Abstract
The genus Quercus comprises important species in forestry not only for their productive value but also for their ability to withstand drought. Hence an evaluation of inter- and intraspecific variation in drought tolerance is important for selecting the best adapted species and provenances for future afforestation. The presence of long vessels makes it difficult to assess xylem vulnerability to embolism in oak. Thanks to the development of an in situ flow centrifuge equipped with a large rotor, we quantified (i) the between species variability of embolism resistance in four native and two exotic species of oaks in Europe and (ii) the within species variability in Quercus petraea. Embolism resistance varied significantly among species, with the pressure inducing 50% loss of hydraulic conductivity (P50) ranging between - 7.0 and -4.2 MPa. Species native to the Mediterranean region were more resistant than pan-European species. In contrast, intraspecific variability in embolism resistance in Q. petraea was low within provenances and null among provenances. A positive correlation between P50 and vessel diameter among the six oak species indicates that the more embolism resistant species had narrower xylem vessels. However, this tradeoff between hydraulic efficiency and safety was not observed between Q. petraea provenances.
Keywords: plant hydraulics, xylem embolism, drought resistance, climate change, oaks
Introduction
Climate change projections predict a significant effect on the growth and survival of forests (Reyer et al., 2014) but the impact on growth rate is species specific. While species in northern latitudes are expected to benefit from a warmer climate (Talhelm et al., 2014), species growing under temperate or more southern latitudes will be negatively affected (Reyer et al., 2014). With the predicted climate change scenario (IPCC 2014), mean annual temperatures are expected to increase and patterns and frequency of rainfall may change considerably. This will probably result in more frequent summer drought events in most parts of Europe. Such drought events will have important implications for vegetation distribution and dynamics as seen from the evidence of drought-induced forest dieback in various parts of the world (Allen et al., 2010; Cailleret et al., 2017) including Europe (Bréda et al., 2006; Anderegg et al., 2016). Understanding the mechanisms leading to such mortality events as well as the capacity of the trees to cope with drought is therefore crucial in predicting the ecological consequences of ongoing climate change.
For trees, drought survival relies on their ability to control the loss of water during an extreme event (McDowell et al., 2008). Drought induced dieback in forest trees is mainly a result of xylem hydraulic failure (Anderegg et al., 2013; Anderegg et al., 2016) caused by the formation of air bubbles (embolism) in the xylem conduits which disrupt the water transport from the roots to the leaves (Tyree & Zimmermann, 2002). Vulnerability to embolism (P50, water potential at which 50% of hydraulic conductivity is lost) and the hydraulic safety margin (difference between P50 and minimum xylem water potential under natural conditions) are key physiological traits linked to tree mortality under severe drought (Brodribb & Cochard, 2009; Brodribb et al., 2010; Barigah et al., 2013; Meinzer et al., 2009; Torres-Ruiz et al., 2017a; Urli et al., 2013). A recent survey on 226 tree species across the world found that more than 30% of the species had narrow hydraulic safety margins, making them susceptible to drought (Choat et al., 2012). Vulnerability curves evaluate the loss of xylem conductance as the xylem pressure decreases thereby providing a valuable method to assess the drought resistance (Cochard et al., 2013). This allows the estimation of hydraulic traits such as P50 which is commonly used to characterize drought tolerance at local and global scales as well as across species (Maherali et al., 2004; Trueba et al., 2017).
Among-species variation in resistance to embolism was earlier reported in conifers (Bouche et al., 2014; Delzon et al., 2010). It is also reported that within-species variation can be considerably less than the among-species variation (David-Schwartz et al., 2016; Lamy et al., 2014). Intraspecific variability in resistance to embolism in conifers can be of larger magnitude than intraspecific variability in angiosperms (Anderegg, 2015) although such studies are very limited in number. The issue is complicated in the long-vesselled angiosperm species, because the commonly used methods to construct vulnerability curves can lead to biased results (Torres-Ruiz et al. 2014; 2017a; Cochard et al. 2013; Ennajeh et al. 2011). This is the case for ring porous species such as oak for which maximum vessel length above 70cm has been typically reported (Cochard and Tyree 1990; Martinez-Vilalta et al. 2002; Jacobsen et al. 2007). The vulnerability to embolism may be overestimated in previous studies due to both the so called “open-vessel” (Cochard et al. 2013; Torres-Ruiz et al. 2014 and 2017a) and “cutting” (Wheeler et al., 2013; Torres-Ruiz et al., 2015) artefacts. The overestimations are especially evident by using centrifuge techniques as the in situ flow centrifuge (Cavitron) in which the samples are spun to gradually expose them to decreasing xylem pressures while monitoring the loss of hydraulic conductivity (Cochard, 2005). These artefacts have therefore questioned the reliability of previous results reported on resistance to embolism of long-vesselled species, complicating the proper evaluation of their function.
The genus Quercus comprises approx. 415 reported species across America, Asia, North Africa and Europe (Oh and Manos, 2008; Hubert et al., 2014). In Europe, Quercus petraea and Q. robur are the two widely distributed species extending from Spain to Scandinavia (Ducousso & Bordacs, 2004). Their pan European distributions suggest their high ability to adapt to very diverse growing conditions and, therefore their suitability for future climates (Epron & Dreyer, 1993; Arend et al., 2011; Eaton et al., 2016). The evergreen oak species, Q. ilex and Q. suber are two of the most common species of the Mediterranean forests and they have demonstrated capacities to withstand drought (David et al., 2007). While drought-induced mortality events are reported for Q. robur (Urli et al., 2014) and Q. petraea (Cochard et al., 1992), other Quercus species have shown a high tolerance to drought (Baquedano & Castillo, 2007) including capacity to recover their canopy after a drought event (Lloret et al., 2004). Such different species responses to drought could be due to inherent differences in morphological and physiological traits of adaptive significance and/or phenotypic plasticity. However, an accurate quantification of the interspecific variation in resistance to embolism within the genus Quercus is still lacking. Moreover, experimental evidence of the adaptive significance of the resistance to embolism is also absent in oaks. Therefore, an evaluation of the species genetic variation in resistance to embolism is crucial in order to anticipate the response of major European Quercus species to climate change.
The aim of the present study was to assess inter- and intraspecific variability in resistance to embolism in the oak genus. First, we quantified the resistance to embolism in six Quercus species widely distributed in Europe by using a newly developed prototype of Cavitron equipped with a 1m-diameter rotor. This reduces the amount of cut-open vessel in the xylem samples and, therefore, prevents artefactual losses in hydraulic conductance by avoiding the ‘open-vessel’ artefact. Secondly we investigated the amount of genetic differentiation among provenances in resistance to embolism, by sampling four Q. petraea provenances growing in a common garden and originating from temperate and Mediterranean latitudes in Europe. Results are expected to provide relevant information not only about the ability of the different Quercus species to withstand the adverse effects of drought events, but also show its capacity to adapt to the new climate conditions imposed by the human-induced climate change.
Materials and Methods
Interspecific variation in xylem vulnerability to embolism for 6 oak species
Vulnerability to embolism was evaluated in six oak species: four were native European species (Quercus petraea, Q. ilex, Q. robur and Q. suber) and two were exotic species introduced from North America (Q. palustris and Q. rubra). Their native distribution range is given in Fig.S1 in supplementary information. For each species, two branches were collected from 5 to 16 healthy mature trees growing in Southern France (INRA and University of Bordeaux campus). All branches were a minimum of two meters long ranging between 18 and 20 mm in diameter, collected in the early morning using a pole pruner from the sunny side of the crown. Sampling was made within a period of six weeks in summer 2015. Once collected, transpiration losses from the branches were prevented by removing the leaves immediately after sampling and wrapping them in moist paper to keep them wet during their transportation to the lab. Once in the lab, branches were stored at 3°C until resistance to embolism was assessed (within three weeks of sampling).
Intraspecific variation in xylem vulnerability to embolism for Quercus petraea
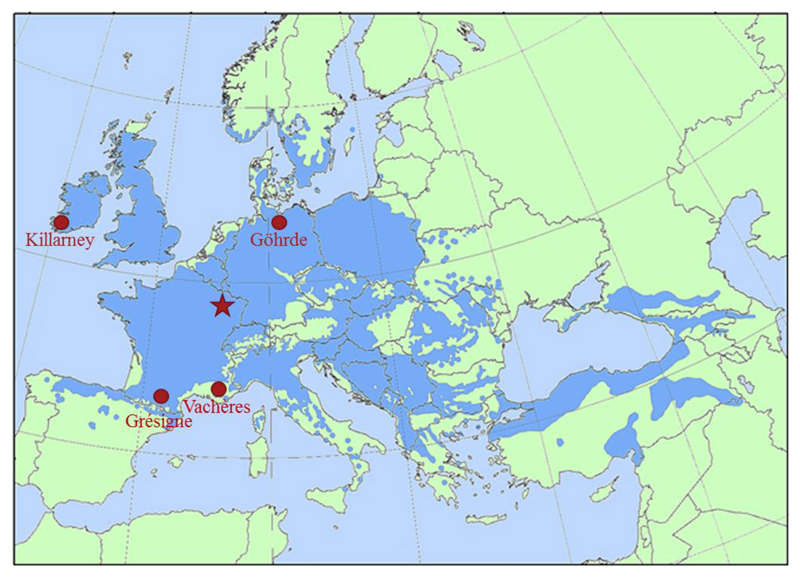
For evaluating the genetic differentiation in vulnerability to embolism among Quercus petraea (sessile oak) provenances, we used a common garden experiment planted in 1986 and 1987 in the Forêt Domaniale de Sillégny (France) which contains in total 107 sessile oak provenances. The initial density of plantation was 1904 individuals per hectare (spacing 3 m × 1.75 m) with each provenance replicated from ten to fifteen times having 24 trees per replicate. Four provenances selected for the present study; Grésigne (Southeastern France), Killarney (Southern Ireland), Vachères (Southwestern France) and Göhrde (Northern Germany) (Fig. 1, Table 1) represent different climatic regions, ranging from dry Mediterranean region in France to continental temperate climate in Germany. For each provenance, the aridity index (AI) was calculated as;
where MAP = Mean Annual Precipitation and MAE = Mean Annual Potential Evapotranspiration.
Fig. 1.
Q. petraea provenances (full circles) planted in the common garden experiment located in the core of the species distribution range (full star) (Map source: EUFORGEN)
Table 1.
Climatic data, location and elevation of the studied Q. petraea provenances
| Provenance | Country | Latitude | Longitude | Altitude (m) | MAT | MAP | MAE | AI |
|---|---|---|---|---|---|---|---|---|
| Grésigne | France | 44.04 | 1.75 | 310 | 11.9 | 806 | 995 | 0.81 |
| Killarney | Ireland | 52.01 | -9.50 | 50 | 10.1 | 1374 | 657 | 2.09 |
| Vachères | France | 43.98 | 5.63 | 650 | 10.7 | 779 | 847 | 0.92 |
| Göhrde | Germany | 53.10 | 10.85 | 85 | 8.4 | 629 | 699 | 0.90 |
MAT = mean annual temperature (°C), MAP = Mean Annual Precipitation (mm), MAE = Mean Annual Potential Evapotranspiration, AI = aridity index
We collected 12 to 15 trees per provenance in the common garden experiment. Trees of each provenance were sampled in five replications. Trees of the common garden were on average 10 m tall, and 2m long branches were cut from upper part of the tree. Branches were wrapped in wet paper before being immediately transported to the lab and then processed as branches collected for the species comparison.
Vulnerability curves
Vulnerability to drought-induced embolism was determined at the Caviplace (University of Bordeaux, Talence, France; http://sylvain-delzon.com/caviplace) with the Cavitron technique (Cochard 2002; Cochard 2005). To prevent artefactual losses in hydraulic conductance due to the presence of open vessels in the samples (Pivovaroff et al., 2016; Torres-Ruiz et al 2017a), branches were recut under water to a 1m-long length, debarked at both ends and then installed in a large cavitron equipped with a 1m-diameter custom-built honeycomb rotor (DGMeca, Gradignan, France). Several samples per species were used to test the presence of open vessels by air injection at 2 bars and none of the studied species presented open vessels in 1 m long branches. Centrifugal force was used to establish negative pressure in the xylem (Pi) and to provoke water stress-induced cavitation. Samples were spun for two minutes at a given speed to decrease the xylem pressure progressively at its center from -0.8 MPa to -10.5 MPa (those pressures correspond to centrifugation rotation from 764 rpm to 2768 rpm respectively). Samples were three to five years old with a diameter varying from 10.3 to 12.2 mm. As this technique enables measuring the hydraulic conductance of the samples under negative pressure, the vulnerability curves were generated by plotting the percentage loss of hydraulic conductivity (PLC) against the different target pressures applied. For each branch, the relationship between PLC and the xylem pressure induced by centrifugation was fitted with the following sigmoidal equation (e.g., Pammenter & Vander Willigen 1998):
where P50 (MPa) is the xylem pressure inducing a 50% loss of conductivity and S (% MPa-1) is the slope of the vulnerability curve at the inflexion point. Mean values of embolism vulnerability parameters (P50 and S) correspond to the average values of 5 to 16 samples per species. The P50 value shows the ability of the branch to maintain its conductance at negative pressures and is commonly used as a proxy for the tree drought resistance. The lower the P50 value, the more drought tolerant the species or the provenance (Delzon 2015).
Anatomical traits
For each oak species and Q. petraea provenance, 30μm to 50μm sections were cut from 5 to 10 of the branches used for vulnerability curve measurements using a sliding microtome (GLS1-microtome, Schenkung Dapples, Switzerland). After sectioning, they were bleached, rinsed with milliQ water and stained with a 1:2 mixture of safranin (0.5% in 50% ethanol, Safranin _ Fisher scientific _ General purpose grade) and alcian blue (1% in water, Alcian Blue 8GX _ Alfa Aesar). After this, sections were washed with milliQ water and five times with 50, 70 and 96% ethanol. Slides of the sections were mounted with Euparal (Euparal 100g _ ROTH) and left to fix at room temperature. After one or two days, slides were placed in the oven at 60°C and the best ones were selected to be scanned. The entire transverse section of each sample was digitized at 20X magnification using a desktop single slide scanner (Nanozoomer 2.0 HT, Hamamatsu). The pictures from the scanner were later processed and analyzed ring by ring to quantify several anatomical traits such as vessel diameter in μm (d), vessel density in n mm-2 (VD), hydraulically weighted vessel diameter in μm (dh, that is the sum of all conduit diameters (Σ d5) divided by the total number of conduits (Σ d4; Sperry et al., 1994) and relative theoretical conductivity in kg m-1 MPa-1 s-1 (Ks). The average of each trait was calculated for the whole cross section (d, dh, VD, Ks) and for the last ring (dring, dh_ring, VDring, Ks_ring).
Statistical Analysis
Before the analyses, we confirmed the normal distribution of values for all variables measured (Shapiro-Wilk test; α = 0.05). To compare vulnerability to embolism between oak species and provenances of Q. petraea, we used one-way analyses of variance with post hoc Tukey’s honest significant difference using 95% confidence intervals to compare hydraulic traits values (P50, P12, P88, S) across species and provenances. Coefficients of variations (CVintra) were estimated within each species and provenance. For Q. petraea, the between-provenance variability (CVinter) was calculated from the between-provenance standard deviation and the overall mean value. Pearson’s correlation analysis was used to test for relationships between hydraulic and anatomical traits between and within species. All analyses were performed in SAS 9.4 (SAS Institute, Cary NC).
Results
Interspecific variation among oak species
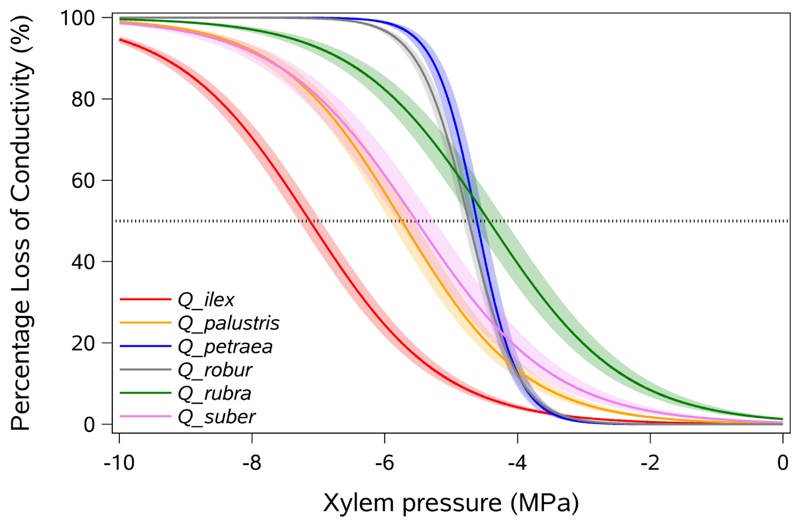
For each species, vulnerability curves showed similar sigmoid shapes (Fig. 2, Fig.3) with the air-entry (P12) ranging from -4.93 in Q. ilex to -2.14 in Q. rubra (Table 2). Values of P50 significantly varied between species (F = 39.12; P < 0.0001), ranging from -7.13 in Q. ilex to -4.43 in Q. rubra (Table 2). Similar significant variation in P12 (F = 7.94; P < 0.0001), and P88 (F = 40.50; P < 0.0001) was observed across species with Q. ilex and Q. rubra at the extremes of the range. The vulnerability curve slope, S (F = 2.15; P < 0.07) was not significantly different among the species. The species with lowest P50 and hence more resistant to xylem embolism were the two Mediterranean species Q. ilex and Q. suber and the North American species Q. palustris whereas the more vulnerable species were Q. rubra, Q. petraea and Q. robur (Fig. 3). The coefficient of variation in P50 (CV) is low and varied from 5.17% in Q. robur to 13.71% in Q. rubra (Table 2).
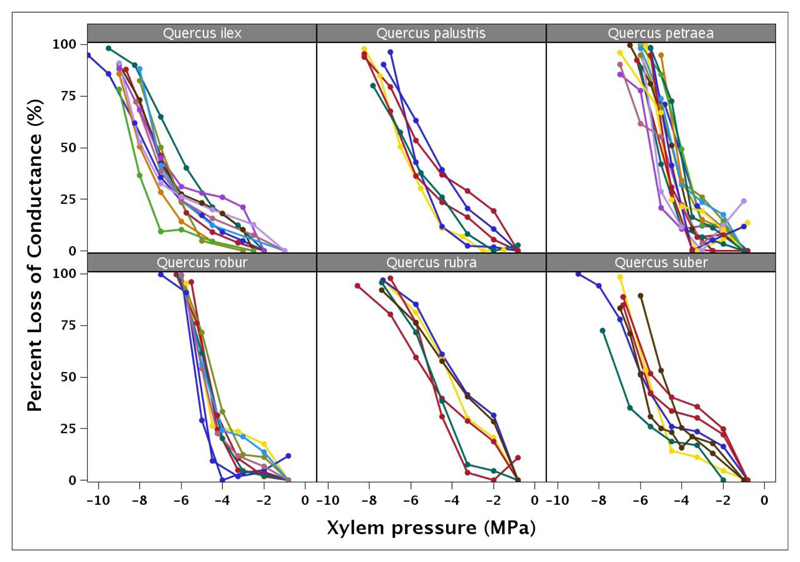
Fig. 2.
Vulnerability curves obtained with the in situ flow centrifuge (Cavitron). They were constructed using the 1 meter diameter rotor in the Cavitron. Each color corresponds to a different individual within each species
Fig. 3.
Mean vulnerability curves for each of the 6 studied oak species showing percentage loss of hydraulic conductance in xylem (%) as a function of xylem pressure (MPa). Shaded bands represent standard errors, and 50% loss in conductance is indicated by a horizontal dotted line
Table 2.
Xylem embolism vulnerability parameters of the six studied oak species. Different letters indicate significant differences between species, (p-value in parenthesis)
| Species | n | P50 | P12 | P88 | S | CV |
|---|---|---|---|---|---|---|
| Quercus ilex | 10 | -7.13 (0.16) c | -4.93 (0.29) c | -9.33 (0.23) d | 24.98(2.24) a | 7.27 |
| Quercus palustris | 5 | -5.74 (0.18) b | -3.53 (0.47) b | -7.95 (0.33) c | 26.87(6.41) a | 6.97 |
| Quercus petraea | 16 | -4.61 (0.11) a | -3.55 (0.19) b | -5.67 (0.17) a | 79.54(23.31) a | 9.78 |
| Quercus robur | 8 | -4.74 (0.09) a | -3.81 (0.20) b | -5.66 (0.09) a | 67.07(14.09) a | 5.17 |
| Quercus rubra | 6 | -4.43 (0.25) a | -2.14 (0.48) a | -6.72 (0.29) b | 24.52(4.08) a | 13.72 |
| Quercus suber | 7 | -5.52 (0.28) b | -3.12 (0.42) b | -7.93 (0.44) c | 24.00(4.07) a | 13.25 |
n = number of samples; P50, P12 and P88 are water potential values at 50%, 12% and 88% loss of conductivity respectively; S = slope of vulnerability curve; CV= coefficient of variation in P50 (%)
Intraspecific variation in Q. petraea
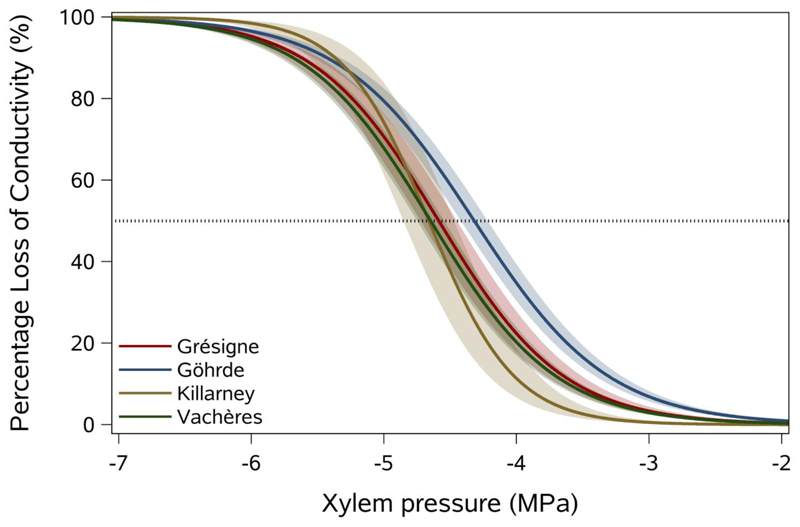
In Q. petraea, all provenances exhibited sigmoidal vulnerability curves (Fig. 4) and no significant differences were found for any embolism resistance trait among the four provenances (Fig. 4; Table 3, Fig. S2). The maximum difference in P50 was only 0.34 MPa (Table 3) and was not significantly different among the provenances (F = 1.53; P = 0.22). Similarly, the maximum differences between provenances were only 0.62 and 0.26 MPa, for P12 and P88, respectively (Table 3) and no significant differences were found for those traits between provenances. The vulnerability curve slope, S (F = 1.90; P = 0.14) was also not significantly different among the provenances (Table 3). The P50 values measured for a given provenance showed a relatively moderate variability with average CVintra for provenances of 10.5% (Table 3). However this within-provenance variability was much larger than the inter provenance variability (CVinter =3.5%).
Fig. 4.
Mean vulnerability curves for each of the 4 studied sessile oak provenances showing percentage loss of hydraulic conductance in xylem (%) as a function of xylem pressure (MPa). Shaded bands represent standard errors, and 50% loss in conductance is indicated by a horizontal dotted line
Table 3.
Xylem embolism vulnerability parameters of the four sessile oak provenances. Different letters indicate significant differences between species, (p-value in parenthesis)
| Species | n | P50 | P12 | P88 | S | CVintra |
|---|---|---|---|---|---|---|
| Grésigne | 12 | -4.58(0.14)a | -3.42(0.19)a | -5.74(0.20)a | 53.05(7.96)a | 10.28 |
| Killarney | 13 | -4.65(0.19)a | -3.72(0.14)a | -5.59(0.31)a | 77.70(15.91)a | 14.78 |
| Vachères | 14 | -4.64(0.10)a | -3.51(0.20)a | -5.78(0.14)a | 52.95(5.80)a | 8.15 |
| Göhrde | 15 | -4.31(0.10)a | -3.10(0.20)a | -5.52(0.14)a | 49.44(5.18)a | 9.00 |
n = number of samples; P50, P12 and P88 are water potential at 50%, 12% and 88% loss of conductivity respectively; S = slope of vulnerability curve; CVintra= coefficient of variation in P50 within provenance (%)
Correlation between P50 and xylem anatomical traits
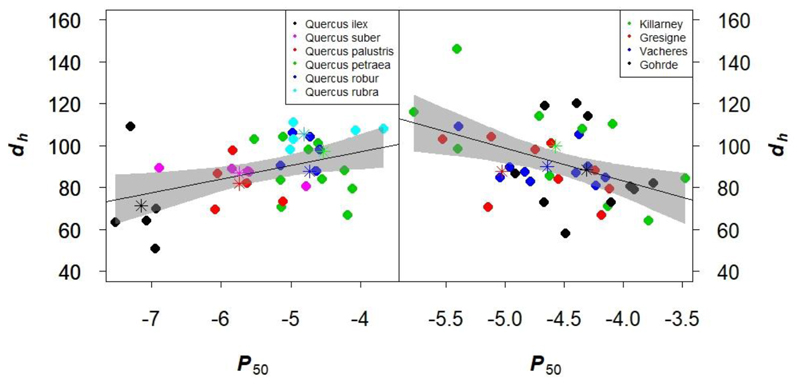
Significant differences were found between species for dring (F = 3.84; P = 0.009), dh (F = 4.52: P = 0.004), dh_ring (F = 9.61; P < 0.0001), Ks (F = 2.57; P = 0.05) and Ks_ring (F = 4.36; P = 0.004) indicating that Mediterranean species (Q.ilex and Q. suber) have smaller vessels than the others. The other analyzed anatomical traits did not show any significant differences between species. Among the six oak species, P50 was positively correlated with dring, dh_ring, dh and Ks_ring (Table 4, Fig. 5). These correlation were calculated at the individual tree level over all species, but the distribution of the species mean values on the regression diagram (Fig.5) indicate that the correlation is mostly driven by the species effect: the more resistant to embolism the species, the narrower the vessels. There were no significant correlations between hydraulic traits and vessel density (VD and VDring).
Table 4.
Pearson correlation coefficient (r) between P50 and various anatomical traits
| Trait | Anatomical traits | r | P- value |
|---|---|---|---|
| Among the 6 oak species | |||
| P50 | VDring | 0.03 | 0.88 |
| VD | 0.17 | 0.33 | |
| dring | 0.47 | 0.004 | |
| d | 0.31 | 0.07 | |
| dh_ring | 0.63 | <0.0001 | |
| dh | 0.42 | 0.01 | |
| Ks_ring | 0.42 | 0.01 | |
| Ks | 0.28 | 0.09 | |
| Within Q. petraea | |||
| P50 | VDring | -0.05 | 0.78 |
| VD | -0.01 | 0.95 | |
| dring | -0.30 | 0.06 | |
| d | -0.41 | 0.01 | |
| dh_ring | -0.35 | 0.03 | |
| dh | -0.45 | 0.004 | |
| Ks_ring | -0.29 | 0.08 | |
| Ks | -0.46 | 0.004 |
VDring = vessel density of the last ring (n mm-2), VD = vessel density (n mm-2), dring = vessel diameter of the last ring (µm), d = vessel diameter (µm), dh = hydraulically weighted vessel diameter (µm), dh_ring = hydraulically weighted vessel diameter of the last ring (µm), Ks = relative theoretical conductivity (kg m-1 MPa-1 s-1), Ks_ring = relative theoretical conductivity of the last ring (kg m-1 MPa-1 s-1), P50 = water potential at 50% loss of conductivity (MPa).
Fig. 5.
Weighted regression between P50 and dh (hydraulically weighted vessel diameter in μm) among the 6 oak species (left) and among the four provenances within Q. petraea (right), mean values per species/provenance is indicated by stars, dh = hydraulically weighted vessel diameter (μm); P50 = water potential at 50% loss of conductivity (MPa)
No significant differences of anatomical traits were observed between provenances within Q. petraea except for VD (F = 4.20; P = 0.01), (Table S3 in supplementary information). However, P50 was negatively correlated with d, dh, dh_ring as well as Ks (Table 4, Fig. 5): the more resistant to embolism the individual, the larger the vessels. As there were no significant differences between provenances, we computed correlation among traits at the within provenance level (individual tree level) by merging the data of all provenances (Fig. 5). Here the correlations are mostly driven by the individual tree effect, rather than by the provenance effect (Fig. 5). There were no significant correlations between hydraulic traits and vessel density (VD and VDring). Quite strikingly regression coefficients between anatomical and hydraulic traits were of opposite signs at the species and provenance level (Table 4).
Discussion
Oak species show large interspecific variation in embolism resistance
Large variation in resistance to embolism was found among the six oak species studied, with a higher resistance in those occurring in the Mediterranean biome, such as Q. ilex, than those occurring in temperate cooler climates. Previous studies have shown that resistance to xylem embolism is a key trait allowing trees to survive in an extreme water scarcity environment (Urli et al., 2013; Brodribb and Cochard 2009, Anderegg et al. 2015). Such variation among species was earlier observed among angiosperms where embolism resistance was negatively correlated with mean annual precipitation of the species distribution range (Maherali et al., 2004; Choat et al., 2012). In addition, Mediterranean species are well-known for being more resistant to embolism than temperate species (Urli et al., 2014; Gimeno et al., 2009). Indeed, we found the temperate species Q. rubra as being the most vulnerable to embolism, even more vulnerable than its European counterparts as previously reported by Cochard et al., (1992). A similar study by Cochard et al. (1992) compared three native European oak species. According to their results, Q. robur was more vulnerable to embolism (P50 of -2.5 MPa) than Q. petraea and Q. pubescens (P50 of -3.3 MPa for both species). Those values are substantially less negative from those obtained in this study but they still show a similar trend among the species. Indeed large discrepancies in P50 values for a given species have been previously reported mainly due to use of different methods for determining their vulnerability to embolism (see Cochard et al. 2013). For instance, Martin St-Paul et al. (2014) showed that the P50 values in Q. ilex varied anywhere between -2 to -8.2 MPa due to the “open-vessel artefact” involved in the traditional methods for obtaining vulnerability curves to cavitation. Also, Venturas et al. 2016 showed very different results in Q. robur with a P50 of -1.4 MPa when vulnerability curves were measured using single vessel air injection technique. To avoid any artefactual results due to presence of open vessel in the samples, a large rotor (1 m in diameter) was used in this study to determine the resistance to embolism for the six Quercus species. Thus, we obtained S-shaped curves for each species, and Quercus robur showed a similar P50 value (-4.7 MPa) as reported by Choat et al. (2015) using the reference microCT technique (-4.2 MPa). This suggests that, when using large diameter rotor (1m), the centrifuge technique reports accurate P50 values for long-vesseled species as Q. robur while it is well known that standard rotors (0.27 m in diameter or less) overestimate their vulnerability to embolism thus supporting open vessel artifact hypothesis (Choat et al., 2010; Cochard et al., 2010; McElrone et al., 2012; Martin-StPaul et al., 2014; Torres-Ruiz et al., 2014,2017). Caution is still advised when applying this technique to species with vessel lengths that exceed the diameter of the rotor used in centrifuge methods.
Lack of genetic differentiation among provenances in resistance to xylem embolism
Within Q. petraea, we found no significant genetic differentiation in resistance to embolism among different provenances originating from different climatic regimes. Exploring the genetics behind plant hydraulics is still a developing area of research (Venturas et al., 2017) and very few studies have been successful in quantifying intraspecific variation in resistance to embolism in angiosperms so far. Lack of genetic differentiation in embolism resistance between provenances for P50 has been already reported for conifer species (Corcuera et al. 2011; Lamy et al. 2011; Sáenz-Romero et al. 2013). For example, Lamy et al. (2011) found a very narrow range in embolism resistance (0.2 MPa) for six Pinus pinaster populations grown in a provenance trial. Narrow ranges of xylem embolism resistance variability between populations of the same tree species suggest a highly canalized response driven by uniform selection of this trait (Lamy et al. 2011; 2014). Studies on Fagus sylvatica provenance trials have also reported low genetic differentiation among populations both in hydraulic as well as anatomical traits (Wortemann et al., 2011; Hajek et al., 2016). Likewise our Q. petraea provenances from different climates and with different evolutionary histories (Petit et al. 2002) shared similar embolism resistance value, suggesting an evolutionary process behind the lack of variability of this trait. Consequently, this low genetic differentiation within species limits the evolution of species through divergent selection especially in its southern margin of distribution range (Kremer et al., 2013).
In our study, the range of within-provenance differentiation is much larger (Table 3) than the range of the among provenance variation (CVinter 3.5%). Interestingly, this moderate genetic differentiation within provenances was maintained even while no genetic differentiation was observed between provenances. Q. petraea is a wind pollinated species and has previously been found to be predominantly outcrossing with random mating (Bacilieri et al. 1996). High levels of gene flow among populations across landscapes have also been reported in the species (Gerber et al. 2014). Sessile oak usually occur in large populations. The presence of moderate genetic differentiation within populations and the absence of genetic differentiation among populations as seen from our results suggest that selection can happen within these populations for adaptive traits (Scotti et al. 2016). Selected genotypes can be maintained within these populations even if there is a high gene flow among these populations (Aitken and Bemmels, 2016; Lowe et al. 2015; Kremer et al. 2012). By working on a common garden trial with provenances coming from contrasting climatic conditions, we were able to quantify genetic differentiation but not the environmental effects. A more elaborate sampling of branches from trees growing in more diverse growing conditions is required to draw more conclusions regarding the phenotypic plasticity of embolism resistance. The need for testing provenances from extreme sites is also recommended in future in order to test whether marginal populations have evolved more embolism resistant xylem.
Relationship between embolism resistance and xylem anatomical traits
The positive correlation observed between P50 and vessel diameter indicates that the more embolism resistant species possess narrower xylem vessels (Table 4, Fig. 5). This agrees with former studies in other angiosperms showing greater vulnerabilities to embolism for those species with wider vessels (Hajek et al., 2014; Cai and Tyree, 2010; Hacke et al., 2006). This was not the case in all genera studied since, in contrast with our results; vessel density and length, rather than vessel diameter (Lens et al., 2010) played a role in embolism resistance in Acer species. Jacobsen et al. (2005) found that the effect of vessel density on embolism resistance is due to its role in modifying fiber properties in wood. A reverse pattern was observed in the genus Cistus, where species with narrow vessels were found to be more prone to xylem embolism in the Mediterranean region (Torres-Ruiz et al., 2017b). Gleason et al. (2016) tested this trade-off across many angiosperms and gymnosperm species and found a positive correlation between hydraulic conductivity and P50 and concluded that there is a weak tradeoff between hydraulic efficiency and safety among species. Unlike the interspecific pattern, we found an opposite trend at the intraspecific level with no significant difference among Q. petraea provenances in P50 value. Regardless of the provenance, individual trees within a provenance having lower P50 tend to have larger vessel sizes. These contrasting trends between P50 and vessel diameter suggest that vulnerability to embolism not only depends on the size of the vessels but also on other traits. In fact, Li et al. (2016) reported recently that the thickness of the pit membranes between two adjoining vessels plays a major role in plant resistance to embolism. To explain the contrasting trends when comparing species or provenances within a given species, it would be necessary to evaluate if changes in pit membrane thickness are related to the diameter of the xylem vessels and how this possible relationship changes within and among species. Indeed, the link between vessel size and vulnerability to embolism might be indirect in angiosperms such as oak, and the ease of air-seeding could be driven by the single tiniest pit membrane of a vessel.
Conclusions
Our results in oaks confirm earlier data obtained in other genera (Lamy et al., 2011, 2014) showing that vulnerability to embolism exhibits significant between-species and substantial within-population variation, but lacks between-population variation. Assuming that the within-population component of variation is partly due to genetic effects, our results suggest that there is potential for divergent evolution between populations. As no population differentiation is actually observed, we may conclude that either differentiation is so narrow that it could not be detected by existing technologies. We may also conclude that selection is constrained and canalized, or that selection is uniform across all populations. Another probable explanation could be that vulnerability to embolism is neutral. Each of these interpretations raises new avenues of research along technological and biological pathways. An immediate concern is to evaluate the adaptive value of the hydraulic parameters derived from the cavitron technique (P50) under controlled conditions, or in nature by assessing their selection gradients.
Supplementary Material
Acknowledgements
We thank the experimental units of Pierroton (UE 0570, INRA, 69 route d’Arcachon, 33612 CESTAS) and Toulenne (UE 0393, INRA, Domaine des Jarres, 33210 Toulenne) for providing material and logistics. We also thank Gaelle Capdeville for technical support. This study was carried out with financial support from the ERC project TREEPEACE (FP7-339728), the Cluster of Excellence COTE (ANR-10-LABX-45) and the Villum Foundation Trees for future forests project (VKR-023063). This work was also supported by the ‘Investments for the Future’ (ANR-10-EQPX-16, XYLOFOREST) programme of the French National Agency for Research.
References
- Aitken SN, Bemmels JB. Time to get moving: assisted gene flow of forest trees. Evolutionary Applications. 2016;9:271–290. doi: 10.1111/eva.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, et al. Cobb N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259:660–684. [Google Scholar]
- Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini AFA, Choat B, Jansen S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proceedings of the National Academy of Sciences. 2016;113(18):5024–5029. doi: 10.1073/pnas.1525678113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg WRL. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytologist. 2015;205:1008–1014. doi: 10.1111/nph.12907. [DOI] [PubMed] [Google Scholar]
- Anderegg WRL, Kane JM, Anderegg LDL. Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change. 2013;3:30–36. [Google Scholar]
- Arend M, Kuster T, Günthardt-Goerg MS, Dobbertin M. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens) Tree Physiology. 2011;31:287–297. doi: 10.1093/treephys/tpr004. [DOI] [PubMed] [Google Scholar]
- Bacilieri R, Ducousso A, Petit RJ, Kremer A. Mating System and Asymmetric Hybridization in a Mixed Stand of European Oaks. Evolution. 1996;50:900–908. doi: 10.1111/j.1558-5646.1996.tb03898.x. [DOI] [PubMed] [Google Scholar]
- Baquedano FJ, Castillo FJ. Drought tolerance in the Mediterranean species Quercus coccifera, Quercus ilex, Pinus halepensis, and Juniperus phoenicea. Photosynthetica. 2007;45:229–238. [Google Scholar]
- Barigah TS, Charrier O, Douris M, Bonhomme M, Herbette S, Améglio T, Fichot R, Brignolas F, Cochard H. Water stress-induced xylem hydraulic failure is a causal factor of tree mortality in beech and poplar. Annals of Botany. 2013;112:1431–1437. doi: 10.1093/aob/mct204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche PS, Larter M, Domec JC, Burlett R, Gasson P, Jansen S, Delzon S. A broad survey of hydraulic and mechanical safety in the xylem of conifers. Journal of Experimental Botany. 2014;65:4419–4431. doi: 10.1093/jxb/eru218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Bowman DJMS, Nichols S, Delzon S, Burlett R. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytologist. 2010;188:533–542. doi: 10.1111/j.1469-8137.2010.03393.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Cochard H. Hydraulic Failure Defines the Recovery and Point of Death in Water-Stressed Conifers. Plant Physiology. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailleret M, Jansen S, Robert EMR, et al. Martínez-Vilalta J. A synthesis of radial growth patterns preceding tree mortality. Global Change Biology. 2017;23:1675–1690. doi: 10.1111/gcb.13535. [DOI] [PubMed] [Google Scholar]
- Cai J, Tyree MT. The impact of vessel size in vulnerability curves:data and models for within-species variability in saplings of aspen, Populus tremuloides Michx. Plant Cell & Environment. 2010;33:1059–1069. doi: 10.1111/j.1365-3040.2010.02127.x. [DOI] [PubMed] [Google Scholar]
- Choat B, Badel E, Burlett R, Delzon S, Cochard H, Jansen S. Non-invasive measurement of vulnerability to drought induced embolism by X-ray microtomography. Plant Physiology. 2015 doi: 10.1104/pp.15.00732. 15.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, et al. Zanne AE. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- Cochard H, Badel E, Herbette S, Delzon S, Choat B, Jansen S. Methods for measuring plant vulnerability to cavitation: a critical review. Journal of Experimental Botany. 2013;64(15):4779–4791. doi: 10.1093/jxb/ert193. [DOI] [PubMed] [Google Scholar]
- Cochard H, Herbette S, Barigah T, Badel E, Ennajeh M, Vilagrosa A. Does sample length influence the shape of xylem embolism vulnerability curves? A test with the Cavitron spinning technique. Plant Cell & Environment. 2010;33:1543–1552. doi: 10.1111/j.1365-3040.2010.02163.x. [DOI] [PubMed] [Google Scholar]
- Cochard H, Damour G, Bodet C, Tharwat I, Poirier M, Améglio T. Evaluation of a new centrifuge technique for rapid generation of xylem vulnerability curves. Physiol Plantarum. 2005;124:410–418. [Google Scholar]
- Cochard H. A technique for measuring xylem hydraulic conductance under high negative pressures. Plant, Cell & Environment. 2002;25:815–819. [Google Scholar]
- Cochard H, Bréda N, Granier A, Aussenac G. Vulnerability to air embolism of three European oak species (Quercus petraea (Matt) Liebl, Q pubescens Willd, Q robur L) Annales des sciences forestieres. 1992;49(3):225–233. [Google Scholar]
- Corcuera L, Cochard H, Gil-Pelegrin E, Notivol E. Phenotypic plasticity in mesic populations of Pinus pinaster improves resistance to xylem embolism (P50) under severe drought. Trees. 2011;25:1033. [Google Scholar]
- David TS, Henriques MO, Kurz-Besson C, et al. David JS. Water-use strategies in two co-occurring Mediterranean evergreen oaks: surviving the summer drought. Tree Physiology. 2007;27:793–803. doi: 10.1093/treephys/27.6.793. [DOI] [PubMed] [Google Scholar]
- David-Schwartz R, Paudel I, Mizrachi M, et al. Cohen S. Indirect evidence for genetic differentiation in vulnerability to embolism in Pinus halepensis. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzon S. New insight into leaf drought tolerance. Functional Ecology. 2015;29:1247–1249. [Google Scholar]
- Delzon S, Urli M, Samalens JC, Lamy JB, Lischke H, Sin F, Zimmermann NE, Porté AJ. Field Evidence of Colonisation by Holm Oak, at the Northern Margin of Its Distribution Range, during the Anthropocene Period. PloS one. 2013;8:e80443. doi: 10.1371/journal.pone.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzon S, Douthe C, Sala A, Cochard H. Mechanism of water-stress induced cavitation in conifers: bordered pit structure and function support the hypothesis of seal capillary-seeding. Plant, Cell & Environment. 2010;33:2101–2111. doi: 10.1111/j.1365-3040.2010.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducousso A, Bordacs S. EUFORGEN Technical Guidelines for genetic conservation and use for Pedunculate and sessile oaks (Quercus robur) and (Quercus petraea) Bioversity International; 2004. [Google Scholar]
- Eaton E, Caudullo G, Oliveira S, de Rigo D. Quercus robur and Quercus petraea in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A, editors. European Atlas of Forest Tree Species. Publication Office of the European Union; Luxembourg: 2016. pp. 160–163. [Google Scholar]
- Ennajeh M, Simões F, Khemira H, Cochard H. How reliable is the double-ended pressure sleeve technique for assessing xylem vulnerability to cavitation in woody angiosperms? Physiologia Plantarum. 2011;142:205–210. doi: 10.1111/j.1399-3054.2011.01470.x. [DOI] [PubMed] [Google Scholar]
- Epron D, Dreyer E. Long-term effects of drought on photosynthesis of adult oak trees [Quercus petraea (Matt.) Liebl. and Quercus robur L.] in a natural stand. New Phytologist. 1993;125:381–389. doi: 10.1111/j.1469-8137.1993.tb03890.x. [DOI] [PubMed] [Google Scholar]
- EUFORGEN. Distribution maps of Sessile oak (Quercus petraea), Pedunculate oak (Quercus robur) and Cork oak (Quercus suber) 2009 www.euforgen.org.
- Gerber S, Chadœuf J, Gugerli F, et al. Kremer A. High Rates of Gene Flow by Pollen and Seed in Oak Populations across Europe. PloS one. 2014;9:e85130. doi: 10.1371/journal.pone.0085130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno TE, Pías B, Lemos-Filho JP, Valladares F. Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiology. 2009;29:87–98. doi: 10.1093/treephys/tpn007. [DOI] [PubMed] [Google Scholar]
- Gleason SM, Westoby M, Jansen S, et al. Zanne AE. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world's woody plant species. New Phytologist. 2016;209:123–136. doi: 10.1111/nph.13646. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiology. 2006;26:689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- Hajek P, Kurjak D, von Wühlisch G, Delzon S, Schuldt B. Intraspecific Variation in Wood Anatomical, Hydraulic, and Foliar Traits in Ten European Beech Provenances Differing in Growth Yield. Frontiers in Plant Science. 2016;7:791. doi: 10.3389/fpls.2016.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Leuschner C, Hertel D, Delzon S, Schuldt B. Trade-offs between xylem hydraulic properties, wood anatomy and yield in Populus. Tree Physiology. 2014;34:744–756. doi: 10.1093/treephys/tpu048. [DOI] [PubMed] [Google Scholar]
- Hoffmann WA, Marchin RM, Abit P, Lau OL. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Global Change Biology. 2011;17:2731–2742. [Google Scholar]
- Hubert F, Grimm GW, Jousselin E, Berry V, Franc A, Kremer A. Multiple nuclear genes stabilize the phylogenetic backbone of the genus Quercus. Systematics and Biodiversity. 2014;12:405–423. [Google Scholar]
- Jacobsen AL, Pratt RB, Ewers FW, Davis SD. Cavitation resistance among twenty-six chaparral species of southern California. Ecological Monographs. 2007;77:99–115. [Google Scholar]
- Jacobsen AL, Ewers FW, Pratt RB, Paddock WA, Davis SD. Do Xylem Fibers Affect Vessel Cavitation Resistance? Plant Physiology. 2005;139:546–556. doi: 10.1104/pp.104.058404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer A, Potts BM, Delzon S. Genetic divergence in forest trees: understanding the consequences of climate change. Functional Ecology. 2014;28:22–36. [Google Scholar]
- Kremer A, Ronce O, Robledo-Arnuncio JJ, et al. Schueler S. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecology Letters. 2012;15:378–392. doi: 10.1111/j.1461-0248.2012.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy JB, Delzon S, Bouche PS, Alia R, Vendramin GG, Cochard H, Plomion C. Limited genetic variability and phenotypic plasticity detected for cavitation resistance in a Mediterranean pine. New Phytologist. 2014;201:874–886. doi: 10.1111/nph.12556. [DOI] [PubMed] [Google Scholar]
- Lamy JB, Bouffier L, Burlett R, Plomion C, Cochard H, Delzon S. Uniform Selection as a Primary Force Reducing Population Genetic Differentiation of Cavitation Resistance across a Species Range. PloS one. 2011;6:e23476. doi: 10.1371/journal.pone.0023476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytologist. 2010;190:709–723. doi: 10.1111/j.1469-8137.2010.03518.x. [DOI] [PubMed] [Google Scholar]
- Li S, Lens F, Espino S, Karimi Z, Klepsch M, Schenk HJ, Schmitt M, Schuldt B, Jansen S. Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA. 2016;37:152–171. [Google Scholar]
- Linton MJ, Sperry JS, Williams DG. Limits to water transport in Juniperus osteosperma and Pinus edulis: implications for drought tolerance and regulation of transpiration. Functional Ecology. 1998;12:906–911. [Google Scholar]
- Little EL. Atlas of United States trees, volume 1, conifers and important hardwoods. U.S. Department of Agriculture Miscellaneous Publication 1146; 1971. p. 9. [Google Scholar]
- Lloret F, Siscart D, Dalmases C. Canopy recovery after drought dieback in holm-oak Mediterranean forests of Catalonia (NE Spain) Global Change Biology. 2004;10:2092–2099. [Google Scholar]
- Lowe AJ, Cavers S, Boshier D, Breed MF, Hollingsworth PM. The resilience of forest fragmentation genetics-no longer a paradox-we were just looking in the wrong place. Heredity. 2015;115:97–99. doi: 10.1038/hdy.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85:2184–2199. [Google Scholar]
- Martin-StPaul NK, Longepierre D, Huc R, Delzon S, Burlett R, Joffre R, Rambal S, Cochard H. How reliable are methods to assess xylem vulnerability to cavitation? The issue of ‘open vessel’ artifact in oaks. Tree Physiology. 2014;34(8):894–905. doi: 10.1093/treephys/tpu059. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Dawson JO. Growth and water use efficiency of Quercus alba, Q. bicolor, Q. imbricaria and Q. palustris seedlings under conditions of reduced soil water availability and solar irradiance. Transactions of the Illinois State Academy of Science. 1990;83:128–148. [Google Scholar]
- McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- McElrone AJ, Brodersen CR, Alsina MM, et al. Choat B. Centrifuge technique consistently overestimates vulnerability to water stress-induced cavitation in grapevines as confirmed with high-resolution computed tomography. New Phytologist. 2012;196:661–665. doi: 10.1111/j.1469-8137.2012.04244.x. [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR. Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Functional Ecology. 2009;23:922–930. [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science. 2006;63:625–644. [Google Scholar]
- Oh SH, Manos PS. Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. Taxon. 2008;57:434–451. [Google Scholar]
- Pammenter NV, Vander Willigen C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology. 1998;18:589–593. doi: 10.1093/treephys/18.8-9.589. [DOI] [PubMed] [Google Scholar]
- Petit RJ, Csaikl UM, Bordács S, et al. Finkeldey R. Chloroplast DNA variation in European white oaks: phylogeography and patterns of diversity based on data from over 2600 populations. Forest Ecology and Management. 2002;156(1):5–26. [Google Scholar]
- Pivovaroff AL, Burlett R, Lavigne B, Cochard H, Santiago LS, Delzon S. Testing the ‘microbubble effect’ using the Cavitron technique to measure xylem water extraction curves. AoB Plants. 2016;8 doi: 10.1093/aobpla/plw011. lw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyer C, Lasch-Born P, Suckow F, Gutsch M, Murawski A, Pilz T. Projections of regional changes in forest net primary productivity for different tree species in Europe driven by climate change and carbon dioxide. Annals of forest science. 2014;71:211–225. [Google Scholar]
- Sade N, Alem G, Moshelion M. Risk-taking plants. Anisohydric behavior as a stress-resistance trait. Plant Signal Behaviour. 2012;7:1–4. doi: 10.4161/psb.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TGM, Pitman R, Broadmeadow MSJ. Species-specific climate response of oaks (Quercus spp.) under identical environmental conditions. iForest. 2013;7:61–69. [Google Scholar]
- Scotti I, González-Martínez SC, Budde KB, Lalagüe H. Fifty years of genetic studies: what to make of the large amounts of variation found within populations? Annals of forest science. 2016;73:69–75. [Google Scholar]
- Sáenz-Romero C, Lamy JB, Loya-Rebollar E, et al. Delzon S. Genetic variation of drought-induced cavitation resistance among Pinus hartwegii populations from an altitudinal gradient. Acta Physiologiae Plantarum. 2013;35:2905. [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of Northern Utah and Interior Alaska. Ecology. 1994;75:1736–1752. [Google Scholar]
- Talhelm AF, Pregitzer KS, Kubiske ME, et al. Karnosky DF. Elevated carbon dioxide and ozone alter productivity and ecosystem carbon content in northern temperate forests. Global Change Biology. 2014;20:2492–2504. doi: 10.1111/gcb.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen JE, Bohrer G, Matheny AM, Ivanov VY, He L, Renninger HJ, Schäfer KV. Contrasting hydraulic strategies during dry soil conditions in Quercus rubra and Acer rubrum in a sandy site in Michigan. Forests. 2013;4:1106–1120. [Google Scholar]
- Torres-Ruiz JM, Cochard H, Choat B, et al. Delzon S. Xylem resistance to embolism: presenting a simple diagnostic test for the open vessel artefact. New Phytologist. 2017a doi: 10.1111/nph.14589. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Cochard H, Fonseca E, Badel E, Gazarini L, Vaz M. Differences in functional and xylem anatomical features allow Cistus species to co-occur and cope differently with drought in the Mediterranean region. Tree Physiology. 2017b doi: 10.1093/treephys/tpx013. In press. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Jansen S, Choat B, McElrone AJ, Cochard H, Brodribb TJ, Badel E, Burlett R, Bouche PS, Brodersen CR. Direct X-ray microtomography observation confirms the induction of embolism upon xylem cutting under tension. Plant Physiology. 2015;167:40–43. doi: 10.1104/pp.114.249706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Cochard H, Mayr S, Beikircher B, Diaz-Espejo A, Rodriguez-Dominguez CM, Badel E, Fernández JE. Vulnerability to cavitation in Olea europaea current-year shoots: further evidence of an open-vessel artifact associated with centrifuge and air-injection techniques. Physiologia Plantarum. 2014;152:465–474. doi: 10.1111/ppl.12185. [DOI] [PubMed] [Google Scholar]
- Trueba S, Pouteau R, Lens F, Feild TS, Isnard S, Olson ME, Delzon S. Vulnerability to xylem embolism as a major correlate of the environmental distribution of rainforest species on a tropical island. Plant Cell& Environment. 2017;40:277–289. doi: 10.1111/pce.12859. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Zimmermann MH. Hydraulic Architecture of Whole Plants and Plant Performance. In: Tyree MT, Zimmermann MH, editors. Xylem Structure and the Ascent of Sap. Springer Berlin Heidelberg; Berlin, Heidelberg: 2002. pp. 175–214. [Google Scholar]
- Urli M, Lamy JB, Sin F, Burlett R, Delzon S, Porté AJ. The high vulnerability of Quercus robur to drought at its southern margin paves the way for Quercus ilex. Plant Ecology. 2014;216:177–187. [Google Scholar]
- Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology. 2013;33:672–683. doi: 10.1093/treephys/tpt030. [DOI] [PubMed] [Google Scholar]
- Venturas MD, Sperry JS, Hacke UG. Plant xylem hydraulics: What we understand, current research, and future challenges. Journal of Integrative Plant Biology. 2017;59:356–389. doi: 10.1111/jipb.12534. [DOI] [PubMed] [Google Scholar]
- Venturas MD, Rodriguez-Zaccaro FD, Percolla MI, Crous CJ, Jacobsen AL, Pratt RB. Single vessel air injection estimates of xylem resistance to cavitation are affected by vessel network characteristics and sample length. Tree Physiology. 2016;36:1247–1259. doi: 10.1093/treephys/tpw055. [DOI] [PubMed] [Google Scholar]
- Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell & Environment. 2013;36:1938–1949. doi: 10.1111/pce.12139. [DOI] [PubMed] [Google Scholar]
- Wortemann R, Herbette S, Barigah T, Fumanal B, Alia R, Ducousso A, Gomory D, Roeckel-Drevet P, Cochard H. Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiology. 2011;31:1175–1182. doi: 10.1093/treephys/tpr101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.