Abstract
Aim
Yokukansan (a Japanese Kampo medicine) has been reported to be safe and useful in treating behavioral and psychological symptoms in dementia patients. This study aimed to investigate the effects of yokukansan on destructive and aggressive behaviors in patients after traumatic brain injury.
Methods
From April 2008 to July 2010, 189 patients who suffered traumatic brain injury were admitted to our tertiary emergency center. Of these, patients with destructive and aggressive behaviors were treated with neuroleptics. Seven patients (five men and two women) who could not be controlled by neuroleptics were given yokukansan (2.5 g powder) three times a day before meals. Main underlying conditions included brain contusion in three patients, acute subdural hematoma in two, and acute epidural hematoma in two. The following assessments were carried out at baseline and 1 and 2 weeks after initiation of treatment: the Glasgow Coma Scale for the assessment of disturbed consciousness after traumatic brain injury; Neuropsychiatric Inventory for the distress of medical staff; Mini‐Mental State Examination for cognitive function; Barthel Index for activities of daily living; Vitality Index for motivation; presence of adverse effects and drug interactions.
Results
After treatment with yokukansan, patients showed significant improvements in Glasgow Coma Scale (P = 0.001), Neuropsychiatric Inventory (P = 0.016), Mini‐Mental State Examination (P = 0.029), Barthel Index (P = 0.043), and Vitality Index (P = 0.013). No adverse effects or drug interactions between yokukansan and Western medicines were observed.
Conclusion
Yokukansan improved the Glasgow Coma Scale, Neuropsychiatric Inventory, Mini‐Mental State Examination, Barthel Index, and Vitality Index without any adverse effects or drug interactions with Western medicines in patients with destructive and aggressive behaviors after traumatic brain injury.
Keywords: Cognitive function, destructive and aggressive behaviors, drug interactions, traumatic brain injury, yokukansan
Introduction
Management of destructive and aggressive behaviors (DAB) in patients after traumatic brain injury (TBI) is challenging and prolonged. In particular, patients with focal ventromedial frontal lobe lesions have a significantly higher frequency of aggressive and violent behaviors compared to controls and patients with lesions located in other parts of the brain.1 The frequency of aggressive and violent behaviors in patients with frontal lobe injury has been reported to range from 3% to 14%.1, 2, 3 Although pharmacological management with beta‐blockers, neuroleptics, antiepileptic agents, antidepressants, neurostimulants, and lithium has been suggested, evidence is lacking to fully support their use.4 Schneider et al. reported that use of atypical antipsychotic drugs as neuroleptics may be associated with a small increase in the risk of death.5
Yokukansan (TJ‐54, Tsumura Co., Tokyo, Japan; yi‐gan san in Chinese) was developed in 1555 by Xue Kai as a remedy for restlessness and agitation in children.6 Several studies have examined the effects of yokukansan in alleviating behavioral and psychological symptoms of dementia (BPSD).6, 7 Destructive and aggressive behavior, a BPSD‐like symptom, is observed in patients after TBI. The aim of this study was to investigate the effects of yokukansan on DAB in patients after TBI.
Patients and Methods
Patient group
A total of 189 patients with TBI were admitted to our tertiary emergency center between April 2008 and July 2010. All patients who suffered DAB following TBI received neuroleptics. Of these, seven patients (five men and two women; mean age, 36 years; range, 20–78 years) who could not be controlled by neuroleptics were evaluated in this study. Main underlying conditions included brain contusion in three patients, acute subdural hematoma in two, and acute epidural hematoma in two.
Evaluation
At baseline, each patient received a uniform evaluation using the Glasgow Coma Scale (GCS) to assess distributed consciousness after TBI, the Neuropsychiatric Inventory (NPI; lower scores indicate better performance)8 for the distress of medical staff, the Mini‐Mental State Examination (MMSE)9 for cognitive function, the Barthel Index (BI; higher scores indicate better performance)10 for activities of daily living, and the Vitality Index (VI; higher scores indicate better performance)11 for motivation. Assessments were also carried out 1 and 2 weeks after initiation of treatment, except in patients 6 and 7. The GCS score was obtained only at baseline in all patients. In addition, 123I‐IMP cerebral scintigraphy was carried out in patient 2, who was the only one to respond to neuroleptic treatment, albeit slightly. Cerebral blood flow was compared before and after 2 weeks of treatment.
Intervention
Yokukansan is a Kampo medicine containing Uncaria hook, Bupleuri radix, Glycyrrhizae radix, Angelicae radix, Hoelen, Atractylodis rhizome, and Cnidii rhizoma, and its use as a remedy for neurosis, insomnia, and irritability in children has been approved by the Ministry of Health, Labour and Welfare of Japan. In this study, patients received 2.5 g yokukansan powder three times a day before meals through a nasal gastric tube. Verbal informed consent was obtained from the families of patients following a detailed explanation about drug interactions and adverse effects.
Outcome measure and statistical analysis
One‐way analysis of variance was used in all analyses. P < 0.05 was considered statistically significant. All statistical analyses were carried out using spss for Windows (SPSS Japan, Tokyo, Japan).
Ethics
This study was designed and performed according to good clinical practice included in the Declaration of Helsinki.
Results
Patient characteristics
The clinical findings for the seven patients are summarized in Table 1. Three patients were involved in motor vehicle collisions, and three patients suffered injuries from falls. All patients suffered severe trauma including TBI, and were also injured in areas other than the brain. The mean Injury Severity Score12 was 35 ± 11. All patients were treated with i.v. haloperidol. In addition, patients 4 and 5 received continuous i.v. infusion of propofol after extubation. Days from admission to treatment with yokukansan varied among patients. Patient 1 suffered meningitis after brain surgery, followed by persistent disturbance of consciousness for approximately 8 months.
Table 1.
Characteristics of patients admitted to a tertiary emergency center with traumatic brain injury (n = 7)
| Patient | Age, years/sex | Cause of injury | GCS on arrival | Brain injury | Other injury | ISS | Time from admission to treatment with yokukansan, days |
|---|---|---|---|---|---|---|---|
| 1 | 20/M | MVC | 7 | SDH, BC, t‐SAH | Upper and lower extremities | 35 | 242 |
| 2 | 23/F | Fall (12 m height) | 9 | BC | Thorax, abdomen | 50 | 20 |
| 3 | 22/M | MVC | 13 | SDH, BC | Thorax, abdomen | 41 | 4 |
| 4 | 32/M | MVC | 6 | EDH, t‐SAH | Face | 17 | 18 |
| 5 | 30/F | Fall (9 m height) | 4 | EDH, BC | Thorax, abdomen, lumbar spine | 40 | 20 |
| 6 | 54/M | Fight | 6 | BC | Face, abdomen | 30 | 19 |
| 7 | 78/M | Fall (3 m height) | 8 | BC, t‐SAH | Face | 29 | 6 |
BC, brain contusion; EDH, epidural hematoma; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MVC, motor vehicle collision; SDH, subdural hematoma; t‐SAH, traumatic subarachnoid hemorrhage.
Efficacy of yokukansan
The efficacy of yokukansan and changes in several markers that are indicative of consciousness status are shown in Tables 2 and 3. Assessments for the NPI, MMSE, BI, and VI were not carried out in patients 6 and 7 because we had not evaluated them using these scales. After yokukansan had been given, patients showed significant improvements in GCS, NPI, MMSE, BI, and VI. The subscales of NPI, such as agitation/aggression and disinhibition, were also significantly improved. Cerebral scintigraphy was carried out in patient 2 before and 2 weeks after treatment, revealing a significant improvement after 2 weeks of treatment (Fig. 1, white arrows). No adverse effects or drug interactions between yokukansan and Western medicines were observed in this study.
Table 2.
Efficacy of yokukansan in patients with traumatic brain injury (n = 7)
| Mean ± SD | P‐value | F‐value | ||
|---|---|---|---|---|
| GCS | 0 | 11.1 ± 2.5 | 0.001 | 36.412 |
| 1 | 14 ± 1.8 | |||
| 2 | 14.6 ± 1.1 | |||
| NPI | 0 | 40.8 ± 17.2 | 0.016 | 9.772 |
| 1 | 17.4 ± 20.3 | |||
| 2 | 6.0 ± 5.8 | |||
| MMSE | 0 | 1.4 ± 3.1 | 0.029 | 10.022 |
| 1 | 16.2 ± 11.4 | |||
| 2 | 17.6 ± 11.0 | |||
| BI | 0 | 9.0 ± 20.1 | 0.043 | 6.833 |
| 1 | 47 ± 41.1 | |||
| 2 | 56 ± 38.5 | |||
| VI | 0 | 2.0 ± 2.3 | 0.013 | 14.247 |
| 1 | 5.8 ± 3.1 | |||
| 2 | 8.0 ± 2.9 |
0, At first treatment; 1, 1 week after commencement of treatment; 2, 2 weeks after commencement of treatment; BI, Barthel Index; GCS, Glasgow Coma Scale; MMSE, Mini‐Mental State Examination; NPI, Neuropsychiatric Inventory; SD, standard deviation; VI, Vitality Index.
Table 3.
Changes in Neuropsychiatric Inventory subscales in patients with traumatic brain injury treated with yokukansan (n = 7)
| Subscale | Mean ± SD | P‐value | F value | |
|---|---|---|---|---|
| Delusions | 0 | 0 ± 0 | 0.374 | 1.000 |
| 1 | 0.6 ± 1.3 | |||
| 2 | 0 ± 0 | |||
| Hallucinations | 0 | 0.6 ± 1.3 | 0.478 | 0.651 |
| 1 | 0.2 ± 0.4 | |||
| 2 | 0 ± 0 | |||
| Agitation/aggression | 0 | 6.2 ± 4.7 | 0.042 | 8.658 |
| 1 | 1.6 ± 3.6 | |||
| 2 | 0 ± 0 | |||
| Dysphoria | 0 | 4.2 ± 4.5 | 0.074 | 4.319 |
| 1 | 2.6 ± 2.6 | |||
| 2 | 0.6 ± 1.3 | |||
| Anxiety | 0 | 6.2 ± 4.7 | 0.063 | 4.223 |
| 1 | 3.2 ± 5.2 | |||
| 2 | 1.0 ± 1.5 | |||
| Euphoria | 0 | 0.6 ± 1.3 | 0.580 | 0.464 |
| 1 | 0.8 ± 1.8 | |||
| 2 | 0 ± 0 | |||
| Apathy | 0 | 3.2 ± 4.4 | 0.180 | 2.591 |
| 1 | 0.4 ± 0.9 | |||
| 2 | 0 ± 0 | |||
| Disinhibition | 0 | 8 ± 4.9 | 0.039 | 6.799 |
| 1 | 2.6 ± 3.4 | |||
| 2 | 2.2 ± 2.0 | |||
| Irritability/lability | 0 | 4.8 ± 4.4 | 0.080 | 5.444 |
| 1 | 2.4 ± 3.6 | |||
| 2 | 0.6 ± 1.3 | |||
| Aberrant motor behavior | 0 | 7.0 ± 5.4 | 0.061 | 6.688 |
| 1 | 3.0 ± 3.3 | |||
| 2 | 1.6 ± 2.2 |
0, At first treatment; 1, 1 week after commencement of treatment; 2, 2 weeks after commencement of treatment.
Figure 1.
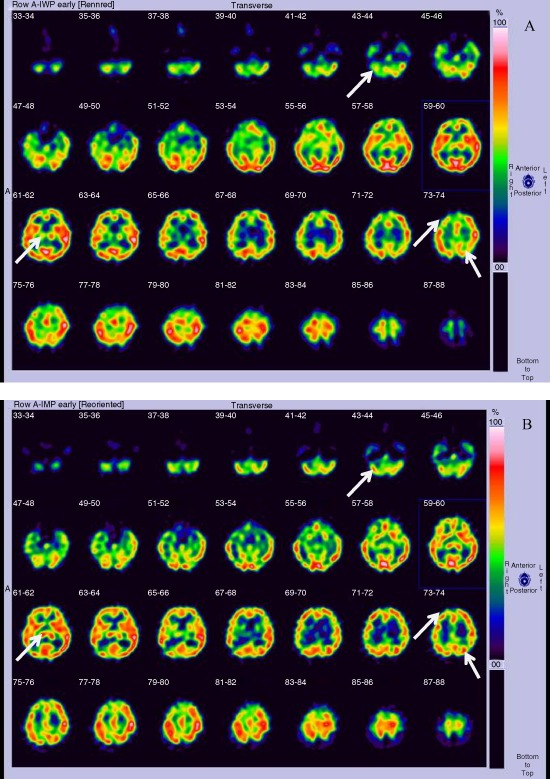
(A) Brain single photon emission computed tomography image of a patient who suffered severe brain trauma, taken before treatment with yokukansan. A decrease in cerebral blood flow is noted in the right cerebellum, right upper‐front lobe, left parietal lobe, and right thalamus (white arrows), where brain contusions were present. (B) Brain single photon emission computed tomography image in the same patient after 2 weeks of treatment with yokukansan, revealing improved cerebral blood flow (white arrows).
Discussion
Efficacy of yokukansan
Yokukansan improved GCS, NPI, MMSE, BI, and VI with no adverse effects or drug interactions in patients with DAB after TBI. Iwasaki et al. and Mizukami et al. reported that yokukansan improved BPSD in a randomized observer‐blind controlled trial and a randomized cross‐over study, respectively.6, 7 Given that DAB after TBI is similar to BPSD, we used yokukansan to treat DAB in TBI patients. Previous reports showed that yokukansan significantly improved NPI, but showed no effects on cognitive function.6, 7, 13 In this study, significant improvements were observed not only in NPI, but also in GCS, MMSE, BI, and VI after treatment with yokukansan. Ikarashi et al. reported that Yokukansan improved memory disturbance, anxiety‐like behaviors, increased aggressive behaviors, and decreased social behaviors in thiamine‐deficient rats, and that degeneration of neuronal and astroglial cells was inhibited in the brain stem, hippocampus, and cortex.14 Furthermore, yokukansan enhanced neurogenesis in young and aged rat brains.15 These reports suggest that yokukansan could have cognitive function improving effects. Several clinical reports have shown no effects on cognitive function, however, the mean age in these studies was higher than 70 years.6, 7, 13 Tasaki et al. reported that age is an important prognostic indicator of favorable neurologic outcomes in patients with severe TBI.16 The lower mean age of the patients of our study may reflect the significant improvements in cognitive function.
In terms of NPI subscales, yokukansan led to alleviation of various symptoms, in particular, agitation/aggression and disinhibition. These symptoms are difficult to manage in patients with DAB after TBI. Yokukansan thus has potential as a first‐line therapy for DAB in TBI patients in the future.
We also carried out 123I‐IMP cerebral scintigraphy in patient 2, revealing that cerebral blood flow was increased after 2 weeks of treatment (Fig. 1). One author reported that yokukansan recovered regional cerebral hypoperfusion, as shown by single photon emission computed tomography, in Charles Bonnet syndrome.17 Thus, yokukansan may also improve cerebral blood flow in damaged tissue.
Adverse effects and drug interactions between yokukansan and Western medicines
No adverse effects or drug interactions between yokukansan and Western medicines were observed in this study. Mild adverse effects have been reported previously; however, these comprise only 20 of 244 cases,6, 7, 13, 18, 19, 20, 21 and moreover, symptoms disappeared quickly after discontinuation of yokukansan. Ito et al. reported that yokukansan is unlikely to cause clinically relevant drug interactions involving the inhibition of major cytochrome P450 isozymes and P‐glycoprotein.22 Therefore, yokukansan can be considered to have favorable usability characteristics in the critical care field.
Mechanism of action
Western medicines such as beta‐blockers and neuroleptics have a single point of action. In contrast, Eastern medicine (or Kampo) is believed to exert its effect through many points of action, as Kampo contains various herbs. Many studies have investigated the mechanism of action of yokukansan. The most important aspect of the mechanism, which is relevant to TBI, is that herbs contained in yokukansan act on the neurotransmission system. Yokukansan has agonistic effects on 5HT1A receptors23 and decreases the expression of 5‐HT2A receptors in the prefrontal cortex.24 Yokukansan also prevents or corrects abnormal glutamate release.25 Moreover, glycyrrhizin and its metabolite, 18 beta‐glycyrrhetinic acid, a constituent herb of yokukansan, ameliorate thiamine deficiency‐induced dysfunction of glutamate transport in cultured rat cortical astrocytes;26 however, they are contained in many other Kampo formulae and are unlikely to have played the primary pharmacological role. Angelicae radix acts on the GABA transmission system,27 whereas Uncaria hook activates the acetylcholine neurotransmitter system.28 Furthermore, Matsumoto et al. reported that Uncaria hook contributed to the behavioral and neurochemical effects of yokukansan in Tg2576 mice, providing behavioral pharmacological evidence for the potential utility of yokukansan in preventing or improving cognitive deficits in Alzheimer patients.29 Yokukansan also improved BPSD in Alzheimer patients,6, 7 clinical symptoms in dementia with Lewy bodies,13 various emotional symptoms in borderline personality disorders,18 tardive dyskinesia and psychotic symptoms in schizophrenia,19, 20 as well as behavioral symptoms in frontotemporal dementia.21 Saito et al. reported the efficacy of yokukansan in a case report,30 with reference to symptoms after TBI. In our study, we evaluated objective indicators including GCS, NPI, MMSE, BI, and VI, and confirmed the efficacy of yokukansan on DAB after TBI in a series of severe trauma patients.
Conclusions
We showed the efficacy of yokukansan on DAB in patients after TBI. Yokukansan improved GCS, NPI, MMSE, BI, and VI scores, with no adverse effects or drug interactions with Western medicines. Yokukansan may be used to modulate the brain environment in TBI patients.
Conflict of Interest
None.
References
- 1. Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 1996; 46: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 2. Lishman WA. Brain damage in relation to psychiatric disability after head injury. Br. J. Psychiatry 1968; 114: 373–410. [DOI] [PubMed] [Google Scholar]
- 3. Virkkunen M, Nuutila A, Huusko S. Effect of brain injury on social adaptability. Acta Psychiatr. Scand. 1976; 53: 168–172. [DOI] [PubMed] [Google Scholar]
- 4. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state‐of‐the‐art review. J. Rehabil. Res. Dev. 2009; 46: 851–879. [DOI] [PubMed] [Google Scholar]
- 5. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials. JAMA 2005; 294: 1934–1943. [DOI] [PubMed] [Google Scholar]
- 6. Iwasaki K, Satoh‐Nakagawa T, Maruyama M et al A randomized, observer‐blind, controlled trial of the traditional Chinese medicine Yi‐Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J. Clin. Psychiatry 2005; 66: 248–252. [DOI] [PubMed] [Google Scholar]
- 7. Mizukami K, Asada T, Kinoshita T et al A randomized cross‐over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioral and psychological symptoms of dementia. Int. J. Neuropsychopharmacol. 2009; 12: 191–199. [DOI] [PubMed] [Google Scholar]
- 8. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–2314. [DOI] [PubMed] [Google Scholar]
- 9. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 10. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med. J. 1965; 14: 61–65. [PubMed] [Google Scholar]
- 11. Toba K, Nakai R, Akishita M et al Vitality Index as a useful tool to assess elderly with dementia. Geriatr. Gerontol. Int. 2002; 2: 23–29. [Google Scholar]
- 12. Baker SP, O'Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974; 14: 187–196. [PubMed] [Google Scholar]
- 13. Iwasaki K, Maruyama M, Tomita N et al Effects of the traditional Chinese herbal medicine Yi‐Gan San for cholinesterase inhibitor‐resistant visual hallucinations and neuropsychiatric symptoms in patients with dementia with Lewy bodies. J. Clin. Psychiatry 2005; 66: 1612–1613. [DOI] [PubMed] [Google Scholar]
- 14. Ikarashi Y, Iizuka S, Imamura S et al Effects of Yokukansan, a traditional Japanese medicine, on memory disturbance and behavioral and psychological symptoms of dementia in thiamine‐deficient rats. Biol. Pharm. Bull. 2009; 32: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka Y, Mizoguchi K. Influence of aging on chondroitin sulphate proteoglycan expression and neural stem/progenitor cells in rat brain and improving effects of an herbal medicine, yokukansan. Neuroscience 2009; 164: 1224–1234. [DOI] [PubMed] [Google Scholar]
- 16. Tasaki O, Shiozaki T, Hamasaki T et al Prognostic indicators and outcome prediction model for severe traumatic brain injury. J. Trauma 2009; 66: 304–308. [DOI] [PubMed] [Google Scholar]
- 17. Miyaoka T, Nagahama M, Tsuchie K et al Charles Bonnet syndrome: successful treatment of visual hallucinations due to vision loss with Yi‐gan san. Prog. Neuropsychopharmacol Biol. Psychiatry 2009; 33: 382–383. [DOI] [PubMed] [Google Scholar]
- 18. Miyaoka T, Furuya M, Yasuda H, Hayashi M, Inagaki T, Horiguchi J. Yi‐gan san for the treatment of borderline personality disorder: an open‐label study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008; 32: 150–154. [DOI] [PubMed] [Google Scholar]
- 19. Miyaoka T, Furuya M, Yasuda H et al Yi‐gan san for the treatment of neuroleptic‐induced tardive dyskinesia: an open‐label study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008; 32: 761–764. [DOI] [PubMed] [Google Scholar]
- 20. Miyaoka T, Furuya M, Yasuda H et al Yi‐gan san as adjunctive therapy for treatment‐resistant schizophrenia: an open‐label study. Clin. Neuropharmacol. 2009; 32: 6–9. [DOI] [PubMed] [Google Scholar]
- 21. Kimura T, Hayashida H, Furukawa H, Takamatsu J. Pilot study of pharmacological treatment for frontotemporal dementia: effect of Yokukansan on behavioral symptoms. Psychiatry Clin. Neurosci. 2010; 64: 207–210. [DOI] [PubMed] [Google Scholar]
- 22. Ito K, Satoh T, Watanabe Y et al Effects of Kampo medicines on CYP and P‐gp activity in vitro . Biol. Pharm. Bull. 2008; 31: 893–896. [DOI] [PubMed] [Google Scholar]
- 23. Kanno H, Sekiguchi K, Yamaguchi T et al Effect of yokukansan, a traditional Japanese medicine, on social and aggressive behaviour of para‐chloroamphetamine‐injected rats. J. Pharm. Pharmacol. 2009; 61: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 24. Egashira N, Iwasaki K, Ishibashi A et al Repeated administration of Yokukansan inhibits DOI‐induced head‐twitch response and decreases expression of 5‐hydroxytryptamine (5‐HT)2A receptors in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008; 32: 1515–1520. [DOI] [PubMed] [Google Scholar]
- 25. Takeda A, Tamano H, Itoh H, Oku N. Attenuation of abnormal glutamate release in zinc deficiency by zinc and Yokukansan. Neurochem. Int. 2008; 53: 230–235. [DOI] [PubMed] [Google Scholar]
- 26. Kawakami Z, Ikarashi Y, Kase Y. Glycyrrhizin and its metabolite 18 beta‐glycyrrhetinic acid in glycyrrhiza, a constituent herb of yokukansan, ameliorate thiamine deficiency‐induced dysfunction of glutamate transport in cultured rat cortical astrocytes. Eur. J. Pharmacol. 2010; 626: 154–158. [DOI] [PubMed] [Google Scholar]
- 27. Liao JF, Jan YM, Huang SY, Wang HH, Yu LL, Chen CF. Evaluation with receptor binding assay on the water extracts of ten CNS‐active Chinese herbal drugs. Proc. Natl Sci. Counc. Repub. China B 1995; 19: 151–158. [PubMed] [Google Scholar]
- 28. Murakami Y, Zhao Q, Harada K, Tohda M, Watanabe H, Matsumoto K. Choto‐san, a Kampo formula, improves chronic cerebral hypoperfusion‐induced spatial learning deficit via stimulation of muscarinic M1 receptor. Pharmacol. Biochem. Behav. 2005; 81: 616–625. [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto K, Zhao Q, Niu Y et al Kampo formulations, chotosan, and yokukansan, for dementia therapy: existing clinical and preclinical evidence. J. Pharmacol. Sci. 2013; 122: 257–269. [DOI] [PubMed] [Google Scholar]
- 30. Saito S, Kobayashi T, Osawa T, Kato S. Effectiveness of Japanese herbal medicine yokukansan for alleviating psychiatric symptoms after traumatic brain injury. Psychogeriatrics 2010; 10: 45–48. [DOI] [PubMed] [Google Scholar]


