Abstract
Purpose
This study aimed at exploring the prevalence of self-reported antenatal and postnatal depressive symptoms by severity across multiple countries and the association between antidepressant treatment in pregnancy and postnatal symptom severity.
Materials and methods
This was a multinational web-based study conducted across 12 European countries (n=8069). Uniform data collection was ensured via an electronic questionnaire. Pregnant women at any gestational week and mothers of children with <1 year of age could participate. We used the Edinburgh Postnatal Depression Scale (EPDS) to measure the prevalence of antenatal and postnatal depressive symptoms according to severity, which were corrected by survey weight adjustment (descriptive analysis). Within mothers with a psychiatric disorder (n=173), we estimated the association between antidepressant treatment in pregnancy and postnatal depressive symptom severity, as standardized EPDS mean scores, via the inverse probability of treatment weight (association analysis).
Results
In the descriptive analysis (n=8069), the period prevalence of moderate-to-very severe depressive symptoms was higher in the western and eastern regions relative to the northern region, both in the antenatal period (6.8%–7.5% vs 4.3%) and in the postnatal period (7.6% vs 4.7%). One in two mothers with psychiatric disorders used an antidepressant in pregnancy (86 of 173). In the association analysis, women medicated at any time during pregnancy (adjusted β=−0.34, 95% confidence interval [CI] =−0.66, −0.02) had a significant postnatal symptom severity reduction compared with the nonmedicated counterpart. This effect was larger (β=−0.74, 95% CI =−1.24, −0.24) when the analysis was restricted to mothers within 6 months after childbirth.
Conclusion
The prevalence of self-reported antenatal and postnatal depressive symptoms differs across European countries. Among women with psychiatric disorders, those who had been on treatment with antidepressants during pregnancy were less likely to report postnatal depressive symptoms, particularly within the 6-month period after childbirth, compared with the nonmedicated counterpart.
Keywords: antidepressants, pharmacotherapy, pregnancy and postpartum, depression, anxiety, web-based
Video abstract
Introduction
Depression is highly prevalent in the perinatal period and often comorbid with anxiety.1,2 During this time, an estimated 1%–6% of women suffer from major depression,3,4 whereas 15%–25% experience subclinical depression-related symptoms.5 Knowledge about the extent of perinatal depression in low- to middle-income countries is scarce,6,7 and comparability of prevalence estimates across high-income countries remains difficult due to dissimilarities in study design, methodology and definition of the disorder, the period of prevalence determination, and varying cultural correlates.6,8
Postnatal depression is often a continuation or recurrence of existing antenatal depression, particularly when the latter has been treated suboptimally.9,10 Pharmacotherapy in pregnancy constitutes a special challenge for pregnant women and clinicians since the effective treatment of the mother has to be assured while harmful effects on the unborn child must be prevented.11 However, whether antidepressant treatment in pregnancy lowers the risk of relapse of maternal depression either during or after pregnancy still remains an unanswered question.
One study12 showed that women discontinuing antidepressants in pregnancy had a fivefold increased hazard of major depression relapse in the antenatal period relative to continuers. While two other studies failed to replicate this association,13,14 one study15 reported a protective effect of psychotropics on perinatal depression overall, but not on depression with postpartum onset. However, these few studies mainly assessed the role of medication on antenatal and very early postnatal maternal mental health, and none of them explored depression in terms of symptom severity and from a (multi)dimensional perspective, as advocated by the National Institute of Mental Health research domain criteria.16
The aim of this study was twofold: 1) to explore the prevalence of antenatal and postnatal depressive symptoms across 12 countries in Europe on the basis of the Edinburgh Postnatal Depression Scale (EPDS) and 2) to investigate, in the postnatal sample, the association between past antidepressant treatment in pregnancy and postnatal depressive symptom severity among women with a psychiatric disorder.
Materials and methods
Study design and data collection
Data were retrieved from the “Multinational Medication Use in Pregnancy Study,” a cross-sectional, web-based study carried out in Europe, North and South America, and Australia from October 2011 to February 2012, to investigate patterns and correlates of medication use in pregnancy.17 Pregnant women at any gestational age and mothers of children under the age of 1 year were eligible for inclusion. Data were collected across 18 countries via an anonymous, self-administered electronic questionnaire (www.questback. com), accessible online for a period of 2 months in each par ticipating country within the period mentioned above. The questionnaire was open to the public through banners posted on two to three pregnancy-related websites in each country, social networks, and/or pregnancy forums. The websites were selected on the basis of the number of daily users. Information about the recruitment tools utilized, the Internet penetration rates in each participating country, and the full questionnaire have been previously published.17
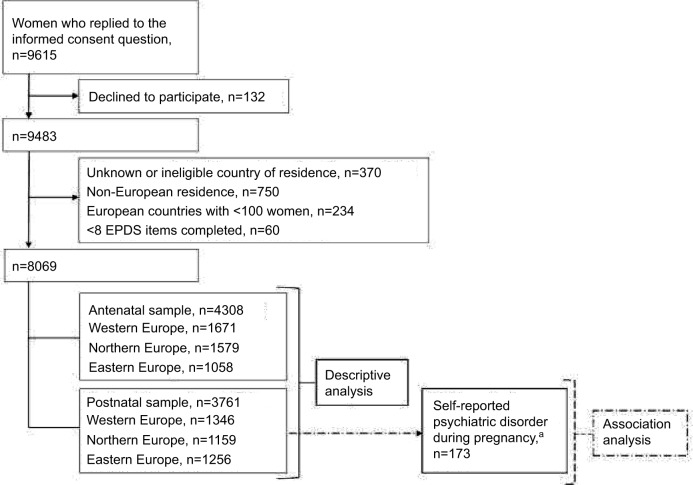
Because the structure of the EPDS responses has been shown to differ between Europe and the USA, but not within European countries,8 only women with the latter residence were included. They were grouped into three regions: 1) Western Europe: France, Italy, Switzerland, and the UK; 2) Northern Europe: Finland, Norway, and Sweden; and 3) Eastern Europe: Croatia, Poland, Russia, Serbia, and Slovenia. Figure 1 outlines the data selection to achieve the final antenatal and postnatal samples. When exploring the period prevalence of depressive symptoms (descriptive analysis), we analyzed the antenatal and postnatal samples separately. When investigating the association between antidepressant use in pregnancy and postnatal depressive symptom severity (association analysis), only the postnatal sample was considered.
Figure 1.
Flow chart to achieve the final antenatal and postnatal samples.
Notes: aPsychiatric disorder refers to self-reported chronic depression, anxiety, bipolar, panic, or personality disorder. Western Europe included France, Italy, Switzerland, and the UK; Northern Europe included Finland, Norway, and Sweden; and Eastern Europe included Croatia, Poland, Russia, Serbia, and Slovenia.
Abbreviation: EPDS, Edinburgh Postnatal Depression Scale.
Antenatal and postnatal depressive symptoms
In the descriptive analysis, the main outcome variables were antenatal and postnatal depressive symptom severity as, respectively, reported by pregnant women and mothers via the EPDS. The EPDS is the most widely used international screening questionnaire for the symptoms of depression during pregnancy and postpartum.18 It is a self-rating 10-item scale validated for major and minor depression in clinical settings, with satisfactory Cronbach’s α reliability (0.87).18 Women were asked to rate whether each item reflected how they had been feeling in the past 7 days. Each item response scored 0–3 on an ordinal scale, producing a total EPDS score of 0–30.18 Higher scores indicated worse symptomatology. Due to the lack of a common cutoff value for probable depression in all the investigated countries,19,20 we used the EPDS as an ordinal variable according to severity level (“no symptoms” [score <10], “mild to moderate” [10–16], “moderate to severe” [17–21], and “very severe” [22–30]) in the prevalence analysis, as in a previous study.21 The EPDS was also explored as a continuous variable to allow comparability with prior research.
In the association analysis, the main outcome variable was postnatal depressive symptom severity. Because several reports have found the EPDS to be multidimensional rather than unidimensional,21–23 we also explored three EPDS subdimensions as in the study by Tuohy and McVey:23 “nonspecific depressive symptoms” (items 7–10), “anxiety symptoms” (items 3–5), and “anhedonia” (items 1 and 2). We used these dimensions since the recruitment strategies in our and the abovementioned study23 were comparable. Mean scores were calculated and then standardized (z score) for the total EPDS and for the three subdimensions when exploring their association with past antidepressant treatment. Lower z scores indicate lower symptom severity, and higher scores indicate the converse. Additional details on the EPDS measure is provided in the Supplementary materials.
Psychiatric disorders and antidepressants
Women were confronted with a list of nine chronic disorders, including depression and anxiety. A free-text field was also available, where any other condition not previously listed could be specified. Because depression is highly comorbid with anxiety and other psychiatric illnesses,2 we considered women reporting depression, anxiety, bipolar, panic, or personality disorders as having a psychiatric disorder. Women were then asked about medication use for each indication as free-text entry, along with the timing of usage (pregnancy weeks 0–12, 13–24, and 25–delivery). Use of medication and timing could also be reported in relation to the treatment of various listed short-term illnesses (eg, nausea and sleeping problems). Drug classification was based on the Anatomical Therapeutic Chemical (ATC) Classification System. Exposure in pregnancy to antidepressants was defined as the use of a drug belonging to the ATC group N06A, including selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors, and tricyclics. As a proxy for pharmacotherapy duration, the number of trimesters of antidepressant use was defined according to how many time intervals were checked in the questionnaires and grouped as follows: “none,” “one,” “two, or three” trimesters. Antidepressant use in pregnancy constituted the exposure variable in the association analysis.
Covariates
Maternal correlates were categorized as presented in Table 1. Pregnancy-related characteristics included time of gestation or weeks since childbirth, previous children, perinatal use of folic acid, and whether the pregnancy was unplanned. Sociodemographic and lifestyle correlates comprised country of residency, age, marital status, employment at the time of conception, education, mother tongue, smoking habit during pregnancy, and alcohol consumption after awareness of pregnancy. The personality trait neuroticism was assessed via the Big Five Inventory24 and measured by eight items. Somatic chronic comorbidity was defined as having asthma, allergy, hypothyroidism, epilepsy, diabetes (type I or II), and rheumatic or cardiovascular diseases, as self-reported within the list of chronic disorders. Women could also indicate whether they had experienced nausea or sleeping problems in pregnancy, within a list of common acute pregnancy-related illnesses. Comedication in pregnancy with acetaminophen, anxiolytics and sedatives, antiepileptics, or antipsychotics was also measured.
Table 1.
Maternal sociodemographic, lifestyle, and health-related characteristics of the antenatal and postnatal samples, overall and by self-reported psychiatric disorder (n=8069)
| Antenatal sample
|
Postnatal sample
|
|||||
|---|---|---|---|---|---|---|
| Total, n=4308 | Psychiatric disorder
|
Total, n=3761 | Psychiatric disorder
|
|||
| No, n=4126 | Yes, n=182 | No, n=3588 | Yes, n=173 | |||
| Pregnancy characteristics | ||||||
| Gestation week (range=1–42), mean (SD) | 22.7 (10.2) | 22.7 (10.2) | 21.6 (10.3) | |||
| Weeks since childbirth, n (%) | ||||||
| 0–12 | 784 (20.9) | 746 (20.8) | 38 (22.0) | |||
| 13–24 | 931 (24.8) | 904 (25.2) | 27 (15.6) | |||
| ≥25 | 2046 (54.4) | 1938 (54.0) | 108 (62.4) | |||
| No previous children, n (%) | 2413 (56.0) | 2311 (56.0) | 102 (56.0) | 1698 (45.2) | 1617 (45.1) | 81 (46.8) |
| No perinatal use of folate,a n (%) | 346 (8.0) | 332 (8.1) | 14 (7.7) | 297 (7.9) | 285 (7.9) | 12 (6.9) |
| Completely unplanned pregnancy, n (%) | 343 (8.0) | 325 (7.9) | 18 (9.9) | 332 (8.8) | 301 (8.4) | 31 (17.9) |
| Sociodemographic characteristics | ||||||
| Residence, n (%) | ||||||
| Western Europe | 1671 (38.8) | 1606 (38.9) | 65 (35.7) | 1346 (35.8) | 1289 (35.9) | 57 (32.9) |
| Northern Europe | 1579 (36.7) | 1488 (36.1) | 91 (50.0) | 1159 (30.8) | 1077 (30.0) | 82 (47.4) |
| Eastern Europe | 1058 (24.6) | 1032 (25.0) | 26 (14.3) | 1256 (33.4) | 1222 (34.1) | 34 (19.7) |
| Maternal age (years; range 15–50), mean (SD) | 29.5 (5.0) | 29.5 (5.0) | 29.3 (5.2) | 30.0 (5.1) | 30.0 (5.0) | 30.4 (6.3) |
| Marital status, n (%) | ||||||
| Married/cohabiting | 4110 (95.4) | 3949 (95.7) | 161 (88.5) | 3556 (94.6) | 3406 (94.9) | 150 (86.7) |
| Other than above | 198 (4.6) | 177 (4.3) | 21 (11.5) | 205 (5.5) | 182 (5.1) | 23 (13.3) |
| Working status at conception, n (%) | ||||||
| Employed | 3132 (72.7) | 3015 (73.1) | 117 (64.3) | 2836 (75.4) | 2746 (76.5) | 90 (52.0) |
| Student | 343 (8.0) | 328 (8.0) | 15 (8.2) | 352 (9.4) | 321 (9.0) | 31 (17.9) |
| Homemaker | 373 (8.7) | 357 (8.7) | 16 (8.8) | 264 (7.0) | 237 (6.6) | 27 (15.6) |
| Job seeker/others | 454 (10.5) | 420 (10.2) | 34 (18.7) | 305 (8.1) | 280 (7.8) | 25 (14.5) |
| Educational attainment, n (%) | ||||||
| Below high school | 179 (4.2) | 160 (3.9) | 19 (10.4) | 152 (4.0) | 124 (3.5) | 28 (16.2) |
| High school | 1741 (40.4) | 1659 (40.2) | 82 (45.1) | 1470 (39.1) | 1398 (39.0) | 72 (41.6) |
| Above high school | 2388 (55.4) | 2307 (55.9) | 81 (44.5) | 2139 (56.9) | 2066 (57.6) | 73 (42.2) |
| Immigrant status (yes),b n (%) | 219 (5.1) | 209 (5.1) | 10 (5.5) | 234 (6.2) | 227 (6.3) | 7 (4.1) |
| Lifestyle characteristics | ||||||
| Alcohol use during pregnancy (yes),c n (%) | 616 (14.3) | 579 (14.0) | 37 (20.3) | 672 (17.9) | 639 (17.8) | 33 (19.1) |
| Smoking during pregnancy (yes), n (%) | 393 (9.1) | 363 (8.8) | 30 (16.5) | 351 (9.3) | 322 (9.0) | 29 (16.8) |
| Neurotic personality traits (range 8–40),d mean (SD) | 22.4 (5.6) | 22.1 (5.5) | 28.4 (5.2) | 22.5 (6.1) | 22.2 (5.9) | 28.5 (5.3) |
| Health-related characteristics | ||||||
| Medical contact due to fertility problems (yes), n (%) | 679 (15.8) | 648 (15.7) | 31 (17.0) | 523 (13.9) | 494 (13.8) | 29 (16.8) |
| Nausea in pregnancy (yes), n (%) | 3234 (75.1) | 3085 (74.8) | 149 (81.9) | 2626 (69.8) | 2492 (69.5) | 134 (77.5) |
| Sleeping problems in pregnancy (yes), n (%) | 2572 (59.7) | 2435 (59.0) | 137 (75.3) | 1978 (52.6) | 1874 (52.2) | 104 (60.1) |
| Chronic somatic comorbidity (yes),e n (%) | 934 (21.7) | 827 (20.0) | 107 (58.8) | 848 (22.6) | 741 (20.7) | 107 (61.9) |
Notes: Missing values were <1.5% for immigrant status, employment, unplanned pregnancy, smoking, fertility problems, folate use, and alcohol use and between 2.5% and 3.0% for neuroticism traits.
Use of folate before and/or during pregnancy;
women having the first language different from the official main language in the country of residence;
alcohol consumption after the awareness of the pregnancy;
measured via the Big Five Inventory personality scale;
defined as self-reported asthma, allergy, hypothyroidism, epilepsy, diabetes (type I or II), and rheumatic or cardiovascular diseases.
Abbreviation: SD, standard deviation.
Data analysis
Descriptive statistics, corrected by survey weight adjustment, were used to characterize the period prevalence of depressive symptom by severity level and mean EPDS scores. The survey weight was based on the auxiliary variables age and education, which are important correlates of study response. Details about the weighting procedure are provided in the Supplementary materials. The EPDS internal consistency was assessed via reliability analysis.25
To estimate the association between exposure to antidepressants during pregnancy and postnatal depressive symptom severity as reported by women with a psychiatric disorder on the EPDS, we applied inverse probability of treatment weighting (IPTW), using the propensity score to survey data, as described by DuGoff et al.26 A logistic regression was first fit to estimate the propensity score, ie, the probability of “exposure” to antidepressants, 1) at any time during pregnancy and 2) in two or three trimesters, given a set of maternal covariates (cf Supplementary materials) and the survey weight. We then derived and normalized the IPTW. Balance of covariates (standardized difference) between medicated and nonmedicated women was assessed after the application of the weights and was considered adequate whenever differences were ≤0.1.27 Lastly, the IPTW was multiplied for the sampling weight to generate a new composite weight, which was applied to fit generalized linear models.26 Because of the small sample size, no IPTW could be computed in relation to antidepressant use in one trimester only. Because the postnatal sample included women with a varying time span since delivery, the weighted analyses were stratified according to the time since childbirth (<25 and ≥25 weeks). Power analysis is outlined in the Supplementary materials.
The crude and adjusted β coefficients with 95% confidence interval (CI) represent the standardized mean difference in postnatal depressive symptoms between women medicated and nonmedicated with antidepressants during pregnancy and can be interpreted as the effect sizes of Cohen’s d, where 0.2, 0.5, and 0.8 are considered a small, medium, and large effect size, respectively.28 Statistical significance was set to p<0.05. All statistical analyses were performed by using Stata Version 14 (StataCorp LP, College Station, TX, USA). We examined the robustness of our findings in a set of sensitivity and exploratory subanalyses, as described in detail in the Supplementary materials.
Ethical approval and informed consent
Informed consent was given by the participants by ticking the answer “yes” to the question “Are you willing to participate in the study?”. The South-East Regional Ethics Committee in Norway granted an ethical approval exemption for the original multinational research survey because of anonymity. As required by the national legislation, in the UK, the original research survey received ethical approval from the University of East Anglia’s Faculty of Medicine and Health Research Ethics Committee. In Italy, the Ethic Board of the health district of Trento was notified about the original research survey. In the remaining European countries, the research survey was exempt from ethical approval because of anonymity. All data were handled and stored anonymously. The data are available to researchers upon the application to the PharmacoEpidemiology and Drug Safety Research Group at the University of Oslo.
Results
This study included 8069 women, whereof 4308 (53.4%) were pregnant and 3761 (46.6%) were mothers of children younger than 1 year at the time of answering the questionnaire. Figure 1 outlines data selection to achieve the final distinct antenatal and postnatal samples for the descriptive and association analyses. Overall, 4.4% (n=355; 173 mothers and 182 pregnant women) of women reported to have had a psychiatric disorder in pregnancy, mainly depression and/or anxiety (n=341); of these, 33.4% reported depression, 27.6% reported anxiety, and 39.0% reported comorbid depression and anxiety. The remaining 14 women had bipolar, panic, or personality disorders. Table 1 lists the sociodemographic, lifestyle, and health-related characteristics of the antenatal and postnatal samples by psychiatric disorder.
Prevalence of antenatal and postnatal depressive symptoms
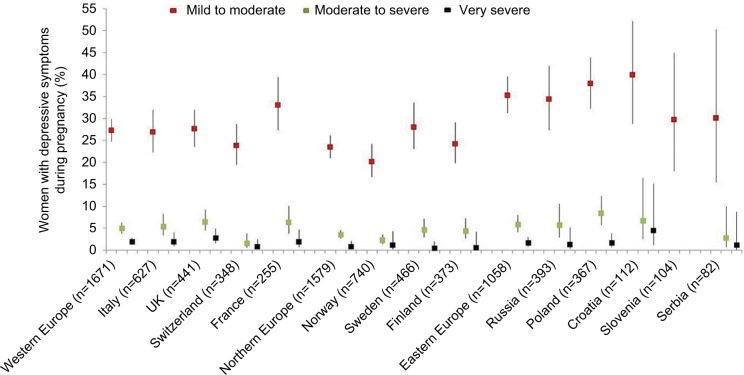
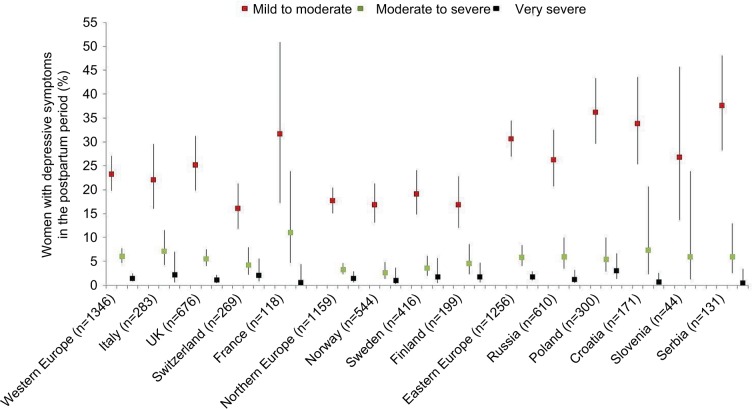
Figures 2 and 3 present the prevalence of antenatal and postnatal depressive symptoms by region/country and by severity level, whereas Figures S1 and S2 present EPDS mean scores, overall and by psychiatric disorder. The period prevalence values of moderate to severe and very severe antenatal depressive symptoms were in the range of 1.6%–8.4% and 0.5%–4.5%, respectively (Figure 2); in the postnatal period, these were correspondingly 2.6%–11.0% and 0.5%–3.0% (Figure 3). In both perinatal periods, the prevalence estimates were higher in eastern and western European countries relative to the northern region. The EPDS had good reliability (Supplementary materials).
Figure 2.
Weighted proportions of women with antenatal depressive symptoms by severity and country of residence.
Notes: Proportions and corresponding 95% CIs were corrected for survey weights based on educational level within age strata for each individual country among women of childbearing age. Population data for 2012 from: European Commission, Eurostat. Population by educational attainment level, sex and age (%) - main indicators; 2017 [updated February 28, 2018; cited November 8, 2017]. Available from: http://ec.europa.eu/eurostat/web/products-datasets/-/edat_lfse_03. Accessed August 17, 2017.49 Symptoms severity assessed as follows: “mild to moderate” (EPDS score=10–16), “moderate to severe” (EPDS score=17–21), and “very severe” (EPDS score=22–30). The proportion of women having no depressive symptoms (EPDS score=0–9) is not represented. In Slovenia, there were no women having moderate to severe or very severe depressive symptoms. The squares in red color indicate the proportion of women with mild to moderate depressive symptoms, the ones in green indicate moderate-to-severe symptoms, and the ones in black indicate very severe depressive symptoms.
Abbreviations: CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale.
Figure 3.
Weighted proportions of women with postnatal depressive symptoms by severity and country of residence.
Notes: Proportions and corresponding 95% CIs were corrected for survey weights based on educational level within age strata for each individual country among women of childbearing age. Population data for 2012 from: European Commission, Eurostat. Population by educational attainment level, sex and age (%) - main indicators; 2017 [updated February 28, 2018; cited November 8, 2017]. Available from: http://ec.europa.eu/eurostat/web/products-datasets/-/edat_lfse_03. Accessed August 17, 2017.49 Symptoms severity assessed as follows: “mild to moderate” (EPDS score =10–16), “moderate to severe” (EPDS score =17–21), and ”very severe” (EPDS score =22–30). The proportion of women having no depressive symptoms (EPDS score =0–9) is not represented. The squares in red color indicate the proportion of women with mild-to-moderate depressive symptoms, the ones in green indicate moderate to severe symptoms, and the ones in black indicate very severe depressive symptoms.
Abbreviations: CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale.
Association between antidepressant treatment during pregnancy and postnatal depressive symptom severity
Of the 173 mothers with a psychiatric disorder, 49.7% (n=86) reported treatment with an antidepressant any time during pregnancy and mainly in two or three trimesters (Table 2). SSRIs were the preferred therapeutic choice and primarily as monotherapy. Table 3 outlines maternal correlates and comedication use in pregnancy by antidepressant exposure for the postnatal sample.
Table 2.
Timing and length of antidepressant use in pregnancy among women with a psychiatric disorder in the postnatal sample (n=173)
| Timing of use
|
Length of use
|
||
|---|---|---|---|
| Anya | n (%) | Anya | n (%) |
| Any time in pregnancy | 86 (49.7) | None | 87 (50.3) |
| 1st trimester | 79 (45.7) | 1 trimester | 18 (10.4) |
| 2nd trimester | 67 (38.7) | 2 or 3 trimesters | 68 (39.3) |
| 3rd trimester | 67 (38.7) | ||
| SSRI | SSRI | ||
| Any time in pregnancy | 75 (46.3) | None | 98 (56.7) |
| 1st trimester | 70 (40.5) | 1 trimester | 15 (8.7) |
| 2nd trimester | 59 (34.1) | 2 or 3 trimesters | 60 (34.7) |
| 3rd trimester | 59 (34.1) | ||
| SNRI | SNRI | ||
| Any time in pregnancy | 11 (6.4) | ||
| 1st trimester | 10 (5.8) | None | 162 (93.6) |
| 2nd trimester | 8 (4.6) | 1 trimester | 2 (1.2) |
| 3rd trimester | 7 (4.1) | 2 or 3 trimesters | 9 (5.2) |
Notes:
It includes 1) three women were on combined therapy with an SSRI plus a tricyclic antidepressant and 2) one woman was on combined therapy with an unspecified antidepressant and an SNRI.
Abbreviations: SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Table 3.
Maternal sociodemographic, lifestyle, and health-related characteristics of the postnatal sample with psychiatric disorders according to antidepressant exposure during pregnancy (n=173)
| Antidepressant in pregnancy
|
||
|---|---|---|
| No, n=87 | Yes, n=86 | |
| Pregnancy characteristics | ||
| Weeks since childbirth, n (%) | ||
| 0–12 | 22 (25.3) | 16 (18.6) |
| 13–24 | 14 (16.1) | 13 (15.1) |
| ≥25 | 51 (58.6) | 57 (66.3) |
| No previous children, n (%) | 42 (48.3) | 39 (45.4) |
| No perinatal use of folate,a n (%) | 5 (5.8) | 7 (8.1) |
| Completely unplanned pregnancy, n (%) | 16 (18.4) | 15 (17.4) |
| Sociodemographic characteristics | ||
| Residence, n (%) | ||
| Western Europe | 27 (31.0) | 30 (34.9) |
| Northern Europe | 31 (35.6) | 51 (59.3) |
| Eastern Europe | 29 (33.3) | 5 (5.8) |
| Maternal age (years; range 15–50), mean (SD) | 29.0 (6.7) | 31.7 (5.5) |
| Marital status, no (%) | ||
| Married/cohabiting | 72 (82.8) | 78 (90.7) |
| Other than above | 15 (17.2) | 8 (9.3) |
| Working status at conception, n (%) | ||
| Employed | 42 (48.3) | 48 (55.8) |
| Student | 19 (61.3) | 12 (38.7) |
| Homemaker | 15 (17.2) | 12 (14.0) |
| Job seeker/others | 11 (12.6) | 14 (16.3) |
| Educational attainment, n (%) | ||
| Below high school | 17 (19.5) | 11 (12.8) |
| High school | 39 (44.8) | 33 (45.8) |
| Above high school | 31 (42.5) | 42 (48.8) |
| Immigrant status (yes),b n (%) | 3 (3.5) | 4 (4.7) |
| Lifestyle characteristics | ||
| Alcohol use during pregnancy (yes),c n (%) | 19 (21.8) | 14 (16.3) |
| Smoking during pregnancy (yes), n (%) | 16 (18.4) | 13 (15.1) |
| Neurotic personality traits (range 8–40),d mean (SD) | 28.8 (5.4) | 28.2 (5.2) |
| Health-related characteristics | ||
| Medical contact due to fertility problems (yes), n (%) | 15 (17.2) | 14 (16.3) |
| Nausea in pregnancy (yes), n (%) | 66 (75.9) | 68 (79.1) |
| Sleeping problems in pregnancy (yes), n (%) | 54 (62.1) | 50 (58.1) |
| Chronic somatic comorbidity (yes),e n (%) | 64 (73.6) | 43 (50.0) |
| Comedication in pregnancy | ||
| Anxiolytics and sedatives | 8 (9.2) | 16 (18.6) |
| Antipsychotics | 7 (8.1) | 9 (10.5) |
| Antiepileptics | 4 (4.6) | 6 (7.0) |
| Acetaminophen | 49 (56.3) | 71 (82.6) |
Notes: Missing values were <1.5% for immigrant status, employment, unplanned pregnancy, smoking, fertility problems, folate use, and alcohol use and between 2.5% and 3.0% for neuroticism traits.
Indicates use of folate before and/or during pregnancy;
women having the first language different from the official main language in the country of residence;
indicates alcohol consumption after awareness of the pregnancy;
measured via the Big Five Inventory personality scale;
defined as self-reported asthma, allergy, hypothyroidism, epilepsy, diabetes (type I or II), and rheumatic or cardiovascular diseases.
Abbreviation: SD, standard deviation.
Table 4 describes the associations between antidepressant exposure in pregnancy and postnatal depressive symptom severity. In the weighted model, mothers who had been medicated at any time during pregnancy (adjusted β=−0.34, 95% CI =−0.66, −0.02), or in two or three trimesters, had a significant postnatal symptom severity reduction compared with the nonmedicated counterpart. The largest effect estimate was observed in relation to postnatal anxiety symptom reduction following treatment with antidepressants in two or three trimesters (adjusted β=−0.44, 95% CI =−0.84, −0.03). In the stratified analysis according to the time since childbirth, treatment with antidepressant at any time or in two or three trimesters was associated with reduced symptom severity (total EPDS) only in the earliest postnatal period (<25 weeks, adjusted β=−0.74 [−1.24, −0.24]; ≥25 weeks, adjusted β=−0.03 [−0.44, 0.39]). Larger effects were detected on the postnatal anxiety and anhedonia EPDS subdimensions (Table 4). Descriptive details of the IPTW and results of the various sensitivity analyses are described in the Supplementary materials. Figures S3 and S4 depict the balance between covariates.
Table 4.
Association between antidepressant use during pregnancy and postnatal depressive symptoms severity as measured by the main and subdimensions of the EPDS (n=173)
| Antidepressant use | Any postnatal time, <1 year since birth
|
Stratification by time since birth
|
||||||
|---|---|---|---|---|---|---|---|---|
| Crude modela
|
Weighted modela,b
|
Weighted modela,b
|
||||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Any time, <1 year (n=173) | <25 weeks (n=65) | ≥25 weeks (n=108) | ||||||
| EPDS, total score | ||||||||
| At any time | −0.38 | −0.71, −0.05** | −0.34 | −0.66, −0.02** | −0.74 | −1.24, −0.24** | −0.03 | −0.44, 0.39 |
| Two or three trimesters | −0.45 | −0.79, −0.11** | −0.38 | −0.72, −0.04** | −0.86 | −1.41, −0.32** | 0.04 | −0.42, 0.50 |
| EPDS, subdimensions | ||||||||
| Nonspecific depressive symptoms | ||||||||
| At any time | −0.25 | −0.59, 0.07 | −0.30 | −0.62, 0.03 | −0.54 | −1.03, −0.04** | −0.11 | −0.55, 0.33 |
| Two or three trimesters | −0.30 | −0.64, 0.03 | −0.31 | −0.65, 0.04 | −0.66 | −1.19, −0.13** | 0.01 | −0.45, 0.47 |
| Anxiety symptoms | ||||||||
| At any time | −0.53 | −0.89, −0.17** | −0.33 | −0.70, 0.04 | −0.75 | −1.34, −0.16** | −0.01 | −0.45, 0.43 |
| Two or three trimesters | −0.64 | −1.02, −0.26* | −0.44 | −0.84, −0.03** | −0.84 | −1.52, −0.15** | −0.07 | −0.55, 0.41 |
| Anhedonia | ||||||||
| At any time | −0.21 | −0.56, 0.15 | −0.31 | −0.68, 0.06 | −0.84 | −1.43, −0.25** | 0.10 | −0.35, 0.56 |
| Two or three trimesters | −0.25 | −0.60, 0.10 | −0.34 | −0.74, 0.05 | −0.94 | −1.53, −0.36** | 0.18 | −0.32, 0.67 |
Notes: Lower z scores indicate lower symptom severity, and higher scores indicate the converse.
p≤0.001,
0.001<p<0.05.
Women not medicated with antidepressants in pregnancy constituted the reference group for all the analyses (n=87);
models weighted with inverse probability of treatment weighting using the propensity score, estimated using maternal covariates and survey weight.
Abbreviations: CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale.
Discussion
This study is the first to report self-reported antenatal and postnatal depressive symptoms in women across 12 European countries using uniform data collection and provides novel insights into the association of antidepressant treatment in pregnancy with postnatal symptom severity. On the basis of the EPDS, we found a substantial disparity in the prevalence of depressive symptoms across the countries. The period prevalence for moderate-to-very severe depressive symptoms, albeit with some amount of uncertainty, was higher in the western and eastern regions relative to the northern region, both in the antenatal period (6.8%–7.5% vs 4.3%) and postnatal period (7.6% vs 4.7%). These estimates are broadly in the range of those of major depression identified in some of the prior research (3%–5% during pregnancy, up to 6% during the first year postpartum).3,4,7,29 However, higher rates (15%–25%) have been identified in women from low- to middle-income countries,9,30,31 which supports our findings in relation to some of the investigated populations. We noted an inverse relationship between reporting a psychiatric disorder during pregnancy and symptom severity indicated by the EPDS score, particularly among women in Eastern Europe. This scenario could certainly reflect a lack of awareness of perinatal mental illnesses and inappropriate treatment and a still existing stigmatization of people with psychiatric diseases in this region.32 Given the deleterious effect of perinatal depression on the mother, child, and family as a whole,33,34 our findings deserve attention and point to a crucial need of resource allocation for screening and treatment of perinatal mental disorders across Europe and particularly in the eastern region.
Our prevalence estimates of mild depressive symptoms were somewhat higher than those found by some5,35 although not all prior studies.36,37 Difference in study design, populations, or EPDS cutoff scores may not account entirely for the disparate findings.19,20,36 We adopted survey weight adjustment, which enabled our estimates to be representative for high-risk women (ie, with younger age and lower education), often underrepresented in prior studies.31,37 The use of a single EPDS measurement,38 or a more honest disclosure of depressive symptoms in a web-based, anonymous questionnaire versus face-to-face interview, may also have contributed. It is difficult to assess whether the varying sociocultural context, the validation status of the EPDS in the different languages, selection of more educated and primiparous women, or the Internet-administered EPDS may have affected our results.8,18,39,40
A key finding of this study was that treatment with antidepressants during pregnancy, mainly SSRIs, may have a preventive, medium size effect on postnatal depressive symptoms. This aligns with some albeit not all prior studies on the detrimental effect of antidepressant discontinuation in pregnancy on maternal perinatal mental health.12–15 Our association was only evident in the early postpartum, ie, the 6-month period since childbirth, and of slightly greater magnitude on the anxiety and anhedonia symptom subdimensions. Putnam et al21 found these two symptom subdimensions to be prominent with early postpartum onset and of notable severity. The elevated pharmacotherapy rate and the high average EPDS score in the psychiatric sample are indeed suggestive of severe mental illness.41 Antidepressants have been shown to have greater benefit in severely ill nonpregnant patients,42 and this may also be true in the perinatal context, although the interplay between the perinatal hormonal changes and the pharmacological action of antidepressants remains elusive. Nevertheless, these maternal correlates may also plausibly reflect a suboptimal treatment of the psychiatric disorder and/or an inadequate dosing of antidepressants. Pregnancy is a major determinant of discontinuation of a needed pharmacotherapy, and the drug doses prescribed during this period are often lower than those effective.41 Thus, if this holds true, the benefit of an optimal treatment with antidepressants in terms of adherence and dosage could be larger than that found in this study.
Bias due to the selection of mothers with more favorable mental health in the early postpartum, and unmeasured factors such as antidepressant treatment in the postnatal period, could possibly explain our findings. Nevertheless, if our observed associations were completely attributable to postnatal antidepressant use, we would have expected similar point estimates for the early and late postnatal period. Our observed associations have to be corroborated or refuted by longitudinal studies, but at present they provide some insights into the importance of exploring perinatal mental health from a (multi)dimensional perspective. Antidepressant treatment during pregnancy, in particular with SSRIs, poses critical challenges to pregnant women and their clinicians given the contradictory findings across multiple studies and the diverged debate on their short-term and long-term reproductive safety.11,43 However, suboptimally treated antenatal depression and anxiety may pose comparable fetal risks,34,44 and they are important risk factors for maternal mental health postpartum.45 An individual-based assessment of both the maternal psychiatric disorder and the benefit–risk ratio of antidepressant pharmacotherapy during pregnancy is essential to prevent harmful effects on both mother and child.
Strengths and limitations
A major strength is the use of a self-reported scale with good reliability designed to measure perinatal mood specifically, validated against clinical interview.18,19 By doing so, we could also explore depressive symptoms from a dimensional perspective and on a subdomain level. Data collection was conducted uniformly in all participating countries via utilization of an anonymous electronic questionnaire, which enabled us to potentially reach a large proportion of the birthing population. This approach allowed us to measure perinatal depression also in low-to middle-income countries and facilitated women’s disclosure of depressive symptoms. We corrected our prevalence estimates and association measures by survey weight adjustment, allowing the findings to be representative of the target population in terms of age and education. The IPTW approach set the difference in baseline characteristics between medicated and nonmedicated women to minimum.
One important limitation is the lack of the temporal component. Treatment with antidepressants was reported retrospectively by mothers, which make their associations with postnatal symptom response valid. The psychiatric disorders were self-reported by the participants and not based on medically confirmed diagnoses, and their time of onset was not captured. However, the psychiatric sample correlates are suggestive of high psychiatric morbidity, and women were specifically asked to indicate whether they had chronic depression or anxiety. We did not have information about the history of psychiatric disorders and prior treatments, past or ongoing nonpharmacological psychotherapy, and use of antidepressant before or after pregnancy. We measured depressive symptoms only once, which could have overestimated our prevalence estimates.19 Information about medication use during pregnancy depended on the accuracy of the woman’s reporting. The small sample size limited the statistical power of country-specific analyses and of the postnatal strata by the time since childbirth. The questionnaire was only available through Internet websites, which did not permit calculation of a conventional response rate, and a selection bias of the target population cannot be ruled out. However, recent epidemiological studies indicate reasonable validity of web-based recruitment methods,46,47 and the overall Internet penetration rate is relatively high among women of childbearing age.48 We have previously assessed the study’s external validity on an individual country and found that, on average, the women in the study had higher education and were slightly more often primiparous than the general birthing populations in various countries.17 It is possible that selection bias may have affected our association measures and potentially underestimated and overestimated the antidepressant effects on the anhedonia and nonspecific depressive symptom subdimensions, respectively.
Conclusion
In this study, we found a differential prevalence of depressive symptoms across the countries in both the antenatal and postnatal periods. Women with more severe depressive symptoms based on the EPDS score less often reported to suffer from a psychiatric disorder during pregnancy. Taken together, this raises concern about unrecognition and suboptimal treatment of perinatal mental illnesses, particularly in some specific countries. Although longitudinal studies are needed to confirm or refute this association, women treated with antidepressant during pregnancy were less likely to report postnatal depressive symptoms, particularly in the early postpartum period, compared with the nonmedicated counterpart. Women should be empowered to develop an evidence-based understanding, not only solely of the potential risks but also of the benefits of antidepressant treatment in pregnancy in order to optimize maternal–child health.
Supplementary materials
Methods
Additional details on “Antenatal and postnatal depressive symptoms”
Information about the use of validated and/or translated versions of the original Edinburgh Postnatal Depression Scale (EPDS) in the current study has been provided elsewhere.1 The EPDS developers were acknowledged in each electronic questionnaire under the section presenting the scale. Imputed values were generated when respondents completed at least eight of the 10 items on the EPDS, using the estimation–maximization algorithm.2 Values were imputed for 3.3% of the women.
Additional details on “Data analysis”
The statistical office of the European Union provided information about the distribution of these variables among women of childbearing age in each participating country, except Russia.3 For the latter, we used average data for the other eastern European countries.3,4 Each woman was assigned a weight, obtained by dividing the population proportion by the corresponding sample proportion in each age-by-education strata.4 Women underrepresented in our sample were assigned a weight >1, while those overrepresented received a weight <1. The survey weight for the entire study sample had a mean of 0.98 (range=0.13–33.05).
The propensity score was estimated using the survey weight and the following maternal covariates: region of residency, age (squared term), education, immigrant status, parity, marital status, unplanned pregnancy, smoking and alcohol use during pregnancy, neurotic traits, comedication in pregnancy with anxiolytics and sedatives, and antipsychotics. These covariates were selected on the basis of subject knowledge, prior research, and characteristics of the resulting propensity score.
Power analysis
The study was adequately powered to detect fairly medium differences in postnatal depressive symptoms. With power analysis for unpaired samples of the difference between two independent means (α=0.05), we had 80% power to detect effect sizes of 0.45.
Sensitivity analyses
Various sensitivity analyses were conducted to assess the robustness of the findings. We excluded women who completed the EPDS within the first 4 weeks postpartum to limit the risk of measuring “baby blues” from the descriptive analysis. The inverse probability of treatment weighting (IPTW) analysis is very sensitive to extreme weights; thus, we progressively truncated the IPTW at 1st/99th and 5th/95th percentile.5 The weighted analyses were also restricted to women on antidepressant monotherapy or solely with depression and/or anxiety. To account for country variation, we replicated the generalized linear models of the primary analyses with the inclusion of a random effect for country of residence, plus weighting by the composite weight.
Results
Additional details on “Results”
The total EPDS and the three subdimensions had good reliability in both the samples (Cronbach’s α =0.80–0.86), also when restricted to women with a psychiatric disorder (0.88–0.89).
The generated IPTW had a mean of 1.04 for both the antidepressant “exposure” groups, with ranges of 0.54–8.17 (any time during pregnancy) and 0.53–11.78 (two or three trimesters).
Sensitivity and stratified analysis
Exclusion of women in the earliest postnatal period (0–4 weeks since childbirth, n=253) did not meaningfully affect the overall and region-specific postnatal mean EPDS scores (1% mean difference).
Exclusion of women on polytherapy (n=4) or with disorders other than depression or anxiety (n=6), or the progressive IPTW truncation, yielded an effect estimate similar to those of the main analysis.
The results of the sensitivity analysis accounting for the random effect by the country of residence did not materially differ from those of the main analysis. The point estimate for antidepressant treatment during pregnancy and overall depressive symptom severity (total EPDS) changed by 3% (adjusted β =−0.33, 95% CI =−0.59, −0.08). In all the EPDS subdimensions, the CI of the association measures did not cross the null effect.
Antenatal EPDS weighted mean score and 95% CI, overall and by self-reported psychiatric disorder (n=4308).
Notes: No EPDS estimate is presented for women with psychiatric disorders in Croatia and Slovenia due to small sample size. Mean values and corresponding 95% CI were survey weight adjusted. The survey weight accounted for educational level within age strata for each individual country among women of childbearing age. Population data for 2012 from European Commission, Eurostat. Population by educational attainment level, sex and age (%) - main indicators; 2017 [updated February 28, 2018; cited November 8, 2017]. Available from: http://ec.europa.eu/eurostat/web/products-datasets/-/edat_lfse_03. Accessed August 17, 2017.3
Abbreviations: CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale.
Postnatal EPDS weighted mean score and 95% CI, overall and by self-reported psychiatric disorder (n=3761).
Notes: No EPDS estimate is presented for women with psychiatric disorders in Croatia due to low numbers. No estimate is presented for women with psychiatric disorders in Slovenia because of small sample size. Mean values and corresponding 95% CI were survey-weight-adjusted. The survey weight accounted for educational level within age strata for each individual country among women of childbearing age. Population data for 2012 from European Commission, Eurostat. Population by educational attainment level, sex and age (%) - main indicators; 2017 [updated February 28, 2018; cited November 8, 2017]. Available from: http://ec.europa.eu/eurostat/web/products-datasets/-/edat_lfse_03. Accessed August 17, 2017.3
Abbreviations: CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale.
Balance of covariates (standardized difference) after the application of the IPTW for antidepressant use at any time during pregnancy.
Abbreviation: IPTW, inverse probability of treatment weight.
Balance of covariates (standardized difference) after the application of the IPTW for antidepressant use in two or three trimesters.
Abbreviation: IPTW, inverse probability of treatment weight.
References
- 1.Lupattelli A, Spigset O, Bjornsdottir I, et al. Patterns and factors associated with low adherence to psychotropic medications during pregnancy – a cross-sectional, multinational web-based study. Depress Anxiety. 2015;32(6):426–436. doi: 10.1002/da.22352. [DOI] [PubMed] [Google Scholar]
- 2.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Series B Stat Methodol. 1977;39(1):1–38. [Google Scholar]
- 3.European Commission Eurostat Population by educational attainment level, sex and age (%) - main indicators. 2017. [Accessed August 17, 2017]. [updated February 28, 2018 cited November 8, 2017] Available from: http://ec.europa.eu/eurostat/web/products-datasets/-/edat_lfse_03.
- 4.Applied Survey Methods – A statistical perspective 2017. [Accessed August 14, 2017]. Available from: http://applied-survey-methods.com/weight.html.
- 5.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
We thank the Steering Committee of the Organization of Teratology Information Services and the Scientific Board of the European Network of Teratology Information Services for reviewing the main study protocol. We are grateful to all the women who took part in this study and to all website providers who contributed to the recruitment phase. We also thank Mårdby AC, Hameen-Anttila K, Passier JLM, and Ingunn Björnsdottir for their contribution to the data collection in Sweden, Finland, the Netherlands, and Iceland. The study has received support from the Foundation for Promotion of Norwegian Pharmacies and the Norwegian Pharmaceutical Society, Oslo, Norway. AL’s postdoctoral research fellowship was funded through the HN’s European Research Council Starting Grant “DrugsInPregnancy” (Grant No. 639377). The funding sources had no involvement in any of the stages from the study design to the submission of the paper for publication.
Footnotes
Author contributions
AL and HN conceived the idea for the study and participated in its design and coordination. AL drafted the manuscript and analyzed the data. All the authors contributed to the data collection, interpreted the results, revised the manuscript critically for important intellectual content, and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Falah-Hassani K, Shiri R, Dennis CL. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med. 2017;47(12):2041–2053. doi: 10.1017/S0033291717000617. [DOI] [PubMed] [Google Scholar]
- 2.Hendrick V. Psychiatric Disorders in Pregnancy and the Postpartum: Principles and Treatment. Totowa, NJ: Humana Press; 2006. [Google Scholar]
- 3.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes. Summary. Evidence Report/Technology Assessment: Number 119. AHRQ Publication Number 05-E006-1. Rockville, MD: Agency for Healthcare Research and Quality; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92. doi: 10.1016/j.jad.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315–323. doi: 10.1192/bjp.bp.116.187179. [DOI] [PubMed] [Google Scholar]
- 8.Di Florio A, Putnam K, Altemus M, et al. The impact of education, country, race and ethnicity on the self-report of postpartum depression using the Edinburgh Postnatal Depression Scale. Psychol Med. 2017;47(5):787–799. doi: 10.1017/S0033291716002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8(1):37–54. [Google Scholar]
- 10.Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1–2):147–157. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Spigset O, Nordeng H. Safety of psychotropic drugs in pregnancy and breastfeeding. In: Spina E, Trifirò G, editors. Pharmacovigilance in Psychiatry. Cham: Springer International Publishing; 2016. pp. 299–319. [Google Scholar]
- 12.Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 13.Yonkers KA, Gotman N, Smith MV, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22(6):848–854. doi: 10.1097/EDE.0b013e3182306847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson SA, Hernandez-Diaz S, Palmsten K, Mogun H, Olfson M, Huybrechts KF. Methodological considerations in assessing the effectiveness of antidepressant medication continuation during pregnancy using administrative data. Pharmacoepidemiol Drug Saf. 2015;24(9):934–942. doi: 10.1002/pds.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimmel M, Hess E, Roy PS, et al. Family history, not lack of medication use, is associated with the development of postpartum depression in a high-risk sample. Arch Womens Ment Health. 2015;18(1):113–121. doi: 10.1007/s00737-014-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Insel TR, Cuthbert BN. Medicine. Brain disorders? Precisely. Science. 2015;348(6234):499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- 17.Lupattelli A, Spigset O, Twigg MJ, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014;4(2):e004365. doi: 10.1136/bmjopen-2013-004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 19.Cox JL, Holden J. Perinatal Mental Health: A Guide to the Edinburgh Postnatal Depression Scale (EPDS) London, UK: RCPsych Publications; 2003. [Google Scholar]
- 20.Kozinszky Z, Dudas RB. Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. J Affect Disord. 2015;176:95–105. doi: 10.1016/j.jad.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 21.Putnam KT, Wilcox M, Robertson-Blackmore E, et al. Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry. 2017;4(6):477–485. doi: 10.1016/S2215-0366(17)30136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odalovic M, Tadic I, Lakic D, Nordeng H, Lupattelli A, Tasic L. Translation and factor analysis of structural models of Edinburgh Postnatal Depression Scale in Serbian pregnant and postpartum women – Web-based study. Women Birth. 2015;28(3):e31–e35. doi: 10.1016/j.wombi.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Tuohy A, McVey C. Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the Edinburgh Postnatal Depression Scale. Br J Clin Psychol. 2008;47(Pt 2):153–169. doi: 10.1111/j.2044-8260.2008.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 24.The Big Five trait taxonomy: history, measurement, and theoretical perspectives. In: John OP, Srivastava S, editors; Pervin LA, John OP, editors. Handbook of Personality: Theory and Research. New York, NY: Guilford; 1999. pp. 102–138. [Google Scholar]
- 25.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. [Google Scholar]
- 26.Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49(1):284–303. doi: 10.1111/1475-6773.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnusson K. Interpreting Cohen’s d effect size – an interactive visualization. 2013. [Accessed May 20, 2017]. [updated February 3, 2014; cited November 7, 2017] Available from: http://rpsychologist.com/d3/cohend/
- 29.Melville JL, Gavin A, Guo Y, Fan MY, Katon WJ. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064–1070. doi: 10.1097/AOG.0b013e3181f60b0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. 2016;3(10):973–982. doi: 10.1016/S2215-0366(16)30284-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher J, Cabral de Mello M, Patel V, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ. 2012;90(2):139G–149G. doi: 10.2471/BLT.11.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon GE, VonKorff M. Recall of psychiatric history in cross-sectional surveys: implications for epidemiologic research. Epidemiol Rev. 1995;17(1):221–227. doi: 10.1093/oxfordjournals.epirev.a036180. [DOI] [PubMed] [Google Scholar]
- 33.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):e321–e341. doi: 10.4088/JCP.12r07968. [DOI] [PubMed] [Google Scholar]
- 34.Gjerde LC, Eilertsen EM, Reichborn-Kjennerud T, et al. Maternal perinatal and concurrent depressive symptoms and child behavior problems: a sibling comparison study. J Child Psychol Psychiatry. 2017;58(7):779–786. doi: 10.1111/jcpp.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Underwood L, Waldie KE, D’Souza S, Peterson ER, Morton SM. A longitudinal study of pre-pregnancy and pregnancy risk factors associated with antenatal and postnatal symptoms of depression: evidence from Growing Up in New Zealand. Matern Child Health J. 2017;21(4):915–931. doi: 10.1007/s10995-016-2191-x. [DOI] [PubMed] [Google Scholar]
- 36.Bergink V, Kooistra L, Lambregtse-van den Berg MP, et al. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011;70(4):385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Chojenta CL, Lucke JC, Forder PM, Loxton DJ. Maternal health factors as risks for postnatal depression: a prospective longitudinal study. PLoS One. 2016;11(1):e0147246. doi: 10.1371/journal.pone.0147246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Underwood L, Waldie K, D’Souza S, Peterson ER, Morton S. A review of longitudinal studies on antenatal and postnatal depression. Arch Womens Ment Health. 2016;19(5):711–720. doi: 10.1007/s00737-016-0629-1. [DOI] [PubMed] [Google Scholar]
- 39.Spek V, Nyklicek I, Cuijpers P, Pop V. Internet administration of the Edinburgh Depression Scale. J Affect Disord. 2008;106(3):301–305. doi: 10.1016/j.jad.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Di Florio A, Jones L, Forty L, et al. Mood disorders and parity – a clue to the aetiology of the postpartum trigger. J Affect Disord. 2014:152–154. 334–339. doi: 10.1016/j.jad.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen I, Gilbert RE, Evans SJ, Man SL, Nazareth I. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from The Health Improvement Network. J Clin Psychiatry. 2011;72(7):979–985. doi: 10.4088/JCP.10m06090blu. [DOI] [PubMed] [Google Scholar]
- 42.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muzik M, Hamilton SE. Use of antidepressants during pregnancy?: what to consider when weighing treatment with antidepressants against untreated depression. Matern Child Health J. 2016;20(11):2268–2279. doi: 10.1007/s10995-016-2038-5. [DOI] [PubMed] [Google Scholar]
- 44.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norhayati MN, Nik Hazlina NH, Asrenee AR, Wan Emilin WM. Magnitude and risk factors for postpartum symptoms: a literature review. J Affect Disord. 2015;175:34–52. doi: 10.1016/j.jad.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 46.Ekman A, Dickman PW, Klint A, Weiderpass E, Litton JE. Feasibility of using web-based questionnaires in large population-based epidemiological studies. Eur J Epidemiol. 2006;21(2):103–111. doi: 10.1007/s10654-005-6030-4. [DOI] [PubMed] [Google Scholar]
- 47.van Gelder MM, Bretveld RW, Roeleveld N. Web-based questionnaires: the future in epidemiology? Am J Epidemiol. 2010;172(11):1292–1298. doi: 10.1093/aje/kwq291. [DOI] [PubMed] [Google Scholar]
- 48.Seybert H. Internet use in households and by individuals in 2011. Eurostat Statistics in focus. 2011. [Accessed November 13, 2015]. Available from: http://ec.europa.eu/eurostat/documents/3433488/5579964/KS-SF-11-066-EN.PDF/090e071f-c3a9-45d8-aa90-9b142251fd3a?version=1.0.
- 49.European Commission Eurostat Population by educational attainment level, sex and age (%) - main indicators. 2017. [Accessed August 17, 2017]. [updated February 28, 2018; cited November 8, 2017] Available from: http://ec.europa.eu/eurostat/web/products-datasets/-/edat_lfse_03.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antenatal EPDS weighted mean score and 95% CI, overall and by self-reported psychiatric disorder (n=4308).
Notes: No EPDS estimate is presented for women with psychiatric disorders in Croatia and Slovenia due to small sample size. Mean values and corresponding 95% CI were survey weight adjusted. The survey weight accounted for educational level within age strata for each individual country among women of childbearing age. Population data for 2012 from European Commission, Eurostat. Population by educational attainment level, sex and age (%) - main indicators; 2017 [updated February 28, 2018; cited November 8, 2017]. Available from: http://ec.europa.eu/eurostat/web/products-datasets/-/edat_lfse_03. Accessed August 17, 2017.3
Abbreviations: CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale.
Postnatal EPDS weighted mean score and 95% CI, overall and by self-reported psychiatric disorder (n=3761).
Notes: No EPDS estimate is presented for women with psychiatric disorders in Croatia due to low numbers. No estimate is presented for women with psychiatric disorders in Slovenia because of small sample size. Mean values and corresponding 95% CI were survey-weight-adjusted. The survey weight accounted for educational level within age strata for each individual country among women of childbearing age. Population data for 2012 from European Commission, Eurostat. Population by educational attainment level, sex and age (%) - main indicators; 2017 [updated February 28, 2018; cited November 8, 2017]. Available from: http://ec.europa.eu/eurostat/web/products-datasets/-/edat_lfse_03. Accessed August 17, 2017.3
Abbreviations: CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale.
Balance of covariates (standardized difference) after the application of the IPTW for antidepressant use at any time during pregnancy.
Abbreviation: IPTW, inverse probability of treatment weight.
Balance of covariates (standardized difference) after the application of the IPTW for antidepressant use in two or three trimesters.
Abbreviation: IPTW, inverse probability of treatment weight.