ABSTRACT
Several families of small GTPases regulate a variety of fundamental cellular processes, encompassing growth factor signal transduction, vesicular trafficking and control of the cytoskeleton. Frequently, their action is hierarchical and complementary, but much of the detail of their functional interactions remains to be clarified. It is well established that Rab family members regulate a variety of intracellular vesicle trafficking pathways. Moreover, Rho family GTPases are pivotal for the control of the actin and microtubule cytoskeleton. However, the interplay between these 2 types of GTPases has been rarely reported. We discuss here our recent findings showing that Rab11, a key regulator of endosomal recycling, and Rac1, a central actin cytoskeleton regulator involved in lamellipodium formation and cell migration, interplay on endosomes through the Rab11 effector FIP3. In the context of the rapidly reactive T lymphocytes, Rab11-Rac1 endosomal functional interplay is important to control cell shape changes and cell symmetry during lymphocyte spreading and immunological synapse formation and ultimately modulate T cell activation.
KEYWORDS: cell shape, cell symmetry, FIP3; immunological synapse, recycling endosomes, T cell activation, Rab11, Rac
Rab proteins constitute the largest family of small GTPases with more than 60 identified members in humans. They localize to particular intracellular membrane compartments, controlling trafficking pathways that drive exchanges between different cellular organelles. Rab GTPases and their effectors ensure a variety of functions, including protein sorting, vesicle mobility and vesicle tethering.1 The Rab11 subfamily comprises Rab11a, Rab11b and Rab11c/Rab25 proteins. Rab11 GTPases, control trafficking through the endosomal recycling compartment (ERC). The ERC is a large tubulo-vesicular compartment, concentrated around the centrosome, and localized proximal to the Golgi apparatus. Rab11 regulates the indirect or ‘long-loop’ endosomal recycling pathway of a variety of cargo proteins and membranes which transit the ERC.2 This trafficking pathway is crucial for numerous cellular functions, ranging from nutrient homeostasis to cell division. Thus, the Rab11 proteins regulate vesicular trafficking pathways that ensure the transport of endosomes from the plasma membrane to the ERC2,3 and from the ERC to the plasma membrane.
Rab11 proteins exert their functions through their interaction with several effector proteins, including the 5 members of the Rab11-family interacting proteins (Rab11-FIPs, or FIPs), Myosin V and others.2-4 The Rab11-FIP proteins are comprised of several protein motifs/domains (Fig. 1), including a Rab binding domain (RBD) which mediates their GTP-dependent interactions with Rab GTPases; (C2 or EF-hand) that confer calcium sensitivity and/or phospholipid binding. The FIPs also bind motor proteins such as Myosin V, Dynein and Kinesin. Additionally, FIPs can form part of the exocyst complex. Depending on their specific protein-, lipid- and calcium-binding abilities, as well as their likely post-translational modification by phosphorylation, the various FIPs likely ensure distinct aspects of the endosomal recycling process.3 In this regard, some major differences are evident in the phenotype resulting from Class II FIPs (particularly FIP3) gain-of-function when compared with Class I FIP gain-of-function in cells during interphase. Specifically, increased FIP3 expression leads to a dramatic accumulation/condensation of the pericentriolar ERC.5,6 While the other Class II FIP (FIP4) also generates a similar albeit weaker phenotype, none of the 3 Class I FIPS (RCP/FIP1C, FIP2 and Rip11/FIP5) give rise to this phenotype. In previous work, we have clarified the mechanism for the FIP3 condensed ERC phenotype; it occurs due to Rab11GTP on peripheral early/sorting endosomes binding FIP3, which binds the cytoplasmic dynein complex. The functional Rab11/FIP3/Dynein regulates the movement of peripheral endosomes along microtubule tracks toward the microtubule organizing center/centrosome generating the ERC.7 While Rab11/FIP3 controlled trafficking from the ERC to the plasma membrane cannot be ruled out, it seems that there is a heavy directionality-bias of Rab11/FIP3 mediated trafficking in an inward cellular direction. The Class I FIPs are the more likely mediators of cargo trafficking from the ERC.
Figure 1.
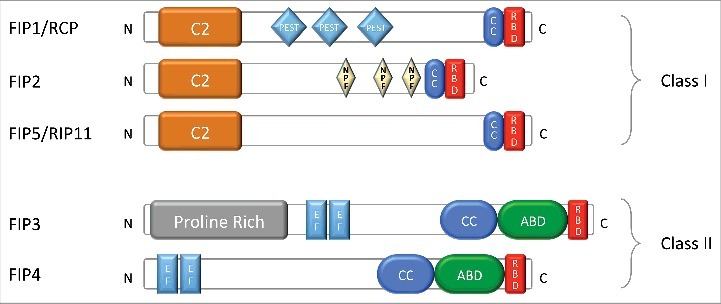
Structure of Rab11-FIPs. Classification and schematic representation of FIPs predicted domain architecture: C2-domain (C2); PEST domain (PEST), coiled-coil domain (CC), Rab-binding domain (RBD), Prolin-Rich domain, EF-hand domain (EF); Arf-binding domain (ABD). According to Horgan and McCaffrey.3
Additional layers of complexity remain to be unravelled regarding Rab11 and FIP controlled vesicular trafficking. One aspect of this is the likelihood that post-translational modification of the FIPs by phosphorylation very likely regulates their function. However, despite the fact that the first report on these proteins in the literature identified FIP5 (Rip11) as a heavily phosphorylated protein (pp75),8 the extent of FIP phosphorylation, identity of kinases and impact of their phosphorylation on vesicular trafficking is largely unclear currently. Furthermore, all 5 FIPs are capable of interacting with all 3 Rab11 subfamily members (Rab11a, Rab11b and Rab11c, also known as Rab25) and some are capable of interacting with other Rabs, for example Rab4 with RCP/FIP1C9 and the Class I FIPs with Rab14.10 These observations beg the question of which FIP is critical for which trafficking pathway, which Rab protein is controlling the event and what specific cargo is being trafficked? Interestingly, data is now beginning to emerge that implicates Rab11b as an important driver of endosomal recycling, while the role of Rab11a appears to be toward the degradative pathway, for certain cargo at least11 (Artemiuk and McCaffrey, unpublished data).
Currently, ERC functions are not precisely defined, but this often-overlooked organelle likely serves various critical roles in cell physiology, acting as a cellular store of protein and bulk membrane for transport to different subcellular locations as required. As such, the ERC likely serves to supply proteins and membrane for recycling back to the plasma membrane, or in a polarized fashion in migrating cells and across epithelial cells during transcytosis. Some examples of the importance of trafficking via this organelle in migrating cells involves trafficking of membrane and adhesion receptors in a polarized fashion to the cell front lamellipodium.12,13 This has both physiological (developmental) and pathophysiological consequences in processes, such as tumor metastasis. Thus, Rab11 and its effector FIP1C (Rab coupling protein RCP) are involved in integrin trafficking that promotes invasive tumor cell invasion. Interestingly, this process is guided by the production of membrane phosphatidic acid that facilitates the tethering of Rab11-RCP vesicles carrying α5β1 integrins to invasive pseudopodes.14 Another striking example of the importance of Rab11/FIP2/Myosin V controlled endosomal recycling is in post-synaptic dendrites involving the calcium triggered delivery of membrane and AMPA-Receptors to dendritic spines.15 This important physiological event underpins memory acquisition—a process known as long-term potentiation.16 Thus Rab11 and its effectors can deliver receptors and signaling molecules in specialized cells, like neurons,16 or lymphocytes.17,18 This mechanism of controlled delivery of signaling components helps to build the activation capacity of neural and immunological synapses.
Finally, small Rho GTPases, may also transit through various types of recycling endosomes and use this mechanism to control cytoskeleton remodeling.5,19,20 Rho GTPases are master regulators of the cytoskeleton. Three subfamilies, Rho, Rac and Cdc42, regulate actin and microtubule cytoskeleton dynamics, controlling cell shape and cell polarity in tissue forming, or in migrating cells.21
Our recent findings in T lymphocytes show that Rac1 traffics through the ERC and that Rac1 endosomal traffic is necessary for the control of its functions on the actin cytoskeleton.5 Traffic of Rac1 to the ERC is controlled by Rab11 and its effector FIP3. Interestingly, at steady state only a minor fraction of Rac1 appears localized at the Rab11+ pericentrosomal ERC. However, when FIP3 was overexpressed, Rac1 massively concentrated at the ERC —a finding very similar to that previously reported by us for several markers of the endosomal recycling pathway.6,7 This indicates that Rac1 traffics to the ERC in a Rab11-FIP3-dependent manner. Further supporting this hypothesis, the overexpression of a FIP3 mutant that cannot interact with Rab11 delocalized Rac1 from the ERC to the plasma membrane and the cytosol. Finally, FIP3 silencing dispersed ERC-localized Rac1 into small vesicles throughout the cytoplasm. Rac1 dispersion had interesting functional consequences in both cortical actin in resting cells, and in dynamic actin remodeling during surface spreading of T cells and in immunological synapse formation. Thus, FIP3-silenced resting T cells displayed reduced cortical rigidity, indicating that endosomal-mediated Rac1 localization controls steady-state cortical actin organization. Moreover, FIP3-silenced cells spread much more on poly-lysine-covered glass surfaces, forming Rac1-dependent extended lamellipodium-like membrane extensions enriched in filamentous actin (F-actin). Altogether, these findings indicate that endosomal Rac1 localization and traffic buffers actin dynamics responsible for cortical rigidity and cell spreading.
T cell antigen receptor (TCR) engagement by peptide antigens associated with major histocompatibility complex (MHC) molecules on antigen presenting cells leads to robust actin cytoskeleton remodeling and the formation of immunological synapses.22 Antibodies directed to TCR complex subunits, like CD3ϵ, can mimic TCR antigen engagement triggering T cell cytoskeleton rearrangements.23 Rho-family GTPases are among the central actin cytoskeleton regulators downstream of the TCR.24 Our recent data support that Rab11-FIP3-mediated traffic of Rac1 is necessary for Rac1 relocalization to the immunological synapse. Thus, concentration of Rac1 at the ERC by overexpression of FIP3, or delocalization of Rac1 from the ERC by overexpression of the FIP3 mutant 1738E, which does not bind to Rab11, inhibited Rac1 clustering at the synapse.5 Interestingly, immunological synapses are symmetric structures25 and this symmetry was significantly altered in FIP3-silenced T cells, despite of the fact that Rac1 could reach the immunological synapse in these cells.5 Therefore, Rab11-FIP3-driven endosomes seem to finely control the spatial and temporal organization of Rac1 at the immunological synapse and, as a consequence, control the balanced actin dynamics that ensures synapse symmetry. Moreover, T cell spreading on anti-CD3 coated surfaces is also controlled by Rab11-FIP3, as indicated by enhanced T cell spreading of FIP3-silenced T cells.5
How does Rac1 associate with Rab11+ endosomes? Rac1 is expected to interact with the cytosolic face of membranes via its geranyl-geranyl C-terminal moieties. Additionally, we unveiled an interaction between FIP3 and Rac1 by co-immunoprecipitation approaches. This interaction was lost only in part when the FIP3 mutant that does not bind to Rab11 was pulled down. These findings suggest that a tripartite Rab11-FIP3-Rac1 complex may be responsible for Rac1 endosomal localization and traffic (Fig. 2). As we demonstrated previously in FIP3-silenced cells, the Rab11+ ERC was dispersed,6 we have recently shown that Rac1 is similarly dispersed under these conditions.5 Moreover, Rab11 and Rac1 did not colocalize in cells lacking FIP3. The most likely interpretation for these findings is that when cells lack FIP3, the Rab11 controlled inward/centrosomal trafficking of peripheral endosomes along microtubule tracks in a dynein dependent manner is abrogated and both Rab11 and Rac1 remain on peripheral vesicles that have associated either Rac1 or Rab11, but not both. FIP3 may be important in linking these 2 types of vesicles.
Figure 2.
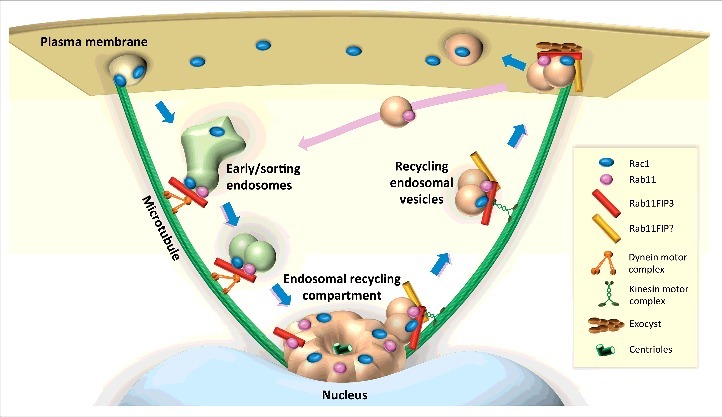
Model for Rab11-Rac1 interplay. After internalization, Rac1 is targeted to early/sorting endosomes, where its traffic is taken in charge by Rab11. Via its interaction with FIP3 and with the help of Rab11 and dynein complex, Rac1 carrying vesicles migrate to the endosomal recycling compartment. Rac1 recycling back to the plasma membrane may involve Rab11 associated to FIP3 or to type I FIPs both interacting with kinesin that drives outward vesicle movement. Finally, interaction of FIP3 with the exocyst complex would facilitate the targeting and release of Rac1 carrying vesicles to the plasma membrane.
FIP3 has several protein interaction domains, including EF-hands rather than C2 domains which are present in the Class I FIPs16 (Fig. 1). i) FIP3 interacts with microtubule-based molecular motors dynein and kinesin.7,26 Our results in T cells are consistent with a predominant effect of dynein, which drives vesicle traffic toward the microtubule minus end, favoring inwards vesicle movement, dependent on FIP3 interaction with Rab11 in its active GTP bound state. Indeed, disruption of FIP3 interaction with Rab11 appears to mediate a kinesin-dependent effect, driving Rac1 vesicles to the plus-end of microtubules and to the plasma membrane (Fig. 2). ii) FIP3 interacts with the small GTPase ARF6,27 also associated with recycling endosomes and capable of regulating intracellular vesicle traffic and actin cytoskeleton dynamics.28 Therefore, FIP3 might form a versatile platform of interplay of the 3 types of small GTPases Rab11, ARF6, and Rac1 that would regulate together T cell spreading and immunological synapse formation. However, our efforts to investigate the involvement of ARF6 in these phenomena did not provide any enlightening result (Bouchet, unpublished). iii) FIP3 interacts with components of the exocyst,27 a protein complex involved in tethering exocytic vesicles at particular areas of the plasma membrane.29
Are other Rho GTPases associated to the Rab11+ ERC? Under the same experimental conditions, we did not observe Cdc42 presence in this compartment in T lymphocytes (Bouchet, unpublished). However, Osmani et al. reported that Cdc42 and its exchange factor βPIX were associated with intracellular vesicles in polarized migrating astrocytes. Moreover, ARF6 was necessary for the polarized recruitment of Cdc42, βPIX, Rac and the Par6-aPKC polarity complex to the leading edge of migrating astrocytes and for microtubule polarization.19 In contrast, under our experimental conditions, microtubule reorganization or polarization at the immunological synapse appeared normal in FIP3-silenced T cells.5 These differences suggest that the ARF6- and the FIP3-dependent processes may be different, despite the reported interaction between FIP3 and ARF6.27
An additional important question is whether endosomal transport would facilitate the localization of Rho GTPase activity in order to fulfill particular cellular functions. Small GTPase activity is controlled by the equilibrium of 3 types of regulatory proteins: guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs) and guanine dissociation inhibitors (GDIs) that drive GTP-GDP exchange. Endosomal traffic may modulate the interaction between the Rho GTPases and their regulatory proteins. In the context of the immunological synapse, Rac1 targeting via endosomes would facilitate the interaction of Rac1 with one of its GEFs, Vav1, which is activated via tyrosine phosphorylation in response to TCR stimulation. Vav1 could locally activate Rac1 to facilitate lamellipodium-like membrane structures rich in F-actin that characterize TCR engagement and synapse formation.23,30 Other GEFs, like DOCK2, Tiam1 and Trio, also regulate Rac1 and may act downstream of the TCR to regulate Rac1 at the synapse.31,32 Consistent with this reasoning, it was shown in other cellular context that Rac1 encounters Tiam1 in Rab5+ endosomes and facilitate Rac1-dependent membrane ruffle formation.20
Do other molecules targeted to the immunological synapse utilize this endosomal transport? The TCR and 2 signaling molecules, the protein tyrosine kinase Lck and the signaling adaptor LAT, are also associated with endosomes that convey them to the synapse. Interestingly, the endosomal subcompartments and the molecular mechanisms involved in targeting these molecules to the synapse are different for the TCRζ, Lck and LAT.33 We observed that the Rab11-FIP3 mediated endosomal transport is used by Lck, but not by TCRζ or LAT (Bouchet et al., unpublished). The src family kinase Lck is the first tyrosine kinase engaged in the TCR signaling cascade upon TCR engagement. Lck was shown to traffic through intracellular vesicles34 that overlap with Rab11.18 Lck endosomal traffic is subverted during human immunodeficiency virus (HIV-1) infection by the viral protein Nef35 in a mechanism that could involve Nef's ability to alter the endosomal recycling compartment.36 We observed that Lck intracellular traffic and targeting to the immunological synapse was altered in FIP3 overexpressing T cells similarly to Rac1.5 Likewise, Lck localization in FIP3-silenced cells was dispersed all over the cytoplasm (Bouchet et al., unpublished). Interestingly, cytoplasmic dispersion of Rac1 and Lck in FIP3-silenced cells led to opposite functional effects. While Rac1 dispersion induced enhanced actin dynamics, Lck dispersion led to a reduced Lck capacity to phosphorylate its main substrates, TCRζ, ZAP70, LAT and PLCγ1 both, at steady state in resting cells and upon TCR stimulation. Therefore, Lck delocalization likely prevents Lck encountering its substrates. Interestingly, reduced TCRζ phosphorylation was concomitant with increased protein levels of TCRζ and higher TCR-CD3 complex cell surface expression (Bouchet et al., unpublished). Of note is that TCRζ levels are controlled by its phosphorylation by Lck.37
In conclusion, by finely regulating the subcellular localization and intracellular traffic of central regulatory molecules, Rab11 and its effector FIP3 modulate central T cell processes, including actin remodeling and cell morphology as well as TCR signal transduction. These findings provide new clues for understanding the functional interplay between intracellular vesicle traffic, actin cytoskeleton remodeling and the TCR signaling machinery into a more complex process of T cell effector functions.
Funding Statement
The authors were supported by grants from the Agence National de Recherche sur le SIDA et les Hepatitis Virales (ANRS), Roux-Institut Pasteur and Sidaction to JB; Agence Nationale de Recherche (ANR, No 11 BSV3 025 01), ANRS (AO 2013-02 CSS1 No 1339/14673), Institut Pasteur, CNRS and INSERM to AA; Science Foundation Ireland Principal Investigator Award (09/IN.1/B2629) to MWM; Fondazione Telethon GGP13254 and AIRC (Italian Association for Cancer Research) IG13524 to AG.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank our laboratory colleagues for stimulating discussions during the development of the mentioned work.
References
- [1].Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 2015; 128:3171-6; PMID:26272922; https://doi.org/ 10.1242/jcs.166074 [DOI] [PubMed] [Google Scholar]
- [2].Kelly EE, Horgan CP, McCaffrey MW. Rab11 proteins in health and disease. Biochem Soc Trans 2012; 40:1360-7; PMID:23176481; https://doi.org/ 10.1042/BST20120157 [DOI] [PubMed] [Google Scholar]
- [3].Horgan CP, McCaffrey MW. The dynamic Rab11-FIPs. Biochem Soc Trans 2009; 37:1032-6; PMID:19754446; https://doi.org/ 10.1042/BST0371032 [DOI] [PubMed] [Google Scholar]
- [4].Lindsay AJ, Jollivet F, Horgan CP, Khan AR, Raposo G, McCaffrey MW, Goud B. Identification and characterization of multiple novel Rab-myosin Va interactions. Mol Biol Cell 2013; 24:3420-34; PMID:24006491; https://doi.org/ 10.1091/mbc.E13-05-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bouchet J, Del Rio-Iniguez I, Lasserre R, Aguera-Gonzalez S, Cuche C, Danckaert A, McCaffrey MW, Di Bartolo V, Alcover A. Rac1-Rab11-FIP3 regulatory hub coordinates vesicle traffic with actin remodeling and T-cell activation. EMBO J 2016; 35:1160-74; PMID:27154205; https://doi.org/ 10.15252/embj.201593274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Horgan CP, Oleksy A, Zhdanov AV, Lall PY, White IJ, Khan AR, Futter CE, McCaffrey JG, McCaffrey MW. Rab11-FIP3 is critical for the structural integrity of the endosomal recycling compartment. Traffic 2007; 8:414-30; PMID:17394487; https://doi.org/ 10.1111/j.1600-0854.2007.00543.x [DOI] [PubMed] [Google Scholar]
- [7].Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci 2010; 123:181-91; PMID:20026645; https://doi.org/ 10.1242/jcs.052670 [DOI] [PubMed] [Google Scholar]
- [8].Wang D, Buyon JP, Zhu W, Chan EK. Defining a novel 75-kDa phosphoprotein associated with SS-A/Ro and identification of distinct human autoantibodies. J Clin Invest 1999; 104:1265-75; PMID:10545525; https://doi.org/ 10.1172/JCI8003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lindsay AJ, Hendrick AG, Cantalupo G, Senic-Matuglia F, Goud B, Bucci C, McCaffrey MW. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J Biol Chem 2002; 277:12190-9; PMID:11786538; https://doi.org/ 10.1074/jbc.M108665200 [DOI] [PubMed] [Google Scholar]
- [10].Kelly EE, Horgan CP, Adams C, Patzer TM, Ni Shuilleabhain DM, Norman JC, McCaffrey MW. Class I Rab11-family interacting proteins are binding targets for the Rab14 GTPase. Biol Cell 2010; 102:51-62; https://doi.org/ 10.1042/BC20090068 [DOI] [PubMed] [Google Scholar]
- [11].Grimsey NJ, Coronel LJ, Cordova IC, Trejo J. Recycling and Endosomal Sorting of Protease-activated Receptor-1 Is Distinctly Regulated by Rab11A and Rab11B Proteins. J Biol Chem 2016; 291:2223-36; PMID:26635365; https://doi.org/ 10.1074/jbc.M115.702993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bretscher MS, Aguado-Velasco . Membrane traffic during cell locomotion. Curr Op Cell Biol 1998; 10:537-41; PMID:9719876; https://doi.org/ 10.1016/S0955-0674(98)80070-7 [DOI] [PubMed] [Google Scholar]
- [13].Paul NR, Jacquemet G, Caswell PT. Endocytic Trafficking of Integrins in Cell Migration. Curr Biol 2015; 25:R1092-105; PMID:26583903; https://doi.org/ 10.1016/j.cub.2015.09.049 [DOI] [PubMed] [Google Scholar]
- [14].Rainero E, Caswell PT, Muller PA, Grindlay J, McCaffrey MW, Zhang Q, Wakelam MJ, Vousden KH, Graziani A, Norman JC. Diacylglycerol kinase alpha controls RCP-dependent integrin trafficking to promote invasive migration. J Cell Biol 2012; 196:277-95; PMID:22270919; https://doi.org/ 10.1083/jcb.201109112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Z, Edwards JG, Riley N, Provance DW Jr., Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, et al.. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 2008; 135:535-48; PMID:18984164; https://doi.org/ 10.1016/j.cell.2008.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kelly EE, Horgan CP, McCaffrey MW, Young P. The role of endosomal-recycling in long-term potentiation. Cell Mol Life Sci 2011; 68:185-94; PMID:20820847; https://doi.org/ 10.1007/s00018-010-0516-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 2004; 20:577-88; PMID:15142526; https://doi.org/ 10.1016/S1074-7613(04)00106-2 [DOI] [PubMed] [Google Scholar]
- [18].Soares H, Henriques R, Sachse M, Ventimiglia L, Alonso MA, Zimmer C, Thoulouze MI, Alcover A. Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. J Exp Med 2013; 210:2415-33; PMID:24101378; https://doi.org/ 10.1084/jem.20130150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol 2010; 191:1261-9; PMID:21173111; https://doi.org/ 10.1083/jcb.201003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 2008; 134:135-47; PMID:18614017; https://doi.org/ 10.1016/j.cell.2008.05.034 [DOI] [PubMed] [Google Scholar]
- [21].Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans 2005; 33:891-5; PMID:16246005; https://doi.org/ 10.1042/BST0330891 [DOI] [PubMed] [Google Scholar]
- [22].Agüera-Gonzalez S, Bouchet J, Alcover A. Immunological Synapse. eLS John Wiley & Sons, Ltd: Chichester: 2015; DOI: 10.1002/9780470015902.a0004027.pub2 [DOI] [Google Scholar]
- [23].Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, Acuto O, Dautry-Varsat A, Alcover A. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity 2001; 15:715-28; PMID:11728334; https://doi.org/ 10.1016/S1074-7613(01)00225-4 [DOI] [PubMed] [Google Scholar]
- [24].Comrie WA, Burkhardt JK. Action and traction: Cytoskeletal control of receptor triggering at the immunological synapse. Front Immunol 2016; 7:68; PMID:27014258; https://doi.org/ 10.3389/fimmu.2016.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, et al.. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell 2007; 129:773-85; PMID:17512410; https://doi.org/ 10.1016/j.cell.2007.03.037 [DOI] [PubMed] [Google Scholar]
- [26].Simon GC, Prekeris R. Mechanisms regulating targeting of recycling endosomes to the cleavage furrow during cytokinesis. Biochem Soc Trans 2008; 36:391-4; PMID:18481966; https://doi.org/ 10.1042/BST0360391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J 2005; 24:3389-99; PMID:16148947; https://doi.org/ 10.1038/sj.emboj.7600803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schweitzer JK, Sedgwick AE, D'Souza-Schorey C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin Cell Dev Biol 2011; 22:39-47; PMID:20837153; https://doi.org/ 10.1016/j.semcdb.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Martin-Urdiroz M, Deeks MJ, Horton CG, Dawe HR, Jourdain I. The Exocyst Complex in Health and Disease. Front Cell Dev Biol 2016; 4:24; PMID:27148529; https://doi.org/ 10.3389/fcell.2016.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal 2004; 16:1-11; PMID:14607270; https://doi.org/ 10.1016/S0898-6568(03)00110-4 [DOI] [PubMed] [Google Scholar]
- [31].Sanui T, Inayoshi A, Noda M, Iwata E, Oike M, Sasazuki T, Fukui Y. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity 2003; 19:119-29; PMID:12871644; https://doi.org/ 10.1016/S1074-7613(03)00169-9 [DOI] [PubMed] [Google Scholar]
- [32].Gronholm M, Jahan F, Marchesan S, Karvonen U, Aatonen M, Narumanchi S, Gahmberg CG. TCR-induced activation of LFA-1 involves signaling through Tiam1. J Immunol 2011; 187:3613-9; PMID:21876037; https://doi.org/ 10.4049/jimmunol.1100704 [DOI] [PubMed] [Google Scholar]
- [33].Niedergang F, Di Bartolo V, Alcover A. Comparative anatomy of phagocytic and immunological synapses. Front Immunol 2016; 7: 18 doi: 10.3389/fimmu.2016.00018; PMID:26858721; https://doi.org/ 10.3389/fimmu.2016.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ehrlich LIR, Ebert PJR, Krummel MF, Weiss A, Davis MM. Dynamics pf p56lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity 2002; 17:809-22; PMID:12479826; https://doi.org/ 10.1016/S1074-7613(02)00481-8 [DOI] [PubMed] [Google Scholar]
- [35].Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 2006; 24:547-61; PMID:16713973; https://doi.org/ 10.1016/j.immuni.2006.02.016 [DOI] [PubMed] [Google Scholar]
- [36].Madrid R, Janvier K, Hitchin D, Day J, Coleman S, Noviello C, Bouchet J, Benmerah A, Guatelli J, Benichou S. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J Biol Chem 2005; 280:5032-44; PMID:15569681; https://doi.org/ 10.1074/jbc.M401202200 [DOI] [PubMed] [Google Scholar]
- [37].D'Oro U, Vacchio MS, Weissman AM, Ashwell JD. Activation of the Lck tyrosine kinase targets cell surface T cell antigen receptors for lysosomal degradation. Immunity 1997; 7:619-28; PMID:9390686; https://doi.org/ 10.1016/S1074-7613(00)80383-0 [DOI] [PubMed] [Google Scholar]


