ABSTRACT
Small GTPases of the Rab protein family control intracellular vesicular trafficking to allow their communication and maintenance. It is a common strategy for intracellular bacteria to exploit these pathways to shape their respective niches for survival. The subversion of Rabs for the generation of an intracellular environment favoring the pathogen has been described almost exclusively for intracellular bacteria that reside within bacterial containing vacuoles (BCVs). However, less is known about Rab subversion for bacteria that rupture the BCV to reach the host cytoplasm. Here, we provide recent examples of Rab targeting by both groups of intracellular bacteria with a special focus on Shigella, the causative agent of bacillary dysentery. Shigella recruits Rab11, the hallmark of the perinuclear recycling compartment to in situ formed macropinosomes at the entry foci via the bacterial effector IpgD. This leads to efficient BCV rupture and cytosolic escape. We discuss the concept of diverted recycling through host Rab GTPases that emerges as a novel pathogen strategy.
KEYWORDS: bacterial containing vacuole, intracellular bacteria, macropinocytosis, Rab subversion, Rab11, Shigella flexneri, vacuolar rupture, vesicular traffic
Introduction
Intracellular bacteria have evolved molecular weapons to subvert host cells for their own benefit. They manipulate eukaryotic pathways for the promotion of efficient cellular invasion within a bacterial containing vacuole (BCV), intracellular survival, evasion of immune responses, and for their own propagation.1 The bacterial weaponry that achieves such complex control is often constituted of an “effector cocktail,” secreted bacterial proteins into the host cytosol through specialized injection devices, like the type-3 secretion system (T3SS). These effectors interplay with host factors involved in the pathogenicity pathways. It has been recognized that the subversion of the vesicular trafficking machinery represents a key strategy by the bacteria in order to physically shape their local intracellular environment.2-4
Rab GTPases in vesicular trafficking
Cell membrane trafficking is regulated by the Rab family of proteins, which is part of the Ras superfamily of small GTPases. In humans, almost 70 different Rab proteins have been identified. They coordinate sequential trafficking steps by switching between their active GTP-bound form and the inactive GDP-bound form, participating at different levels for example during vesicle formation, motility, tethering or docking and fusion.5
Vesicles carrying internalized cargo from different entry pathways, such as clathrin and caveolae-mediated endocytosis, macropinocytosis and phagocytosis traffic to lysosomes for degradation. The pair Rab5-Rab7 is considered as the master regulator of endosomal trafficking and maturation. Rab5 determines the identity of early endosomal compartments.6 One of its effectors, the phosphatidylinositol 3-kinase catalytic subunit type (PI3KC3), produces phosphatidylinositol 3-phosphate (PI3P) on endosome membranes, which in turn exerts a positive feedback loop resulting in increased Rab5 recruitment.7 Rabex5, a GDP/GTP exchange factor (GEF) for Rab5, contributes to the positive loop by stabilizing Rab5 on the membranes.8 More than 60 downstream effectors of Rab5 have been identified,9 but their functions remain most often poorly understood. The progressive replacement of Rab5 by Rab7 is essential for proper maturation of early endosomes into lysosomes.10-12 Upon Rab7 recruitment and activation, Rab5 is released allowing the maturation of early endosomes into late endosomes.10 Retrograde transport of late endosomes along microtubules is necessary for efficient fusion with lysosomes. It is mediated by the binding of active Rab7 to Rab-interacting lysosomal protein (RILP), which induces the subsequent interaction with dynein-dynactin motor complexes.13 In addition, the protein Hook has been shown to be involved in endosome maturation; its N-terminus interacts with microtubules and its C-terminus with membranes.14
Alternatively to catabolic degradation, and as a requirement for cell surface homeostasis, a part of the endosomal membrane and cargo are recycled back to the plasma membrane.15 The membrane recycling system is highly dynamic; multiple pathways for entry and exit from the endocytic recycling compartment have been described with Rab11 present in most of them. Rab11 attracts effectors responsible for the trafficking of recycling endosomes.16 It mediates vesicular transport along microfilaments by binding the globular tail domain of myosin V (MyoV).17 Rab11 also interacts with the C-terminal of MyoV forming a ternary complex through Rab11 family-interacting protein 2 (FIP2).18 In addition, Rab11 employs kinesins (KIFs) for anterograde transport along microtubules. FIP5 drives the interaction of Rab11 with KIF3.19 Rab11 can bind directly KIF13 promoting the formation of tubules.20 The effector protrudin directs the interactions of Rab11 with KIF5. Intriguingly, protrudin interacts selectively with GDP-Rab11.21 This changes the concept of Rab11 being switched off in its GDP form, and could explain the large promiscuity of Rab11 functions. FIP3 regulates the retrograde transport of Rab11 endosomes along microtubules mediated by dynein. This has been proposed for the sorting of peripheral endosomes to the perinuclear recycling compartment.22 In addition, Rab11 is involved in the tethering and fusion of recycling endosomes with the plasma membrane.23,24
Although Rab11 is the hallmark of the slow recycling pathway, its highly versatile nature and its complex localization profile in different cell types have led to numerous hypotheses about its roles. Importantly, only one Rab11 GEF, Crag, has been recently identified in Drosophila.25 In mitosis, Rab11 has a role in organizing the mitotic spindle and spindle poles, and centriole distribution.26 It is involved in ciliogenesis, cytokinesis, neuritogenesis, and oogenesis.16 It controls the maintenance of microvilli and apical/basolateral specialization in epithelial cells,27 and it is a regulator of autophagosome maturation.28
Besides the 3 Rabs, Rab5, Rab7 and Rab11, described in some detail above, other Rabs have been analyzed in the context of several local trafficking pathways. Exemplarily, Rab1 and Rab2 mediate endoplasmic reticulum (ER)-Golgi trafficking, Rab3 controls exocytic events, Rab32 is involved in the biogenesis of lysosome-related organelles (LROs) and mitochondrial fission, Rab6 regulates intra-Golgi trafficking, Rab9 and Rab7L1 mediate retrograde trafficking from late endosomes and lysosomes to the trans-Golgi network (TGN), Rab4 is involved in the fast recycling pathway, as has been reviewed in detail by Mizuno-Yamasaki and cols.29 In addition, many of the Rabs have been described to be frequently involved in more than one single pathway.
Manipulation of Rabs by intracellular bacteria
Intracellular bacterial pathogens use different mechanisms to hijack Rab proteins (Table 1), especially those that reside within BCVs. They can mimic Rab GEFs and GTP-hydrolysis activating proteins (GAPs) through their effector proteins, either favoring recruitment to or displacement of certain Rabs from their vacuoles. For instance, the phosphatase activity of the Salmonella enterica effector SopB is required for Rab5 recruitment to the BCV. SopB produces PI3P at the BCV membrane, which prolongs the association of Rab5 allowing BCV maturation. In addition, active Rab5 associates with the PI3KC3, which is responsible for augmented PI3P formation on the BCV membrane.30 SopE, another Salmonella effector, has been found to act as a GEF for Rab5, recruiting non-prenylated Rab5 on the BCV.31 Mycobacterium tuberculosis also interferes with Rab5. The glycolipid lipoarabinomannan (LAM), released from the bacterial membrane into the phagosomal membrane, inactivates the PI3KC3 impairing Rab5 recruitment.32 Concomitantly, the Mycobacterium phosphatase SapM depletes PI3P contributing to the arrest of BCV maturation.33
Table 1.
Examples of Rab subversion by different intracellular bacterial pathogens.
| Pathogen | Effector | Mechanism of subversion | Effect on Rabs | Advantages for infection | References |
|---|---|---|---|---|---|
| Salmonella enterica | SopB | Inositol phosphate phosphatase. Converts PIP3 to PI3P | Prolongs and increases Rab5 recruitment on the BCV. | Delays interaction of BCVs with late endosomes | Mallo et al. (2008) |
| SopE | GEF for Rab5. Acts on non-prenylated Rab5 | Prolongs and increases Rab5 recruitment on the BCV. | Delays interaction of BCVs with late endosomes | Mukherjee et al. (2001) | |
| SifA | Forms a complex with SKIP, which binds Rab9 | Induces recruitment of Rab9 to BCVs and SIFS | Prevents the delivery of hydrolytic enzymes to the BCV | Brumell et al. (2002) McGourty et al. (2012) | |
| Salmonella enterica sv. Typhimurium | GtgE | Rab32 protease | Avoids Rab32 recruitment to the BCV | Prevents the delivery of antimicrobial molecules to the BCV | Spanò and Galan (2012) |
| SopD2 | GAP for Rab32 | Contributes to Rab32 removal from BCV membranes | Prevents the delivery of antimicrobial molecules to the BCV | Spanò et al. (2016) | |
| Binds to Rab7 | Stabilizes Rab7 on SCVs and inhibits GTPase cycling. | Contributes to the evasion of lysosomal degradation, promotes the formation of SIFs | D'Costa et al. (2015) | ||
| Micobacterium tuberculosis | LAM | Inacivates 3PI3PKC3 inhibiting PI3P production | Impairs recruitment of Rab5 to the BCV | Arrests phagosome maturation and degradation | Fratti et al. (2001) |
| SapM | Acid phosphatase. Dephosphorylates PI3P | Depletes PI3P on BCVs avoiding Rab5 recruitment | Arrests phagosome maturation and degradation | Vergne et al. (2005) | |
| Legionella pneumophila | VipD | Binds to Rab5 and Rab22 | Prevents interaction of Rab5 and Rab22 with their effectors | Decreases bacterial degradation by general impairment of endosomal maturation | Ku et al. (2012) Gaspar et al. (2014) |
| DrrA | GEF for Rab1. Modifies Rab1 by AMPylation | AMPylated Rab1 is inert to GAPs and becomes constitutively active on BCVs | Establishment of a replicative niche with ER characteristics. Tight modulation of the different effectors is necessary to control BCV trafficking during the different phases of infection | Murata et al. (2006) Müller et al. (2010) | |
| SidD | DeAMPylates Rab1 | Opposed function of DrrA | Tan and Luo (2011) | ||
| LidA | Binds GDI-free Rab1 previously activated by DrrA | Involved in accumulating activated Rab1 on BCVs | Machner and Isberg (2007) | ||
| LepB | GAP for Rab1 | Deactivates Rab1 on the BCV | Gazdag et al. (2013) | ||
| AnkX | Transfers phosphocholine to Rab1 | Phosphocholinated Rab1 is inert to GEFs and GDIs on BCV membranes | Tan et al. (2011) | ||
| Listeria monocytogenes | GADPH | ADP-ribosylates Rab5 | Recruits Rab5 to BCVs and inhibits the activation of GEFs | Blocks maturation of the phagosomes | Alvarez-Dominguez et al. (2008) |
| Brucella abortus | RicA | Binds to Rab2 | Tethers Rab2 to the BCV | Helps to establish a replicative BCV with ER characteristics | de Barsy et al. (2011) |
| Chlamydia trachomatis | ? | ? | Recruitment of Rab6, Rab11, Rab14 and Rab39 to the BCV | Scavenge sphingomyelin for bacterial nutrition from Golgi and MVBs | Rejman Lipinski et al. (2009) Capmany and Damiani (2010) Gambarte Tudela et al. (2015) |
| Yersinia pestis | ? | ? | Recruitment of Rab1 to the BCV | Avoids acidification of BCVs to establish a replicative niche | Connor et al. (2015) |
| Shigella flexneri | VirA | GAP for Rab1 | Deactivates Rab1 on Rab1 positive endosomes | Inhibits ER-to-Golgi transport and impairs autophagy | Dong et al. (2012) |
| IpgD | Inositol phosphate phospatase. Converts PI(4,5)P to PI5P | Induces the recruitment of Rab11 to macropinosomes | Enhances vacuolar rupture and bacterial escape into the cytosol | Mellouk et al. (2014) Weiner et al. (2016) |
Alternatively, some bacterial effectors bind Rabs and thus prevent subsequent interactions with their cognate host effectors. For instance, the Legionella effector VipD forms a complex with Rab5 and Rab22 preventing the interaction with their effectors, such as Rabaptin-5 and early endosome antigen 1 (EEA1).34 This leads to a generalized impairment in endosomal maturation and, consequently, in pathogen degradation.35 Another example is the complex formed by the Salmonella effector SifA and host protein pleckstrin homology domain-containing family M member 2 (SKIP). The SifA-SIKP complex induces the formation of Salmonella induced filaments (SIFs).36 It also binds and recruits Rab9 to the BCV and SIFs. Sequestration of Rab9 by SifA-SKIP avoids the recycling of mannose phosphate receptors (MPRs), which prevents the delivery of lysosomal hydrolytic enzymes to the BCV and SIFs.37 In addition, the C-terminus of SifA, which shows similar structure of other bacterial GEFs, binds RhoA although it does not change its GTPase status.38 SIF formation is indirectly supported through another Salmonella effector, SopD2, which binds directly to Rab7. This effector inhibits the nucleotide exchange of this Rab. It impairs the recruitment of the Rab effectors to the BCV, and subsequenly avoids degradation within lysosomes, thus allowing BCV maturation.39
Other bacterial effectors are able to covalently modify Rab proteins and therefore completely change their properties. It has been described that the Legionella effector DrrA, which is a GEF for Rab1,40 covalently modifies Rab1 by AMPylation of its Switch II region. Then, the AMPylated Rab1 restricts the access of GAPs, becoming constitutively active at the BCV.41 With opposed function, SidD reverses Rab1 AMPylation.42 The effector LidA has also an auxiliary role on Rab1 recruitment thought the action of DrrA,43 and the effector LepB functions as a Rab1 GAP.44 Another Legionella effector named AnkX also covalently modifies Rab1. It transfers a phosphocoline group from CDP-choline to a serine also in the Swich II region, leading to a strong inhibition of its interaction with GEFs and Rab GDP dissociation inhibitors (GDIs).45 Therefore, as phosphocolinated Rab1 cannot be solubilized by the GDI, it remains membrane bound even in the GDP form. The Listeria monocytogenes enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ADP-ribosylates Rab5. This covalent modification renders Rab5 unresponsive for activation by GEFs, and in turn it blocks further maturation into Rab7.46
Some effectors can even degrade Rabs by proteolytic cleavage. This is the case of the Salmonella effector GtgE, which is expressed in the broad-host bacterium Salmonella enterica serovar Typhimurium (S. Typhimurium) and helps to overcome the host restriction barrier. This bacterial effector is specific for Rab32 and Rab7L1. In mouse macrophages, GtgE avoids Rab32 recruitment to the BCV preventing delivery of antimicrobial molecules to the BCV, and allowing bacterial survival and replication. Conversely, the human specific serovars S. Typhi and S. Parathypi do not express GtgE. Rab32 is therefore recruited to the BCVs, bringing antimicrobial capacity to the BCVs and killing intravacuolar bacteria.47 The Salmonella GAP for Rab32, SopD2, contributes to Rab32 removal from the BCV in S. Typhimurium.48 However, in S. Typhi SopD2 is a pseudogene.49 In addition, the absence of GtgE in the human-adapted S. Typhi and S. Parathypi allows Rab7L1 recruitment to the BCV, which is required for the export of typhoid toxin, a unique virulence factor for human-adapted serovars.50
The different examples of manipulation of Rab GTPases by pathogens described above have been characterized in some detail at the molecular level. However, this has not yet been achieved for many other cases. For example, the Brucella abortus effector RicA tethers Rab2 to its BCV by an unknown mechanism. In vitro, RicA binds preferentially GDP-Rab2 but does not possess GEF activity.51 Chlamydia trachomatis recruits Rab6, Rab11 and Rab14 to the BCV in order to scavenge sphingomyelin from the Golgi.52,53 It also recruits Rab39, which is involved in multi-vesicular bodies (MVBs) trafficking, for inclusion growth and bacterial development.54 The specific bacterial effectors responsible of such subversions are still unknown. Finally, Yersinia pestis promotes the recruitment of Rab1 to the BCVs.55 It is thought that this may be a mechanism to control vacuolar pH but, again, neither bacterial effectors responsible nor precise molecular events have been identified so far.
Subversion of Rabs by Shigella: Role in vacuolar rupture
For the causative agent of bacillary dysentery in humans, Shigella flexneri, Rab subversion has only been studied very recently. It has been shown that the Shigella effector VirA functions as a GAP for Rab1, mediating the disruption of ER-Golgi trafficking and suppressing host autophagy, which contributes to intracellular bacterial persistence.56 Even though not directly acting on Rabs, IpaJ cleaves the Arf1 and Arf2 N-myristoylated host GTPases, which regulate Golgi trafficking, inhibiting thus the host secretory pathway.57
The scarce information about the usage of host Rabs by Shigella may be due to the intricate lifestyle of this pathogen. Shigella is a cytosolic bacterium that ruptures its BCV very rapidly after invasion,58 replicates in the cytosol, and propagates into the neighboring cells.59 The events occurring at the initial steps of infection are highly transient, making them difficult to be studied in detail.60 In fact, in both reports mentioned above, the effects of the Shigella effectors were studied at late infection times (around 3 hours post-infection). At that stage bacteria are cytosolic and spread inter-cellularly. Therefore, the possible implication of VirA and IpaJ on Rab subversion at the early stages, such as bacterial entry and vacuolar escape, remains unclear.
As mentioned before, the step of vacuolar escape is a key event for Shigella virulence, but the implication of vesicular trafficking and Rab subversion in the process was never evaluated. Membrane damage, and thus vacuolar rupture, has been thought to occur mainly through the insertion of the T3SS injectisome on the BCV membrane.61,62 Contrary to the established ideas, our group has demonstrated that BCV rupture requires host factors involved in vesicular trafficking, including Rab GTPases, to take place in an efficient manner.63,64 In particular, Shigella hijacks Rab11 to newly formed macropinosomes leading to efficient BCV rupture. This relation was initially discovered characterizing data from a high-content siRNA library screen of host membrane trafficking factors involved in Shigella BCV rupture. BCV escape was monitored by automated microscopy using the CCF4 FRET probe as a reporter of vacuolar rupture.65 This screen provided further Rab proteins to be potentially involved in vacuolar escape, such as Rab1, Rab3, Rab4, Rab5, Rab7L1 along with Rab11.63
Our live imaging revealed that Rab5, Rab7 and Rab11 were recruited to the Shigella entry sites upon bacterial invasion. While Rab5 was transiently recruited, Rab11 was massively and permanently accumulated.63 Then, in order to evaluate the specific role of Rab11 in vacuolar rupture, we used fluorescently tagged galectin-3 as live cell marker for loss of endomembrane integrity.66 In Rab11 knockdown cells, Shigella vacuolar escape is dramatically delayed, but it does not affect bacterial entry. Importantly, the GTPase activity of Rab11 is necessary for efficient BCV rupture, since expression of GDP-locked64 and GTP-locked Rab11 (non published results) led to a strong delay in vacuolar rupture. We found that Rab11 recruitment to the entry foci was entirely controlled by the bacterial effector IpgD,63 an inositol phosphate phosphatase that converts phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) to phosphatidylinositol 5-phosphate (PI5P).67 In agreement with its implication in Rab11 recruitment, the Shigella effector IpgD exhibited a strong delay in vacuolar rupture. In addition, we demonstrated that IpgD was also involved in the regulation of macropinosome formation during bacterial invasion. The IpgD mutant, which entered without any delay into epithelial cells, was dramatically impaired for macropinosome formation during the time of invasion.63 Previously, IpgD, through the action of PI5P production was reported to be involved in the shaping of the entry foci.68
Canonical macropinocytosis has been described as clathrin-independent non-selective endocytosis (see Fig. 1 for a comparison of Shigella induced macropinosomes). By macropinocytosis, cells are able to internalize considerable volumes of extracellular fluid for the uptake of soluble molecules as well as particles such as viruses, bacteria and apoptotic cell fragments. Antigen presenting cells are capable of constitutive macropinocytosis, while in macrophages, lymphocytes, fibroblast and epithelial cells macropinosomes appear upon applying a variety of external stimuli.69 Macropinosomes are directed toward the lysosomal pathway though the Rab5/Rab7 cascade, Lamp1 acquisition and acidification.70 The recycling of them has only been suggested by a few studies, furthermore the molecular mechanism of this and its regulation remains unclear. Intriguingly, in some non-professional phagocytic cells macropinosomes have been reported to recycle to the plasma membrane, with little or no interaction with endosomal vesicles.71 Our recent study on Shigella induced macropinosomes has been the first that proposed a direct implication of Rab11.
Figure 1.
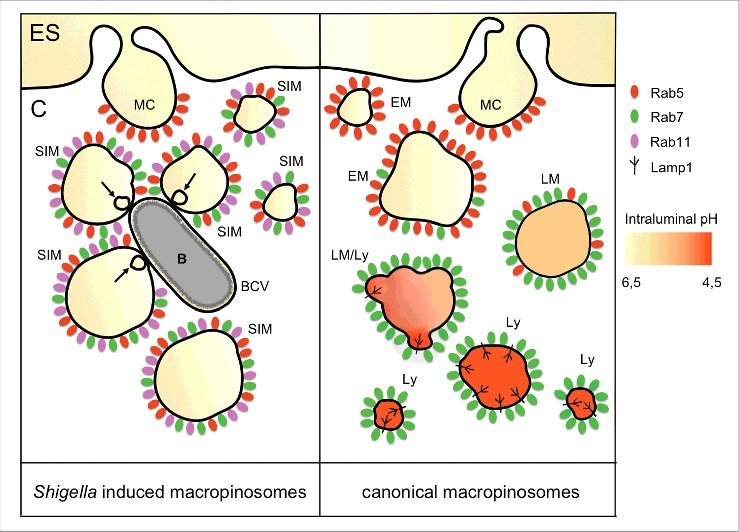
Schematic representation of macropinosome-like vesicles induced by Shigella infection in comparison to the canonical macropinocytic pathway. On the right side, canonical macropinosomes traffic along the endolysosomal pathway, where they are eventually degraded. Rab5 (red) is first recruited to nascent macropinosomes, similar to its recruitment to early endosomes. Then, it is replaced by Rab7 (green). Acquisition of Rab7 implies retrograde transport along microtubules and subsequent fusion with lysosomes. Upon fusion with lysosomes, macropinosomes acquire lysosomal markers such as Lamp1 and hydrolytic enzymes leading to their acidification. In contrast, macropinosome-like vesicles induced by Shigella infection block their maturation before their fusion with lysosomes (left side). Rab11 (magenta) is instead recruited by the bacterial effector IpgD. Bacterial subversion of Rab11, and its recruitment to Shigella-induced macropinosomes, promotes efficient vacuolar escape. ES, extracellular space; C, cytosol; MC, macropinocytic cup; SIM, Shigella induced macropinosome; BCV, bacteria containing vacuole; B, bacteria; EM, early macropinosome; LM, late macropinosome; LM/Ly, late macropinosome-lysosome; Ly, lysosome; arrow, intramacropinosomal vesicle.
Classically, it has been considered that Shigella takes advantage of ruffling for its own internalization within macropinosome-like vesicles.72 In contrast to this paradigm, our results from multidimensional live cell confocal microscopy showed that the recruited Rab5, Rab7 and Rab11 were located exclusively at the membranes of the surrounding macropinosomes (Fig. 1), but never at the forming BCV.64 At the ultrastructural level, the different identities of both compartments was confirmed by focused ion beam scanning electron microscopy (FIB/SEM), an emerging technique for tomography of large volumes,73 using a correlative approach. This showed clearly that Shigella enters in a tight vacuole distinct from the surrounding macropinosomes. In addition, FIB/SEM revealed the presence of contact sites between the BCV and macropinosomes, with the appearance of smaller intramacropinosomal vesicles right at their interface during vacuolar rupture.64 Although Rab5 and Rab7 are recruited to Shigella induced macropinosomes, they do not recruit Lamp1 nor do they show acidification (non published results). Instead, they are blocked for further maturation and hijack Rab11 for virulence purposes. These observations suggest that BCV rupture may be mediated by direct physical contacts between Rab11-positive macropinosomes and the BCV. Such contacts may be an outcome of “misdirected” trafficking toward the BCV instead of their recycling to the plasma membrane, which could be considered as an “internal recycling” pathway.
In the scenario of “internal recycling” the exocyst (a protein complex made of 8 distinct proteins) may play a role. Rab11-positive recycling endosomes are delivered to the apical cell surface along radial actin filaments by MyoV.17 Then, they are tethered to the plasma membrane by binding of MyoV and Rab11 to the exocyst component Sec15 to form a tripartite docking complex. Afterwards, Sec15 interacts with SNARE complexes to mediate vesicle fusion with the plasma membrane.23,24 Knocking down constituents of the exocyst inhibited the process of Shigella internalization (our own unpublished experiments), but the precise step of inhibition needs to be elucidated.
Since the GDP/GTP status of Rab11 is important for BCV destabilization, another possible role for Rab11 in BCV rupture could be as a shuttle, providing the nascent macropinosomes with downstream molecules involved in contact formation. It has been described in Drosophila that under fed conditions the protein Hook has a negative regulatory role in autophagosome maturation. Hook anchors late endosomes to microtubules and thus impairs their fusion with amphisomes, delaying their maturation. In this context Rab11 is prominently located at recycling endosomes. In starving conditions, Rab11 regulates the relocalization of Hook protein in a GTP-dependent manner binding and recruiting Hook to autophagic structures. Late endosomes are then not anchored anymore to microtubules, and their fusion with amphisomes is favored, allowing a faster maturation and catabolic degradation.28
Interestingly, Rab 11 has been also reported to be involved in expulsion of pore forming toxins from the surface of challenged host cells.74 A similar function has been described for the ESCRT machinery, which is involved in local membrane deformation and scission, and in repairing plasma membrane wounds.75 It could be considered that Rab11 is recruited together with the ESCRT complex to the BCV via macropinosomes to repair the damage caused by the insertion of the T3SS translocon complex, pinching off small intraluminal vesicles at the contact interphase, and inducing in turn vacuolar rupture.
Finally, the notion that endosomal compartments function as multifunctional platforms on which unique sets of molecular machines are assembled have emerged recently.76 Therefore, Shigella induced macropinosomes may represent a platform for localized production of specific signaling lipids and docking of certain protein complexes promoting vacuolar scape.
Conclusion and perspective
In summary, as a result of Rab subversion, bacterial effectors induce or avoid multiple interactions between the BCV and other host cell compartments modifying the BCV or its membrane composition. Therefore, bacteria establish an intracellular replicative niche through the generation of a sort of hybrid organelle.3,4
Most of the currently described Rab modifications by pathogens lead to an impairment of BCV degradation for bacteria survival. This is achieved either by blocking phagosome maturation, by avoiding fusion with lysosomes or by redirecting BCV to alternative trafficking pathways such as the ER. In addition, intracellular bacteria also subvert vesicular trafficking in order to gain access to nutrients or scavenge building blocks for intracellular replication (see Table 1 for an overview).
From our findings about the cytosolic bacterium Shigella we propose that the concept of Rab subversion is not an exclusive strategy for BCV-contained bacteria, but also for cytosolic ones. We have discovered a new role for recycling endosomes, via Rab11, during early stages of Shigella infection. This provides a different concept about bacterial vacuolar rupture. Intriguingly, none of the different Rabs recruited to the entry foci (Rab5, Rab7 and Rab11) were located on BCV membranes, but only on macropinosomes. Further research on Rab manipulation by cytosolic bacteria will help to fully understand the process of vacuolar rupture, a key event for their virulence.
Funding Statement
This work was supported by the LabEx consortium IBEID, and by the Institut Pasteur CARNOT-MIE program. J.E also acknowledges support of an ERC starting grant (Rupteffects, Nr. 261166).
Abbreviations
- BCVs
Bacterial containing vacuoles
- EEA1
Early endosome antigen 1
- ER
Endoplasmic reticulum
- FIB/SEM
Focused ion beam scanning electron microscopy
- GEF
GDP/GTP exchange factor
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GAPs
GTP-hydrolysis activating proteins
- KIFs
Kinesin superfamily proteins, kinesins
- LAM
Lipoarabinomannan
- LROs
Lysosome-related organelles
- MPRs
Mannose phosphate receptors
- MVBs
Multi-vesicular bodies
- MyoV
Myosin V
- PI3KC3
Phosphatidylinositol 3-kinase catalytic subunit type
- PI3P
Phosphatidylinositol 3-phosphate
- PI(4,5)P2
Phosphatidylinositol 4,5-bisphosphate
- PI5P
Phosphatidylinositol 5-phosphate
- SKIP
Pleckstrin homology domain-containing family M member 2
- GDIs
Rab GDP dissociation inhibitors
- RILP
Rab-interacting lysosomal protein
- FIP
Rab11 family-interacting protein
- SIFs
Salmonella induced filaments
- TGN
Trans-Golgi network
- T3SS
Type-3 secretion system
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature 2007; 449:827-34; PMID:17943119; https://doi.org/ 10.1038/nature06247 [DOI] [PubMed] [Google Scholar]
- [2].Cossart P, Craig RR. Manipulation of host membrane machinery by bacterial pathogens. Curr Opin Cell Biol 2010; 22:547-54; PMID:20542678; https://doi.org/ 10.1016/j.ceb.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sherwood RK, Roy CR. A Rab-centric perspective of bacterial pathogen-occupied vacuoles. Cell Host Microbe 2013; 14:256-68; https://doi.org/ 10.1016/j.chom.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stein MP, Müller MP, Wandinger-Ness A. Bacterial pathogens commandeer Rab GTPases to establish intracellular niches. Traffic 2012; 13:1565-88; PMID:22901006; https://doi.org/ 10.1111/tra.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 2015; 128:3171-6; PMID:26272922; https://doi.org/ 10.1242/jcs.166074 [DOI] [PubMed] [Google Scholar]
- [6].Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, Epstein-Barash H, Kuchimanchi S, Peng CG, Ruda VM, et al.. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 2012; 485:465-70; PMID:22622570; https://doi.org/ 10.1038/nature11133 [DOI] [PubMed] [Google Scholar]
- [7].Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, et al.. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol 2005; 170:607-18; PMID:16103228; https://doi.org/ 10.1083/jcb.200505128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M. Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell 2001; 12:2219-28; PMID:11452015; https://doi.org/ 10.1091/mbc.12.7.2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mottola G. The complexity of Rab5 to Rab7 transition guarantees specificity of pathogen subversion mechanisms. Front Cell Infect Microbiol 2014; 4:180; PMID:25566515; https://doi.org/ 10.3389/fcimb.2014.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005; 122:735-49; PMID:16143105; https://doi.org/ 10.1016/j.cell.2005.06.043 [DOI] [PubMed] [Google Scholar]
- [11].Poteryaev D, Fares H, Bowerman B, Spang A. Caenorhabditis elegans SAND-1 is erssential for RAB-7 function in endosomal traffic. EMBO J 2007; 26:301-12; PMID:17203072; https://doi.org/ 10.1038/sj.emboj.7601498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huotari J, Helenius A. Endosome maturation. EMBO J 2011; 30:3481-500; PMID:21878991; https://doi.org/ 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol Cell Biol 2003; 23:6494-506; PMID:12944476; https://doi.org/ 10.1128/MCB.23.18.6494-6506.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luiro K, Yliannala K, Ahtiainen L, Maunu H, Järvelä I, Kyttälä A, Jalanko A. Interconnections of CLN3, Hook1 and Rab proteins link Batten disease to defects in the endocytic pathway. Hum Mol Genet 2004; 13:3017-27; PMID:15471887; https://doi.org/ 10.1093/hmg/ddh321 [DOI] [PubMed] [Google Scholar]
- [15].Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 2009; 10:597-608; PMID:19696797; https://doi.org/ 10.1038/nrm2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Welz T, Wellbourne-Wood J, Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol 2014; 24:407-15; PMID:24675420; https://doi.org/ 10.1016/j.tcb.2014.02.004 [DOI] [PubMed] [Google Scholar]
- [17].Pylypenko O, Attanda W, Gauquelin C, Lahmani M, Coulibaly D, Baron B, Hoos S, Titus MA, England P, Houdusse AM. Structural basis of myosin V Rab GTPase-dependent cargo recognition. Proc Natl Acad Sci U S A 2013; 110:20443-8; PMID:24248336; https://doi.org/ 10.1073/pnas.1314329110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem 2002; 277:50415-21; PMID:12393859; https://doi.org/ 10.1074/jbc.M209270200 [DOI] [PubMed] [Google Scholar]
- [19].Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, Prekeris R. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci 2008; 121:3824-33; PMID:18957512; https://doi.org/ 10.1242/jcs.032441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Delevoye C, Miserey-Lenkei S, Montagnac G, Gilles-Marsens F, Paul-Gilloteaux P, Giordano F, Waharte F, Marks MS, Goud B, Raposo G. Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A. Cell Rep 2014; 6:445-54; https://doi.org/ 10.1016/j.celrep.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shirane M, Nakayama KI. Protrudin induces neurite formation by directional membrane trafficking. Science 2006; 314:818-21; PMID:17082457; https://doi.org/ 10.1126/science.1134027 [DOI] [PubMed] [Google Scholar]
- [22].Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci 2010; 123:181-91; PMID:20026645; https://doi.org/ 10.1242/jcs.052670 [DOI] [PubMed] [Google Scholar]
- [23].Takahashi S, Kubo K, Waguri S, Yabashi A, Shin HW, Katoh Y, Nakayama K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J Cell Sci 2012; 125:4049-57; PMID:22685325; https://doi.org/ 10.1242/jcs.102913 [DOI] [PubMed] [Google Scholar]
- [24].Guichard A, Nizet V, Bier E. Rab11-mediated trafficking in host-pathogen interactions. Nat Rev Microbiol 2014; 12:624-34; PMID:25118884; https://doi.org/ 10.1038/nrmicro3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xiong B, Bayat V, Jaiswal M, Zhang K, Sandoval H, Charng WL, Li T, David G, Duraine L, Lin YQ, et al.. Crag is a GEF for Rab11 required for rhodopsin trafficking and maintenance of adult photoreceptor cells. PLoS Biol 2012; 10:e1001438; PMID:23226104; https://doi.org/ 10.1371/journal.pbio.1001438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Das S, Hehnly H, Doxsey S. A new role for Rab GTPases during early mitotic stages. Small GTPases 2014; 5:e29565; PMID:24921241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Knowles BC, Weis VG, Yu S, Roland JT, Williams JA, Alvarado GS, Lapierre LA, Shub MD, Gao N, Goldenring JR. Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes. J Cell Sci 2015; 128:1617-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Szatmári Z, Kis V, Lippai M, Hegedus K, Faragó T, Lorincz P, Tanaka T, Juhász G, Sass M. Rab11 facilitates cross-talk between autophagy and endosomal pathway through regulation of Hook localization. Mol Biol Cell 2014; 25:522-31; PMID:24356450; https://doi.org/ 10.1091/mbc.E13-10-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem 2012; 81:637-59; PMID:22463690; https://doi.org/ 10.1146/annurev-biochem-052810-093700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mallo GV, Espina M, Smith AC, Terebiznik MR, Alemán A, Finlay BB, Rameh LE, Grinstein S, Brumell JH. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol 2008; 182:741-52; https://doi.org/ 10.1083/jcb.200804131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mukherjee K, Parashuraman S, Raje M, Mukhopadhyay A. SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J Biol Chem 2001; 276:23607-15; PMID:11316807; https://doi.org/ 10.1074/jbc.M101034200 [DOI] [PubMed] [Google Scholar]
- [32].Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol 2001; 154:631-44; PMID:11489920; https://doi.org/ 10.1083/jcb.200106049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2005; 102:4033-8; PMID:15753315; https://doi.org/ 10.1073/pnas.0409716102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ku B, Lee KH, Park WS, Yang CS, Ge J, Lee SG, Cha SS, Shao F, Heo WD, Jung JU, et al.. VipD of Legionella pneumophila targets activated Rab5 and Rab22 to interfere with endosomal trafficking in macrophages. PLoS Pathog 2012; 8:e1003082; PMID:23271971; https://doi.org/ 10.1371/journal.ppat.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gaspar AH, Machner MP. VipD is a Rab5-activated phospholipase A1 that protects Legionella pneumophila from endosomal fusion. Proc Natl Acad Sci U S A 2014; 111:4560-5; PMID:24616501; https://doi.org/ 10.1073/pnas.1316376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brumell JH, Goosney DL, Finlay BB. SifA, a type III secreted effector of Salmonella typhimurium, directs Salmonella-induced filament (Sif) formation along microtubules. Traffic 2002; 3:407-15; PMID:12010459; https://doi.org/ 10.1034/j.1600-0854.2002.30604.x [DOI] [PubMed] [Google Scholar]
- [37].McGourty K, Thurston TL, Matthews SA, Pinaud L, Mota LJ, Holden DW. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science 2012; 338:963-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhao W, Moest T, Zhao Y, Guilhon AA, Buffat C, Gorvel JP, Méresse S. The Salmonella effector protein SifA plays a dual role in virulence. Sci Rep 2015; 5:12979; PMID:26268777; https://doi.org/ 10.1038/srep12979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].D'Costa VM, Braun V, Landekic M, Shi R, Proteau A, McDonald L, Cygler M, Grinstein S, Brumell JH. Salmonella disrupts host endocytic trafficking by SopD2-mediated inhibition of Rab7. Cell Rep 2015; 12:1508-18; PMID:26299973; https://doi.org/ 10.1016/j.celrep.2015.07.063 [DOI] [PubMed] [Google Scholar]
- [40].Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol 2006; 8:971-7; PMID:16906144; https://doi.org/ 10.1038/ncb1463 [DOI] [PubMed] [Google Scholar]
- [41].Müller MP, Peters H, Blümer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science 2010; 329:946-9; PMID:20651120; https://doi.org/ 10.1126/science.1192276 [DOI] [PubMed] [Google Scholar]
- [42].Tan Y, Luo ZQ. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature 2011; 475:506-9; PMID:21734656; https://doi.org/ 10.1038/nature10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Machner MP, Isberg RR. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science 2007; 318:974-7; PMID:17947549; https://doi.org/ 10.1126/science.1149121 [DOI] [PubMed] [Google Scholar]
- [44].Gazdag EM, Streller A, Haneburger I, Hilbi H, Vetter IR, Goody RS, Itzen A. Mechanism of Rab1b deactivation by the Legionella pneumophila GAP LepB. EMBO Rep 2013; 14:199-205; PMID:23288104; https://doi.org/ 10.1038/embor.2012.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tan Y, Arnold RJ, Luo ZQ. Legionella pneumophila regulates the small GTPase Rab1 activity by reversible phosphorylcholination. Proc Natl Acad Sci U S A 2011; 108:21212-7; PMID:22158903; https://doi.org/ 10.1073/pnas.1114023109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alvarez-Dominguez C, Madrazo-Toca F, Fernandez-Prieto L, Vandekerckhove J, Pareja E, Tobes R, Gomez-Lopez MT, Del Cerro-Vadillo E, Fresno M, Leyva-Cobián F, et al.. Characterization of a Listeria monocytogenes protein interfering with Rab5a. Traffic 2008; 9:325-37; PMID:18088303; https://doi.org/ 10.1111/j.1600-0854.2007.00683.x [DOI] [PubMed] [Google Scholar]
- [47].Spanò S, Galán JE. A Rab32-dependent pathway contributes to Salmonella typhi host restriction. Science 2012; 338:960-3; PMID:23162001; https://doi.org/ 10.1126/science.1229224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Spanò S, Gao X, Hannemann S, Lara-Tejero M, Galán JE. A bacterial pathogen targets a host rab-family GTPase defense pathway with a GAP. Cell Host Microbe 2016; 19:216-26; PMID:26867180; https://doi.org/ 10.1016/j.chom.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, Churcher C, Mungall KL, Bentley SD, Holden MT, et al.. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 2001; 413:848-52; PMID:11677608; https://doi.org/ 10.1038/35101607 [DOI] [PubMed] [Google Scholar]
- [50].Spanò S, Liu X, Galán JE. Proteolytic targeting of Rab29 by an effector protein distinguishes the intracellular compartments of human-adapted and broad-host Salmonella. Proc Natl Acad Sci U S A 2011; 108:18418-23; PMID:22042847; https://doi.org/ 10.1073/pnas.1111959108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].de Barsy M, Jamet A, Filopon D, Nicolas C, Laloux G, Rual JF, Muller A, Twizere JC, Nkengfac B, Vandenhaute J, et al.. Identification of a Brucella spp. secreted effector specifically interacting with human small GTPase Rab2. Cell Microbiol 2011; 13:1044-58; PMID:21501366; https://doi.org/ 10.1111/j.1462-5822.2011.01601.x [DOI] [PubMed] [Google Scholar]
- [52].Rejman Lipinski A, Heymann J, Meissner C, Karlas A, Brinkmann V, Meyer TF, Heuer D. Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent Golgi fragmentation. PLoS Pathog 2009; 5:e1000615; PMID:19816566; https://doi.org/ 10.1371/journal.ppat.1000615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Capmany A, Damiani MT. Chlamydia trachomatis intercepts Golgi-derived sphingolipids through a Rab14-mediated transport required for bacterial development and replication. PLoS One 2010; 5:e14084; PMID:21124879; https://doi.org/ 10.1371/journal.pone.0014084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gambarte Tudela J, Capmany A, Romao M, Quintero C, Miserey-Lenkei S, Raposo G, Goud B, Damiani MT. The late endocytic Rab39a GTPase regulates the interaction between multivesicular bodies and chlamydial inclusions. J Cell Sci 2015; 128:3068-81; PMID:26163492; https://doi.org/ 10.1242/jcs.170092 [DOI] [PubMed] [Google Scholar]
- [55].Connor MG, Pulsifer AR, Price CT, Abu Kwaik Y, Lawrenz MB. Yersinia pestis requires host Rab1b for survival in macrophages. PLoS Pathog 2015; 11:e1005241; PMID:26495854; https://doi.org/ 10.1371/journal.ppat.1005241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dong N, Zhu Y, Lu Q, Hu L, Zheng Y, Shao F. Structurally distinct bacterial TBC-like GAPs link Arf GTPase to Rab1 inactivation to counteract host defenses. Cell 2012; 150:1029-41; PMID:22939626; https://doi.org/ 10.1016/j.cell.2012.06.050 [DOI] [PubMed] [Google Scholar]
- [57].Burnaevskiy N, Fox TG, Plymire DA, Ertelt JM, Weigele BA, Selyunin AS, Way SS, Patrie SM, Alto NM. Proteolytic elimination of N-myristoyl modifications by the Shigella virulence factor IpaJ. Nature 2013; 496:106-9; PMID:23535599; https://doi.org/ 10.1038/nature12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ray K, Bobard A, Danckaert A, Paz-Haftel I, Clair C, Ehsani S, Tang C, Sansonetti P, Tran GV, Enninga J. Tracking the dynamic interplay between bacterial and host factors during pathogen-induced vacuole rupture in real time. Cell Microbiol 2010; 12:545-56; PMID:20070313; https://doi.org/ 10.1111/j.1462-5822.2010.01428.x [DOI] [PubMed] [Google Scholar]
- [59].Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol 2009; 7:333-40; PMID:19369949; https://doi.org/ 10.1038/nrmicro2112 [DOI] [PubMed] [Google Scholar]
- [60].Mellouk N, Enninga J. Cytosolic access of intracellular bacterial pathogens: The shigella paradigm. Front Cell Infect Microbiol 2016; 6:35; PMID:27092296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].High N, Mounier J, Prévost MC, Sansonetti PJ. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J 1992; 11:1991-9; PMID:1582426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Du J, Reeves AZ, Klein JA, Twedt DJ, Knodler LA, Lesser CF. The type III secretion system apparatus determines the intracellular niche of bacterial pathogens. Proc Natl Acad Sci U S A 2016; 113:4794-9; PMID:27078095; https://doi.org/ 10.1073/pnas.1520699113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mellouk N, Weiner A, Aulner N, Schmitt C, Elbaum M, Shorte SL, Danckaert A, Enninga J. Shigella subverts the host recycling compartment to rupture its vacuole. Cell Host Microbe 2014; 16:517-30; PMID:25299335; https://doi.org/ 10.1016/j.chom.2014.09.005 [DOI] [PubMed] [Google Scholar]
- [64].Weiner A, Mellouk N, Lopez-Montero N, Chang YY, Souque C, Schmitt C, Enninga J. Macropinosomes are key players in early Shigella invasion and vacuolar escape in epithelial cells. PLoS Pathog 2016; 12:e1005602; PMID:27182929; https://doi.org/ 10.1371/journal.ppat.1005602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nothelfer K, Dias Rodrigues C, Bobard A, Phalipon A, Enninga J. Monitoring Shigella flexneri vacuolar escape by flow cytometry. Virulence 2011; 2:54-7; PMID:21317555; https://doi.org/ 10.4161/viru.2.1.14666 [DOI] [PubMed] [Google Scholar]
- [66].Keller C, Mellouk N, Danckaert A, Simeone R, Brosch R, Enninga J, Bobard A. Single cell measurements of vacuolar rupture caused by intracellular pathogens. J Vis Exp 2013; 12:e50116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Niebuhr K, Giuriato S, Pedron T, Philpott DJ, Gaits F, Sable J, Sheetz MP, Parsot C, Sansonetti PJ, Payrastre B. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J 2002; 21:5069-78; PMID:12356723; https://doi.org/ 10.1093/emboj/cdf522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Niebuhr K, Jouihri N, Allaoui A, Gounon P, Sansonetti PJ, Parsot C. IpgD, a protein secreted by the type III secretion machinery of Shigella flexneri, is chaperoned by IpgE and implicated in entry focus formation. Mol Microbiol 2000; 38:8-19; PMID:11029686; https://doi.org/ 10.1046/j.1365-2958.2000.02041.x [DOI] [PubMed] [Google Scholar]
- [69].Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol 2011; 89:836-43; PMID:21423264; https://doi.org/ 10.1038/icb.2011.20 [DOI] [PubMed] [Google Scholar]
- [70].Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol 1993; 121:1011-20; PMID:8099075; https://doi.org/ 10.1083/jcb.121.5.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hamasaki M, Araki N, Hatae T. Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. Anat Rec A Discov Mol Cell Evol Biol 2004; 277:298-306; PMID:15052657 [DOI] [PubMed] [Google Scholar]
- [72].Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 2004; 304:242-8; PMID:15073367; https://doi.org/ 10.1126/science.1090124 [DOI] [PubMed] [Google Scholar]
- [73].Murphy GE, Narayan K, Lowekamp BC, Hartnell LM, Heymann JA, Fu J, Subramaniam S. Correlative 3D imaging of whole mammalian cells with light and electron microscopy. J Struct Biol 2011; 176:268-78; PMID:21907806; https://doi.org/ 10.1016/j.jsb.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Los FC, Kao CY, Smitham J, McDonald KL, Ha C, Peixoto CA, Aroian RV. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe 2011; 9:147-57; PMID:21320697; https://doi.org/ 10.1016/j.chom.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. ESCRT machinery is required for plasma membrane repair. Science 2014; 343:1247136; PMID:24482116; https://doi.org/ 10.1126/science.1247136 [DOI] [PubMed] [Google Scholar]
- [76].Gould GW, Lippincott-Schwartz J. New roles for endosomes: from vesicular carriers to multi-purpose platforms. Nat Rev Mol Cell Biol 2009; 10:287-92; PMID:19277045; https://doi.org/ 10.1038/nrm2652 [DOI] [PMC free article] [PubMed] [Google Scholar]


