion over regional left ventricular function and coronary stenosis by cardiac CT for the detection of acute coronary syndromes in high-risk patients: a subgroup analysis of the ROMICAT trial. J Cardiovasc Comput Tomogr. 2011;5:382–91. doi: 10.1016/j.jcct.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Lessick J, Ghersin E, Dragu R, Litmanovich D, Mutlak D, Rispler S, Agmon Y, Engel A, Beyar R. Diagnostic accuracy of myocardial hypoenhancement on multidetector computed tomography in identifying myocardial infarction in patients admitted with acute chest pain syndrome. J Comput Assist Tomogr. 2007;31:780–8. doi: 10.1097/rct.0b013e318033d6fc. [DOI] [PubMed] [Google Scholar]
- 35.Nagao M, Matsuoka H, Kawakami H, Higashino H, Mochizuki T, Uemura M, Shigemi S. Myocardial ischemia in acute coronary syndrome: assessment using 64-MDCT. AJR Am J Roentgenol. 2009;193:1097–106. doi: 10.2214/AJR.08.1965. [DOI] [PubMed] [Google Scholar]
- 36.Branch KR, Busey J, Mitsumori LM, Strote J, Caldwell JH, Busch JH, Shuman WP. Diagnostic performance of resting CT myocardial perfusion in patients with possible acute coronary syndrome. AJR Am J Roentgenol. 2013;200:W450–7. doi: 10.2214/AJR.12.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–97. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 38.Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis A, Lin FY, Dunning AM, Budoff MJ, Malpeso J, Leipsic J, Min JK. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ Cardiovasc Imaging. 2013;6:881–9. doi: 10.1161/CIRCIMAGING.113.000297. [DOI] [PubMed] [Google Scholar]
- 39.Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, Erglis A, Lin FY, Dunning AM, Apruzzese P, Budoff MJ, Cole JH, Jaffer FA, Leon MB, Malpeso J, Mancini GB, Park SJ, Schwartz RS, Shaw LJ, Mauri L. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308:1237–45. doi: 10.1001/2012.jama.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


