Abstract
Previous studies have attributed to the cocoa powder the capacity to attenuate the immune response in a rat oral sensitization model. To gain a better understanding of cocoa-induced mechanisms at small intestinal level, 3-week-old female Lewis rats were fed either a standard diet or a diet containing 10% cocoa for 4 weeks with or without concomitant oral sensitization with ovalbumin (OVA). Thereafter, we evaluated the lymphocyte composition of the Peyer’s patches (PPL), small intestine epithelium (IEL) and lamina propria (LPL). Likewise, gene expression of several immune molecules was quantified in the small intestine. Moreover, histological samples were used to evaluate the proportion of goblet cells, IgA+ cells and granzyme+ cells as well. In cocoa-fed animals, we identified a five time reduction in the percentage of IgA+ cells in intestinal tissue together with a decreased proportion of TLR4+ IEL. Analyzing the lymphocyte composition, almost a double proportion of TCRγδ+ cells and an increase of NK cell percentage in PPL and IEL were found. In addition, a rise in CD25+, CD103+ and CD62L- cell proportions was observed in CD4+ PPL from cocoa-fed animals, along with a decrease in gene expression of CD11b, CD11c and IL-10. These results suggest that changes in PPL and IEL composition and in the gene expression induced by the cocoa diet could be involved, among other mechanisms, on its tolerogenic effect.
Keywords: cholera toxin, cocoa, ovalbumin, TCRγδ+ cells, tolerance
1. Introduction
The intestinal tract is the largest surface of the body protecting the internal towards the external environment. The primary function of the intestine is digestion and absorption of nutrients [1], but it is also recognized as the major immune organ, with the gut-associated lymphoid tissue (GALT) playing a central role in immune system homeostasis [2]. The GALT comprises approximately 70% of immune cells from the entire immune system [2], protecting the enormous intestinal surface (200 m2 in humans) [3], which is in contact every day with a vast number of potentially harmful antigens [4]. The GALT is structurally and functionally classified into two different compartments: the organized inductive site and the diffuse effector site. The organized compartment is composed of Peyer’s patches (PP), mesenteric lymph nodes (MLN) and isolated lymphoid follicles, whereas the diffuse compartment is formed by lamina propria lymphocytes (LPL) and intraepithelial lymphocytes (IEL). PP lymphocytes (PPL) and MLN lymphocytes (MLNL) are considered to be responsible for inducing oral tolerance or initiating immune response to antigens [5,6]. Situated in the lamina propria, LPL also contribute to oral tolerance, respond to antigen uptake and initiate migration of dendritic cells (DC) to the MLN [7]. Located between the epithelial cells, IEL regulate intestinal homeostasis, defend against infection and protect the integrity of the epithelial barrier [8,9].
Food allergies are currently considered a major public health concern due to their increasing prevalence. Food allergies affect approximately 5% of the general population and 8% of children worldwide [10]. According to current understanding, multiple pathways, cells and molecules are involved in the generation of an allergic response. Upon oral sensitization, allergens resisting denaturation and digestion in the gastrointestinal tract [11] reach the intestinal epithelium in an intact form and penetrate the first barrier of defense, the mucus layer, produced by the goblet cells, and then the intestinal epithelial cell barrier. Afterwards, DC, mainly found in PP, acting as antigen-presenting cells, pick up the allergen and present it to T helper (Th) cells in PP or MLN [12]. Later, Th cells proliferate and differentiate into Th1, Th2, Th17 or regulatory T (Treg) cells according to different cytokine patterns [13]. In most food allergies, an imbalance is evident towards Th2 response [14]. The immune response ends with the activation of effector cells such as B cells, which later turn into IgA-secreting cells. Nevertheless, it still remains a matter of debate which cells are the driving forces for initiating sensitization in the gut.
In a healthy immune response, ingestion of food proteins results in the development of oral tolerance, that is, the suppression of an immune response [7,10]. This immune unresponsiveness affects different immune compartments and is associated with the suppression of antibody production [7]. In contrast, a food allergy develops when there is either a failure in the induction or a breakdown of oral tolerance [15,16]. Due to its increasing frequency, researchers worldwide are focusing on new food allergy preventive measures with increasing awareness of a potential beneficial role of nutraceuticals.
Previous studies have indicated the ability of a cocoa-enriched diet to influence the GALT functionality in rats. Accordingly, we have shown that cocoa consumption modifies the PPL composition in rats [17,18]. The tolerogenic influence of cocoa in a rat oral sensitization model has recently been demonstrated [19]. A 10% cocoa-enriched diet inhibited the synthesis of serum specific anti-ovalbumin (OVA) antibodies and attenuated intestinal IgA. Additionally, this nutritional intervention induced changes in the lymphocyte composition and gene expression of MLN [19]. Specifically, in MLN, a cocoa diet increased the proportion of TCRγδ+ cells, playing a crucial role in the tolerance to oral antigens, and CD8+CD103+ cells, associated with regulatory functions. A decrease in CD4+CD62L+ and CD8+CD62L+ cell percentage was additionally observed, indicating a reduced influx of lymphocytes in MLN [19]. Together, these results show the capacity of a cocoa diet to induce oral tolerance and its potential role as a nutraceutical in food allergies.
Despite these previous studies, the influence of cocoa at the intestinal level remains unknown. We hypothesized that cocoa-enriched diet might regulate intestinal lymphoid tissue because it is the first compartment of the immune system in contact, and these changes could thereby contribute to the avoidance of the oral sensitization in rats. On the basis of this hypothesis, the present study aimed to analyze the effects of a cocoa diet on the small intestinal immune system in a rat oral sensitization model. Therefore, intestinal samples from rats orally sensitized with OVA were evaluated for lymphocyte composition in three functional compartments of the small intestinal immune system (PP, IEL and LPL) and also for the effect of a cocoa diet on representative molecules produced by GALT.
2. Materials and Methods
2.1. Chemicals, reagents and diets
Albumin from chicken egg white (OVA; grade V), cholera toxin (CT), collagenase, 1,4-dithiothreitol (DTT), ethylenediaminetetraacetic acid (EDTA), fetal bovine serum (FBS), Hanks balanced salt solution (HBSS), Roswell Park Memorial Institute (RPMI), Mayer’s hematoxylin solution, eosin Y solution, Percoll®, Trizma® base (Tris Base) and Tween 20 were purchased from Sigma-Aldrich (Madrid, Spain). Donkey serum was purchased from Jackson Immuno Research Laboratories (West Grove, PA, USA). NaN3, p formaldehyde, tri-sodium citrate dihydrate and citric acid were provided by Merck (Darmstadt, Germany) and RNAlater® by Ambion (Applied Biosystems, Austin, TX, USA). Xylene and 4',6-diamidino-2-phenylindole (DAPI) was purchased from Thermo Fisher Scientific (Vienna, Austria) and ethanol absolute from VWR (Vienna, Austria). Fluoromont G was provided by SouthernBiotech (Birmingham, AL, USA). Ketamine was obtained from Merial Laboratories S.A. (Barcelona, Spain) and xylazine from Bayer A.G. (Leverkusen, Germany). Natural Forastero cocoa was obtained from Idilia Foods SL (formerly Nutrexpa S.L., Barcelona, Spain) and AIN-93M diet and basal mix from Harlan Teklad (Madison, Wisconsin, USA).
2.2. Animals and experimental food intervention
Female Lewis rats were obtained from Janvier Labs (Saint Berthevin Cedex, France) and maintained under conditions controlled for temperature and humidity in a 12-h light/dark cycle. The present experimental design and procedure were approved by the local Ethical Committee for Animal Experimentation of the University of Barcelona (CEEA/UB ref.5988).
After a week of acclimatization at the Faculty of Pharmacy animal facilities (UB), 3-week-old rats were housed in cages (three per cage) and given ad libitum access to water and solid food during the 28 days of the study. The rats were randomly distributed into four experimental groups: reference group (RF/R), reference group fed cocoa diet (RF/C), OVA-sensitized group (OVA/R) and OVA-sensitized group fed cocoa diet (OVA/C) (n=9 each). The RF/R and the OVA/R groups were fed a standard diet (AIN-93M) whereas the RF/C and the OVA/C groups were fed an isoenergetic diet containing 10% cocoa (Table 1). The OVA/R and OVA/C groups were orally sensitized as described [19,20], receiving 50 mg/mL of OVA plus 30 µg of CT in distilled water by oral gavage three times per week (days 0, 2, 4, 7, 9, 11, 14, 16, 18 and 21). The RF/R and OVA/R groups received 1 mL of distilled water accordingly.
Table 1.
Composition of the experimental diets. All values are expressed as g/kg of diet.
| Components | Standard diet1 | 10% cocoa diet2 | |
|---|---|---|---|
| Basal mix | Cocoa powder | ||
| Proteins | 140.73 | 118.27 | 23.05 |
| Lipids | 38.71 | 27.06 | 11.53 |
| Carbohydrates | 721.93 | 692.41 | 16.76 |
| Soluble fiber | - | - | 8.91 |
| Insoluble fiber | 50.00 | 24.52 | 26.72 |
| Minerals | 35.86 | 27.83 | 6.29 |
| Vitamins | 10.20 | 7.92 | 0.04 |
| Choline bitartrate | 2.56 | 1.98 | - |
| Antioxidant | 0.01 | 0.01 | - |
| Theobromine | - | - | 2.50 |
| Phenolic compounds3 | - | - | 4.02 |
| Total | 1000.0 | 1000.0 | |
All values are expressed as g/kg of diet.
AIN-93M formula was used as standard diet.
The 10% cocoa diet was prepared from a basal mix in which 100 g cocoa/kg was added.
Reversed-phase high performance liquid chromatography coupled to a diode array detector revealed that cocoa phenolic compounds were epicatechin (2.34 mg/g), catechin (0.4 mg/g) and procyanidins
2.3. Sample collection and processing
On day 28, the animals were euthanized and the small intestine was carefully collected. After discarding the duodenum, the intestine was rinsed with phosphate buffer saline (PBS) solution in order to remove fecal content. A 0.5 cm portion of the middle of the intestine was immediately conserved in RNAlater®, and the consecutive following segment was placed in cassettes and fixed in 4% paraformaldehyde. The rest of the intestine was opened lengthwise along the mesenteric line; PP were collected and stored in RPMI medium. Finally, the remaining intestine was cut into 2 cm pieces and immersed in HBSS supplemented with 10% heat-inactivated FBS to isolate IEL and LPL.
2.4. Peyer’s patches lymphocyte isolation
PP were incubated with 1 mM DTT in RPMI medium under continuous agitation (55 u/min, 5 min, 37 °C). Consecutively, DTT medium was discarded, and PP were washed and passed through a sterile 70 µm mesh with a syringe plunger. The suspension obtained was centrifuged (538 g, 5 min, 4 °C) and resuspended with RPMI-10% FBS medium. Thereafter, cells were counted and viability was determined by staining with trypan blue using a Countess™ Automated Cell Counter (Invitrogen, Thermo Fisher Scientific).
2.5. Intraepithelial and lamina propria lymphocyte isolation
IEL and LPL isolation was carried in accordance with previous studies [21,22]. Briefly, small pieces of intestine, without PP, were incubated with a 5 mM DTT solution in HBSS-10% FBS under continuous agitation (55 u/min, 20 min, 37 °C). The first supernatants were then collected by decanting the tubes. Afterwards, a solution of 5 mM EDTA in HBSS-10% FBS was added to the remaining intestinal tissue and incubated twice (55 u/min, 15 min, 37 °C). The supernatants were collected together with the first ones and centrifuged (538 g, 5 min, 4 °C). The resulting cell suspensions were subjected to IEL purification.
For LPL collection, the remaining intestinal tissue from the above incubation was washed with RPMI-10% FBS (55 u/min, 20 min, 37 °C). The supernatants were discarded, and intestinal samples were cut into small pieces for 60 min of incubation with 300 U/mL of collagenase in RPMI-10% FBS at 85 u/min and 37 °C. Afterwards, 10 mL of medium was added to each sample to stop the reaction. The supernatants were filtered through a stainless steel mesh. Finally, the suspensions containing LPL were centrifuged (538 g, 5 min, 4 °C).
The resulting suspensions of both IEL and LPL were subjected to a cell purification gradient using 44 67.5% Percoll. Lymphocytes were re-suspended in medium and cell number and viability were determined using a Countess™ Automated Cell Counter.
2.6. Flow cytometry analysis
For flow cytometric analysis, 5x105 PPL, IEL and LPL were labelled with mouse anti-rat monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridininchlorophylla protein (PerCP) or allophycocyanin (APC). The antibodies used were anti-CD4, anti-CD8α, anti-CD8β, anti-TCRαβ, anti-TCRγδ, anti-NKR-P1A, anti-CD25 (BD Biosciences, Oxford, UK), anti-CD62L, anti-CD103 (Biolegend, San Diego, CA, USA) and anti-TLR4 (Novus Biologicals, Littleton, CO, USA). The cells were stained as previously described [19]. Briefly, lymphocytes were incubated with saturating amounts of antibodies in PBS-0.2% FBS-0.1% NaN3 (darkness, 20 min, 4 °C). Consecutively, the cells were washed, and fixed with 0.5% p-formaldehyde (darkness, until analysis, 4 °C). A negative control staining was included in each cell sample. Analyses were performed with a Gallios Cytometer (Beckman Coulter, Miami, FL, USA) in the Scientific and Technological Centres of the University of Barcelona (CCiTUB).
2.7. Gene expression in small intestine
As previously described [20], intestinal samples conserved in RNA later® were transferred into a lysing matrix tube (MP Biomedicals, Illkirch, France) for 30 s of homogenization by a FastPrep-24 (MP Biomedicals). RNA was obtained using an RNeasy® mini kit (Qiagen, Madrid, Spain) following the manufacturer’s instructions. RNA purity and concentration were determined by a NanoPhotometer (BioNova Scientific, S.L. Fremont, CA, USA). Subsequently, cDNA was obtained in a thermal cycler PTC 100 Programmable Thermal Controller using TaqMan® Reverse Transcription Reagents (Applied Biosystems, AB, Weiterstadt, Germany).
The specific PCR TaqMan® primers (AB) used to perform the PCR quantitative assay (ABI Prism 7900 HT, AB) were IgA (331943, made to order), TGF-β1 (Rn00572010_m1, Inventoried (I)), CD11c (Rn01511082_m1, I), CD11b (Rn00709342_m1, I), OX40L (Rn00585582_m1, I), IL-10 (Rn00563409_m1, I), FoxP3 (Rn01525092_m1, I) and Muc2 (Rn01498195_m1, I). The relative gene expression of genes of interest was normalized with the housekeeping genes β-actin (Rn00667869_m1, I) or HPRT1 (Rn01527840_m1, I) using the 2-ΔΔCt method [20]. For FoxP3, IL-10 and OX40L, the gene expression of HPRT1 was used as housekeeping gene, whereas for the rest of the gens, β-actin expression was used. Results are expressed as percentage of values of each experimental group normalized to the mean value obtained for the reference group, which was set at 100%.
2.8. Hematoxylin eosin and periodic acid Schiff stainings
Fixed intestinal tissues were dehydrated, paraffin-embedded and cut into 4 µm sections using a microtome (Thermo Scientific Microtome® HM355-S). Subsequently, the sections were mounted on glass slides and dried overnight at 37 °C.
For hematoxylin eosin (HE) staining, the samples were deparaffinized and rehydrated. Afterwards, intestinal tissues were stained with hematoxylin for 7 min, washed with running tap water and stained with eosin 5% for 4 min. Slides were again washed twice in distilled water and mounted with coverslips using Fluoromount-G.
For Periodic Acid Schiff (PAS) staining, deparaffinized and rehydrated samples were immersed in a 0.5% periodic acid solution for 5 min and washed in distilled water. Afterwards, sections were submerged in Schiff’s reagent for 15 min, washed in running tap water and counterstained with Mayer’s hematoxylin solution for 1 min. To finish, slides were rinsed in running tap water and in distilled water and mounted with coverslips.
After histological staining, microscopy analysis was performed using the TissueFaxs technology platform (TissueGnostics, Vienna, Austria). By means of the TissueFAXS 4.6.6245.1019 BF software, acquisition was done with a 200x magnification objective. Afterwards, PAS staining samples were analyzed using HistoQuest v4.04.0131 software (TissueGnostics, Vienna, Austria). Five microvilli of each intestine were selected as regions of interest (ROIs). The number of goblet cells was counted manually. Goblet cells are expressed as PAS-positive cells per mm2 of area. All analyses were done twice, examining different sections and different microvilli to obtain representative results. The counting of goblet cells was done by two independent investigators.
2.9. Immunofluorescence staining of small intestinal tissue
For immunofluorescence (IF) staining, sections were deparaffinized and rehydrated. Successively, antigen retrieval was done followed by permeabilization with PBS-0.2% Tween and a blocking with serum. Sections were incubated for 1 h with the primary antibody and, consecutively, washed and incubated with the corresponding secondary antibody for 1 h. After washing, the samples were incubated for 10 min with DAPI to stain the nuclei and finally mounted with fluoromount-G.
For IgA IF, 10 mM Tris-1mM EDTA (pH 9) was used for antigen retrieval and 5% donkey serum for blocking. Polyclonal goat anti-rat IgA α chain (Abcam, Cambridge, UK) was used as primary antibody and Alexa Fluor 568 donkey anti-goat IgG (Life Technologies, Austin, TX, USA) as secondary.
For granzyme B (GzmB) IF staining, 10 mM citrate buffer (pH 6) was used for antigen retrieval, and 10% of normal goat serum for blocking. The primary antibody used was polyclonal rabbit anti-rat GzmB (Novus Biologicals), with Alexa Fluor 647 goat anti-rabbit IgG (Life Technologies) being used as secondary antibody. In both stainings, a negative control was done omitting the primary antibody.
After IF stainings, pictures were acquired by TissueFaxs 4.2.6245.1019 FL software using a 200x magnification objective. Stainings were analyzed using TissueQuest 4.0.1.0128 software (TissueGnostics). Five microvilli of each intestine were selected as ROIs to be analyzed together and to obtain the scattergram of positive cells. All analyses were done twice, examining different sections and different microvilli for representative results.
2.10. Statistical analysis
Data are expressed as means ± standard errors. All statistical analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS, version 22.0, Chicago, IL, USA).
Prior to the analysis, the Levene’s and Kolmogorov-Smirnov test were performed in order to assess variance equality and normal distribution, respectively. When all the results indicated equality of variance and normal distribution, a two-way ANOVA test was performed (oral sensitization and diet). When the interaction between oral sensitization and diet was statistically significant, a one-way ANOVA with Bonferroni’s post hoc test was carried out to detect differences among groups.
The results that had different variance and/or different distribution were analyzed by non-parametric tests; Kruskal-Wallis followed by Mann-Whitney U tests were performed. In all cases, significant differences were accepted when P≤0.05.
3. Results
At the end of the study, the body weight increase of the animals fed standard diet was about 186%, whereas animals fed cocoa showed an increase of almost 157% [19]. This lower body weight increase, also observed in previous studies [17–18], was not associated with less food intake. Considering the average food consumption throughout the study and the amount of cocoa in the food, the daily amount of cocoa powder ingested was 11.75 g/kg.
3.1. Effect of a cocoa diet on the intestinal structure and the proportion of goblet cells in orally sensitized rats
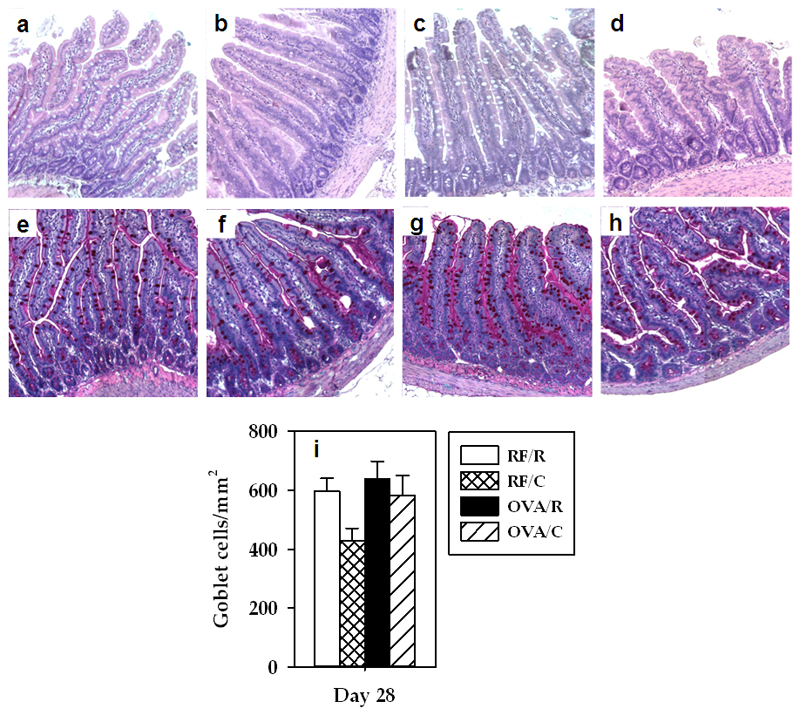
HE staining (Figures 1a–1d) did not reveal morphological changes due to the nutritional intervention with cocoa or with the administration of OVA plus CT. In addition, no significant variations were detected among groups concerning the proportion of goblet cells (Figures 1e–1i).
Figure 1. Hematoxylin-eosin and PAS staining.
HE staining of small intestine section from a representative rat belonging to (a) RF/R, (b) RF/C, (c) OVA/R, and (d) OVA/C groups. PAS staining showing goblet cells of small intestine from a representative rat belonging to (e) RF/R, (f) RF/C, (g) OVA/R and (h) OVA/C groups. (i) Goblet cells number/mm2 (values are expressed as mean ± standard error, n=6).
3.2. Effect of a cocoa diet on Peyer’s patches lymphocyte composition in orally sensitized rats
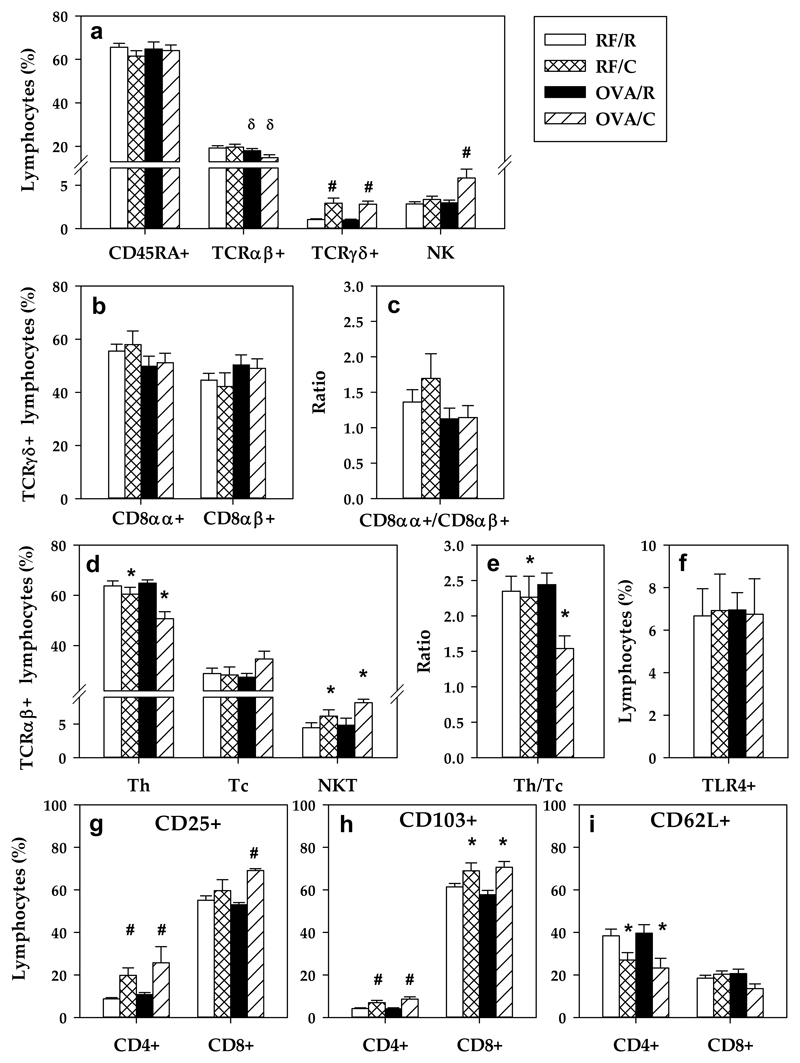
PP from the RF/R group were characterized by having about 70% CD45RA+ cells, 20% TCRαβ+ cells, and less than 3% TCRγδ+ lymphocytes and NK cells (Figure 2a). Likewise, in the RF/R group, there was a similar proportion of TCRγδ+CD8αα+ and TCRγδ+CD8αβ+ cells (Figures 2b–2c), and, among all TCRαβ+ cells, 65% were Th (TCRαβ+CD4+), 30% Tc lymphocytes (TCRαβ+CD8+) and about 5% NKT cells (Figures 2d–2e). Oral sensitization decreased the total proportion of TCRαβ+ cells (Figure 2a) without modifying their main subsets (Th, Tc and NKT cells) (Figures 2d–2e). On the other hand, a cocoa-enriched diet increased the proportion of TCRγδ+ cells (both CD8αα+ and CD8αβ+) and NK cells (Figures 2a–2c). Although the total proportion of TCRαβ+ cells was not influenced by a cocoa-enriched diet, there was an increase in the percentage of NKT cells and a decrease in the proportion of Th lymphocytes (Figure 2d), producing a reduced Th/Tc ratio (Figure 2e).
Figure 2. PP lymphocyte composition.
(a) Percentage of the main lymphocyte subsets; (b) percentage of TCRγδ+ PPL subsets (c) CD8αα+/CD8αβ+ ratio in TCRγδ+ cells; (d) percentage of TCRαβ+ PPL subsets; (e) Th/Tc ratio; (f) percentage of TLR4+ PPL; proportion of (g) CD25+, (h) CD103+ and (i) CD62L+ cells in CD4+ and CD8+ PPL. Values are expressed as mean ± standard error (n=6-9). Statistical differences: δ p<0.05 (two-way ANOVA) induced by the oral sensitization *p<0.05 (one-way or two-way ANOVA) induced by the diet; # p<0.05 (Mann-Whitney U) vs the corresponding reference group.
The percentage of cells bearing toll-like receptor 4 (TLR4) on PP was also established, but it did not reveal any modifications by oral sensitization or a cocoa diet (Figure 2f).
Further analysis of CD4+ and CD8+ PPL with the activation marker (CD25+), the marker of intestinal homing (CD103+) and the marker of secondary lymphoid tissue homing (CD62L+) was carried out. Rats fed a cocoa diet showed an increase in the proportion of CD25+ (Figure 2g) and CD103+ (Figure 2h) cells at intestinal level. Moreover, a decrease in the proportion of Th CD62L+ lymphocytes (Figure 2i) was detected, indicating an increase in CD4+CD62L- cell percentage.
3.3. Effect of a cocoa diet on intraepithelial lymphocyte composition in orally sensitized rats
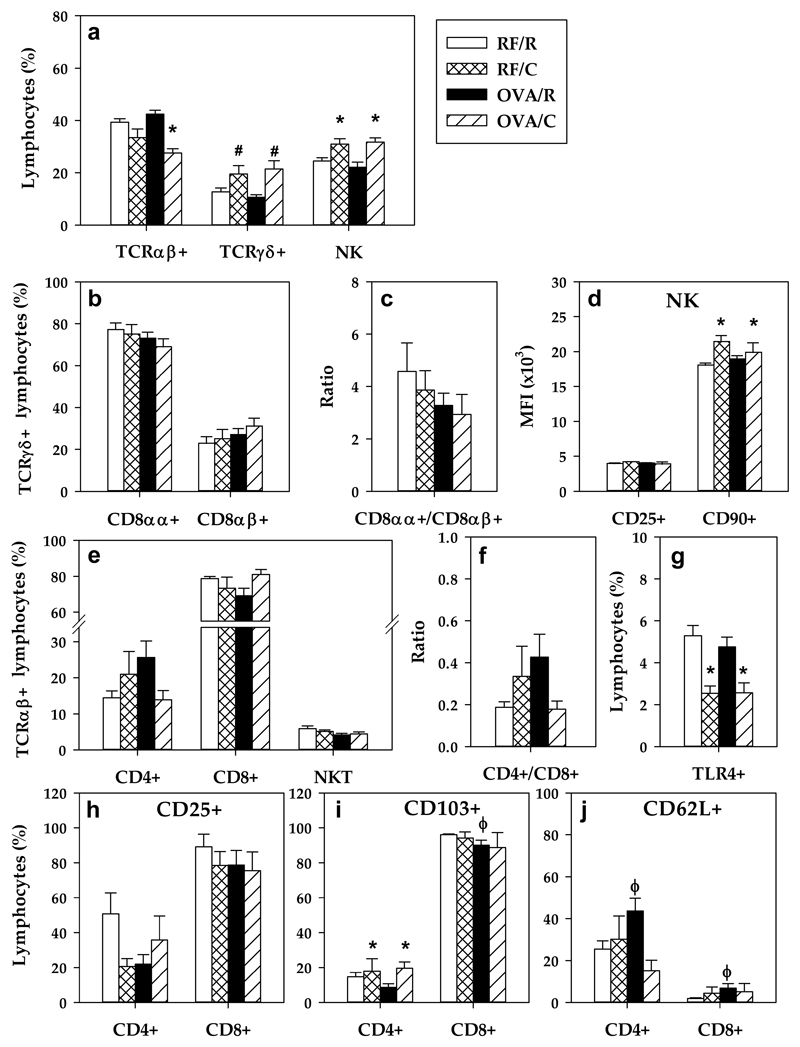
In the RF/R animals, the small intestinal IEL population was composed of about 40% TCRαβ+, 25% NK cells and 15% TCRγδ+ lymphocytes (Figure 3a). Almost 80% TCRγδ were CD8αα+ (Figures 3b–3c). Within the TCRαβ+ IEL, we found 79% CD8+, 14% CD4+ and 6% NKT cells (Figure 3e). Oral sensitization with OVA did not induce significant changes in these IEL proportions, in spite of a tendency toward a higher Th/Tc ratio (Figure 3f). However, similarly to PPL, an increase in the percentage of TCRγδ+ and NK cells was observed as a result of being fed a cocoa diet. The increase in TCRγδ+ IEL was due to both the CD8αα+ major population and CD8αβ+ minor population (Figures 3b–3c). A decrease in the TCRαβ+ cell proportion occurred in the OVA/C group (Figure 3a), however, these changes could not be attributed to any of the Th, Tc and NKT subsets considered (Figure 3e).
Figure 3. IEL lymphocyte composition.
(a) Percentage of the main lymphocyte subsets; (b) percentage of TCRγδ+ IEL subsets (c) CD8αα+/CD8αβ+ ratio in TCRγδ+ cells; (d) Mean fluorescence intensity (MFI) of CD25+ and CD90+ in NK cells; (e) percentage of TCRαβ+ IEL subsets; (f) Th/Tc ratio; (g) percentage of TLR4+ IEL; proportion of (h) CD25+ (i) CD103+ (j) and CD62L+ cells in CD4+ and CD8+ IEL. Values are expressed as mean ± standard error (n=6-9). Statistical differences: *p<0.05(one-way or two-way ANOVA) induced by the diet; # p<0.05 (Mann-Whitney U) vs the corresponding reference group; ϕ p<0.05 (Mann-Whitney U) vs RF/R group.
With regard to NK cells, the cocoa diet induced an increase in the MFI of CD90+ (Thy-1) expression of NK cells whereas no changes were observed in the MFI of CD25 in this cellular type (Figure 3d).
With respect to TLR4+ IEL, the cocoa diet reduced their proportion by up to a half (Figure 3g), affecting cells both with high and low TLR4+ expression (data not shown).
Evaluating both the activation and the homing marker expressions in CD4+ and CD8+ IEL, no significant differences were detected in the CD4+CD25+ and CD8+CD25+ subsets in OVA-sensitized animals (Figure 3h). As expected, most of the CD8+ IEL expressed the intestinal CD103 marker, whereas only about 2% were CD62L+. In contrast, in the smaller fraction of CD4+ IEL, less than 15% were CD103+ and about 25% were CD62L+. In CD8+ IEL, oral sensitization reduced the proportion of CD103+ cells and increased that of CD62L+ cells. Similarly, the sensitization procedure induced a higher percentage of CD62L+ cells in CD4+ IEL which was prevented by the cocoa diet (Figure 3j). An increase in CD4+CD103+ cells was observed due to the cocoa diet.
3.4. Effect of a cocoa diet on lamina propria lymphocyte composition in orally sensitized rats
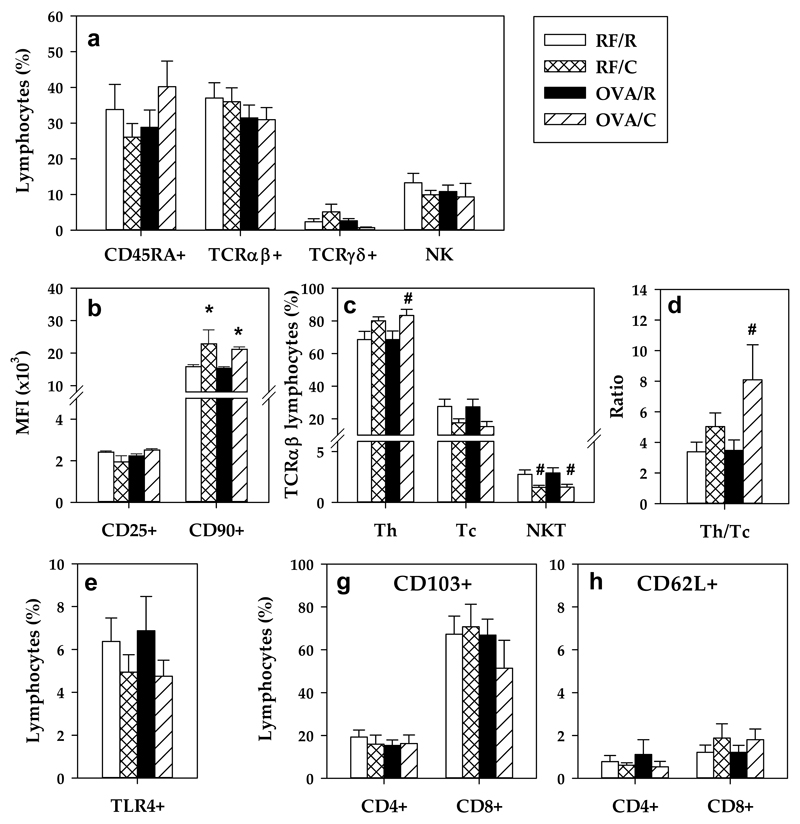
Focusing on the LPL composition in the RF/R group, the distribution of the main lymphocyte subsets was about 40% TCRαβ+ cells, 35% CD45RA+ lymphocytes, 15% NK cells and less than 3% TCRγδ+ cells (Figure 4a). In addition, in the total TCRαβ+ LPL population, about 69% were Th cells, 28% were Tc cells and 3% were NKT cells (Figure 4c). No significant differences were detected after oral sensitization. However, the OVA/C group had higher proportion of Th LPL (Figures 4c–4d). Moreover, the cocoa diet decreased the percentage of NKT cells in TCRαβ+ LPL.
Figure 4. LPL lymphocyte composition.
(a) Percentage of the main lymphocyte subsets; (b) Mean fluorescence intensity (MFI) of CD25 and CD90 MFI in NK cells; (c) percentage of TCRαβ+ LPL subsets; (d) Th/Tc ratio; (e) percentage of TLR4+ LPL; proportion of (f) CD103+ and (g) CD62L+ cells in CD4+ and CD8+. Values are expressed as mean ± standard error (n=6-9). Statistical differences: # p<0.05(Mann-Whitney U) vs the corresponding reference group.
A cocoa-enriched diet was associated with an increase in the expression of CD90 in NK cells, and no changes were observed in the expression of the CD25 molecule (Figure 4b).
CD25 expression was highly variable on CD4+ and CD8+ cells from IEL (data not shown). No changes were observed due to the cocoa diet either through the sensitization procedure in the expression of TLR4 (Figure 4e), CD103 (Figure 4f) and CD62L (Figure 4g) in LPL.
3.5. Effect of a cocoa diet on small intestine IgA+ cells in orally sensitized animals
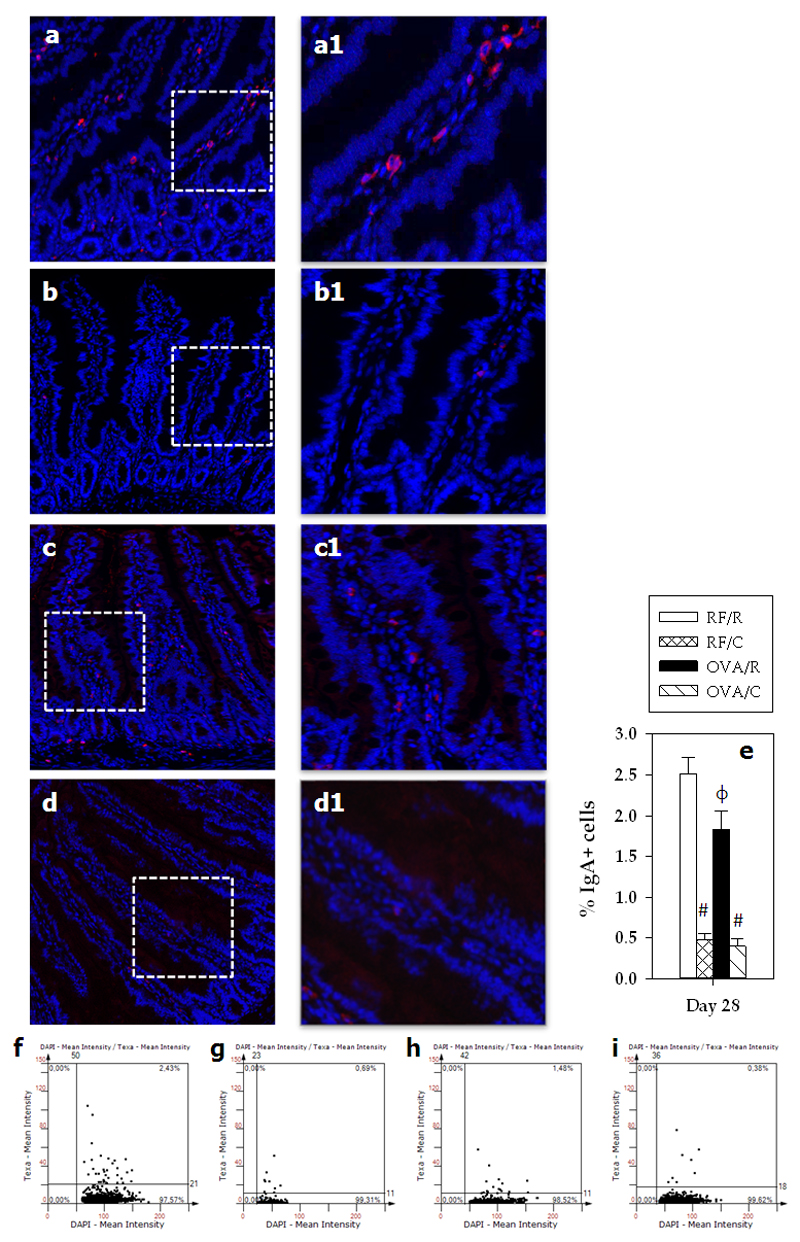
RF/R rats showed a percentage of about 2.5% of IgA+ cells located in the lamina propria (Figure 5). Oral sensitization decreased this proportion up to 15%. A cocoa-enriched diet was associated with a reduction in that percentage to 0.5% IgA+ LP cells both in the RF/C and the OVA/C groups (Figures 5a–5i).
Figure 5. Percentage of IgA+ cells.
Immunofluorescence IgA staining of sections from small intestinal samples from a representative rat belonging to (a) RF/R, (b) RF/C, (c) OVA/R, or (d) OVA/C groups. A magnification of the specified area for each representative sample is shown (a1, b1, c1 and d1). The images show IgA+ cells in red (Texas red). The DAPI blue fluorescence indicates the nuclei. (e) Percentage of IgA+ cells in small intestine of the rats (values are expressed as mean ± standard error, n=6-9). Statistical differences: # p<0.05 (Mann-Whitney U) respect to the corresponding reference group; ϕ p<0.05 (Mann-Whitney U) vs RF/R group. Scattergrams of the expression of IgA+ from a representative rat of each group of study: (f) RF/R, (g) RF/C, (h) OVA/R and (i) OVA/C.
3.6. Effect of a cocoa diet on small intestine granzyme B+ cells in orally sensitized animals
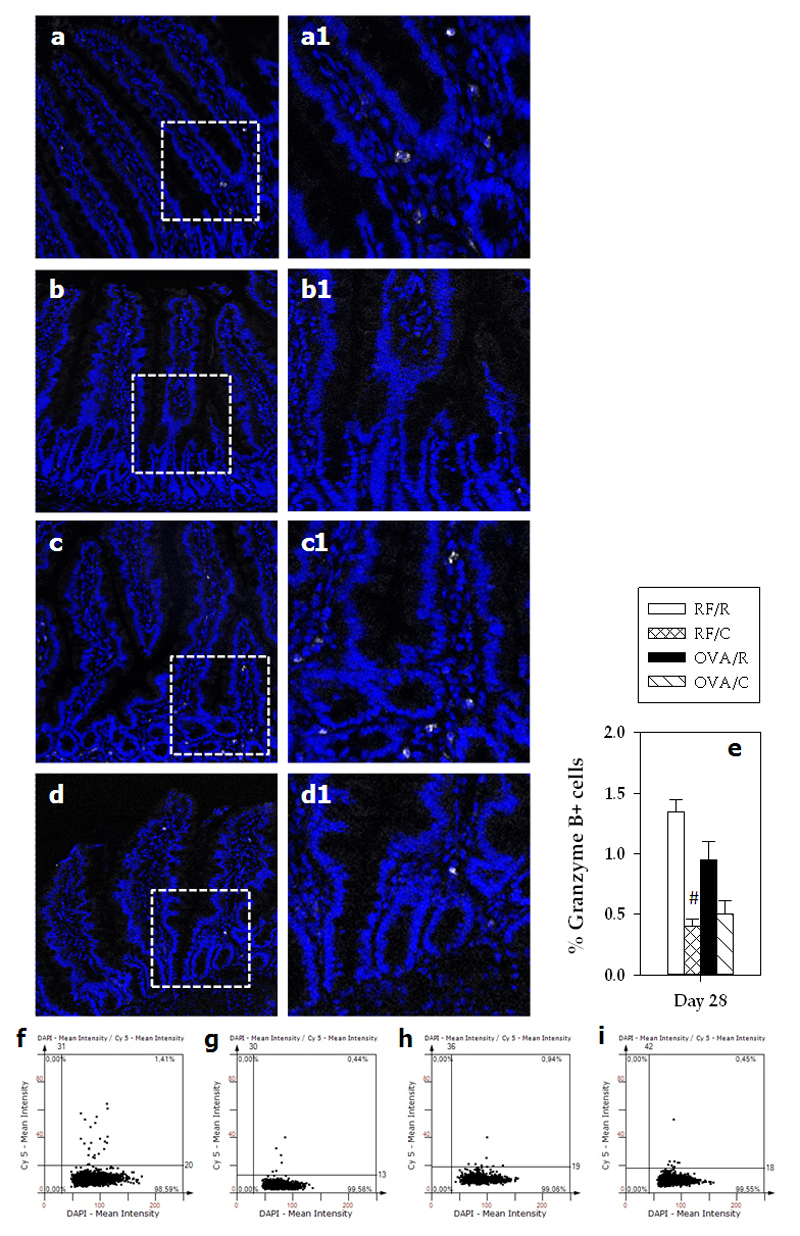
In the RF/R group, the percentage of GzmB+ cells in LP was about 1.3% (Figure 6). No differences were observed after sensitization; however, the cocoa-enriched diet induced a decrease in the GzmB+ cell percentage to 0.4% in the RF/C group, whereas changes in the OVA/C group did not achieve statistical significance.
Figure 6. Percentage of GzmB+ cells.
Immunofluorescence GzmB staining of sections from small intestinal samples from a representative rat belonging to (a) RF/R, (b) RF/C, (c) OVA/R, or (d) OVA/C group. A magnification of the specified area for each representative sample is shown (a1, b1, c1 and d1). The images show GzmB+ cells in white (Cy5). The DAPI blue fluorescence indicates the nuclei. (e) Percentage of GzmB+ in small intestine of the rats (values are expressed as mean ± standard error, n=6-9). Statistical difference: # p<0.05(Mann-Whitney U) vs RF/R group. Scattergrams of the expression of GzmB+ from a representative rat of each group of study: (f) RF/R, (g) RF/C, (h) OVA/R and (i) OVA/C.
3.7. Effect of a cocoa diet on the small intestine gene expression of orally sensitized animals
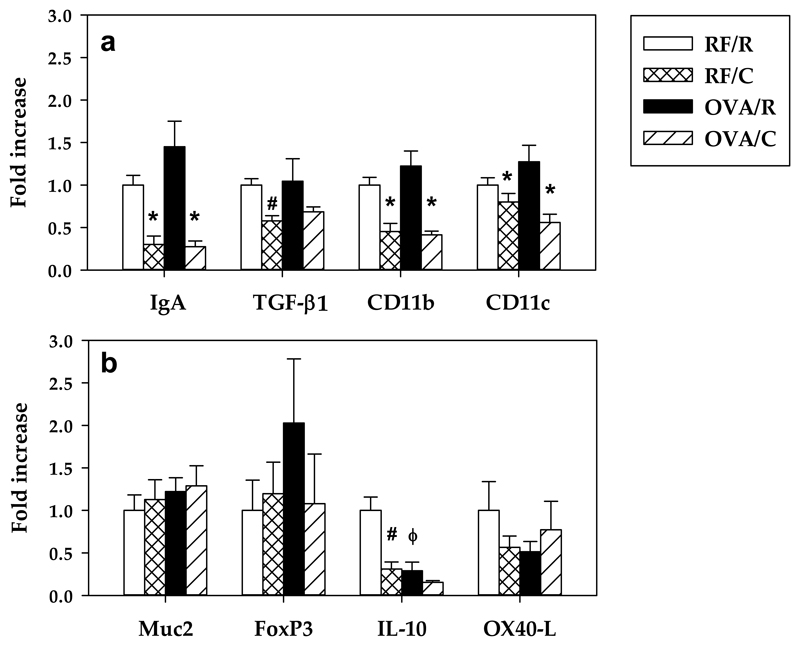
The gene expression of IgA, TGF-β1, CD11b and CD11c was not modified by oral sensitization but was reduced for IgA, CD11b and CD11c in the RF/C and OVA/C groups. TGF-β1 expression was decreased only in the RF/C group.
On the other hand, oral OVA sensitization did not modify the gene expression of Muc2, FoxP3 and OX40-L but decreased that of IL-10. Additionally, cocoa diet intake was associated with reduced levels of IL-10 mRNA (Figure 7).
Figure 7. Gene expression of some molecules in small intestinal samples.
The relative mRNA gene expression was calculated assigning the value of 1 to the mean of the rats from the RF/R group. Values are expressed as mean ± standard error (n=6). Statistical differences: *p<0.05 (two-way ANOVA) induced by the cocoa diet compared with its reference group, # p<0.05 (Mann-Whitney U) vs RF/R group, ϕ p<0.05 (Mann-Whitney U) vs RF/R group.
4. Discussion
In a previous study, we demonstrated that a cocoa diet prevents antibody synthesis and modifies mesenteric lymph node composition and functionality using the same rat oral sensitization model [19]. These results prompted us to study the influence of a cocoa diet on intestinal tissue composition. The data presented here demonstrate that a cocoa diet modifies the small intestinal compartment mainly by increasing the presence of TCRγδ+ cells and NK cells in PPL and IEL. Moreover, a cocoa diet increased the proportion of CD4+CD25+, CD4+CD103+, CD8+CD103+ and CD4+CD62L- cells in PP. These changes were accompanied by a decrease in the proportion of IgA+ cells and GzmB+ cells in LP and in the gene expression of IgA, TGF-β1, CD11b, CD11c and IL-10 in the small intestine.
In contrast with other studies using CT as intestinal adjuvant that reported an increase in intestinal IgA levels [23,24], the oral sensitization procedure used here decreased the proportion of intestinal IgA+ cells in LP, although no differences were seen at the secreted intestinal IgA levels in these conditions [19]. Furthermore, with this sensitization protocol, no changes were found in the gene expression of IgA and TGF-β1 in line with previous studies [20]. In the current study, a cocoa diet was also associated with decreased numbers of intestinal IgA+ cells. Previously, we demonstrated that a 10% cocoa diet attenuated intestinal IgA levels at different ages and in different rat strains, such as Wistar [25,26], Brown Norway [18] and Lewis [19,27]. With the current results regarding IgA+ cells on LP, we can conclude that the reduction of intestinal IgA was due to a lower number of IgA+ cells at this level. These results are in line with the lower IgA mRNA levels found in this work as well as in a previous study [26]. Multiple cytokines such as TGF-β1 [28] and a vast number of cell interactions are required to activate and differentiate B cells in PP [16], and to promote their migration to the MLN and the eventual gut homing [29]. The reduction in TGF-β1 gene expression at small intestinal level suggests that, among other possibilities, a 10% cocoa diet influences IgA+ B cell differentiation, thereby involving a reduction of IgA+ B cells in LP. These results are in line with changes in the chemokines CCR9, CCL25, CCL28, RARα and RARβ, required for gut homing, as previously demonstrated after cocoa feeding [25]. On the other hand, in the current study, a cocoa-enriched diet was also associated with a decrease in the proportion of TLR4+ IEL, the receptor binding the bacterial endotoxin lipopolysaccharide (LPS). This reduction in TLR4+ IEL was in line with previous data detecting a decrease in TLR4 gene expression in the small intestine due to cocoa diet [25], and could be related to changes in intestinal microbiota as previously reported in cocoa-fed rats [30]. TLR4 signaling also has implication in the production of intestinal IgA as activation of TLR4 pathways is associated with an increase of diverse chemokines (mainly CCL20, CCL28 and CXCL16) related to the recruitment and differentiation of IgA+ B cells to the intestine [31]. Accordingly, the decrease in the TLR4+ IEL in animals fed a 10% cocoa diet could be seen in the context of lower numbers of LP IgA+ cells and, the consequently, lower intestinal IgA levels.
In the current study, neither 10% cocoa diet nor the administration of OVA plus CT modified the intestinal structure. In order to initiate the immune response, the first barrier that antigens must penetrate is the mucus layer. This barrier in the intestine prevents food allergy, enhancing gut homeostasis and oral tolerance [32]. To determine whether oral sensitization and the cocoa diet were interacting at that point, evaluation of goblet cells and one of their products, Muc2, was considered. Neither the sensitization procedure nor the cocoa diet modified the goblet cell proportion and Muc2 gene expression. These results are in partial agreement with those obtained in a food allergy model in Brown Norway rats fed with two different cocoa-enriched diets with unchanged Muc2 gene expression but lower numbers of goblet cells [33]. Overall, these results lead us to suggest that the tolerogenic effect of a cocoa diet is not due to a modification of that barrier.
Even though no changes were detected at the structural level of small intestine, several modifications were found in the proportions of lymphocyte populations isolated from PP, IEL and LPL, representing essential sites involved in oral tolerance induction [7,34]. A cocoa diet induced an increase in the proportions of TCRγδ+, both CD8αα+ and CD8αβ+, cells in PPL and IEL, essential subsets for the induction of oral tolerance. Our results are in line with those of Akiyama et al. [35], who reported that unripe apple polyphenols inhibit oral sensitization associated with a rise in the proportion of TCRγδ+ IEL. In line with these studies, it was described that a decrease in the number of TCRγδ+ cells induced by anti-TCRγδ antibody treatment facilitated oral sensitization in mice [36].
Furthermore, a cocoa diet increased the NK cell percentage in both PP and IEL compartments. Although NK cells are primarily involved in innate immunity, they also have regulatory functions and can contribute to the inhibition of allergic disease [37,38]. Several subsets of NK cells have been defined in humans and mice [39,40] but less information about rat NK cells is available. In a recent study focused on mice NK cells [41], activation of NK cells involved, among other changes, a lower expression of granzyme B, CD11b and CD11c but a higher expression of CD90 in these cells. It is worth noting that the increase in NK IEL due to a cocoa diet found here was accompanied by a higher CD90 surface expression. In addition, cocoa diet decreased GzmB+ cell percentage in the intestinal LP and downregulated CD11b and CD11c intestinal gene expression. Although further studies are needed for an in depth evaluation of the NK subset and function associated with a cocoa diet, the current results suggest that some intestinal NK cells (perhaps related to high CD90 surface expression, low GzmB content and low CD11b and CD11c gene expression) might contribute to the tolerogenic effect of a cocoa diet. Additionally, the decrease in CD11b and CD11c gene expression may be related to a lower presence of dendritic cells in the intestinal wall. These cells could migrate from LP to MLN, where we have previously observed higher CD11c gene expression [19] and where they could promote tolerance as suggested [12].
A cocoa diet additionally modified the proportion of CD25+ cells in CD4+ and CD8+ PPL. The CD25 molecule is, among others, a marker of Treg cells, which induce tolerance against dietary antigens [42]. Thus, the increased proportion of CD25+ cells in PP cells after consumption of a cocoa diet might contribute to the tolerogenic effect of cocoa.
Consuming cocoa also changed the proportions of cells expressing CD103 and CD62L molecules in PPL. Cocoa-fed animals showed a higher proportion of CD103+ cells in CD4+ and CD8+ PPL, and a lower proportion of CD62L+ (and consequently a higher CD62L- percentage) in CD4+ PPL. CD103 (also known as αE integrin) is a marker of gut homing cells [8] with a role in controlling the homeostasis of the intestinal immune system and inducing the expansion of Treg cells [43]. Therefore, the increase of CD103+ cells in the CD4+ and CD8+ PPL subsets could also contribute to the tolerogenic effect of the cocoa diet. As a marker of lymph node homing, CD62L is constitutively expressed in naive lymphocytes and is downregulated after cell activation [44,45]. CD62Llow Treg cells found in secondary lymphoid tissues guide T cells to migrate to non-lymphoid tissues in order to maintain immune homeostasis [46]. Our results suggest that a cocoa diet induced more cell activation and, consequently, more effector cells were retained in the intestinal compartment with the potential to enhance the tolerogenic response. Taking all these results into consideration, we suggest that a cocoa diet induces the activation of tolerogenic cells migrating to the intestinal PP compartment, thereby avoiding oral sensitization.
Another modification found here was the downregulation of IL-10 gene expression in intestinal tissue both in orally sensitized animals and in cocoa-fed rats. IL-10 has been shown to induce Treg cells mostly of the Tr1 type [47] which agrees with lower IL-10 gene expression in sensitized animals, thereby promoting the loss of oral tolerance as reported in a murine model of food allergy [48]. However, cocoa-fed animals also downregulated IL-10 gene expression. Despite these contradictory data, our results are in line with those reported for flavonoids such as quercitrin, flavones and those found in an apple extract in orally sensitized mice [49–51]. Therefore, it might be concluded that flavonoids modulated immune response without enhancing IL-10 tolerogenic effects.
In the current study, several markers of healthy immune status of GALT were considered. Unexpectedly, in the applied experimental design, not so many changes were seen due to the oral sensitization procedure used, although it was able to induce the production of Th2-antibodies, as previously described [19]. This could be due to the implication of other mechanisms such as specific dendritic cells at intestinal level that would enhance antigen presentation, the up-regulation of some makers in the lymphocytes enhancing either the antigen presentation or the activation of plasma cells, among others. Moreover, the time point in which of these biomarkers were studied could be too late to observe their alterations.
In conclusion, the data presented here showed that consumption of a diet containing 10% of cocoa for four weeks either in healthy conditions or in a rat oral sensitization model was associated with similar substantial changes in small intestinal lymphocyte subsets located in Peyer’s patches, epithelium and lamina propria. A cocoa-enriched diet induces a rise in the proportion of TCRγδ+ cells and NK PPL and IEL, suggesting a contribution to the prevention of oral sensitization. In line with this, the nutritional intervention with cocoa induces an increase of CD25+, CD103+ and CD62L- cells in PP and reduces CD11b, CD11c and IL-10 gene expression, together with a lower number of IgA+ LP cells. In summary, these changes might contribute to enhancing oral tolerance, thereby underlining the role of cocoa in preventing oral sensitization.
Acknowledgements
The authors would like to thank Erika Bajna and Denise Heiden (Medical University of Vienna) for their excellent technical assistance with TissueFAXs. We also thank Idilia Foods S.L. for providing the cocoa powder and Dr. J Comas from the “Centres Científics i Tecnològics” from the University of Barcelona (CCiT-UB) for his expert assistance in the cytometry service.
Funding: This study was financially supported by funding from the Spanish Ministry of Economy and Competitiveness (AGL2011-24279). MCB is the recipient of a fellowship from the University of Barcelona (APIF2014). The collaboration with the University of Vienna was supported by grants from the Fundation Agustí Pedro i Pons (UB) and the Nutrition and Food Safety Research Institute (INSA-UB) as well as grants KLI284 and WKP039 from the Austrian Science Fund (to EU).
Footnotes
Conflicts of interest
None of the authors have any conflicts of interest to declare.
References
- [1].Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–25. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- [2].Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153:3–6. doi: 10.1111/j.1365-2249.2008.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- [4].Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- [5].Jung C, Hugot J-P, Barreau F. Peyer’s patches: the immune sensors of the intestine. Int J Inflam. 2010;2010 doi: 10.4061/2010/823710. 823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–9. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–56. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sheridan BS, Lefrançois L. Intraepithelial lymphocytes: To serve and protect. Curr Gastroenterol Rep. 2010;12:513–21. doi: 10.1007/s11894-010-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johnston LK, Chien KB, Bryce PJ. The Immunology of Food Allergy. J Immunol. 2014;192:2529–34. doi: 10.4049/jimmunol.1303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. 2016;137:984–97. doi: 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2009;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang H, Kong H, Zeng X, Guo L, Sun X, He S. Subsets of regulatory T cells and their roles in allergy. J Transl Med. 2014;12:125. doi: 10.1186/1479-5876-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Helm RM, Burks AW. Mechanisms of food allergy. Curr Opin Immunol. 2000;12:647–53. doi: 10.1016/s0952-7915(00)00157-6. [DOI] [PubMed] [Google Scholar]
- [15].Burks AW, Laubach S, Jones SM. Oral tolerance, food allergy, and immunotherapy: Implications for future treatment. J Allergy Clin Immunol. 2008;121:1344–50. doi: 10.1016/j.jaci.2008.02.037. [DOI] [PubMed] [Google Scholar]
- [16].van Wijk F, Knippels L. Initiating mechanisms of food allergy: Oral tolerance versus allergic sensitization. Biomed Pharmacother. 2007;61:8–20. doi: 10.1016/j.biopha.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [17].Ramiro-Puig E, Pérez-Cano FJ, Ramos-Romero S, Pérez-Berezo T, Castellote C, Permanyer J, et al. Intestinal immune system of young rats influenced by cocoa-enriched diet. J Nutr Biochem. 2008;19:555–65. doi: 10.1016/j.jnutbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [18].Massot-Cladera M, Abril-Gil M, Torres S, Franch À, Castell M, Pérez-Cano FJ. Impact of cocoa polyphenol extracts on the immune system and microbiota in two strains of young rats. Br J Nutr. 2014;112:1944–54. doi: 10.1017/S0007114514003080. [DOI] [PubMed] [Google Scholar]
- [19].Camps-Bossacoma M, Abril-Gil M, Saldaña-Ruiz S, Franch À, Pérez-Cano FJ, Castell M. Cocoa diet prevents antibody synthesis and modifies lymph node composition and functionality in a rat oral sensitization model. Nutrients. 2016;8:242. doi: 10.3390/nu8040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Camps-Bossacoma M, Abril-Gil M, Franch À, Pérez-Cano FJ, Castell M. Induction of an oral sensitization model in rats. Clin Immunol Endocr Metab Drugs. 2014;1:89–101. [Google Scholar]
- [21].Pérez-Cano FJ, Castellote C, González-Castro AM, Pelegrí C, Castell M, Franch A. Developmental changes in intraepithelial T lymphocytes and NK cells in the small intestine of neonatal rats. Pediatr Res. 2005;58:885–91. doi: 10.1203/01.pdr.0000182187.88505.49. [DOI] [PubMed] [Google Scholar]
- [22].Pérez-Cano FJ, Castellote C, Marín-Gallén S, Franch A, Castell M. Neonatal immunoglobulin secretion and lymphocyte phenotype in rat small intestine lamina propria. Pediatr Res. 2005;58:164–9. doi: 10.1203/01.PDR.0000156367.60769.36. [DOI] [PubMed] [Google Scholar]
- [23].Gagliardi MC, Sallusto F, Marinaro M, Vendetti S, Riccomi A, De Magistris MT. Effects of the adjuvant cholera toxin on dendritic cells: stimulatory and inhibitory signals that result in the amplification of immune responses. Int J Med Microbiol. 2002;291:571–5. doi: 10.1078/1438-4221-00169. [DOI] [PubMed] [Google Scholar]
- [24].Macpherson AJ, McCoy KD, Johansen F-E, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- [25].Pérez-Berezo T, Franch A, Castellote C, Castell M, Pérez-Cano FJ. Mechanisms involved in down-regulation of intestinal IgA in rats by high cocoa intake. J Nutr Biochem. 2012;23:838–44. doi: 10.1016/j.jnutbio.2011.04.008. [DOI] [PubMed] [Google Scholar]
- [26].Pérez-Berezo T, Franch A, Ramos-Romero S, Castellote C, Pérez-Cano FJ, Castell M. Cocoa-enriched diets modulate intestinal and systemic humoral immune response in young adult rats. Mol Nutr Food Res. 2011;55(Suppl 1):S56–66. doi: 10.1002/mnfr.201000588. [DOI] [PubMed] [Google Scholar]
- [27].Massot-Cladera M, Franch A, Castellote C, Castell M, Pérez-Cano FJ. Cocoa flavonoid-enriched diet modulates systemic and intestinal immunoglobulin synthesis in adult Lewis rats. Nutrients. 2013;5:3272–86. doi: 10.3390/nu5083272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mora JR, von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- [29].Hieshima K, Kawasaki Y, Hanamoto H, Nakayama T, Nagakubo D, Kanamaru A, et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. 2004;173:3668–75. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- [30].Massot-Cladera M, Pérez-Berezo T, Franch A, Castell M, Pérez-Cano FJ. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch Biochem Biophys. 2012;527:105–12. doi: 10.1016/j.abb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- [31].Shang L, Fukata M, Thirunarayanan N, Martin AP, Maussang D, Berin C, et al. TLR signaling in small intestinal epithelium promotes B cell recruitment and IgA production in lamina propria. 2009;135:529–38. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shan M, Gentile M, Yeiser JR, Walland AC, Victor U, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2014;342:447–53. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Abril-Gil M, Garcia-Just A, Pérez-Cano FJ, Franch À, Castell M. Effect of a cocoa-enriched diet on immune response and anaphylaxis in a food allergy model in Brown Norway rats. J Nutr Biochem. 2016;27:317–26. doi: 10.1016/j.jnutbio.2015.09.022. [DOI] [PubMed] [Google Scholar]
- [34].Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, et al. Peyer’s patches are required for oral tolerance to proteins. Proc Natl Acad Sci USA. 2001;98:3310–5. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Akiyama H, Sato Y, Watanabe T, Nagaoka MH, Yoshioka Y, Shoji T, et al. Dietary unripe apple polyphenol inhibits the development of food allergies in murine models. FEBS Lett. 2005;579:4485–91. doi: 10.1016/j.febslet.2005.07.019. [DOI] [PubMed] [Google Scholar]
- [36].Okunukt H, Teshima R, Sa Y, Nakamura R, Akiyama H, Maitani T, et al. The hyperresponsiyeness of W/WV mice to oral sensitization is associated with a decrease in TCRγδ-T cells. Biol Pharm Bull. 2005;28:584–90. doi: 10.1248/bpb.28.584. [DOI] [PubMed] [Google Scholar]
- [37].Deniz G, Akdis M. NK cell subsets and their role in allergy. Expert Opin Biol Ther. 2011;11:833–41. doi: 10.1517/14712598.2011.572549. [DOI] [PubMed] [Google Scholar]
- [38].Deniz G, Van De Veen W, Akdis M. Natural killer cells in patients with allergic diseases. J Allergy Clin Immunol. 2013;132:527–35. doi: 10.1016/j.jaci.2013.07.030. [DOI] [PubMed] [Google Scholar]
- [39].Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- [40].Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- [41].Müller JR, Waldmann TA, Dubois S. Loss of cytotoxicity and gain of cytokine production in murine tumor-activated NK cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–36. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ruane DT, Lavelle EC. The role of CD103+ dendritic cells in the intestinal mucosal immune system. Front Immunol. 2011;2:1–6. doi: 10.3389/fimmu.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yang S, Liu F, Wang QJ, Rosenberg SA, Morgan RA. The shedding of CD62L (L-selectin) regulates the acquisition of lytic activity in human tumor reactive T lymphocytes. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hengel RL, Thaker V, Pavlick MV, Metcalf JA, Dennis G, Yang J, et al. Cutting Edge: L-Selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J Immunol. 2003;170:28–32. doi: 10.4049/jimmunol.170.1.28. [DOI] [PubMed] [Google Scholar]
- [46].Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259:103–14. doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lutz MB. Induction of CD4(+) Regulatory and Polarized Effector/helper T Cells by Dendritic Cells. Immune Netw. 2016;16:13–25. doi: 10.4110/in.2016.16.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Frossard CP, Tropia L, Hauser C, Eigenmann PA. Lymphocytes in Peyer patches regulate clinical tolerance in a murine model of food allergy. J Allergy Clin Immunol. 2004;113:958–64. doi: 10.1016/j.jaci.2003.12.017. [DOI] [PubMed] [Google Scholar]
- [49].Cruz EA, Da-Silva SAG, Muzitano MF, Silva PMR, Costa SS, Rossi-Bergmann B. Immunomodulatory pretreatment with Kalanchoe pinnata extract and its quercitrin flavonoid effectively protects mice against fatal anaphylactic shock. Int Immunopharmacol. 2008;8:1616–21. doi: 10.1016/j.intimp.2008.07.006. [DOI] [PubMed] [Google Scholar]
- [50].Zuercher AW, Holvoet S, Weiss M, Mercenier A. Polyphenol-enriched apple extract attenuates food allergy in mice. Clin Exp Allergy. 2010;40:942–50. doi: 10.1111/j.1365-2222.2010.03460.x. [DOI] [PubMed] [Google Scholar]
- [51].Yano S, Umeda D, Yamashita T, Ninomiya Y, Sumida M, Fujimura Y, et al. Dietary flavones suppresses IgE and Th2 cytokines in OVA-immunized BALB/c mice. Eur J Nutr. 2007;46:257–63. doi: 10.1007/s00394-007-0658-7. [DOI] [PubMed] [Google Scholar]