Abstract
Objective
To investigate white matter microstructure compromise in Veterans with a history of traumatic brain injury (TBI) and its possible contribution to post-traumatic stress disorder (PTSD) symptomatology and neuropsychological functioning via diffusion tensor imaging (DTI).
Participants and Methods
38 Veterans with mild (n = 33) and moderate (n = 5) TBI and 17 Military Control (MC) participants without TBI completed neuropsychological testing and psychiatric screening and underwent MRI scanning an average of 4 years following their TBI event(s). Fractional anisotropy (FA) and diffusivity measures were extracted from 9 white matter tracts.
Results
Compared to MCs, TBI participants reported higher levels of PTSD symptoms and performed worse on measures of memory and psychomotor processing speed. TBI was associated with lower FA in the genu of the corpus callosum and left cingulum bundle. FA negatively correlated with processing speed and/or executive functions in 7 of the 8 tracts. Regional FA did not correlate with memory or PTSD symptom ratings.
Conclusion
Results suggest that current PTSD symptoms are independent of TBI-related white matter alterations, as measured by DTI. Additionally, white matter microstructural compromise may contribute to reduced processing speed in our sample of participants with history of neurotrauma. Findings of the current study add insight into the factors associated with complicated recovery from mild to moderate TBI.
Introduction
Traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD) are highly comorbid in Veterans of the recent conflicts in Iraq and Afghanistan.1 Veterans with histories of TBI endorse higher rates of PTSD symptoms than those with other injuries,1 and deployment-related TBI has been shown to predict the onset or exacerbation of PTSD symptoms, even beyond other contributing factors such as combat intensity.2,3 Additionally, the presentation of PTSD symptoms is reportedly of greater intensity in Veterans with histories of TBI compared to those without reported neurotrauma.4 Such findings echo those of civilian TBI studies which show heightened psychiatric distress following a TBI,5 and they suggest that disruptions in brain function as a consequence of a TBI event may contribute to the manifestation of PTSD symptoms in Veterans.6
In samples without history of TBI, PTSD symptoms have been associated with structural alterations within cortical and subcortical regions within the frontal and temporal lobes including prefrontal cortices7 the anterior cingulate (ACC),8 the temporal cortex,9 the hippocampus and amygdala.10–12 Such findings support the theory that disrupted fronto-subcortical circuitry in systems that mediate emotional regulation may contribute to the manifestation of PTSD symptoms.13 Thus, damage within the white matter tracts interconnecting those frontal and limbic brain regions may also contribute to or exacerbate PTSD symptoms following a traumatic event. Although available studies linking white matter microstructure and PTSD symptoms have been limited, PTSD has been associated with microstructural damage within the cingulum bundle, a white matter tract connecting limbic regions (e.g., cingulate cortex and hippocampus).14,15 PTSD has also been tied to altered frontal white matter microstructure in frontal lobe regions (e.g., prefrontal cortex; precentral gyrus) and in the internal capsule.15 An examination of white matter pathways that may impact PTSD is particularly relevant in the context of TBI as many of these same brain regions (i.e., the prefrontal cortex) are consistently shown to be susceptible to TBI effects16,17—with brain white matter being particularly vulnerable to TBI even in its mild forms.18–20
The pathophysiological processes contributing to white matter compromise in mild TBI (mTBI) are complex. Generally, tensile forces upon white matter fibers at the initial time of the injury are thought to initiate damage that provokes a secondary cascade of neurochemical and neurotoxic processes responsible for the diffuse axonal injury often observed in TBI.21 Traditional structural MRI techniques are not sensitive to the presence mild forms of axonal injury, due to the relative homogeneity of the T1 signal within white matter. Researchers have turned to diffusion tensor imaging (DTI), a magnetic resonance imaging (MRI) modality that is sensitive to the movement of water molecules within brain structures. In highly organized tissue, such as neural white matter, these patterns of molecular water movement can be used to describe the neuronal integrity of the tissue, a common index of which is fractional anisotropy (FA).22 FA values range from zero in voxels where the diffusion is equal in all directions, to one, in regions with a high degree of directional uniformity. Thus, higher FA values are indicative of healthy tissue with uniform structure, while relatively lower values suggest a disruption of this structure and tissue damage.22 Reductions in FA may result from a decrease in axial diffusivity (AD) (diffusion along the principal diffusion direction [along the axon]), an increase in radial diffusivity (diffusion perpendicular to the primary diffusion direction), or an additive or synergistic effect of the two. Although there is some debate as to the specific meaning of the component diffusion measures,23,24 AD has most commonly been interpreted as describing axonal integrity, and RD has been described as a proxy for myelin integrity.25 Many DTI studies have found evidence for disrupted white matter microstructure in frontal and limbic white matter regions in both mild and severe TBI non-Veteran populations.26–34 A growing number of studies of Veterans with histories of mTBI have shown evidence of white matter abnormalities as well,35–37 though such an effect is not always found.38
The purpose of this study was to investigate whether TBI-related damage to white matter tracts contributes to PTSD symptom severity and concomitant reduced cognitive performance. Tracts selected for analysis included specific pathways previously shown to be related to PTSD symptoms (e.g., cingulum bundle and internal capsule) and those that interconnect frontal regions also implicated in PTSD (i.e., genu and body divisions of the corpus callosum). We hypothesized that (1) a positive history of TBI would be associated with compromised microstructure in white matter pathways, including tracts that connect structures involved in emotional regulation (e.g., cingulum bundle) and (2) greater disruption of the white matter would be associated with poorer cognitive performance and increased current PTSD symptom severity in a well-characterized sample of military Veterans with history of mild to moderate TBI.
Methods
Participants
Data for this project were gathered from ongoing studies of TBI in returning OEF/OIF Veterans being conducted at the VA San Diego Healthcare System (VASDHS), with Institutional Review Board approval from the VASDHS and the University of California, San Diego. TBI participants were recruited from outpatient TBI treatment clinics, from study advertisements within the VASDHS, and word of mouth. Forty-nine Veterans with reported TBI histories were originally screened for participation in this study. Following application of exclusionary criteria, including exclusion of those with effort test results below published cutpoints,39,40 a final sample of 38 Veterans with TBI participated. Another age-matched control group of OEF/OIF veterans with no reported history of TBI (n = 17) was also recruited for participation via study advertisements within the VASDHS and word of mouth.
TBI group inclusion/exclusion criteria
OEF/OIF veterans diagnosed with a mild or moderate closed head TBI (n = 38) from either blast exposure (i.e., secondary to IED, land mine, or rocket grenade) or mechanical force (i.e., motor vehicle accident, or other closed head injury [blunt trauma]) were included in the study. The criteria defined by the Department of Defense and Department of Veterans Affairs Traumatic Brain Injury Task Force were used for classifying injury severity in TBI.41 Specifically, mild TBI (n = 33) was defined as: alteration (AOC) or loss of consciousness (LOC) ≤ 30 minutes, if available an initial Glasgow Coma Scale (GCS)42 score between 13–15, a post-traumatic amnesia (PTA) ≤ 24 hours, and no visible lesions on MRI or CT scan. Moderate TBI (n = 5) was defined as: LOC between 30 minutes and 6 hours, an initial GCS score between 9–12, and PTA of less than 7 days. Of the five participants meeting criteria for moderate TBI, four had reported LOC of greater than 30 min and one reported a PTA of greater than 24 hours. No participants included in this study showed focal lesions on conventional structural MRI.
Exclusion criteria included severe head injury (GCS ≤ 8); a prior history of other neurological disorder (e.g., multiple sclerosis, tumor, seizure disorder); developmental learning disability; current (within past 30 days) substance or alcohol abuse according to DSM-IV criteria; pre-injury metabolic or other diseases known to affect CNS functions; or contraindication to scanning (e.g., claustrophobia, shrapnel). As noted, participants with poor performance on tests of effortful engagement were excluded from analyses.
Military Control group inclusion/exclusion criteria
OEF/OIF veterans who did not meet criteria for TBI as described above comprised the Military Control (MC) group. Exclusion criteria included a history of concussion or other neurological disorder; developmental learning disability; current substance or alcohol abuse according to DSM-IV criteria; presence of a psychotic disorder or bipolar disorder as defined by DSM-IV criteria, poor effort, or metabolic or other diseases known to affect CNS functions.
Measures
Neuropsychological testing was performed by trained research assistants. Administration time for the neuropsychological battery was approximately 2 hours and administration time for the mood and functional measures took approximately 15 minutes.
Neuropsychological testing
The Wide Range Achievement Test-4th Edition, Reading subtest,43 was used as an estimate of premorbid verbal intellectual ability. The California Verbal Learning Test-II (CVLT-II)44 was used to assess verbal memory. Variables included Trials 1–5 Total Correct, Long Delay Free Recall Total Correct, and the Recognition Discriminability Index. Visual memory was evaluated using the Rey-Osterrieth Complex Figure Test recall total score.45 Executive functions were assessed via the total number of errors on the Wisconsin Card Sorting Test-64;46 Delis-Kaplan Executive Function System (D-KEFS)47 Verbal Fluency Switching total number of responses, and D-KEFS Trail Making Test letter number switching total time.47 The Wechsler Adult Intelligence Scale-III (WAIS-III)48 and Wechsler Adult Intelligence Scale-IV (WAIS-IV)49 Digit Symbol subtests were used to derive a processing speed variable. Due to changes in the testing protocols, some participants received the WAIS-III version (n = 9) and others completed the WAIS-IV version. The basis for combining these measures lies in these two tests being almost identical in terms of task demands, a .85 Pearson correlation between versions, and no significant differences in mean age-corrected performance between the two measures.50 Z-scores were calculated for each domain using the grand mean and standard deviation of each test. The Test of Memory Malingering39 and the Forced-Choice Recognition Trial of the CVLT-II40 were used to assess effortful engagement in testing.
Psychological/Psychosocial Assessment
The PTSD Checklist—Military Version (PCL-M)51 was used to rate the frequency and intensity of PTSD-related symptoms. Levels of depressive symptoms were assessed using the Beck Depression Inventory II (BDI-II).52
TBI Interview
Each participant was asked detailed questions regarding the characteristics of their TBI event, including the number of TBIs they sustained, the number of blasts to which they were exposed, and whether or not they lost consciousness with each TBI event. Self-reported duration of loss of consciousness (LOC) for any TBI event was also queried. Only five participants self-reported a LOC duration or PTA consistent with a moderate TBI classification, while the vast majority of TBI participants (n = 33) were classified as mild.
Imaging Procedures
All participants underwent structural MRI and DTI on 3T General Electric (GE) MRI scanners housed within the UCSD Center for Functional Magnetic Resonance Imaging (CFMRI) on the UCSD La Jolla campus. Forty-three participants were scanned using the Excite HDx platform and, following the FMRI Center’s scanner upgrade, data on 10 subjects were acquired with the scanner running the MR750 platform.
Structural scanning
A sagittally-acquired high-resolution 3D T1-weighted anatomical MRI was collected with the following parameters: FOV 24 cm, 256 × 256 × 192 matrix, 0.94 × 0.94 × 1 mm voxels, 176 slices, TR=20 ms, TE=4.8 ms; flip angle 12°. Total scan time was roughly 7 minutes.
Diffusion Tensor Imaging
DTI images were collected with a dual spin echo EPI acquisition53 with the following parameters: FOV = 240 mm, slice thickness = 3 mm, matrix size 128 × 128, in-plane resolution = 1.875 × 1.875, TR = 10900 ms, TE = 93 ms. The ten scans from the MR750 platform used identical scanning parameters though TR was shortened to 8000 ms to reduce scan time without affecting image quality. This modification likely did not impact image signal-to-noise ratio or contrast since the TR at eight seconds remained many times greater (> five times) than the T1 value of the brain tissue (see Gelman et al54). Indeed, prior analysis of DTI signal equation modeling found that the SNR difference in white matter between TR 8000 ms and TR 10900 ms is only 0.007%.55 Across scanners, 34 slices were acquired with 61 diffusion directions distributed on the surface of a sphere according to the electrostatic repulsion model56 and a b-value of 1500 s/mm2, as well as one T2 image with no diffusion weighting (b = 0). Two field maps with the same spatial parameters as those of the DTI scan were collected in order to correct for distortions due to magnetic field inhomogeneities. Total DTI acquisition time with field mapping was roughly 12–16 minutes.
Image Processing
Diffusion Tensor Imaging Data
The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Package (FSL)57 was used for diffusion imaging processing. The two field maps were used to unwarp the DTI acquisitions. Additionally, a linear alignment tool to reduce the effects of gradient coil eddy currents and a six-degrees of freedom affine motion correction for head motion was completed. Each image was visually inspected for quality and none were rejected. The FSL program dtifit was used on the corrected data to calculate diffusion eigenvalues and FA on a voxel-by-voxel basis. Axial diffusivity (AD) was defined as the amount of diffusion corresponding to the principal diffusion direction. Radial diffusivity (RD) was defined as the average of the two eigenvalues orthogonal to the principal diffusion direction. AD and RD are often used as proxies for estimating damage to neuronal and myelin structures, respectively.25 Thus, increases in RD are associated with worse myelin integrity, while reductions in AD may indicate worse axonal integrity.25 Mean diffusivity (MD), an average of the three eigenvalues and an indicator of bulk diffusivity within a voxel, was not examined in this study in favor of the directional diffusivity measurements of AD and RD described above as these measures are more informative in terms of white matter properties which may modify measures of anisotropy.58
Fiber tracts were generated in TrackVis59 following the FACT method.60 To produce the fiber tracts, regions of interest (ROIs) were drawn and used as “seed points” for tractography. One rater (SFS), blind to each image’s group status, drew a seed ROI (S-ROI) within each subject’s color-map image for each tract. The color-map uses a color-coded scheme to display the main orientation of diffusion within each voxel. To help reduce partial voluming effects of bordering gray matter, tracking was restricted to include only those voxels with an FA value greater than .20.61 Additionally, to restrict aberrant tracking an angle threshold of 41.4 degrees was used.61 This restriction limited contiguous tracking to only those voxels wherein the difference in the angle of the principal eigenvectors is less than 41.4 degrees. FA, RD and AD values from each of the tracts produced were then extracted for each subject for statistical analysis. Depictions of the tracts are shown in Figure 1.
Figure 1. Diffusion Tensor Imaging Tracts.
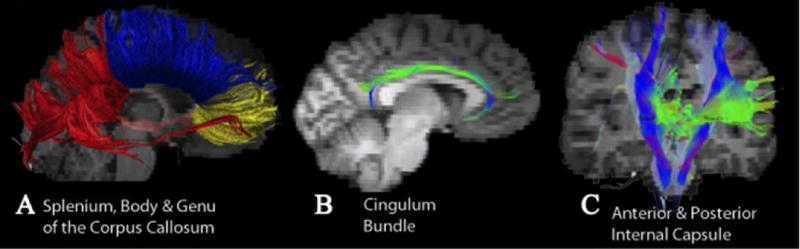
Depiction of the white matter tracts on a representative TBI participant. A) Splenium (red), Body (blue), Genu (yellow); B) Cingulum Bundle; C) anterior (green) and posterior (blue) internal capsule.
Corpus Callosum
The whole of the corpus callosum (CC) was tracked by placing S-ROIs along the length of the CC, in red-colored voxels, in the midsagittal slice.62 CC sub-divisions were identified using an adapted classification method based on cortical connectivity derived from DTI fiber tracking.63 The posterior border of the genu was defined by a perpendicular line coursing through the anterior most point of the inner convexity. CC voxels anterior to this line (including the rostrum and the anterior sixth of the length of the CC) represented the S-ROI for fiber tracking. The splenium was defined as the posterior fourth of the whole CC with the whole length of the CC defined as the distance from the anterior end to the posterior end. The body of the CC consisted of the middle portion bordered by the genu and the splenium as described above.
Internal Capsule
S-ROIs were placed following published methods.62 For the anterior internal capsule, the S-ROI was placed on the color-map image in the axial plane in green-colored voxels between the putamen and the caudate. For the posterior internal capsule, the S-ROI was placed in the axial plane in blue-colored voxels medial to the lenticular nucleus (putamen and pallidum) and lateral to the thalamus.
Cingulum
The cingulum bundle appears in the coronal plane as green voxels inferior to the cingulum gyrus and superior to the corpus callosum. To produce each cingulum tract, separate S-ROI were placed in the anterior portion, the middle, and the posterior portion following the description of Concha, Gross, & Beaulieu.64
Intra-rater reliabilities indicated strong reliability with intraclass correlation coefficients for FA ranged from .70 – .99. Eight regions had ICCs higher than .85, and 7 were above .90. The lowest ICC value (.70) was for the left anterior internal capsule.
Statistical Analysis
Separate hierarchical regression analyses were conducted for each cognitive domain and tract to test for an effect of PTSD or TBI. Age, years of education, and BDI total score were entered in the first step, PCL-M scores were entered in the second step, and the TBI grouping variable was entered in the third step of the model. Partial correlation, chi-square analysis and ANCOVAs were used as indicated below. All analyses were conducted using SPSS version 22 (SPSS Inc, Chicago, Illinois).
Results
Sample Characteristics
As shown in Table 1, TBI and MC participants did not significantly differ with respect to most demographic characteristics except for years of education (p = .05). However, the groups did not differ in WRAT-4 Reading, suggesting that the difference in education was not related to differences in premorbid intellectual functioning. The TBI sample reported significantly higher levels of psychiatric distress including higher ratings on depression and PTSD symptom severity. TBI injury characteristics are also shown in Table 1. Within the TBI group, most reported experiencing more than one TBI event, half reported sustaining a head injury that was combat related, half reported being exposed to blast waves, and most reported LOC associated with any one TBI.
Table 1.
Sample Characteristics of the Military Control (MC) and TBI Groups
| MC | TBI | ||
|---|---|---|---|
| n | 17 | 38 | |
| Age (years) | 33.7 (8.7) | 31.2 (9.1) | |
| WRAT-4 Reading (SS) | 105.8 (9.1) | 105.6 (12.2) | |
| Years of Education** | 14.7 (2.0) | 13.4 (1.4) | |
| % Male | 77% | 90% | |
| % Caucasian | 77% | 55% | |
| BDI-II *** | 5.1 (8.4) | 17.8 (12.5) | |
| PCL-M*** | 22.4 (10.9) | 43.0 (17.5) | |
| Re-experiencing*** | 5.7 (2.2) | 12.1 (5.6) | |
| Avoidance/Numbing*** | 9.2 (4.9) | 17.3 (8.1) | |
| Arousal*** | 7.8 (4.0) | 14.4 (5.1) | |
| Months Since TBI | - | 48.3 (31.8) | |
| Mean Number of TBIs | - | 2.7 (2.2) | |
| % > 1 TBI | - | 68% | |
| % Combat TBI | - | 50% | |
| % Reporting Any LOC at TBI | - | 63% | |
| % Reporting Blast Related TBI | - | 54% | |
| No. of Blasts Exposed | - | 6.9 (23.7) | |
| No. of Times Dazed from Blasts | - | 2.2 (2.9) | |
Note: values = mean (SD), count, or percentage (as indicated); SS = standard score; LOC = loss of consciousness; WRAT-4 = Wide Range Achievement Test, Fourth Edition; AUDIT = Alcohol Use Disorders Identification Test; NSI = Neurobehavioral Symptom Inventory; BDI-II = Beck Depression Inventory-2; PCL-M = Post-traumatic Stress Disorder Check List- Military Version.
p<.01,
p<.001
DTI Tractography Associations
Mean FA for each group across tracts is shown in Figure 2, and results for significant predictors of regional DTI values from the hierarchical regression analyses are shown in Table 2. After adjusting for important confounds (i.e., age, education, and depression), and PCL-M score, TBI was a significant predictor of lower FA values in both the genu of the corpus callosum (p = .03) and in the left cingulum bundle (p = .01).
Figure 2.
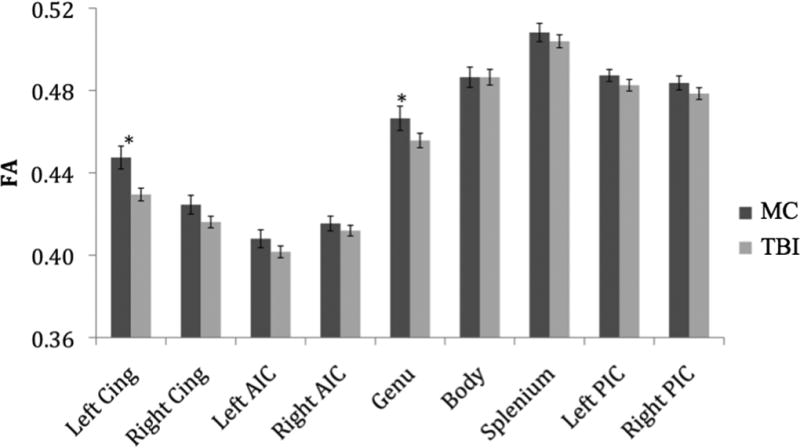
Regional FA Values by Group. Note: Cing = Cingulum, AIC = Anterior Internal Capsule, PIC = Posterior Internal Capsule. Error bars represent standard error. *Groups significantly differed on hierarchical regression analysis (p<.05).
Table 2.
Regional DTI indices associated with TBI
| Region | F (1,49) | R2Δ | |
|---|---|---|---|
| Fractional Anisotropy | Genu | 5.12* | .09 |
| Cingulum Left | 7.28** | .12 | |
| Cingulum Right | 3.29t | .06 | |
|
| |||
| Radial Diffusivity | Genu | 7.29** | .12 |
| Cingulum Left | 7.73** | .12 | |
| Cingulum Right | 4.64* | .07 | |
p<.01,
p<.05,
p<.10
Results of hierarchical regression predicting regional DTI values (level one: years of education, Beck Depression Inventory-II scores; level two: PTSD Check-List scores; level three: TBI vs. Control).
Mean RD for each group across tracts is shown in Figure 3. After adjusting for age, education, depression and PCL-M score, TBI history was a significant predictor of higher RD in the left cingulum bundle (p = .01), right cingulum bundle (p = .04), and the genu (p = .01). PCL-M total score was not a significant predictor of DTI values in any region in models with or without the TBI grouping variable included (p’s > .23).
Figure 3.
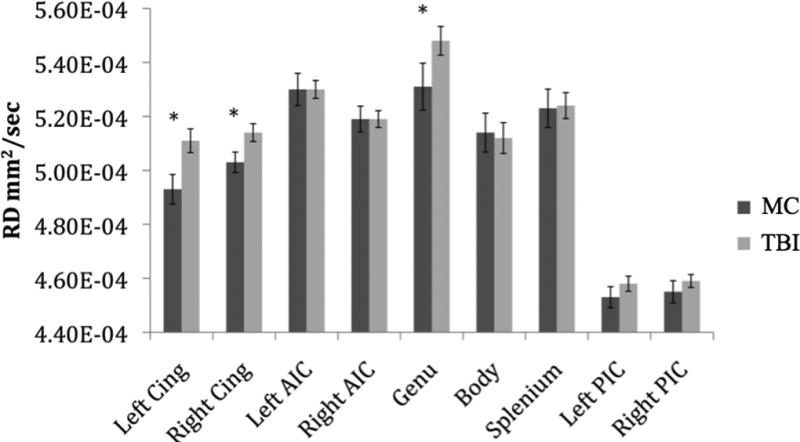
Regional Radial Diffusivity Values by Group. Note: Cing = Cingulum, AIC = Anterior Internal Capsule, PIC = Posterior Internal Capsule. Error bars represent standard error. *Groups significantly differed on hierarchical regression analysis (p < .05).
Associations Among TBI Injury Characteristics and Regional DTI Values
Adjusting for age, education, BDI and PCL-M scores, the number of TBIs reported was significantly negatively associated with FA in the left AIC (β̂ = −.0027, t = −2.13, p = .04), and right AIC (β̂ = −.0025, t = −2.08, p = .05). Number of blasts reported and presence of LOC were not significantly associated with regional FA, RD or AD values (all p-values > .05).
Correlations with PCL-M Scores within the TBI Group
Partial correlations, adjusting for age, demonstrated no significant correlations between FA and total PCL-M scores, or PCL-M symptom subtype scores (all p’s > .05). Contrary to expectations, RD significantly negatively correlated with PCL-M scores in the genu (r = −.35, p = .04). AD was significantly negatively correlated with PCL-M scores in the genu (r = −.33, p = .05). Neuropsychological domain scores were not associated with PCL-M scores (all p-values > .05). Number of TBIs reported did not significantly correlate with PCL-M scores (p > .86).
Neuropsychological Test Performances
Z-scores for the cognitive domains by group and means for individual tests by group are shown in Table 3. TBI history was significantly associated with poorer performances on both memory (p = .02) and coding (p = .03) measures, after adjusting for age, years of education, and BDI and PCL-M scores. In no individual models did the association between PCL-M scores and cognitive domain scores reach significance (all p’s > .05). There were also no significant PCL-M by TBI interactions (all p-values >.10), and no significant associations between number of TBIs or number of blasts with any of the neuropsychological domain scores (all p-values > .10).
Table 3.
Neuropsychological Domain and Individual Test Z-Scores by Group
| MC | TBI | F(1,49) | R2Δ | ||
|---|---|---|---|---|---|
| Memory | .31 (.59) | −.11 (.72) | 5.72* | .10 | |
| CVLT-II 1–5 Total | .45 (1.03) | −.21 (.93) | |||
| CVLT-II Long Delay Free Recall | .38 (.83) | −.17 (1.03) | |||
| CVLT-II Recognition Discriminability Index | .14 (1.14) | −.06 (.94) | |||
| Rey- Osterrieth Delay Recall | .09 (.53) | −.04 (1.16) | |||
|
| |||||
| Processing Speed | .47 (.57) | −.24 (1.07) | 5.32* | .09 | |
| WAIS-IV Coding | .54 (.53) | −.33 (1.08) | |||
| WAIS-III Coding | −.53 | .07 (1.05) | |||
|
| |||||
| Executive Functions | .24 (.44) | −.13 (.77) | 1.03 | .02 | |
| WCST Total Errors | .03 (.71) | −.01 (1.11) | |||
| D-KEFS Verbal Switching | .50 (.81) | −.24 (1.00) | |||
| D-KEFS Trails Switching | .21 (.65) | −.10 (1.12) | |||
Results of hierarchical regression predicting regional DTI values (level one: years of education, Beck Depression Inventory-II scores; level two: PTSD Check-List scores; level three: TBI vs. Control). Note: Values represent mean (SD); CVLT-II = California Verbal Learning Test, Second Edition; WAIS = Wechsler Adult Intelligence Scale; WCST = Wisconsin Card Sorting Test; D-KEFS = Delis-Kaplan Executive Functioning System.
p<.05
Correlations among Neuropsychological Domain Scores and Imaging Variables
Significant partial correlations between neuropsychological domain scores and regional DTI variables across groups are presented in Table 4. Both processing speed and executive functions significantly correlated with FA values from multiple tracts, including those that differed between TBI and MC groups. There were no significant associations between DTI scores and the memory composite (p’s > .05). As can be seen in Table 4, RD associations with processing speed scores were non-specific with strong associations across 8 of the 9 tracts.
Table 4.
Significant Partial Correlations between Neuropsychological Domain Scores and DTI
| Executive Functions | Processing Speed | ||
|---|---|---|---|
| FA | AIC Right | .27* | ns |
| Cingulum Left | .38** | .38** | |
| Cingulum Right | .27* | .33** | |
| Genu | ns | .44** | |
| Body | .37** | .43** | |
| Splenium | .36** | .31* | |
| PIC Left | .51*** | .50*** | |
| PIC Right | .49*** | .27* | |
|
| |||
| RD | AIC Left | ns | −.27* |
| Cingulum Left | ns | −.40** | |
| Cingulum Right | ns | −.37** | |
| Genu | ns | −.45** | |
| Body | −.30* | −.48*** | |
| Splenium | ns | −.36** | |
| PIC Left | −.30* | −.48*** | |
| PIC Right | −.27* | −.28* | |
|
| |||
| AD | Body | ns | −.29* |
| PIC Right | .32* | ns | |
Results of partial correlation adjusting for age
p<.05,
p<.01,
p<.001,
ns = not significant
Discussion
This study investigated white matter microstructure in Veterans who reported a history of mild to moderate TBI and examined whether disrupted microstructure was associated with PTSD symptom severity and cognition. DTI findings indicated that TBI history as associated with white matter damage with disrupted microstructure in the cingulum bundles and the genu of the corpus callosum. Additionally, successive mild TBIs were associated with reduced FA in bilateral anterior internal capsule regions suggesting a dose effect of TBI. These results are consistent with the frontal susceptibility hypothesis of TBI,16,17 and they add to the growing number of DTI studies linking milder grades of TBI with disrupted white matter microstructure in both civilian27,65–67 and military samples.35–37 Importantly, observed reductions in DTI metrics shown in the TBI group were independent of demographic differences or psychiatric symptoms, including PTSD and depression.
Although our results demonstrated that the TBI group showed far greater PTSD symptom severity than MCs, evidence in support of an association between PTSD symptom severity and disrupted white matter pathways was lacking. Current PTSD symptom ratings did not correlate with FA in any regression model, with or without inclusion of the TBI grouping variable. Additionally, correlations between higher PTSD ratings and worse DTI values within the TBI group were not observed. Our results contrast with those of Bazarian and colleagues68 and Schuff and colleagues15 who reported associations between PTSD and DTI values in Veterans (i.e., increased diffusivity or decreased FA corresponding to increased PTSD symptoms, respectively). Differences between the DTI analytic methods of this study and those of Bazarian and colleagues68 may, in part, account for this discrepancy. For example, Bazarian and colleagues found the highest percentile (i.e., 1st percentile) of mean diffusivity sampled across the whole of the white matter significantly correlated with PTSD severity. Such an approach may increase sensitivity to focal but spatially variable white matter variations, but is limited as it does not identify where in the white matter greater diffusivity significantly corresponded with PTSD severity. The present study identified a priori identified tracts known to be affected in mTBI or with tentative links to PTSD symptoms. Schuff and colleagues15 reported associations between the diagnosis of PTSD and FA in the anterior cingulate and white matter within the prefrontal cortex. However, their study did not stringently control for possible comorbid mild TBI or blast exposure, which could also account for reduced FA in those regions, as shown in the present study. Overall, our findings align with other recent studies that have found white matter degradations associated with TBI in OEF/OIF Veterans in multiple white matter regions, including those identified in the present study (i.e., the genu of the corpus callosum and cinglum white matter), but failed to find any association between regional white matter integrity and current PTSD symptoms post-TBI.36,37
Reductions in FA in the context of TBI are non-specific and may be related to demyelination, axonal degeneration, or both.69 Our findings of increased RD suggest that microstructural alterations in the TBI group are related to reductions in myelin integrity25 and are consistent with similar reports in this regard. For example, in a sample of Veterans with blast-related mild TBI, MacDonald and colleagues35 found elevated RD in the cingulum bundle within 90 days of blast injury compared to controls. Study authors described a relative normalization of RD upon a follow-up scan 6–12 months later, though a trend toward increased RD relative to controls persisted (p = .07). Our study results, however, portray a more permanent alteration in RD given our longer 4-year interval between the TBI event(s) and scanning. In another study, using macromolecular proton fraction mapping (MPF), an MRI technique sensitive to myelin, Petrie and colleagues37 reported a diffuse pattern of abnormal myelin content associated with blast-related mild TBI. Taken together, these studies suggest dynamic changes in myelin properties following mTBI with incomplete return to baseline.
Persisting reductions in myelin integrity may explain some of the observed cognitive deficits shown in the TBI group. White matter disruption may fragment neural networks responsible for cognitive processing and therefore reduce functional connections among cortical and subcortical gray matter.20 Indeed, participants with history of head trauma performed poorly on a processing speed task that requires synchronized and speeded output of many cortical and subcortical regions. The robust correlations observed between increased RD and slower processing speed suggests that myelin compromise and concomitant slowed propagation of neuronal signaling may then contribute to reduced processing speed. However, other cognitive findings were mixed. For example, executive functions did not significantly differ between TBI and MC groups, but did correlate with DTI indices across many ROIs. Additionally, reduced memory performance in the TBI group compared to MCs appears to be unrelated to white matter integrity in our sample. Such findings contrast with Levin and colleagues38 who found that worse DTI values correlated with poorer performance on a slightly more complicated word memory task than the one used in our study. It is possible that the differing task demands between the two measures may account for this discrepancy.
The findings of poorer processing speed and memory performances in the TBI group relative to MCs is somewhat inconsistent with the typical model of recovery following milder forms of TBI which is characterized by a return to the normal levels within a few months post-injury,70–73 although it has been suggested that a small percentage (10–15%) of mTBI individuals may experience mild but permanent cognitive deficits.74,75 Importantly, all analyses as part of this study were conducted again with the exclusion of the five participants classified as having a “moderate” TBI and the results were unchanged. Thus, any contribution of greater severity, at this level of injury, was not sufficient to account for the observed differences in cognitive functioning between MC and TBI groups in this study. Other factors, such as comorbid psychiatric distress, have been tied to complicated recovery following mTBI.76,77 Along these lines, some studies have found that any association between mTBI and post-concussive symptom complaints and/or cognitive performance in Veteran samples is lost after accounting for psychiatric symptoms (e.g., PTSD).1,78 In the present study, the observed neuropsychological effects of TBI were independent of comorbid psychiatric distress and suggest that other factors (e.g., the degree of white matter damage secondary to head injury) may be contributing to worse cognitive performance in this population.
One of the strengths of this study is that we investigated a well-characterized group of military personnel using a comprehensive cognitive battery and an imaging protocol designed to be sensitive to the effects of mTBI. Additionally, we carefully excluded participants with suboptimal effort since inclusion of individuals with sub-threshold scores on effort testing may exaggerate group differences on neuropsychological tests and could attenuate possible group differences on biological markers. The cognitive findings in particular have potential to inform clinical care as they suggest some Veteran’s may indeed experience reductions in cognitive functions that persist well beyond the typical time frame associated with spontaneous recovery of about one year. Follow-up longitudinal studies would be necessary to determine the resiliency of these findings over time. Importantly, the findings suggest that treatment of psychiatric symptoms alone, while vital to a Veteran’s health, may not be sufficient to address lingering TBI-associated cognitive reductions. Thus, additional interventions that may assist Veterans to compensate for relative reductions in cognitive abilities (e.g., compensatory memory strategies)79 may be warranted to more fully address the complex symptom profiles in this cohort of Veterans.
However, there are some limitations that warrant discussion. For example, 10 participants were scanned after the GE scanner upgrade; however, SNR was likely unaffected by the upgrade (as described above) and a follow-up comparison of regional FA values between those 10 participants scanned post-upgrade and 10 age-matched participants scanned pre-upgrade found no significant differences. This analysis did find differences in AD and RD within the Genu between scanner platforms, however, the findings for this region remained after including scanner as a predictor in the regression models. Additionally, the generalizability of our findings to single-event TBIs is limited as most of our participants endorsed multiple TBI events. Moreover, since our sample was on average four years removed from their most recent mTBI event, our finding may not extend to persons with more recent mTBI. Our assessment of PTSD symptoms also carries limitations. While the reliability of the PCL-M has been demonstrated,80 symptom endorsement may have differed from those of a clinician-guided assessment of PTSD symptoms. Both MC and TBI groups endorsed deployments during OEF/OIF operations, however the severity of their combat exposure was available for group comparisons. This could account for the fairly large discrepancy in the PTSD severity observed. This limitation was not thought to have greatly affected the findings of this study there was no correlation between PTSD symptoms and DTI values within the TBI group or when the regression analyses included both groups. Similarly, in a follow-up analysis we found that the PTSD and depression ratings of Veterans who sustained TBIs in combat (~50% of the sample) did not significantly differ from those who reported TBIs sustained in non-combat environments (p’s .47 – .92). Finally, the relationship between RD and myelin integrity or, membrane permeability, is indirect and susceptible to further uncertainty within highly complex white matter bundles within a voxel.24 How white matter disruption may impact the presentation of psychiatric symptoms, including PTSD, remains an area of ongoing study and DTI is but one of many available measures of white matter properties. To aid in clarifying the relationship between DTI measures and white matter properties, future studies could incorporate imaging methods more directly related to myelin content.81,82
Conclusion
This study investigated whether TBI-related white matter alterations, as measured via DTI, contribute to heightened PTSD symptoms in Veterans of the recent conflicts in Iraq and Afghanistan. Our findings suggest that while TBI-related alterations of frontal white matter pathways are associated with reductions in cognitive processes (e.g., processing speed), such alterations do not appear to contribute to current PTSD symptom severity. Additionally, cumulative mTBIs were associated with reduced white matter integrity in bilateral anterior internal capsule regions suggesting a dose effect of TBI. These results further support the burgeoning literature linking milder forms of head trauma to disrupted white matter microstructure. Importantly, observed reductions in DTI metrics shown in the TBI group were independent of demographic differences or psychiatric symptoms, including PTSD and depression. Taken together, these findings suggest that persisting cognitive symptoms following a history of TBI are related to white matter pathology that may be independent of comorbid psychiatric illness.
Acknowledgments
The authors sincerely thank the OEF/OIF (Operation Enduring Freedom/Operation Iraqi Freedom) veterans who volunteered to participate in this study. In addition, they are grateful to the Veterans Affairs Center of Excellence in Stress and Mental Health (CESAMH) at the Veterans Affairs San Diego Healthcare System for their organizational assistance.
Source of Funding: This work was supported by grants awarded by the Veterans Affairs (Career Development Awards [CDA]: L.D.-W., D.S.; Merit Award, L.D.-W.) as well as the Department of Defense (Investigator-Initiated Research Grant [IIRG]: L.D.-W.). This material is further supported with resources of the Veterans Affairs Center of Excellence for Stress and Mental Health (CESAMH: L.D.-W.).
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
References
- 1.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from iraq. N Engl J Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 2.Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in iraq and afghanistan: Persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. 2008;167(12):1446–1452. doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- 3.Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. 2014;71(2):149–157. doi: 10.1001/jamapsychiatry.2013.3080. [DOI] [PubMed] [Google Scholar]
- 4.Ragsdale KA, Neer SM, Beidel DC, Frueh BC, Stout JW. Posttraumatic stress disorder in OEF/OIF veterans with and without traumatic brain injury. J Anxiety Disord. 2013;27(4):420–426. doi: 10.1016/j.janxdis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Koponen S, Taiminen T, Portin R, et al. Axis I and II psychiatric disorders after traumatic brain injury: A 30-year follow-up study. Am J Psychiatry. 2002;159(8):1315–1321. doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- 6.Vasterling JJ, Verfaellie M, Sullivan KD. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: Perspectives from cognitive neuroscience. Clin Psychol Rev. 2009;29(8):674–684. doi: 10.1016/j.cpr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: A critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 8.Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 9.Lindauer RJ, Booij J, Habraken JB, et al. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: A randomized clinical trial. Psychol Med. 2008;38(4):543–554. doi: 10.1017/S0033291707001432. [DOI] [PubMed] [Google Scholar]
- 10.Bremner JD. Brain imaging in anxiety disorders. Expert Rev Neurother. 2004;4(2):275–284. doi: 10.1586/14737175.4.2.275. [DOI] [PubMed] [Google Scholar]
- 11.Protopopescu X, Pan H, Tuescher O, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57(5):464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 13.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Kim MJ, Lyoo IK, Kim SJ, et al. Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport. 2005;16(10):1049–1053. doi: 10.1097/00001756-200507130-00004. [DOI] [PubMed] [Google Scholar]
- 15.Schuff N, Zhang Y, Zhan W, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. Neuroimage. 2011;54(Suppl 1):S62–8. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Avants B, Patel S, et al. Structural consequences of diffuse traumatic brain injury: A large deformation tensor-based morphometry study. Neuroimage. 2008;39(3):1014–1026. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine B, Kovacevic N, Nica EI, et al. The toronto traumatic brain injury study: Injury severity and quantified MRI. Neurology. 2008;70(10):771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- 18.Bigler ED. Quantitative magnetic resonance imaging in traumatic brain injury. J Head Trauma Rehabil. 2001;16(2):117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: A diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29(3):514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging Behav. 2012;6(2):108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- 21.Buki A, Povlishock JT. All roads lead to disconnection?--traumatic axonal injury revisited. Acta Neurochir (Wien) 2006;148(2):181–93. doi: 10.1007/s00701-005-0674-4. discussion 193-4. [DOI] [PubMed] [Google Scholar]
- 22.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 23.Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26(7):874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler-Kingshott CA, Cercignani M. About "axial" and "radial" diffusivities. Magn Reson Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 25.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 27.Bendlin BB, Ries ML, Lazar M, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage. 2008;42(2):503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25(3):370–376. [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–19. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 30.Lipton ML, Gellella E, Lo C, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008;25(11):1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- 31.Levin HS, Wilde EA, Chu Z, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidaros A, Engberg AW, Sidaros K, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain. 2008;131(Pt 2):559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 33.Wilde EA, Chu Z, Bigler ED, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- 34.Yuan W, Holland SK, Schmithorst VJ, et al. Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR Am J Neuroradiol. 2007;28(10):1919–1925. doi: 10.3174/ajnr.A0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mac Donald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morey RA, Haswell CC, Selgrade ES, et al. Effects of chronic mild traumatic brain injury on white matter integrity in iraq and afghanistan war veterans. Hum Brain Mapp. 2013;34(11):2986–2999. doi: 10.1002/hbm.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrie EC, Cross DJ, Yarnykh VL, et al. Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in iraq and afghanistan war veterans. J Neurotrauma. 2014;31(5):425–436. doi: 10.1089/neu.2013.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin HS, Wilde E, Troyanskaya M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma. 2010;27(4):683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- 39.Tombaugh TN. Test of memory malingering (TOMM) Toronto, Ontario, Canada: Multi-Health Systems, Inc.; 1996. [Google Scholar]
- 40.Moore BA, Donders J. Predictors of invalid neuropsychological test performance after traumatic brain injury. Brain Inj. 2004;18(10):975–984. doi: 10.1080/02699050410001672350. [DOI] [PubMed] [Google Scholar]
- 41.Traumatic Brain Injury Task Force. Report to the surgeon general: Traumatic brain injury task force. 2008 retrieved from http://Www.armymedicine.army.mil/reports/tbi/TBITaskForceReportJanuary2008.pdf.
- 42.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson GS, Robertson GJ. Wide range achievement test--fourth edition. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 44.Delis D, Kramer JH, Kaplan E, Ober B. The california verbal learning test: Second edition. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 45.Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms and commentary. second. New York: Oxford University Press; 1998. [Google Scholar]
- 46.Heaton RK. Wisconsin card sorting test manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- 47.Delis D, Kaplan E, Kramer JH. The delis-kaplan executive function system. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 48.Wechsler D. Wechsler adult intelligence scale-third edition (WAIS-III) San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 49.Wechsler D. Wechsler adult intelligence Scale–Fourth edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 50.Wechsler D. Wechsler adult intelligence Scale–Fourth edition: Technical and interpretive manual. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 51.Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD checklist (PCL): Reliability, validity, and diagnostic utility; paper presented at the annual meeting of the inter-national society for traumatic stress studies; san antonio, TX. 1993. [Google Scholar]
- 52.Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 53.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49(1):177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 54.Gelman N, Ewing JR, Gorell JM, Spickler EM, Solomon EG. Interregional variation of longitudinal relaxation rates in human brain at 3.0 T: Relation to estimated iron and water contents. Magn Reson Med. 2001;45(1):71–79. doi: 10.1002/1522-2594(200101)45:1<71::aid-mrm1011>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 55.Sorg SF, Delano-Wood L, Luc N, et al. White matter integrity in veterans with mild traumatic brain injury: Associations with executive function and loss of consciousness. J Head Trauma Rehabil. 2014;29(1):21–32. doi: 10.1097/HTR.0b013e31828a1aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42(3):515–525. [PubMed] [Google Scholar]
- 57.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 58.Beaulieu C. The biological basis of diffusion anisotropy. In: Johansen-Berg H, Behrens TE, editors. Diffusion MRI: From quantitative measurement to in vivo neuroanatomy. First. London: Elsevier; 2009. p. 105. [Google Scholar]
- 59.Wang R, Benner T, Soensen AG, Wedeen VJ. Diffusion toolkit: A software package for diffusion imaging data processing and tractography (abstract presented at ISMRM) Proc. Intl. Soc. Mag. Reson. Med. 2007;15 [Google Scholar]
- 60.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Mori S, van Zijl PC. Fiber tracking: Principles and strategies - a technical review. NMR Biomed. 2002;15(7–8):468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 62.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 63.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 64.Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26(9):2267–2274. [PMC free article] [PubMed] [Google Scholar]
- 65.Huang MX, Theilmann RJ, Robb A, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma. 2009;26(8):1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- 66.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J Neurosurg. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 67.Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29(5):967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bazarian JJ, Donnelly K, Peterson DR, Warner GC, Zhu T, Zhong J. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during operations enduring freedom and iraqi freedom: A diffusion tensor imaging study. J Head Trauma Rehabil. 2012 doi: 10.1097/HTR.0b013e318256d3d3. [DOI] [PubMed] [Google Scholar]
- 69.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using fourier analysis of stained tissue sections. Brain. 2011;134(Pt 8):2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Binder LM, Rohling ML, Larrabee GJ. A review of mild head trauma. part I: Meta-analytic review of neuropsychological studies. J Clin Exp Neuropsychol. 1997;19(3):421–431. doi: 10.1080/01688639708403870. [DOI] [PubMed] [Google Scholar]
- 71.Frencham KA, Fox AM, Maybery MT. Neuropsychological studies of mild traumatic brain injury: A meta-analytic review of research since 1995. J Clin Exp Neuropsychol. 2005;27(3):334–351. doi: 10.1080/13803390490520328. [DOI] [PubMed] [Google Scholar]
- 72.Rohling ML, Binder LM, Demakis GJ, Larrabee GJ, Ploetz DM, Langhinrichsen-Rohling J. A meta-analysis of neuropsychological outcome after mild traumatic brain injury: Re-analyses and reconsiderations of binder et al. (1997), frencham et al. (2005), and pertab et al. (2009) Clin Neuropsychol. 2011;25(4):608–623. doi: 10.1080/13854046.2011.565076. [DOI] [PubMed] [Google Scholar]
- 73.Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry. 2003;15(4):341–349. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- 74.Ruff R. Two decades of advances in understanding of mild traumatic brain injury. J Head Trauma Rehabil. 2005;20(1):5–18. doi: 10.1097/00001199-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Ruff RM, Camenzuli L, Mueller J. Miserable minority: Emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 1996;10(8):551–565. doi: 10.1080/026990596124124. [DOI] [PubMed] [Google Scholar]
- 76.Dikmen S, Machamer J, Temkin N. Psychosocial outcome in patients with moderate to severe head injury: 2-year follow-up. Brain Inj. 1993;7(2):113–124. doi: 10.3109/02699059309008165. [DOI] [PubMed] [Google Scholar]
- 77.Ettenhofer ML, Abeles N. The significance of mild traumatic brain injury to cognition and self-reported symptoms in long-term recovery from injury. J Clin Exp Neuropsychol. 2009;31(3):363–372. doi: 10.1080/13803390802175270. [DOI] [PubMed] [Google Scholar]
- 78.Vasterling JJ, Brailey K, Proctor SP, Kane R, Heeren T, Franz M. Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in iraq-deployed US army soldiers. Br J Psychiatry. 2012;201(3):186–192. doi: 10.1192/bjp.bp.111.096461. [DOI] [PubMed] [Google Scholar]
- 79.Twamley EW, Jak AJ, Delis DC, Bondi MW, Lohr JB. Cognitive symptom management and rehabilitation therapy (CogSMART) for veterans with traumatic brain injury: Pilot randomized controlled trial. J Rehabil Res Dev. 2014;51(1):59–70. doi: 10.1682/JRRD.2013.01.0020. [DOI] [PubMed] [Google Scholar]
- 80.Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596–606. doi: 10.1002/da.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. 2008;60(6):1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- 82.Yarnykh VL, Bowen JD, Samsonov A, et al. Fast whole-brain three-dimensional macromolecular proton fraction mapping in multiple sclerosis. Radiology. 2014:140528. doi: 10.1148/radiol.14140528. [DOI] [PMC free article] [PubMed] [Google Scholar]


