ABSTRACT
The abuse of anabolic androgenic steroids (AAS) has remained on the rise despite their well-known deleterious effects. We describe a case of AAS-induced multisystem failure following an extensive history of abuse in a 41-year-old bodybuilder. Furthermore, we review pertinent literature and discuss the different pathophysiologic mechanisms through which AAS affect the heart and other organs. This case points to the possibility of multiorgan involvement and severe cardiac effects of AAS abuse in young individuals who may not have any past medical history.
KEYWORDS: Acute coronary syndrome, acute myocardial infarction, anabolic steroid abuse, multiorgan failure, subclinical congestive heart failure, testosterone replacement therapy
The use of anabolic androgenic steroids (AAS) has moved from the elite athlete and competitive bodybuilder to the average fitness enthusiast. We report a case of a 41-year-old man who developed multisystem organ failure that complicated an extensive 20-year history of AAS abuse.
CASE PRESENTATION
A 41-year-old male fitness trainer presented with sudden-onset chest pain at rest 3 hours prior to presentation. He had no significant past medical, surgical, or family history. He abused AAS for >20 years in significant amounts. At presentation, the electrocardiograph was consistent with inferior ST segment myocardial infarction with a heart rate of 54 beats/min. His troponin level initially was 0.100 ng/mL and quickly rose to 95.200 ng/mL. Consequently, an angiogram showed a totally occluded right coronary artery. Thrombectomy and percutaneous coronary intervention with insertion of a drug-eluting stent were performed. His high-density lipoprotein was 30 mg/dL. Liver enzymes were initially mildly elevated (aspartate transaminase, 74 IU/L; alkaline phosphatase, 117 IU/L; alanine transaminase, 83 IU/L). His total bilirubin was 1.8 mg/dL with an international normalized ratio of 1.7; brain natriuretic peptide was 1626 pg/mL; blood urea nitrogen was elevated at 41 mg/dL; and creatinine was 3.14 mg/dL with an estimated glomerular filtration rate of 22 mL/min/1.73 m2. Urinalysis showed mild proteinuria and a moderate amount of blood. The remaining workup was negative.
Chest x-ray was consistent with cardiomegaly with no evidence of congestive heart failure (Figure 1). Cardiac echocardiography demonstrated a severely dilated right atrium, right ventricle, and left atrium; a normal-sized left ventricle with moderate concentric left ventricular hypertrophy; and a severely reduced left ventricular ejection fraction of <20%. Akinesis was noted from the basal to mid inferior septal and basal to apical inferior wall, consistent with right coronary artery disease.
Figure 1.
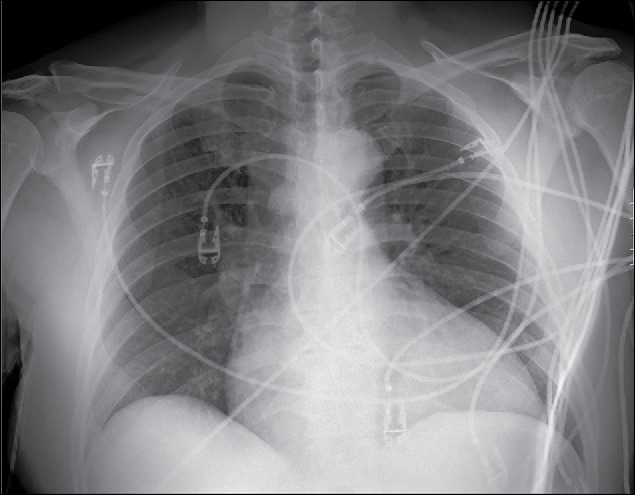
Chest x-ray (anteroposterior view) showing cardiomegaly with no evidence of congestive heart failure.
His postprocedure course was complicated by arrhythmias with variable atrioventricular blocks (mostly third-degree block), and by hospital day 2, he developed worsening acute kidney injury, an abnormal coagulation profile, hyperbilirubinemia, and acute liver injury with aspartate transaminase and alkaline phosphatase levels reaching 2073 IU/L and 1837 IU/L, respectively. Based on assessments by a gastroenterologist and nephrologist, the patient had acute ischemic injury to both kidney and liver secondary to decreased cardiac output. He was given low-infusion-rate intravenous dextrose for the acute liver injury while both liver and kidney function was closely monitored. He was given furosemide with strict input and output measurements.
After approximately 7 days of hospitalization, the patient showed remarkable improvement. Repeat echocardiography showed improved left ventricular function with an increase in left ventricular ejection fraction (35%). Liver and kidney function improved as well. His arrhythmias improved and he remained stable at first-degree atrioventricular block with a normal heart rate. At discharge, he had lost almost 30 pounds, which was attributed to both his AAS abstinence and his diuresis. He was discharged on hospital day 8 in stable condition to outpatient follow-up with cardiology, nephrology, and gastroenterology.
DISCUSSION
AAS are a family of hormones that exhibit anabolic (muscle-building) and androgenic (masculinizing) properties.1 They include the natural male hormone testosterone as well as other synthetic hormones.1 Several studies have shown that the use of anabolic steroids remains on the rise.2 Our patient described a chronic use of large amounts of various types of AAS, which could very well explain his presentation and findings.
The lifetime prevalence of AAS among male adolescents in Western countries is 5% to 10%.3 Although there is a need for more substantiation and current evidence is mostly anecdotal, emerging consensus supports an association of AAS abuse with an increased risk of sudden cardiac death, myocardial infarction, abnormal lipid profile, and cardiac hypertrophy.4 Cardiovascular responses to AAS are due to certain myocardial receptors that have transcriptional regulatory functions. AAS-induced hypertrophy appears to be due to direct action on cardiac androgen receptors.5–7 In addition, the direct toxicity of AAS could result in fibrosis and intimal hyperplasia of the intramural coronary arteries, resulting in chronic ischemic damage.8 Vasospasm may result from AAS's effect on the vascular endothelial cells.4 Alterations in lipid profiles have been consistently demonstrated in the literature, and these changes produce a significant increased risk of atheromatous coronary artery disease.9 Higher doses of AAS are also associated with increased platelet aggregation, yielding a procoagulant state, through a dose-dependent prothrombotic profile.10 An autopsy study done in Sweden involving 34 male AAS users revealed chronic pathologic changes, specifically left ventricular hypertrophy, cardiac fibrosis, and coronary artery disease.8,11
We emphasize here the subclinical presentation of a cardiomyopathy without the typical symptoms of congestive heart failure. We attribute this, as well as the low ejection fraction, to compensated cardiomyopathy. This case suggests an acute prothrombotic event resulting in right coronary artery infarct, in addition to left ventricular dysfunction secondary to chronic dilated cardiomyopathy as a result of AAS abuse. This unique and concerning subclinical presentation is consistent with other previously published isolated case reports on AAS abuse in the literature.12–15
Given the diverse spectrum of steroid profiles and unclear risk of each particular agent, in addition to the variable dosing, it is difficult to risk-stratify this particular patient population. Furthermore, given the off-label use of animal and human compounds, in addition to the lack of regulation of concentration doses with production, the time course of abuse is subject to clinical risk interpretation. Review of the literature demonstrates a fairly consistent level of clinical severity and pathological consequence among patients abusing AAS ranging from the age of 18 to 50 with no significant past medical history for a time course under just 1 year and up to as many as 20 years.1,12,16–20 It is our recommendation that any significant exposure to AAS, given its propensity for subclinical presentation, should warrant aggressive cardiovascular, hepatic, and renal screening.
References
- 1.Shamloul RM, Aborayah AF, Hashad A, Abd-Allah F. Anabolic steroids abuse–induced cardiomyopathy and ischaemic stroke in a young male patient. BMJ Case Rep . 2014;2014. doi: 10.1136/bcr-2013-203033. PMID:24574525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Sullivan AJ, Kennedy MC, Casey JH, Day RO, Corrigan B, Wodak AD. Anabolic–androgenic steroids: medical assessment of present, past and potential users. Med J Aust. 2000;173:323–327. PMID:11061405. [DOI] [PubMed] [Google Scholar]
- 3.Thiblin I, Petersson A. Pharmacoepidemiology of anabolic androgenic steroids: a review. Fundam Clin Pharmacol . 2005;19:27–44. doi: 10.1111/j.1472-8206.2004.00298.x. PMID:15660958. [DOI] [PubMed] [Google Scholar]
- 4.Melchert RB, Welder AA. Cardiovascular effects of androgenic–anabolic steroids. Med Sci Sports Exerc . 1995;27:1252–1262. doi: 10.1249/00005768-199509000-00004. PMID:8531623. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn CM. Anabolic steroids. Recent Prog Horm Res . 2002;57:411–434. doi: 10.1210/rp.57.1.411. PMID:12017555. [DOI] [PubMed] [Google Scholar]
- 6.Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation . 1998;98:256–261. doi: 10.1161/01.CIR.98.3.256. PMID:9697826. [DOI] [PubMed] [Google Scholar]
- 7.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev . 2003;24:313–340. doi: 10.1210/er.2003-0005. PMID:12788802. [DOI] [PubMed] [Google Scholar]
- 8.Montisci M, El Mazloum R, Cecchetto G, et al. Anabolic androgenic steroids abuse and cardiac death in athletes: morphological and toxicological findings in four fatal cases. Forensic Sci Int . 2012;217:e13–e18. doi: 10.1016/j.forsciint.2011.10.032. PMID:22047750. [DOI] [PubMed] [Google Scholar]
- 9.Dickerman RD, McConathy WJ, Zachariah NY. Testosterone, sex hormone–binding globulin, lipoproteins, and vascular disease risk. J Cardiovasc Risk . 1997;4:363–366. doi: 10.1097/00043798-199710000-00008. PMID:9865668. [DOI] [PubMed] [Google Scholar]
- 10.Winkler UH. Effects of androgens on haemostasis. Maturitas . 1996;24:147–155. doi: 10.1016/S0378-5122(96)82004-4. PMID:8844628. [DOI] [PubMed] [Google Scholar]
- 11.Thiblin I, Lindquist O, Rajs J. Cause and manner of death among users of anabolic androgenic steroids. J Forensic Sci . 2000;45:16–23. doi: 10.1520/JFS14635J. PMID:10641914. [DOI] [PubMed] [Google Scholar]
- 12.Bispo M, Valente A, Maldonado R, et al. Anabolic steroid–induced cardiomyopathy underlying acute liver failure in a young bodybuilder. World J Gastroenterol . 2009;15:2920–2922. doi: 10.3748/wjg.15.2920. PMID:19533818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fussell KM, Awad JA, Ware LB. Case of fulminant hepatic failure due to unrecognized peripartum cardiomyopathy. Crit Care Med . 2005;33:891–893. doi: 10.1097/01.CCM.0000158517.25962.8E. PMID:15818120. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman BJ, Pate MB, Marsh WH, Lee WM. Cardiomyopathy unrecognized as a cause of hepatic failure. J Clin Gastroenterol . 1990;12:306–309. doi: 10.1097/00004836-199006000-00015. PMID:2362100. [DOI] [PubMed] [Google Scholar]
- 15.Wiesen S, Reddy KR, Jeffers LJ, Schiff ER. Fulminant hepatic failure secondary to previously unrecognized cardiomyopathy. Dig Dis . 1995;13:199–204. doi: 10.1159/000171502. PMID:8548983. [DOI] [PubMed] [Google Scholar]
- 16.Sonmez E, Turkdogan KA, Yilmaz C, Kucukbuzcu S, Ozkan A, Sogutt O. Chronic anabolic androgenic steroid usage associated with acute coronary syndrome in bodybuilder. Turk J Emerg Med . 2016;16:35–37. doi: 10.1016/j.tjem.2014.11.001. PMID:27239638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poorzand H, Jafarzadeh Esfehani R, Hosseinzadeh P, Vojdanparast M. Acute myocardial infarction in a young male wrestler: a case report. ARYA Atheroscler . 2015;11:366–369. PMID:26862345. [PMC free article] [PubMed] [Google Scholar]
- 18.Frati P, Busardò FP, Cipolloni L, Dominicis ED, Fineschi V. Anabolic androgenic steroid (AAS) related deaths: autoptic, histopathological and toxicological findings. Curr Neuropharmacol . 2015;13:146–159. doi: 10.2174/1570159X13666141210225414. PMID:26074749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unai S, Miessau J, Karbowski P, Baram M, Cavarocchi NC, Hirose H. Caution for anabolic androgenic steroid use: a case report of multiple organ dysfunction syndrome. Respir Care . 2013;58:e159–e163. doi: 10.4187/respcare.02338. PMID:23611866. [DOI] [PubMed] [Google Scholar]
- 20.Herlitz LC, Markowitz GS, Farris AB, et al. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol . 2010;21:163–172. doi: 10.1681/ASN.2009040450. PMID:19917783. [DOI] [PMC free article] [PubMed] [Google Scholar]


