Abstract
Background
Cutaneous human papillomaviruses (HPVs) increase the risk of non-melanoma skin cancer in sun-exposed skin. We examined the role of beta-HPV in the development of male external genital lesions (EGLs), a sun-unexposed site.
Methods
In this nested case-control study (67 men with pathologically-confirmed EGLs and 134 controls), exfoliated cells collected from the surface of lesions and normal genital skin 0, 6, and 12 months preceding EGL development were tested for beta-HPV DNA using a type-specific multiplex genotyping assay. Beta-HPV prevalence was estimated and conditional logistic regression was used to evaluate the association with condyloma, the most common EGL.
Results
While beta-HPV prevalence among controls remained stable, the prevalence among cases was lowest on the surface of lesion. Detecting beta-HPV on the normal genital skin was not associated with the presence or development of condyloma.
Conclusions
Cutaneous beta-HPV does not appear to be contributing to pathogenesis in male genital skin.
Keywords: Beta-HPV, condyloma, cutaneous HPV, genital lesions, HIM Study
Introduction
Human papillomaviruses (HPV) are comprised of more than 200 types representing five major genera: alpha-, beta-, gamma-, mu-, and nu-papillomavirus.[1, 2] HPVs that infect epithelial cells of mucosal surfaces belong to the genus alpha and HPVs infecting cutaneous skin represent all five genera. Different HPV types have different biological characteristics and disease associations.[1, 3] The majority of research has focused on alpha types,[2] including their oncogenic potential (e.g., HPV 16, which causes cervical, anogenital, and oropharyngeal cancers) and role in the development of benign anogenital lesions (e.g., HPV 6, which causes condyloma);[1] however, efforts are ongoing to understand the role of HPV types belonging to other genera in the development of benign and malignant lesions of the skin.
Beta-HPV types are commonly detected on the surface of healthy skin,[4] oral cavity,[5] and genitals[6] in otherwise healthy individuals, and can persist over several years.[4] However, in the presence of ultraviolet radiation (UVR), beta-HPV may increase the risk of non-melanoma skin cancer (NMSC), including squamous cell carcinoma (SCC) and basal cell carcinoma.[7–11] Rather than directly contribute to oncogenesis, it has been hypothesized that beta-HPVs promote tumorigenesis through destabilization of the host genome, including the inhibition of DNA repair genes that are responsible for correcting UVR-induced DNA damage.[9]
The role of cutaneous beta-HPV in UVR-unexposed skin remains unknown. Few studies have examined beta-HPV at anatomic sites not typically exposed to UVR (i.e., the anogenital region),[6, 12–15] none of which have used a longitudinal design to assess a temporal association between beta-HPV and the development of external genital lesions (EGLs). Thus, the aim of this study was to examine the potential role of beta-HPV in the development of male EGLs.
Materials and Methods
This case-control study was nested within the HPV Infection in Men (HIM) Study, a multinational cohort study of HPV natural history among men.[16] Briefly, over 4,000 adult men were recruited in the United States (US), Brazil, and Mexico. Every six months, for up to four years, participants underwent a clinical examination and completed a risk factor questionnaire. All participants provided written informed consent, and the human subjects committees of participating institutions approved all study procedures.
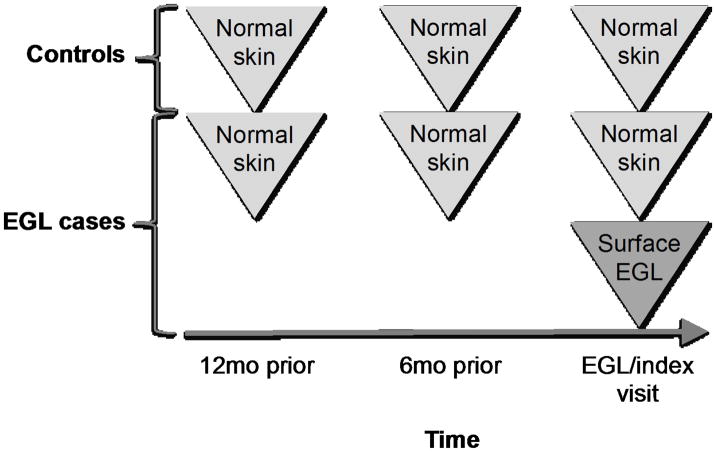
In the current study, men were included as cases if they developed an incident, pathologically confirmed EGL between February 2009 and May 2011.[6] Two controls (men who did not develop an EGL throughout the study period) were individually matched to each case on age, country, and length of follow-up. The study visit at which a case developed an EGL is hereinafter referred to as the index visit. Four genital specimens were evaluated for each case (Figure 1): surface of lesion at the index visit, normal skin at the index visit, normal skin six months prior, and normal skin twelve months prior. Three genital specimens were evaluated for each control: normal skin at the index visit, normal skin six months prior, and normal skin twelve months prior.
Figure 1.
Study design and timeline of specimen collection for EGL cases and controls in the HPV Infection in Men Study
Specimen collection and processing
Details of the HIM Study clinical protocol have been published previously.[6, 17] Briefly, visual inspection of the genital skin was conducted using 3× light magnification and exfoliated cells were collected from the genital skin. The normal genital skin was swabbed, including the penile head, shaft, and scrotum.[16] If an EGL was identified and thought to be HPV-related, exfoliated cells from the surface of the lesion were collected prior to the normal genital skin sampling and archived separately.[6, 17] EGLs that appeared visually distinct were biopsied and evaluated by two trained pathologists for the presence of inflammatory, infectious, or neoplastic conditions trained pathologists.[17] Formalin-fixed paraffin-embedded tissue sections were used for DNA extraction.
DNA extraction and HPV genotyping
Genital swab specimens (normal skin and surface of lesion) underwent robotic DNA extraction using the QIAamp Media MDx Kit (Qiagen, Gaithersburg, MD). All swab specimens were tested for the presence of mucosal alpha-HPV types using Linear Array (Roche Molecular Diagnostics, Alameda, CA) and cutaneous beta-HPV using a type-specific multiplex genotyping (TS-MPG) assay (IARC, Lyon, France), which combines multiplex PCR[18–20] with a bead-based Luminex technology.[21, 22] The TS-MPG assay detects 25 beta-HPV types (species β1: 5, 8, 12, 14, 19, 20, 21, 24, 25, 36, 47, 93; species β2: 9, 15, 17, 22, 23, 37, 38, 80; species β3: 49, 75, 76; species β4: 92; and species β5: 96). Biopsies were evaluated for the presence of alpha-HPV types using INNO-LiPA HPV Genotyping Extra (Fujirebio, Ghent, Belgium); however, tissue specimens were not available for beta-HPV DNA analysis. In addition to negative controls (distilled water), primers for the amplification of β-globin were included in each HPV assay to provide a positive control for the quality of the template DNA.
Statistical analysis
Separate analyses were conducted for all EGLs, combined, and for subsets of diagnoses, including 1) condyloma and suggestive of condyloma (hereinafter referred to as condyloma), 2) penile intraepithelial neoplasia (PeIN) grades I–III, and 3) other. Lesions suggestive of condyloma included those that appeared to be suggestive but not diagnostic of HPV or condyloma, such as benign squamous keratosis and benign squamous papilloma. Other diagnoses included skin conditions thought to be unrelated to mucosal HPV infection, such as seborrheic keratosis and skin tags.
Overall, species-specific, and type-specific beta-HPV DNA prevalence was estimated from the normal genital skin of controls at three time points (index visit, six months prior, and twelve months prior), from the normal genital skin of cases at three time points (index visit, six months prior, and twelve months prior), and from the surface of lesion (index visit); prevalence was estimated separately for all EGLs, condyloma, PeIN, and other diagnoses. As condyloma is the most common, clinically relevant EGL, modeling was conducted for this group only. Conditional logistic regression was used to estimate the risk associated with condyloma development for beta-HPV (overall and type-specific) detected concurrently or prior to condyloma diagnosis (six and twelve months). Models were also adjusted for the simultaneous presence of alpha-HPV 6/11 DNA, the mucosal HPV types known to cause condyloma. In exploratory analyses, associations were evaluated among HPV 6/11-negative lesions. Analyses were performed using SAS version 9.3.
Results
At the time of this analysis, 67 men developed incident, pathologically confirmed EGLs within the HIM Study and were selected as cases, and 134 men were selected as controls. Of the 67 EGLs, 27 (40.3%) were condyloma, 16 (23.9%) suggestive of condyloma, 5 (7.5%) PeIN, and 19 (28.4%) other diagnoses. No statistically significant differences in participant characteristics were observed between cases and controls (Table 1). After accounting for six EGLs with invalid or missing alpha-HPV results, 56 EGLs (91.8%) were positive for ≥1 alpha-HPV types within tissue biopsies, with HPV 6 (39.3%) and 11 (29.5%) being the most common types, followed by 16 (8.2%) and 52 (6.6%). Other HPV types detected include HPV 26, 31, 33, 39, 40, 51, 53, 58, and 74 (each <5%).
Table 1.
Select participant characteristics of EGL cases and controls in the HPV Infection in Men Study
| Characteristic | Controls n=134 No. (%) |
Cases | |||
|---|---|---|---|---|---|
|
| |||||
| All EGLs n=67 No. (%) |
Pathological diagnoses | ||||
|
| |||||
| Condyloma/Suggestive of Condyloma n=43 No. (%) |
PeIN n=5 No. (%) |
Other EGLs n=19 No. (%) |
|||
| Country of residence | |||||
| USA | 46 (34.3) | 23 (34.3) | 18 (41.9) | 2 (40.0) | 3 (15.8) |
| Brazil | 50 (37.3) | 25 (37.3) | 16 (37.2) | 1 (20.0) | 8 (42.1) |
| Mexico | 38 (28.4) | 19 (28.4) | 9 (20.9) | 2 (40.0) | 8 (42.1) |
| Age, years | |||||
| Median (range) | 31 (18–52) | 31 (19–50) | 29 (19–50) | 26 (23–35) | 31 (21–47) |
| 18–30 | 65 (48.5) | 33 (49.3) | 22 (51.2) | 3 (60.0) | 8 (42.1) |
| 31–44 | 52 (38.8) | 26 (38.8) | 16 (37.2) | 2 (40.0) | 8 (42.1) |
| 45–73 | 17 (12.7) | 8 (11.9) | 5 (11.6) | 0 (0) | 3 (15.8) |
| Race | |||||
| White | 60 (44.8) | 34 (50.7) | 26 (60.5) | 2 (40.0) | 6 (31.6) |
| Black | 26 (19.4) | 14 (20.9) | 8 (18.6) | 1 (20.0) | 5 (26.3) |
| Asian/PI/AI/AN | 5 (3.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other/mixed race | 42 (31.3) | 18 (26.9) | 8 (18.6) | 2 (40.0) | 8 (42.1) |
| Missing data | 1 (0.7) | 1 (1.5) | 1 (2.3) | 0 (0) | 0 (0) |
| Marital status | |||||
| Single or never married | 67 (52.3) | 29 (44.6) | 18 (41.9) | 2 (40.0) | 9 (52.9) |
| Married | 38 (29.7) | 15 (23.1) | 11 (25.6) | 1 (20.0) | 3 (17.6) |
| Cohabitating | 13 (10.2) | 15 (23.1) | 10 (23.3) | 0 (0) | 5 (29.4) |
| Divorced/separated/widowed | 10 (7.8) | 6 (9.2) | 4 (9.3) | 2 (40.0) | 0 (0) |
| Education, years | |||||
| ≤12 | 46 (34.3) | 24 (35.8) | 14 (32.6) | 3 (60.0) | 7 (36.8) |
| 13–15 | 32 (23.9) | 25 (37.3) | 18 (41.9) | 1 (20.0) | 6 (31.6) |
| ≥16 | 52 (38.8) | 15 (22.4) | 11 (25.6) | 1 (20.0) | 3 (15.8) |
| Missing data | 4 (3.0) | 3 (4.5) | 0 (0) | 0 (0) | 3 (15.8) |
| Circumcision | |||||
| No | 81 (60.4) | 39 (58.2) | 21 (48.8) | 3 (60.0) | 15 (78.9) |
| Yes | 53 (39.6) | 28 (41.8) | 22 (51.2) | 2 (40.0) | 4 (21.1) |
| Current smoker | |||||
| Current | 24 (17.9) | 18 (26.9) | 12 (27.9) | 1 (20.0) | 5 (26.3) |
| Former | 21 (15.7) | 14 (20.9) | 5 (11.6) | 3 (60.0) | 6 (31.6) |
| Never | 89 (66.4) | 35 (52.2) | 26 (60.5) | 1 (20.0) | 8 (42.1) |
| Sexual orientation | |||||
| MSW | 113 (84.3) | 56 (83.6) | 38 (88.4) | 3 (60.0) | 15 (78.9) |
| MSM | 8 (6.0) | 4 (6.0) | 3 (7.0) | 1 (5.3) | |
| MSWM | 6 (4.5) | 4 (6.0) | 1 (2.3) | 1 (20.0) | 2 (10.5) |
| Missing data | 7 (5.2) | 3 (4.5) | 1 (2.3) | 1 (20.0) | 1 (5.3) |
| Lifetime female sex partners | |||||
| 0–1 | 12 (9.2) | 6 (9.2) | 3 (7.1) | 1 (20.0) | 2 (11.1) |
| 2–9 | 59 (45.0) | 18 (27.7) | 9 (21.4) | 1 (20.0) | 8 (44.4) |
| 10–19 | 24 (18.3) | 13 (20.0) | 8 (19.0) | 1 (20.0) | 4 (22.2) |
| ≥20 | 36 (27.5) | 28 (43.1) | 22 (52.4) | 2 (40.0) | 4 (22.2) |
| Female sex partners in past 3–6 months | |||||
| 0 | 33 (24.6) | 12 (17.9) | 7 (16.3) | 1 (20.0) | 4 (21.1) |
| 1 | 56 (41.8) | 24 (35.8) | 17 (39.5) | 1 (20.0) | 6 (31.6) |
| 2 | 17 (12.7) | 6 (9.0) | 5 (11.6) | 0 (0) | 1 (5.3) |
| ≥3 | 19 (14.2) | 20 (29.9) | 13 (30.2) | 2 (40.0) | 5 (26.3) |
| Missing data | 9 (6.7) | 5 (7.5) | 1 (2.3) | 1 (20.0) | 3 (15.8) |
| Male anal sex partners in past 3 months | |||||
| 0 | 107 (80.5) | 52 (80.0) | 34 (81.0) | 4 (80.0) | 14 (77.8) |
| ≥1 | 26 (19.5) | 13 (20.0) | 8 (19.0) | 1 (20.0) | 4 (22.2) |
Data are n (%).
Asian/PI/AI/AN: Asian, Pacific Islander, American Indian, Alaskan Native; MSW: men who have sex with women; MSM: men who have sex with men; MSWM: men who have sex with women and men.
Note: No statistically significant differences were observed between cases and controls
Genital beta-HPV prevalence among controls varied by type but was stable over the one-year follow-up period. In contrast, beta-HPV prevalence among the cases varied over time prior to lesion detection, with the lowest HPV prevalence consistently observed on the surface of the lesion (Table 2). Similar results were observed among men with condyloma (Table 3). While beta-HPV prevalence remained stable over time among controls, it declined among condyloma cases; 37 (86.0%) men were positive for ≥1 beta-HPV types on the normal genital skin twelve months prior to condyloma diagnosis, 36 (83.7%) were positive six months prior to diagnosis, 33 (76.7%) were positive at the time of diagnosis (i.e., index visit), and only 28 (65.1%) were positive at the surface of the lesion. In contrast, the prevalence of causative alpha-HPV types 6 and 11 increased over time among condyloma cases and was highest when detected on the surface of the lesion, with 26 (60.5%) men positive for alpha-HPV 6/11. At the index visit, the most common beta-HPV types observed on the normal skin of controls were 24 (26.7%), 5 (25.6%), 22 and 38 (24.4% each), and 47 (23.3%), whereas the most common types observed on the normal skin of condyloma cases were 47 (33.3%), 5 (28.2%), 22 (25.6%), and 12, 24, 17 (23.1% each). The simultaneous presence of mucosal and cutaneous HPV types was observed among 18 cases (41.9%). On the surface of lesion, types 38 (18.6%), 5 (16.3%), 14 (16.3%), 8 (11.6%), and 17 (11.6%) were the most prevalent. Co-infection with multiple beta-HPV types was frequently observed at all time points among cases and controls.
Table 2.
Prevalence of beta (β)-HPV DNA detected on the surface of all EGL cases and the normal genital skin of EGL cases and controls, at the time of lesion detection (index visit), 6, and 12 months prior to lesion detection
| HPV species/type | Controls n=134 |
Any EGLa n=67 |
|||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Normal genital skin | Normal genital skin | EGL | |||||
|
|
|
|
|||||
| 12mo prior | 6mo prior | Index visit | 12mo prior | 6mo prior | Index visit | Index visit | |
|
|
|
|
|||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Any β-HPV type | 113 (84.3) | 117 (87.3) | 111 (82.8) | 58 (86.6) | 57 (85.1) | 54 (80.6) | 43 (64.2) |
| β1 | |||||||
| Any β1 | 88 (67.2) | 86 (64.2) | 87 (65.4) | 48 (71.6) | 44 (67.7) | 42 (68.9) | 34 (50.7) |
| HPV5 | 35 (26.7) | 35 (26.1) | 30 (22.6) | 21 (31.3) | 21 (32.3) | 18 (29.5) | 11 (16.4) |
| HPV8 | 27 (20.6) | 23 (17.2) | 23 (17.3) | 9 (13.4) | 10 (15.4) | 11 (18.0) | 6 (9.0) |
| HPV12 | 21 (16.0) | 20 (14.9) | 25 (18.8) | 18 (26.9) | 17 (26.2) | 15 (24.6) | 8 (11.9) |
| HPV14 | 9 (6.9) | 9 (6.7) | 9 (6.8) | 10 (14.9) | 10 (15.4) | 8 (13.1) | 7 (10.4) |
| HPV19 | 1 (0.8) | 1 (0.7) | 0 (0) | 1 (1.5) | 1 (1.5) | 1 (1.6) | 0 (0) |
| HPV20 | 0 (0) | 2 (1.5) | 4 (3.0) | 2 (3.0) | 4 (6.2) | 6 (9.8) | 3 (4.5) |
| HPV21 | 5 (3.8) | 12 (9.0) | 14 (10.5) | 8 (11.9) | 7 (10.8) | 4 (6.6) | 6 (9.0) |
| HPV24 | 35 (26.7) | 28 (20.9) | 37 (27.8) | 15 (22.4) | 17 (26.2) | 17 (27.9) | 4 (6.0) |
| HPV25 | 2 (1.5) | 1 (0.7) | 3 (2.3) | 1 (1.5) | 0 (0) | 0 (0) | 0 (0) |
| HPV36 | 17 (13) | 17 (12.7) | 13 (9.8) | 6 (9.0) | 8 (12.3) | 7 (11.5) | 5 (7.5) |
| HPV47 | 42 (32.1) | 39 (29.1) | 35 (26.3) | 6 (9.0) | 7 (10.8) | 19 (31.1) | 4 (6.0) |
| HPV93 | 2 (1.5) | 1 (0.7) | 5 (3.8) | 1 (1.5) | 2 (3.1) | 0 (0) | 1 (1.5) |
| β2 | |||||||
| Any β2 | 68 (51.9) | 69 (51.5) | 71 (53.4) | 49 (73.1) | 47 (72.3) | 32 (52.5) | 24 (35.8) |
| HPV9 | 13 (9.9) | 16 (11.9) | 10 (7.5) | 8 (11.9) | 3 (4.6) | 6 (9.8) | 5 (7.5) |
| HPV15 | 11 (8.4) | 9 (6.7) | 6 (4.5) | 6 (9.0) | 10 (15.4) | 6 (9.8) | 2 (3.0) |
| HPV17 | 16 (12.2) | 18 (13.4) | 16 (12.0) | 15 (22.4) | 19 (29.2) | 13 (21.3) | 8 (11.9) |
| HPV22 | 21 (16.0) | 22 (16.4) | 30 (22.6) | 14 (20.9) | 19 (29.2) | 13 (21.3) | 7 (10.4) |
| HPV23 | 23 (17.6) | 20 (14.9) | 22 (16.5) | 21 (31.3) | 14 (21.5) | 11 (18.0) | 5 (7.5) |
| HPV37 | 10 (7.6) | 12 (9.0) | 9 (6.8) | 8 (11.9) | 2 (3.1) | 3 (4.9) | 1 (1.5) |
| HPV38 | 24 (18.3) | 26 (19.4) | 26 (19.5) | 21 (31.3) | 25 (38.5) | 11 (18.0) | 12 (17.9) |
| HPV80 | 6 (4.6) | 16 (11.9) | 8 (6.0) | 12 (17.9) | 12 (18.5) | 5 (8.2) | 6 (9.0) |
| β3 | |||||||
| Any β3 | 7 (5.3) | 7 (5.2) | 5 (3.8) | 11 (16.4) | 16 (24.6) | 6 (9.8) | 3 (4.5) |
| HPV49 | 4 (3.1) | 3 (2.2) | 3 (2.3) | 5 (7.5) | 8 (12.3) | 2 (3.3) | 1 (1.5) |
| HPV75 | 3 (2.3) | 4 (3.0) | 1 (0.8) | 8 (11.9) | 8 (12.3) | 3 (4.9) | 3 (4.5) |
| HPV76 | 1 (0.8) | 1 (0.7) | 2 (1.5) | 5 (7.5) | 2 (3.1) | 2 (3.3) | 0 (0) |
| β4 | |||||||
| HPV92 | 3 (2.3) | 5 (3.7) | 4 (3.0) | 3 (4.5) | 4 (6.2) | 3 (4.9) | 1 (1.5) |
| β5 | |||||||
| HPV96 | 9 (6.9) | 9 (6.7) | 10 (7.5) | 8 (11.9) | 7 (10.8) | 2 (3.3) | 5 (7.5) |
| Multiple β-HPV types | |||||||
| ≥2 types | 77 (57.5) | 78 (58.2) | 79 (59.0) | 47 (70.1) | 45 (67.2) | 35 (52.2) | 26 (38.8) |
Note: Sample sizes change depending on specimen availability and validity of HPV results.
Includes all pathologically confirmed external genital lesions (EGLs).
Table 3.
Prevalence of beta (β)-HPV DNA and alpha (α)-HPV DNA detected on the surface of condyloma cases and the normal genital skin of condyloma cases and controls, at the time of lesion detection (index visit), 6, and 12 months prior to lesion detection
| HPV species/type | Controls n=86 |
Condyloma/Suggestive of Condyloma n=43 |
|||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Normal genital skin | Normal genital skin | Condyloma | |||||
|
|
|
|
|||||
| 12mo prior | 6mo prior | Index visit | 12mo prior | 6mo prior | Index visit | Index visit | |
|
|
|
|
|||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Any β-HPV type | 73 (84.9) | 75 (87.2) | 72 (83.7) | 37 (86.0) | 36 (83.7) | 33 (76.7) | 28 (65.1) |
| β1 | |||||||
| Any β1 | 53 (63.1) | 52 (60.5) | 56 (65.1) | 30 (69.8) | 26 (61.9) | 27 (69.2) | 23 (53.5) |
| HPV5 | 21 (25.0) | 21 (24.4) | 22 (25.6) | 14 (32.6) | 13 (31.0) | 11 (28.2) | 7 (16.3) |
| HPV8 | 15 (17.9) | 13 (15.1) | 12 (14.0) | 5 (11.6) | 5 (11.9) | 7 (17.9) | 5 (11.6) |
| HPV12 | 11 (13.1) | 11 (12.8) | 14 (16.3) | 10 (23.3) | 9 (21.4) | 9 (23.1) | 4 (9.3) |
| HPV14 | 6 (7.1) | 4 (4.7) | 4 (4.7) | 5 (11.6) | 7 (16.7) | 7 (17.9) | 7 (16.3) |
| HPV19 | 1 (1.2) | 1 (1.2) | 0 (0) | 1 (2.3) | 1 (2.4) | 1 (2.6) | 0 (0) |
| HPV20 | 0 (0) | 1 (1.2) | 4 (4.7) | 0 (0) | 1 (2.4) | 3 (7.7) | 2 (4.7) |
| HPV21 | 3 (3.6) | 6 (7.0) | 10 (11.6) | 6 (14.0) | 5 (11.9) | 2 (5.1) | 2 (4.7) |
| HPV24 | 19 (22.6) | 18 (20.9) | 23 (26.7) | 6 (14.0) | 9 (21.4) | 9 (23.1) | 3 (7.0) |
| HPV25 | 0 (0) | 0 (0) | 1 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HPV36 | 13 (15.5) | 9 (10.5) | 9 (10.5) | 4 (9.3) | 5 (11.9) | 6 (15.4) | 3 (7.0) |
| HPV47 | 24 (28.6) | 23 (26.7) | 20 (23.3) | 4 (9.3) | 5 (11.9) | 13 (33.3) | 3 (7.0) |
| HPV93 | 1 (1.2) | 0 (0) | 2 (2.3) | 0 (0) | 1 (2.4) | 0 (0) | 0 (0) |
| β2 | |||||||
| Any β2 | 45 (53.6) | 47 (54.7) | 50 (58.1) | 29 (67.4) | 29 (69.0) | 21 (53.8) | 17 (39.5) |
| HPV9 | 9 (10.7) | 12 (14.0) | 6 (7.0) | 2 (4.7) | 1 (2.4) | 4 (10.3) | 2 (4.7) |
| HPV15 | 8 (9.5) | 8 (9.3) | 5 (5.8) | 5 (11.6) | 7 (16.7) | 5 (12.8) | 2 (4.7) |
| HPV17 | 11 (13.1) | 11 (12.8) | 12 (14.0) | 8 (18.6) | 11 (26.2) | 9 (23.1) | 5 (11.6) |
| HPV22 | 13 (15.5) | 14 (16.3) | 21 (24.4) | 9 (20.9) | 13 (31.0) | 10 (25.6) | 4 (9.3) |
| HPV23 | 15 (17.9) | 11 (12.8) | 14 (16.3) | 13 (30.2) | 8 (19.0) | 8 (20.5) | 4 (9.3) |
| HPV37 | 9 (10.7) | 11 (12.8) | 8 (9.3) | 4 (9.3) | 1 (2.4) | 1 (2.6) | 0 (0) |
| HPV38 | 18 (21.4) | 21 (24.4) | 21 (24.4) | 13 (30.2) | 19 (45.2) | 8 (20.5) | 8 (18.6) |
| HPV80 | 3 (3.6) | 12 (14.0) | 4 (4.7) | 8 (18.6) | 9 (21.4) | 3 (7.7) | 4 (9.3) |
| β3 | |||||||
| Any β3 | 4 (4.8) | 5 (5.8) | 4 (4.7) | 4 (9.3) | 11 (26.2) | 5 (12.8) | 1 (2.3) |
| HPV49 | 2 (2.4) | 2 (2.3) | 2 (2.3) | 2 (4.7) | 5 (11.9) | 2 (5.1) | 0 (0) |
| HPV75 | 1 (1.2) | 3 (3.5) | 1 (1.2) | 3 (7.0) | 5 (11.9) | 3 (7.7) | 1 (2.3) |
| HPV76 | 1 (1.2) | 1 (1.2) | 2 (2.3) | 1 (2.3) | 1 (2.4) | 1 (2.6) | 0 (0) |
| β4 | |||||||
| HPV92 | 1 (1.2) | 3 (3.5) | 2 (2.3) | 1 (2.3) | 2 (4.8) | 1 (2.6) | 1 (2.3) |
| β5 | |||||||
| HPV96 | 7 (8.3) | 8 (9.3) | 7 (8.1) | 3 (7.0) | 4 (9.5) | 1 (2.6) | 2 (4.7) |
| Multiple β-HPV types | |||||||
| ≥2 types | 49 (57.0) | 50 (58.1) | 52 (60.5) | 28 (65.1) | 28 (65.1) | 25 (58.1) | 18 (41.9) |
| α-HPV typea | |||||||
| HPV6/11 | 2 (2.3) | 2 (2.3) | 4 (4.7) | 16 (37.2) | 16 (37.2) | 18 (41.9) | 26 (60.5) |
Note: Sample sizes change depending on specimen availability and validity of HPV results.
Surface of normal genital skin and EGL specimens were evaluated for α-HPV using Linear Array.
Despite a small sample size, similar trends were observed among five men with PeIN (Table 4). Four (80.0%) were positive for ≥1 beta-HPV types on the normal genital skin twelve months prior to diagnosis and all were positive six months prior to and at the time of diagnosis. Beta-HPV prevalence was substantially lower on the surface of the PeIN lesion, with two (40.0%) men positive for ≥1 beta-HPV types at the surface of the lesion. The prevalence of causative alpha-HPV types 16 and 18 increased over time among PeIN cases and was highest at the time of lesion detection, with 2 (40.0%) men positive for alpha-HPV 16/18 on the normal genital skin and 2 (40.0%) on the surface of lesion. At the index visit, the most common HPV type observed on the normal genital skin of controls was HPV47 (30.0%), whereas the most common type on the surface of PeIN was HPV21 (40.0%).
Table 4.
Beta (β)-HPV DNA prevalence detected on the surface of PeIN I–III cases and the normal genital skin of PeIN cases and controls, at the time of lesion detection (index visit), 6, and 12 months prior to lesion detection
| HPV species/type | Controls n=10 |
PeIN n=5 |
|||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Normal genital skin | Normal genital skin | PeIN | |||||
|
|
|
|
|||||
| 12mo prior | 6mo prior | Index visit | 12mo prior | 6mo prior | Index visit | Index visit | |
|
|
|
|
|||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Any β-HPV type | 8 (80.0) | 8 (80.0) | 5 (50.0) | 4 (80.0) | 5 (100) | 5 (100) | 2 (40.0) |
| β1 | |||||||
| Any β1 | 7 (77.8) | 6 (60.0) | 4 (40.0) | 4 (80.0) | 4 (80.0) | 3 (60.0) | 2 (40.0) |
| HPV5 | 4 (44.4) | 3 (30.0) | 2 (20.0) | 2 (40.0) | 3 (60.0) | 3 (60.0) | 0 (0) |
| HPV8 | 4 (44.4) | 3 (30.0) | 2 (20.0) | 1 (20.0) | 2 (40.0) | 1 (20.0) | 0 (0) |
| HPV12 | 2 (22.2) | 1 (10.0) | 1 (10.0) | 2 (40.0) | 1 (20.0) | 1 (20.0) | 0 (0) |
| HPV14 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) |
| HPV19 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HPV20 | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HPV21 | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | 2 (40.0) |
| HPV24 | 2 (22.2) | 1 (10.0) | 1 (10.0) | 1 (20.0) | 0 (0) | 0 (0) | 0 (0) |
| HPV25 | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HPV36 | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HPV47 | 5 (55.6) | 6 (60.0) | 3 (30.0) | 1 (20.0) | 0 (0) | 1 (20.0) | 0 (0) |
| HPV93 | 1 (11.1) | 1 (10.0) | 2 (20.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| β2 | |||||||
| Any β2 | 8 (88.9) | 5 (50.0) | 3 (30.0) | 4 (80.0) | 5 (100) | 3 (60.0) | 2 (40.0) |
| HPV9 | 2 (22.2) | 2 (20.0) | 2 (20.0) | 1 (20.0) | 1 (20.0) | 0 (0) | 1 (20.0) |
| HPV15 | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) |
| HPV17 | 1 (11.1) | 1 (10.0) | 0 (0) | 2 (40.0) | 3 (60.0) | 1 (20.0) | 1 (20.0) |
| HPV22 | 3 (33.3) | 1 (10.0) | 2 (20.0) | 1 (20.0) | 1 (20.0) | 0 (0) | 0 (0) |
| HPV23 | 2 (22.2) | 2 (20.0) | 2 (20.0) | 2 (40.0) | 2 (40.0) | 2 (40.0) | 0 (0) |
| HPV37 | 1 (11.1) | 1 (10.0) | 1 (10.0) | 1 (20.0) | 0 (0) | 0 (0) | 0 (0) |
| HPV38 | 3 (33.3) | 1 (10.0) | 2 (20.0) | 1 (20.0) | 2 (40.0) | 2 (40.0) | 1 (20.0) |
| HPV80 | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 1 (20.0) |
| β3 | |||||||
| Any β3 | 2 (22.2) | 1 (10.0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 0 (0) |
| HPV49 | 1 (11.1) | 1 (10.0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 0 (0) |
| HPV75 | 2 (22.2) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 0 (0) |
| HPV76 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| β4 | |||||||
| HPV92 | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | 0 (0) |
| β5 | |||||||
| HPV96 | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 0 (0) |
| Multiple β-HPV types | |||||||
| ≥2 types | 7 (70.0) | 5 (50.0) | 3 (30.0) | 4 (80.0) | 4 (80.0) | 2 (40.0) | 1 (20.0) |
| α-HPV typea | |||||||
| HPV16/18 | 1 (10.0) | 1 (10.0) | 1 (10.0) | 1 (20.0) | 1 (20.0) | 2 (40.0) | 2 (40.0) |
Note: Sample sizes change depending on specimen availability and validity of HPV results.
Surface of normal genital skin and EGL specimens were evaluated for α-HPV using Linear Array
Of the 19 cases with other diagnoses (Table 5), 17 (89.5%) were positive for beta-HPV on the normal skin twelve months prior to diagnosis and 16 (84.2%) were positive six months prior to diagnosis. Overall beta-HPV prevalence remained steady on the normal genital skin but then declined to 13 (68.4%) at the surface of the lesion. The most common HPV types on the surface of these lesions were 5 and 12 (21.1%, each).
Table 5.
Beta (β)-HPV DNA prevalence detected on the surface of other EGL cases and the normal genital skin of other EGL cases and controls, at the time of lesion detection (index visit), 6, and 12 months prior to lesion detection
| HPV species/type | Controls n=38 |
Other EGLa n=19 |
|||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Normal genital skin | Normal genital skin | Other EGL | |||||
|
|
|
|
|||||
| 12mo prior | 6mo prior | Index visit | 12mo prior | 6mo prior | Index visit | Index visit | |
|
|
|
|
|||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Any β-HPV type | 32 (84.2) | 34 (89.5) | 34 (89.5) | 17 (89.5) | 16 (84.2) | 16 (84.2) | 13 (68.4) |
| β1 | |||||||
| Any β1 | 28 (73.7) | 28 (73.7) | 27 (73.0) | 14 (73.7) | 14 (77.8) | 12 (70.6) | 9 (47.4) |
| HPV5 | 10 (26.3) | 11 (28.9) | 6 (16.2) | 5 (26.3) | 5 (27.8) | 4 (23.5) | 4 (21.1) |
| HPV8 | 8 (21.1) | 7 (18.4) | 9 (24.3) | 3 (15.8) | 3 (16.7) | 3 (17.6) | 1 (5.3) |
| HPV12 | 8 (21.1) | 8 (21.1) | 10 (27.0) | 6 (31.6) | 7 (38.9) | 5 (29.4) | 4 (21.1) |
| HPV14 | 3 (7.9) | 5 (13.2) | 5 (13.5) | 5 (26.3) | 3 (16.7) | 0 (0) | 0 (0) |
| HPV19 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| HPV20 | 0 (0) | 0 (0) | 0 (0) | 2 (10.5) | 3 (16.7) | 3 (17.6) | 1 (5.3) |
| HPV21 | 2 (5.3) | 6 (15.8) | 4 (10.8) | 1 (5.3) | 1 (5.6) | 1 (5.9) | 2 (10.5) |
| HPV24 | 14 (36.8) | 9 (23.7) | 13 (35.1) | 8 (42.1) | 8 (44.4) | 8 (47.1) | 1 (5.3) |
| HPV25 | 2 (5.3) | 1 (2.6) | 1 (2.7) | 1 (5.3) | 0 (0) | 0 (0) | 0 (0) |
| HPV36 | 4 (10.5) | 7 (18.4) | 4 (10.8) | 2 (10.5) | 3 (16.7) | 1 (5.9) | 2 (10.5) |
| HPV47 | 13 (34.2) | 10 (26.3) | 12 (32.4) | 1 (5.3) | 2 (11.1) | 5 (29.4) | 1 (5.3) |
| HPV93 | 0 (0) | 0 (0) | 1 (2.7) | 1 (5.3) | 1 (5.6) | 0 (0) | 1 (5.3) |
| β2 | |||||||
| Any β2 | 15 (39.5) | 17 (44.7) | 18 (48.6) | 16 (84.2) | 13 (72.2) | 8 (47.1) | 5 (26.3) |
| HPV9 | 2 (5.3) | 2 (5.3) | 2 (5.4) | 5 (26.3) | 1 (5.6) | 2 (11.8) | 2 (10.5) |
| HPV15 | 2 (5.3) | 1 (2.6) | 1 (2.7) | 1 (5.3) | 2 (11.1) | 1 (5.9) | 0 (0) |
| HPV17 | 4 (10.5) | 6 (15.8) | 4 (10.8) | 5 (26.3) | 5 (27.8) | 3 (17.6) | 2 (10.5) |
| HPV22 | 5 (13.2) | 7 (18.4) | 7 (18.9) | 4 (21.1) | 5 (27.8) | 3 (17.6) | 3 (15.8) |
| HPV23 | 6 (15.8) | 7 (18.4) | 6 (16.2) | 6 (31.6) | 4 (22.2) | 1 (5.9) | 1 (5.3) |
| HPV37 | 0 (0) | 0 (0) | 0 (0) | 3 (15.8) | 1 (5.6) | 2 (11.8) | 1 (5.3) |
| HPV38 | 3 (7.9) | 4 (10.5) | 3 (8.1) | 7 (36.8) | 4 (22.2) | 1 (5.9) | 3 (15.8) |
| HPV80 | 3 (7.9) | 4 (10.5) | 4 (10.8) | 3 (15.8) | 3 (16.7) | 2 (11.8) | 1 (5.3) |
| β3 | |||||||
| Any β3 | 1 (2.6) | 1 (2.6) | 1 (2.7) | 6 (31.6) | 5 (27.8) | 1 (5.9) | 2 (10.5) |
| HPV49 | 1 (2.6) | 0 (0) | 1 (2.7) | 2 (10.5) | 3 (16.7) | 0 (0) | 1 (5.3) |
| HPV75 | 0 (0) | 1 (2.6) | 0 (0) | 4 (21.1) | 3 (16.7) | 0 (0) | 2 (10.5) |
| HPV76 | 0 (0) | 0 (0) | 0 (0) | 4 (21.1) | 1 (5.6) | 1 (5.9) | 0 (0) |
| β4 | |||||||
| HPV92 | 2 (5.3) | 2 (5.3) | 2 (5.4) | 1 (5.3) | 1 (5.6) | 1 (5.9) | 0 (0) |
| β5 | |||||||
| HPV96 | 2 (5.3) | 1 (2.6) | 3 (8.1) | 4 (21.1) | 3 (16.7) | 1 (5.9) | 3 (15.8) |
| Multiple β-HPV types | |||||||
| ≥2 types | 21 (55.3) | 23 (60.5) | 24 (63.2) | 15 (78.9) | 13 (68.4) | 8 (42.1) | 6 (31.6) |
Note: Sample sizes change depending on specimen availability and validity of HPV results.
Includes various skin conditions thought to be unrelated to HPV infection, such as seborrheic keratosis and skin tags.
Conditional logistic regression models were used to determine whether beta-HPV was associated with the presence and development of condyloma (Table 6). At the index visit, men with condyloma were as likely as controls to have beta-HPV (any type) on the normal genital skin (OR: 0.62, 95% CI: 0.21–1.82). In contrast, men with condyloma were significantly less likely to have beta-HPV on the surface of the lesion than controls were to have beta-HPV on the normal genital skin (OR: 0.37, 95% CI: 0.14–0.94); however, after controlling for the presence of condyloma-causing alpha-HPV types 6 and 11, this association was no longer statistically significant (OR: 0.62, 95% CI: 0.12–3.09). When examined prospectively, the detection of beta-HPV on the normal genital skin was not associated with the subsequent risk of developing condyloma six or twelve months later (OR: 0.75, 95% CI: 0.23–2.54 and OR: 1.11, 95% CI: 0.33–4.01, respectively), even after accounting for alpha-HPV 6/11 (OR: 0.85, 95% CI: 0.19–4.09 and OR: 0.98, 95% CI: 0.20–5.13, respectively). Similar results were observed when the associations were assessed among alpha-HPV 6/11-negative men, with odds ratios attenuated towards the null (data not shown).
Table 6.
Associations between condyloma and beta (β)-HPV DNA detected on the surface of condyloma cases and the normal genital skin of condyloma cases and controls, at the time of lesion detection (index visit), 6, and 12 months prior to lesion detection
| HPV species/type | Condyloma/Suggestive of Condyloma n=43 |
|||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Normal genital skin | Condyloma/Suggestive of Condyloma | |||||||
|
|
|
|||||||
| 12mo prior | 6mo prior | Index visit | Index visit | |||||
|
|
|
|||||||
| ORa | aOR (95% CI)b | ORa | aOR (95% CI)b | ORa | aOR (95% CI)b | ORa | aOR (95% CI)c | |
| Any β-HPV type | 1.11 | 0.98 (0.20–5.13) | 0.75 | 0.85 (0.19–4.09) | 0.62 | 0.49 (0.11–2.03) | 0.37 | 0.62 (0.12–3.09) |
| β1 | ||||||||
| Any β1 | 1.32 | 1.19 (0.43–3.46) | 1.05 | 0.79 (0.28–2.26) | 1.14 | 0.97 (0.28–3.46) | 0.60 | 0.55 (0.13–2.12) |
| HPV5 | 1.44 | 0.99 (0.30–3.05) | 1.33 | 0.82 (0.24–2.54) | 1.14 | 1.12 (0.35–3.39) | 0.53 | 0.86 (0.12–5.67) |
| HPV8 | 0.62 | 0.50 (0.07–2.53) | 0.75 | 0.42 (0.07–1.82) | 1.52 | 0.83 (0.17–3.60) | 0.82 | 2.64 (0.36–21.26) |
| HPV12 | 1.97 | 1.66 (0.43–6.47) | 1.89 | 2.99 (0.70–15.2) | 1.88 | 2.52 (0.48–15.59) | 0.48 | 0.42 (0.02–5.76) |
| HPV14 | 1.67 | 1.78 (0.32–9.53) | 4.22 | 2.17 (0.34–15.53) | 4.22 | 1.74 (0.22–14.95) | 4.22 | 3.55 (0.42–42.89) |
| HPV19 | NE | NE | 2.00 | NE | NE | NE | NE | NE |
| HPV20 | NE | NE | NE | NE | 1.50 | 0.78 (0.04–15.49) | 1.00 | 1.31 (0.04–30.14) |
| HPV21 | 4.00 | 4.77 (0.58–63.93) | 2.00 | 1.11 (0.15–6.77) | 0.44 | 0.08 (0–1.27) | 0.40 | 1.12 (0.09–9.47) |
| HPV24 | 0.41 | 0.42 (0.06–1.91) | 1.08 | 1.01 (0.24–3.89) | 0.84 | 0.88 (0.19–3.55) | 0.09 | 0.05 (0.01–0.77) |
| HPV25 | NE | NE | NE | NE | 2.00 | 2.00 (0–38.00) | NE | NE |
| HPV36 | 0.62 | 0.93 (0.18–3.78) | 1.12 | 0.75 (0.11–3.71) | 1.36 | 0.81 (0.17–3.29) | 0.67 | 0.30 (0.02–2.92) |
| HPV47 | 0.25 | 0.09 (0–0.73) | 0.32 | 0.24 (0.04–1.00) | 1.66 | 1.17 (0.35–3.73) | 0.20 | 0.57 (0.04–4.59) |
| HPV93 | NE | NE | 2.00 | 2.00 (0.11–NE) | 2.00 | 11.39 (0–216.45) | NE | NE |
| β2 | ||||||||
| Any β2 | 1.79 | 2.22 (0.78–7.09) | 1.77 | 1.46 (0.58–3.97) | 0.83 | 0.72 (0.26–1.94) | 0.51 | 1.28 (0.36–5.15) |
| HPV9 | 0.44 | 0.24 (0.01–1.94) | 0.17 | 0.24 (0.01–1.86) | 1.60 | 1.13 (0.13–9.79) | 0.67 | 0.39 (0.01–14.07) |
| HPV15 | 1.25 | 1.21 (0.21–5.85) | 1.94 | 1.83 (0.40–8.61) | 2.91 | 4.74 (0.61–60.35) | 0.80 | 0.39 (0.01–13.87) |
| HPV17 | 1.43 | 1.60 (0.33–7.58) | 2.29 | 1.30 (0.34–4.85) | 1.57 | 1.17 (0.27–4.63) | 0.81 | 1.77 (0.19–16.12) |
| HPV22 | 1.41 | 1.82 (0.50–6.97) | 2.21 | 1.87 (0.62–5.84) | 1.24 | 1.11 (0.31–3.87) | 0.28 | 0.58 (0.07–3.42) |
| HPV23 | 1.94 | 2.99 (0.84–12.22) | 1.49 | 1.70 (0.48–5.97) | 1.17 | 1.00 (0.22–3.90) | 0.55 | 0.79 (0.07–7.03) |
| HPV37 | 0.84 | 0.93 (0.13–5.13) | 0.16 | 0.15 (0–2.24) | 0.25 | 0.51 (0–3.04) | 0.15 | 2.97 (0–26.27) |
| HPV38 | 1.66 | 1.84 (0.57–6.12) | 2.44 | 2.03 (0.74–5.93) | 0.73 | 0.78 (0.20–2.76) | 0.71 | 1.59 (0.31–8.83) |
| HPV80 | 5.33 | 3.05 (0.50–22.97) | 1.57 | 1.37 (0.36–4.94) | NE | NE | 2.26 | 2.61 (0.18–39.95) |
| β3 | ||||||||
| Any β3 | 1.84 | 2.23 (0.31–16.42) | 5.16 | 2.92 (0.63–15.83) | 2.91 | 3.92 (0.57–33.84) | NE | NE |
| HPV49 | 2.00 | 2.00 (0.14–27.59) | 5.00 | 3.05 (0.23–59.76) | 2.00 | 2.97 (0.19–48.98) | NE | NE |
| HPV75 | NE | NE | 3.33 | 1.90 (0.18–25.03) | NE | NE | NE | NE |
| HPV76 | 1.41 | 0.37 (0–44.48) | NE | NE | 1.00 | 0.55 (0–24.84) | NE | NE |
| β4 | ||||||||
| HPV92 | NE | NE | 1.33 | 0.52 (0–29.37) | NE | NE | NE | NE |
| β5 | ||||||||
| HPV96 | 0.86 | 0.96 (0.12–5.46) | 1.00 | 0.99 (0.10–7.52) | 0.29 | 0.09 (0–1.36) | 0.54 | 0.66 (0.03–8.50) |
| Multiple β-HPV types | ||||||||
| ≥2 types | 1.41 | 1.62 (0.59–4.67) | 1.32 | 0.95 (0.37–2.49) | 0.90 | 1.25 (0.43–3.74) | 0.45 | 1.37 (0.33–6.66) |
NE: Not estimable
Note: Sample sizes change depending on specimen availability and validity of HPV results.
Unadjusted model; bolded OR indicates a statistically significant association (p<0.05).
Adjusted for presence of alpha-HPV 6/11 DNA on the normal genital skin of cases and controls at the corresponding visit; surface of normal genital skin and EGL specimens were evaluated for α-HPV using Linear Array.
Adjusted for presence of alpha-HPV 6/11 DNA within EGL tissue of cases and normal genital skin of controls at the index visit; EGL tissue biopsies were evaluated for α-HPV using INNO-LiPA and the surface of normal genital skin specimens were evaluated for α-HPV using Linear Array.
In type-specific analyses conducted at the index visit (Table 6), men with condyloma were significantly less likely to have beta-HPV 24 and 47 detected on the surface of the lesion than controls were to have beta-HPV 24 and 47 on the normal genital skin (OR: 0.09, 95% CI: 0–0.60 and OR: 0.20, 95% CI: 0.02–0.87, respectively). When detected on the normal genital skin, HPV47 was associated with a reduced risk of developing condyloma six and twelve months later (OR: 0.32, 95% CI: 0.07–1.04 and OR: 0.25, 95% CI: 0.05–0.87, respectively), even after controlling for alpha-HPV 6/11 (OR: 0.24, 95% CI: 0.04–1.00 and OR: 0.09, 95% CI: 0.01–0.73, respectively). Conversely, HPV38 was significantly associated with an increased risk of condyloma six months later (OR: 2.44, 95% CI: 1.06–5.83), but not after adjusting for alpha-HPV 6/11.
Discussion
In this study, we demonstrate that cutaneous beta-HPV types were not significantly associated with the presence or development of external genital lesions in men and do not appear to be involved in pathogenesis at UVR-unexposed genital skin. One type, beta-HPV47, was associated with a reduced risk of developing condyloma. Examining the presence of beta-HPV types on the surface of lesions, as well as the normal genital skin preceding lesion development, enabled us to evaluate the temporal association between beta-HPV and EGLs. Furthermore, we were able to account for the established causal role of alpha-HPV infections in the pathogenesis of condyloma.
A subset of cutaneous beta-HPV types appear to significantly increase the risk of NMSC at sun-exposed sites;[7–11] however, it remains unknown whether beta-HPV is associated with the risk of benign and malignant lesions at non-sun-unexposed sites, such as the male genitals. Few studies have evaluated the association between beta-HPVs and male EGLs, including penile SCC,[14, 15] precancerous penile lesions,[6, 12] and condyloma,[6, 13] most of which were cross-sectional and limited to a small number of beta-HPV types. All of the abovementioned studies identified a high prevalence of alpha-HPV 16 on or within male EGLs and none provided sufficient evidence to support a causal role for cutaneous beta-HPV infection, independent of alpha mucosal HPV, in the development of HPV-related genital lesions.
Examining patterns in HPV prevalence across the spectrum of disease progression provides important insights into the role of HPV in pathogenesis. If beta-HPVs were actively involved in EGL development, we would expect their prevalence to be highest on the lesions and lower on the normal genital skin preceding lesion development, as has been observed for alpha-HPV types known to cause cancer and genital warts. Among women who develop cervical lesions, oncogenic HPV prevalence increases significantly with increasing lesion severity, whereas the prevalence of non-oncogenic types remains constant or decreases with increasing lesion severity.[23, 24] In the current study, alpha-HPV 6/11 prevalence on male genital skin increased over time and was highest on the surface of the lesion. However, the contrasting pattern observed for beta-HPV in terms of EGL development suggests that cutaneous beta-HPVs are not actively involved in EGL pathogenesis.
This is the first study to prospectively evaluate the role of cutaneous beta-HPV in the development of male EGLs. The nested case-control design provided the opportunity to conduct longitudinal HPV sampling among men who went on to develop EGLs as well as men who did not develop lesions (i.e., otherwise healthy controls). The addition of EGL-free controls in this study provides one of the largest populations from which to estimate type-specific beta-HPV prevalence of male genital skin at multiple time points. Furthermore, we utilized the TS-MPG assay, which allowed us to detect a large number of beta-HPV types compared with previously published studies. However, the following limitations must be considered. First, small sample sizes limited our ability to examine the role of beta-HPV in the development of PeIN, the precursor to penile cancer. Second, HPV DNA detected on the surface of a lesion may not represent types present throughout biopsy tissue. In the HIM Study, moderate to high agreement between sampling methods was found for genus alpha-HPV types[25] but remains unknown for genus beta. Lastly, misclassification of pathological diagnosis may have occurred, though it would have been minimized by the use of a pathology panel.
Conclusions
The ubiquitous presence of beta-HPV on the normal genital skin and substantially lower prevalence on the surface of both condyloma and non-condyloma lesions suggests that beta-HPVs do not play a causal role in EGL development. Findings from this study along with others have shown that beta-HPV typically occurs as a co-infection with alpha-HPV types that are known to cause EGLs and exhibit lower viral loads than those alpha-HPV types.[26, 27] In agreement with the published literature,[28, 29] cutaneous beta-HPVs appear to be a normal part of the commensal microbiome of the cutaneous epithelium. Whether these viruses play an antagonistic (i.e., competition with alpha-HPV) or passive role has yet to be determined. Further research is needed to definitively evaluate the role of beta-HPVs in the development of skin lesions at UVR-unexposed sites, including mechanistic studies assessing HPV transcriptional activity and host interactions.
Acknowledgments
The authors thank the HIM Study participants and teams in the USA (Moffitt Cancer Center, Tampa, FL), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina Instituto do Câncer do Estado de São Paulo, Ludwig Institute for Cancer Research, São Paulo), and Mexico (Instituto Mexicano del Seguro Social, Instituto Nacional de Salud Pública, Cuernavaca. The authors also thank the Tissue Core Facilities at the Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center, supported under NIH grant P30 CA076292, as well as Dr. Jorge Salmerón for proofreading the manuscript.
Abbreviations
- HPV
Human papillomavirus
- UVR
ultraviolet radiation
- NMSC
non-melanoma skin cancer
- SCC
squamous cell carcinoma
- EGL
external genital lesion
- HIM Study
HPV Infection in Men Study
- US
United States
- PeIN
penile intraepithelial neoplasia
Footnotes
Ethical Approval: Ethical approval was given by the University of South Florida (IRB# 102660), Ludwig Institute for Cancer Research, Centro de Referência e Treinamento em Doenças Sexualmente Transmissíveis e AIDS, and Instituto Nacional de Salud Pública de México.
Presentation at a prior meeting: Preliminary results of this study were presented, in part, as an oral presentation during the Emerging Oncogenic Viruses Workshop in San Pietro in Bevagna, Manduria, Italy in June 2014.
Conflict of Interest: ARG receives research funding from Merck Sharp & Dohme Corp. LLV and ARG serve as consultants to Merck Sharp & Dohme Corp. for HPV vaccines. MHS is a consultant in clinical trial development and performance and/or expert pathologist in HPV related clinical trials for Merck, Roche/Ventana Medical Systems, Becton Dickinson(BD), Hologic/Gen Probe, Cepheid and Inovio Pharmaceuticals. None of the other authors have conflicts of interest to report.
References
- 1.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25(Suppl 1):2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard HU. Taxonomy and phylogeny of papillomaviruses: an overview and recent developments. Infect Genet Evol. 2013;18:357–61. doi: 10.1016/j.meegid.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Hazard K, Karlsson A, Andersson K, Ekberg H, Dillner J, Forslund O. Cutaneous human papillomaviruses persist on healthy skin. J Invest Dermatol. 2007;127:116–9. doi: 10.1038/sj.jid.5700570. [DOI] [PubMed] [Google Scholar]
- 5.Bottalico D, Chen Z, Dunne A, Ostoloza J, McKinney S, Sun C, et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J Infect Dis. 2011;204:787–92. doi: 10.1093/infdis/jir383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce Campbell CM, Messina JL, Stoler MH, Jukic DM, Tommasino M, Gheit T, et al. Cutaneous human papillomavirus types detected on the surface of male external genital lesions: a case series within the HPV Infection in Men Study. J Clin Virol. 2013;58:652–9. doi: 10.1016/j.jcv.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akgul B, Cooke JC, Storey A. HPV-associated skin disease. J Pathol. 2006;208:165–75. doi: 10.1002/path.1893. [DOI] [PubMed] [Google Scholar]
- 8.Viarisio D, Decker KM, Aengeneyndt B, Flechtenmacher C, Gissmann L, Tommasino M. Human papillomavirus type 38 E6 and E7 act as tumour promoters during chemically induced skin carcinogenesis. J Gen Virol. 2013;94:749–52. doi: 10.1099/vir.0.048991-0. [DOI] [PubMed] [Google Scholar]
- 9.Wallace NA, Robinson K, Galloway DA. Beta human papillomavirus E6 expression inhibits stabilization of p53 and increases tolerance of genomic instability. J Virol. 2014;88:6112–27. doi: 10.1128/JVI.03808-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Aldabagh B, Yu J, Arron ST. Role of human papillomavirus in cutaneous squamous cell carcinoma: a meta-analysis. J Am Acad Dermatol. 2014;70:621–9. doi: 10.1016/j.jaad.2014.01.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howley PM, Pfister HJ. Beta genus papillomaviruses and skin cancer. Virology. 2015;479–480:290–6. doi: 10.1016/j.virol.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wieland U, Jurk S, Weissenborn S, Krieg T, Pfister H, Ritzkowsky A. Erythroplasia of queyrat: coinfection with cutaneous carcinogenic human papillomavirus type 8 and genital papillomaviruses in a carcinoma in situ. J Invest Dermatol. 2000;115:396–401. doi: 10.1046/j.1523-1747.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 13.Sturegard E, Johansson H, Ekstrom J, Hansson BG, Johnsson A, Gustafsson E, et al. Human papillomavirus typing in reporting of condyloma. Sex Transm Dis. 2013;40:123–9. doi: 10.1097/OLQ.0b013e31827aa9b3. [DOI] [PubMed] [Google Scholar]
- 14.Heideman DA, Waterboer T, Pawlita M, Delis-van Diemen P, Nindl I, Leijte JA, et al. Human papillomavirus-16 is the predominant type etiologically involved in penile squamous cell carcinoma. J Clin Oncol. 2007;25:4550–6. doi: 10.1200/JCO.2007.12.3182. [DOI] [PubMed] [Google Scholar]
- 15.Humbey O, Cairey-Remonnay S, Guerrini JS, Algros MP, Mougin C, Bittard H, et al. Detection of the human papillomavirus and analysis of the TP53 polymorphism of exon 4 at codon 72 in penile squamous cell carcinomas. Eur J Cancer. 2003;39:684–90. doi: 10.1016/s0959-8049(02)00835-3. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingles DJ, Pierce Campbell CM, Messina JA, Stoler MH, Lin HY, Fulp WJ, et al. Human papillomavirus virus (HPV) genotype- and age-specific analyses of external genital lesions among men in the HPV Infection in Men (HIM) Study. J Infect Dis. 2015;211:1060–7. doi: 10.1093/infdis/jiu587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gheit T, Billoud G, de Koning MN, Gemignani F, Forslund O, Sylla BS, et al. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. J Clin Microbiol. 2007;45:2537–44. doi: 10.1128/JCM.00747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannacone MR, Gheit T, Waterboer T, Giuliano AR, Messina JL, Fenske NA, et al. Case-control study of cutaneous human papillomaviruses in squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2012;21:1303–13. doi: 10.1158/1055-9965.EPI-12-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollison DE, Pawlita M, Giuliano AR, Iannacone MR, Sondak VK, Messina JL, et al. Measures of cutaneous human papillomavirus infection in normal tissues as biomarkers of HPV in corresponding nonmelanoma skin cancers. Int J Cancer. 2008;123:2337–42. doi: 10.1002/ijc.23795. [DOI] [PubMed] [Google Scholar]
- 21.Iannacone MR, Gheit T, Waterboer T, Giuliano AR, Messina JL, Fenske NA, et al. Case-control study of cutaneous human papillomavirus infection in Basal cell carcinoma of the skin. J Invest Dermatol. 2013;133:1512–20. doi: 10.1038/jid.2012.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol. 2006;44:504–12. doi: 10.1128/JCM.44.2.504-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S3/26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–59. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 25.Anic GM, Messina JL, Stoler MH, Rollison DE, Stockwell H, Villa LL, et al. Concordance of human papillomavirus types detected on the surface and in the tissue of genital lesions in men. J Med Virol. 2013;85:1561–6. doi: 10.1002/jmv.23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, et al. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol. 2005;125:93–7. doi: 10.1111/j.0022-202X.2005.23733.x. [DOI] [PubMed] [Google Scholar]
- 27.Weissenborn SJ, Wieland U, Junk M, Pfister H. Quantification of beta-human papillomavirus DNA by real-time PCR. Nature protocols. 2010;5:1–13. doi: 10.1038/nprot.2009.153. [DOI] [PubMed] [Google Scholar]
- 28.Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol. 2000;74:11636–41. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wieland U, Kreuter A, Pfister H. Human papillomavirus and immunosuppression. Curr Probl Dermatol. 2014;45:154–65. doi: 10.1159/000357907. [DOI] [PubMed] [Google Scholar]