Abstract
Objective
Direct electrical stimulation (DES) is a clinical gold standard for human brain mapping and readily evokes conscious percepts, yet the neurophysiological changes underlying these percepts are not well understood.
Approach
To determine the neural correlates of DES, we stimulated the somatosensory cortex of ten human participants at frequency-amplitude combinations that both elicited and failed to elicit conscious percepts, meanwhile recording neural activity directly surrounding the stimulation site. We then compared the neural activity of perceived trials to that of non-perceived trials.
Main results
We found that stimulation evokes distributed high gamma activity, which correlates with conscious perception better than stimulation parameters themselves.
Significance
Our findings suggest that high gamma activity is a reliable biomarker for perception evoked by both natural and electrical stimuli.
Keywords: DES, electrocorticography, functional mapping, neural prosthesis
1. Introduction
Direct electrical stimulation (DES)—the application of electrical current to the cortical surface—has been used clinically since the early 1900s to map human functional anatomy.[1–5] DES mapping is routinely used to guide surgical decisions by identifying patient-specific brain regions critical for perceptual or cognitive functions such as sensation, movement, language and more.[6–14] The functional maps created by DES are then used to prevent neurological morbidity after surgical resection. Recent work has provided strong evidence linking stimulation site and intensity to properties of a patient’s subjective experience of naturally and artificially evoked percepts[15,16]; however, the neural effects of DES remain unknown.[17,18] Most studies of the effects of DES are limited to behavioral reports of overt percepts elicited by stimulation.[14–16,19–21] The paucity of electrophysiological data in these studies relates to technical challenges associated with simultaneous stimulation and recording of neural activity, including stimulation artifact and amplifier saturation.
The goal of the present study was to systematically investigate the neurophysiological effects of DES in order to better understand the relationships between stimulation properties, neural activity, and behavioral outcomes. Pioneering electrocorticography (ECoG) studies by Crone and colleagues discovered that high gamma (75–100Hz) neural activity in humans, which is correlated with local multi-unit neural firing rate[22], focally increases in relation to natural somatosensory perception in humans.[23] High gamma increase in response to sensory stimuli has been recapitulated in the case of controlled natural stimulation during brain mapping and in the case of peripheral nerve stimulation.[24,25] We hypothesized that the artificial percepts induced by DES would share this underlying electrophysiological signature. Moreover, we hypothesized that high gamma activity was specifically related to the conscious perception of electrical stimulation, independent of the stimulation parameters.
To test these hypotheses, we recorded neural activity before, during, and after electrical stimulation in somatosensory cortex of 10 human participants undergoing chronic subdural recordings for epilepsy localization and clinical brain mapping. To overcome the aforementioned technical challenges, we used a high dynamic range amplifier that did not saturate during stimulation, along with artifact rejection signal processing methods, while recording from the area directly adjacent to the site of stimulation and the wider surrounding cortex. For each subject, we collected behavioral reports and neurophysiological responses at a range of clinically relevant stimulation parameters (manipulating pulse frequency and amplitude). Finally, we defined each subject’s perceptual threshold and applied DES repeatedly to examine trial-by-trial neural response properties that differed as a function of perceptual outcome when stimulation parameters were fixed. Our results indicate that stimulation-evoked high gamma power is an independent marker of somatosensory perception.
2. Methods
2.1 Experimental setup
2.1.1 Participants
Experimental procedures were approved by the Committee for Human Research at the University of California, San Francisco, and all participants provided written informed consent. Patients undergoing ECoG grid implantation for epilepsy focus localization (N=10, 6 males; 20 – 55 years old) volunteered to participate in this research study (Supplementary Table 1). Patients were implanted with 256 channel high-density subdural electrode arrays (AdTech, Racine, WI or Integra, Plainsboro, NJ) with 4mm center-to-center spacing (1.17mm exposed diameter). Electrode arrays were positioned purely according to clinical indications. Participants were included whose coverage included the ventral sensorimotor cortex (pre- and post-central gyri). An electrode in the sub-galeal space served as the reference electrode and ground for recording.
2.1.2 Tasks and stimuli
A neurologist (VRR or RCK) operated a clinical bipolar stimulator (Nicolet™ Cortical Stimulator, Natus Medical Inc) while monitoring ECoG signal for electrical abnormalities throughout testing. Stimulation pulse train parameters were as follows: biphasic, charge balanced, cathode-leading, pulse width=100 µs, duration = 1sec. In a typical experimental session, the clinician tested several bipolar pairs individually by stimulating with typical clinical parameters and asking the subject to report any sensations evoked by electrical stimulation. The site that produced the most discrete sensory percept was chosen as the test site for the rest of the experimental session. Most of these sites produced percepts in the hand or lower face areas. The subject was instructed to press a button when they felt the percept and to not move otherwise. The subject did not know when stimulation was occurring, as they could not see the operator and there was no sound associated with stimulation. The typical 3×3 table of parameters was then tested with at least five repetitions of stimulation pulse trains for each current and frequency combination. Because the testing would have taken too long if the clinician had changed the parameters for each trial, the five repetitions were always completed back to back. However, each patient had a pseudorandom order of presentation of the current and frequency combinations. Pulse trains were presented by the operator with several seconds between pulse trains. Across patients, the timing of button press following a perceived stimulus was well correlated with timing of stimulation pulse trains, and false positives were rare. Six subjects also participated in a second experiment during the same session during which stimulation was presented at a constant “threshold” level, and the subject was again instructed to press the button when he or she perceived stimulation. A mid-range level of current delivered at 50Hz was chosen for this threshold test and titrated until the experimenter determined that the patient was perceiving the stimuli approximately half of the time.
2.1.3 Button press control
Throughout all of the testing sessions, subjects were instructed to press the button using their ipsilateral (to the implanted grid) thumb. A button press control task was performed by each subject in the absence of electrical stimuli. The subject was cued by the researcher to press and hold the button. The same statistical analysis that was used to test for increased high gamma following stimulation pulse trains was used to test for high gamma activity over baseline corresponding with the button press. These electrodes were then excluded from further analyses (n=107 of 1824 total electrodes across patients).
2.1.4 Sensory control
A sensory control task was performed by 6 subjects in the absence of electrical stimuli. In anatomical locations for which it was feasible, the researcher applied light tactile pressure to the anatomical area in which the subject reported feeling paresthesias. The sensory control was time-aligned using a corresponding button press by an observing researcher. Because this control was used only to identify the location of neural representation of the sensory area in question, the exact pressure applied was not analyzed. The same statistical analysis that was used to test for increased high gamma following stimulation pulse trains was used to test for high gamma activity over baseline corresponding with the sensory stimulus. Electrodes that displayed high gamma activity above baseline were then designated as part of the “natural sensory network.”
2.2 Recording and data processing
Signals were acquired at a sampling rate of 3051.8 Hz and were amplified and digitized using a Tucker-Davis Technologies (Alachua, FL, USA) neural recording system (RZ2 DSP combined with a 256-channel PZ2 amplifier). Manual artifact and channel rejection was performed, followed by common median referencing.[26]
The signal from each electrode channel was filtered into 40 frequency bands using an FFT followed by a Hilbert transform with logarithmically increasing center frequencies (range of CF = 4.07 Hz – 193.8 Hz). The analytic amplitude, also known as the envelope, and the phase were calculated for each [27]. The high gamma analytic amplitude was calculated by averaging filtered frequencies between 70 and 150 Hz. A z-score of the filtered frequencies was calculated with respect to a rest block recorded during the same session by following the method of Canolty, et al.[28] The anatomical location of electrodes was determined with the aid of co-registered brain CT and MRI images.
2.3 Analytical methods
2.3.1 Basic analyses
To avoid contamination by stimulation artifact, we excluded 100ms of data on either side of the stimulation pulse train. Due to forward and reverse filtering, any artifact from the stimulation is symmetrical at the onset and offset of stimulation. This interval was adequate for artifact rejection for all parameters used, as determined by comparison of z-scored signals prior to stimulation and following stimulation across stimulation parameters, and was corroborated by visual inspection.
The stimulating channel pair was excluded from analyses. Z-scored signals were tested for significance from baseline and corrected for false discovery rate using the method of Canolty et al.[28] It was found that the only consistent signal across subjects that differed from baseline was the increased amplitude of the high gamma band following stimulation. The time course of high gamma increase was determined by counting the number of time points of the neural spectrogram from 0.1 to 2 seconds post pulse train with significantly greater high gamma amplitude than during the baseline period. Across all electrodes, over two thirds of the significant time points occurred in the time period between 0.1 and 0.3 s following stimulation. Thus, the average high gamma power during this time frame is used for following analyses.
2.3.2 Logistic regression on behavior
A logistic regression was performed to predict whether or not a subject perceived a given trial (binary value) as a function of stimulation frequency and amplitude, and their interaction. Random intercepts were included in the model, allowing the regression to capture differences among subjects in their perceptual thresholds.
2.3.3 Linear regression on HG amplitude
A linear regression was performed for each electrode for the single subject that was able to complete most of a 5×4 matrix of stimulation parameter values (current values of 1.5, 3, 4.5, 6, 7.5mA and frequencies of 10, 30, 50, and 100Hz). This extended matrix of parameters was tested because, in this rare case, the team had more time to collect data prior to the patient’s surgery. This afforded an opportunity to determine what factors best predict increases in high gamma amplitude, and whether there exist subpopulations of electrodes that respond to some parameters and not others. Parameters that went into the regression were frequency, current, “charge per time” (defined as frequency multiplied by current), percept (binary: 0 or 1), and a full model with all predictors included. Electrodes that never displayed an adjusted R2 of 0.1 or over were discarded from the analysis. Two additional outliers with abnormally high R2 values were identified and removed.
2.3.4 LDA classification of perceived trials
In the same subject, and for each stimulation parameter pair, two linear discriminant analysis classifiers were constructed to predict whether a conscious percept was elicited. In one model, the stimulation frequency, amplitude, and charge per time were the predictors. In the second model, the mean high gamma z-score from 100 to 300ms post-pulse train for all significant electrodes were the predictors. The classifiers were evaluated with cross-validation (trained on data from all parameter sets except two, which served as the held-out parameters to test). The same paradigm was then repeated over 100 iterations to define distributions for the accuracy of stimulation parameters and of high gamma in predicting perception. The result of each model was a percent correct over all predictions, obtained by dividing the number correct by the total number of predictions.
2.3.5 Testing for differences between HG after and during stimulation at threshold
For data after stimulation, the previous methods were used to determine which electrodes exceeded baseline levels in the high gamma range in the time period of 100–300ms post pulse train. A student’s t-test was performed for each of these electrodes to determine if there was a significant difference between high gamma values in perceived versus nonperceived trials from the threshold experiment. Electrodes with a significant difference in high gamma are plotted for a single example subject in Fig3B. For data during stimulation, the Lomb-Scargle algorithm was used to estimate power in the high gamma band with missing data points. Its input was raw data recorded 100ms after the start of a pulse train to 100ms before the end of a pulse train, with a total of 7.2ms of data missing in the time surrounding each stimulation pulse. A student’s t-test was performed on power estimates of high gamma amplitude for each trial obtained with the Lomb-Scargle algorithm. Electrodes that displayed above-baseline activity are plotted for a single example subject in Fig3C. In both cases, a paired t-test was used to test for differences in electrodes’ activity combined across patients in the case of perceived versus non-perceived stimuli.
Figure 3.
With stimulation parameters held constant, high gamma (HG) amplitude increases on perceived trials, as compared to non-perceived trials. A) Schematic: 3D MRI brain reconstruction with electrode array placement and stimulating pair is shown for one example subject who was stimulated with threshold level stimulation for 48 stimulation pulse trains. 22 were perceived and 26 were not perceived. Schematic shows the time included for during and after stimulation in relation to a stimulation pulse train. B) Most sites show greater HG activity after perceived trials. The T statistic is plotted for each electrode that increased its activity statistically above baseline after stimulation as determined using the same methods as in prior figures and that showed a significant difference between perceived and non-perceived trials as determined using a t-test. A positive T statistic (blue electrodes) denotes that the mean HG power after stimulation was higher in the case of perception over no perception. Across subjects, electrodes display significantly higher HG amplitudes during stimulation for perceived trials over non-perceived trials (paired t-test, p=6.74×10−4). C) Most sites show greater HG activity during perceived trials. The T statistic is plotted for each electrode that increased its activity at least 2 standard deviations above baseline during stimulation. A positive T statistic (blue electrodes) denotes that the mean HG power during stimulation was higher in the case of perception over no perception (t-test on Lomb-Scargle processed data). Across subjects, electrodes display significantly higher HG amplitudes during stimulation for perceived trials over non-perceived trials (paired t-test, p=0.0098).
Some readers may wonder if the response that we describe during stimulation is actually neural activity, or if it may be attributed to artifact due to electrical ringing or data filtration. This question may be addressed in three ways. First, our filtering methods take advantage of forward and reverse filters, so artifacts due to data processing/filtering would be expected to show up symmetrically on either side of any offending electrical artifact: that is, both before and after stimulation in terms of time. This is not the case, as shown in Figure 1C. Second, ringing artifacts are expected to be observed directly following stimulation offset (expected latency <1ms) and to decay exponentially, a phenomenon that we do not see, especially considering our data of interest arises at relatively long latency (>0.1second post stimulation). [29] Third, threshold trial data (Figure 3) show differences in neural activity even though stimulation and processing parameters were exactly the same. If the effect were due to artifact, one would expect that all trials would show the same high gamma increase. The fact that we find significantly greater high gamma power on consciously perceived trials than on otherwise identical unperceived trials demonstrates that the measured neural effects cannot be attributed to stimulation artifact.
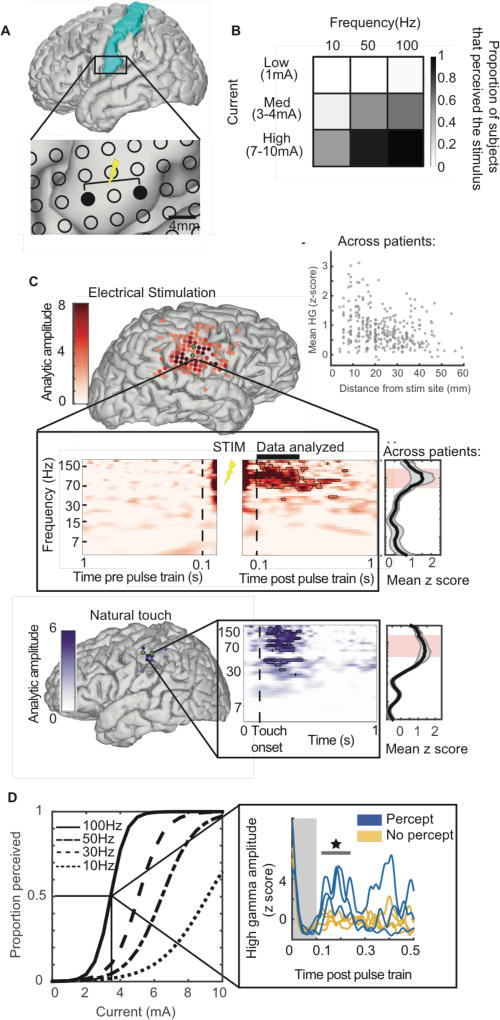
Figure 1.
Direct electrical stimulation (DES) evoked high gamma (HG) activation is correlated with conscious perception. A) Stimulation schematic. Somatosensory cortex (highlighted in blue) is the target site of stimulation for all patients. Inset: Bipolar stimulation occurs across two electrodes spaced 8mm apart. Neural activity is recorded throughout the experiment from electrodes surrounding the stimulation site. B) Proportion of subjects that perceived stimulation increases with frequency and amplitude. Proportion shown for each of the nine stimulation parameter combinations. C) HG increases following perceived stimulation. Mean HG activation 100–300ms post pulse train is mapped onto the cortical surface for an example subject following stimulation with a parameter set in which the subject reported feeling a percept. Stimulation electrodes are shown in green. Inset: neural spectrogram 1 second before and after stimulation for an example electrode. Neural frequency is on the y-axis in log space. Dashed lines mark 100ms on either side of the stimulation pulse train, denoting the conservative window of time that was discarded from analyses to avoid spectral bleed from stimulation artifact. Data points that were determined to be significantly different than baseline activity are outlined in black (z score with respect to baseline distribution, p < 0.01). Right panel: the amplitude during the time window of 100–300ms after stimulation is averaged across electrodes and patients and shown as the dark black line, with the standard error of the mean in gray. Red shaded region demarcates the HG range. Above: Scatterplot shows mean HG versus distance from stimulating pair for each significant electrode across patients. The HG amplitude 100–300ms post stimulation offset determines significant electrodes. Natural touch: Electrodes that significantly respond to natural touch are shown on the brain of an example subject in purple, with respect to stimulation electrodes in green. Inset shows the neural spectrogram of an example electrode with respect to onset of natural touch. Right panel: The amplitude after touch is averaged across electrodes and patients and shown as the dark black line, with the standard error of the mean in light gray. Red shaded region demarcates the HG range. D) Proportion of stimuli perceived is predicted by stimulation parameters and by HG activity. Logistic regression best-fit lines calculated across subjects are shown for each frequency of stimulation as a function of increasing current. Inset: Single trials of HG post pulse train are shown for an example electrode. 0–100ms post offset is shown in gray. To avoid stimulation artifact contamination, this part of the data is not included in analyses. Perceived trials are blue lines, and non-perceived trials are yellow lines. Gray bar demarcates time points in which perceived trials are significantly different than non-perceived trials (t-test, p<0.05).
3. Results
For each participant, we chose a single cortical site that had been determined through clinical mapping to evoke a somatosensory percept. We applied bipolar stimulation using a pair of electrodes at that site spaced 8mm apart (typical electrode spacing used clinically) (Fig1A). To determine the effect of stimulation parameters on perception and on neural activity, we employed a 3×3 stimulation pulse train paradigm that included at least five trials each of low (1mA), medium (3–4mA), and high (7–10mA) current amplitudes at frequencies of 10, 50, and 100 Hz. Stimulation pulse trains lasted one second and pulse width was 100µs. The participants were instructed to push a button with their ipsilateral thumb if they felt the stimulation. Subjects were blind to the timing of stimulation, and false positives were rare. Electrodes that were responsive to button press in isolation were excluded from further analyses. Across subjects, the proportion of trials that elicited a percept increased, as expected, with increasing charge delivered per unit time (defined here as stimulation frequency × current, Fig1B).
We next examined the neural recordings associated with stimulation pulse trains in the 3×3 paradigm. To avoid examining data contaminated with stimulation artifact, we analyzed data 100–300ms following the offset of stimulation. Across subjects, the only consistent evoked activity was in the 65–200Hz range (Fig1C), typically referred to as the high gamma range[22,28,30]. The frequency of the evoked response (high gamma) did not vary with stimulation frequency (as indicated by non-significant t-tests between average neural responses grouped by stimulation frequencies of 50Hz and 100Hz). In a minority of sites for some subjects, lower frequency bands showed significant deviations from baseline, but these changes were not observed for the majority of subjects in response to stimulation.
Focal bipolar stimulation was associated with high gamma activation of a relatively large cortical area surrounding the stimulation site. For sites that showed significant high gamma modulation in response to stimulation, the amplitude of their high gamma increase was, on average, related to their proximity to the stimulation site, but the variability in this relationship was high (Fig 1C, scatterplot, R2 of best fit=0.098). This spatial spread function is inconsistent with a simple volume conduction model, as distant sites regularly exhibit greater high gamma values than some directly adjacent sites. This spatial spread is therefore likely driven by cortico-cortical evoked potentials.
For a subset of subjects, we used the same analytical methods to quantify the neural response to natural touch in the absence of electrical stimulation (Fig1C, ‘Natural touch’). We applied light tactile pressure to the site that the patient reported their evoked percept while recording their neural response. As has been previously shown[31], natural touch reliably evoked high gamma activity in these subjects.
We used mixed-effects logistic regression across patients to quantify the separate effects of stimulation amplitude and frequency on the probability of causing a conscious percept (Figure 1D). Stimulation frequency multiplied by current, defined in this work using the term ‘charge per time,’ was also entered as a predictor of perception. Increased amplitude and increased charge per time were both associated with higher rates of reported perception (p < 10−4 and p < 10−6, respectively). Some parameter combinations produced a “threshold response” such that the patient felt the stimulation on approximately half of the trials. At such threshold parameter combinations in the 3×3 paradigm, we noted increased high gamma activity for perceived trials over non-perceived trials (Fig1D inset, t-test, p<0.05).
To test whether increased high gamma activity and conscious perception were consistently related, we compared perceptual reports with high gamma activity at stimulation-responsive sites (Fig2A). We found a pronounced co-occurrence of perceptual reports with increased high gamma, and a significant shift to greater high gamma activity for perceptual trials over non-perceptual trials in the 3×3 paradigm (Fig2B).
Figure 2.
High gamma (HG) amplitude predicts perception better than stimulation parameters. A) Neural data mirrors behavioral reports of perceived stimuli. Behavioral and neural data are shown for 3 example subjects. Although the goal was to obtain behavioral and neural data for a full 3 × 3 parameter set, some participants did not tolerate the higher stimulation intensities. At least five trials of each stimulation parameter pair were completed. Dark blue boxes indicate that the subject reported feeling the stimulus 5/5 or 100% of the trials unless otherwise noted by text in white. Yellow boxes indicate that the subject reported feeling the stimulus 0/5 trials. For the corresponding neural data, each box denotes a post-stimulus HG z score averaged over electrodes that showed significantly different activity from baseline in the HG band for any stimulation parameter pair, whether or not it was perceived. B) Across subjects, the HG z score of significant electrodes shows a shift toward higher values for perceived trials (the two distributions have significantly different means, p<0.05 in a paired t-test). C) Neither current, frequency, nor charge per time perfectly predict perception. An expanded experimental paradigm and its corresponding number of perceived trials are shown for one subject where additional stimulation parameters could be tested. Matched pairs of total charge within the extended matrix are outlined and denoted by their total charge (mA × Hz). D) Whether the stimulus was perceived predicts HG activity better than stimulation parameters at single electrodes. Adjusted R2 values for linear regressions predicting HG amplitude are shown for significant electrodes. Predictors are frequency (F), current (C), charge per time (CT), percept (P, input as 0 or 1), and a full model with all parameters (F,C,FxC,P). Filled dots are adjusted R2 values for each electrode, predicted by the parameter(s) above which they are centered. Gray lines connect the dots to denote the trajectory of the model fit for the same electrode predicted in each of the 5 cases. Neighboring groups were tested pairwise for differences in their means. Stars and brackets demarcate neighboring groups that show a significant difference (Student’s t-test, p<0.0125). E) HG predicts perception better than stimulation parameters predict perception in a classification analysis. LDA classification results for predicting perception at each stimulation parameter pair, bootstrapped over 100 iterations. Gray bars show the relative performance of stimulation parameters and HG across all electrodes above baseline as inputs to the classifier. HG predicts perception better than stimulation parameters of frequency, charge per time, and current (student’s t-test, p<10−5).
Patients tended to perceive stimulation during trials with pulse trains of high stimulation amplitude, high stimulation frequency, or a combination of the two. We expanded our 3×3 table of stimulation frequencies and amplitudes so that there was more continuity in tested levels of frequency and amplitude, and so that there were matched pairs of charge per time for a single subject (e.g. 10 Hz at 7.5 mA and 50 Hz at 1.5 mA, both of which inject 75 Hz × mA of charge; matched pairs are outlined in black and labeled in Fig2C). In some cases, even though equal amounts of charge per time are injected, the perceptual outcomes are different. This is exemplified by the two stimulation parameter combinations that result in a charge per time of 300Hz × mA, but whose perceptual outcomes are highly disparate (Fig2C). There are also examples of a higher charge per time resulting in fewer positive percepts than a lower charge per time. For instance, at 100Hz and 3mA ([300 Hz × mA]) the subject in Fig2C felt the stimulation in approximately 20% of the trials, yet at 50Hz and 4.5mA ([225 Hz × mA]), the subject felt the stimulation in 100% of trials. These differences in outcome from matched pairs of charge illustrate that it is not simply the charge per time that leads to a percept, nor is it solely the stimulation frequency or stimulation amplitude. Increasing stimulation parameters boosts the chance of evoking a conscious percept, but the stimulation parameters themselves are not the sole determinants of the perceptual outcome of stimulation.
So far, we have shown that stimulation parameters, high gamma activation, and perception are all related, but that stimulation parameters alone are not sufficient to predict either perceptual outcomes or the extent or amplitude of high gamma activation. These findings suggest that high gamma amplitude and conscious perception are correlated. We evaluated the best predictors of high gamma change by conducting a series of linear regression analyses for each recording site. For each site, the trial-by-trial high gamma activity following stimulation was modeled using frequency, current, charge per time, or percept as sole predictors. We compared the model fits to each other and to a full model that incorporated all of the factors (Fig2D). For the majority of electrodes, whether or not the stimulus was perceived was the best indicator of high gamma activity, and, moreover, for most electrodes, this simple binary model based on percept alone did approximately as well as the full model (mean increase in adjusted R2 was 0.018). That is, perception alone predicts high gamma as well as perception with all other stimulation parameters (the full model was not significantly different from that of the percept model, p = 0.56). Finally, we used an LDA classifier to predict whether the subject perceived the stimulation based on either stimulation parameters or high gamma activity across significant sites (Fig2E). We found that more trials were accurately classified using high gamma activity than stimulation parameters (93% versus 78%). Across these different methods of analysis, our results strongly suggest that the cortical high gamma response to stimulation predicts perception more accurately than stimulation parameters.
If high gamma evoked by DES is a reliable predictor of a subject’s behavioral response to stimulation, a compelling demonstration would be to show that high gamma activity is consistently greater for perceived than non-perceived trials, even when stimulation parameters are held constant. As such, we conducted a controlled psychophysical stimulation experiment with a subset of the experimental subjects, wherein we calibrated stimulation parameters to find each subject’s sensory threshold, defined as the stimulus level at which subjects perceive the stimulus about 50% of the time (Fig3A). By holding stimulation current and frequency constant at a subject’s threshold over many trials and comparing the recorded neural signal on perceived and non-perceived trials, it is possible to isolate biomarkers that reflect differences in the underlying neural processing of a stimulus, rather than differences in external factors such as stimulation parameters or stimulation artifact. Using electrical stimulation fixed at perceptual threshold, we still observe that the evoked high gamma is greater in perceived trials than non-perceived trials following stimulus presentation (Fig3B). For all patients that completed the threshold stimulation task, high gamma amplitude following stimulation is higher in perceived trials over non-perceived trials (Fig3B, paired t-test, t(42)=3.67, p = 6.74×10−4).
Up to this point, we have limited our analysis to evoked neural signals following electrical stimulation to avoid the concern of stimulation artifact during stimulation pulses. Threshold trials enable us to confidently estimate the differences in high gamma signal between perceived and non-perceived trials during stimulation, since the electrical stimulus (and any stimulation artifact) is identical for both conditions. The Lomb-Scargle algorithm[32,33] estimates the power spectrum of a time series with missing data points. The algorithm was used to compare the mean high gamma activity during stimulation at each electrode (see Methods). Many more electrodes in the vicinity of the stimulation site show increased HG during perceived trials as compared to non-perceived trials at threshold stimulation parameters (Fig 3C). For all patients that completed the threshold stimulation task, normalized high gamma amplitude during stimulation is higher in perceived trials over non-perceived trials (Fig3C, paired t-test, t(57)=2.67, p = 0.0098).
4. Discussion
Understanding the effects of cortical stimulation in humans is instrumental to the interpretation of both clinical and scientific studies that employ stimulation in assigning functions to cortical areas. In this study, we used clinically relevant stimulation parameters to evoke sensory percepts while measuring the neural response at cortical sites surrounding the stimulation site. Several experimental features enabled us to draw conclusions about neural correlates of effective cortical stimulation. In contrast to other cortical stimulation studies, we were able not only to record neural activity throughout stimulation using a high dynamic range amplifier, but also to densely sample this activity over centimeters of cortex directly adjacent to the stimulation site.
We have shown that: 1) DES evokes neural activity primarily in the high gamma frequency range, consistent with the neural response observed during natural touch; 2) high gamma increases in response to stimulation are found in cortical regions beyond the focal site of stimulation; and 3) evoked high gamma activity is significantly greater for stimulation that evokes conscious perception, both after stimulation and during stimulation at threshold stimulation levels. These responses were evident across participants, and the conclusions are independent of which anatomical sites in somatosensory cortex were stimulated.
One important implication of this work is that DES recapitulates normal physiology, evoking conscious perception via the same mechanism as natural stimuli. The characteristic increase in high gamma following stimulation that we found here is consistent with animal work describing increased neural firing in response to electrical microstimulation using pulse trains.[34] Activity in the high gamma range in response to natural stimuli has been observed in multiple sensory cortices [35–44], and it was explicitly distinguished from lower frequency bands in studies of human subjects using ECoG.[45,46] It has since proven to be a strong correlate of sensory, motor, and cognitive events.[23,28,31,45,47–50] High gamma activity correlates not only with the functional magnetic resonance imaging blood-oxygen level-dependent signal[51–53], but also with multi-unit firing rate[22], making it a reasonable proxy for evoked neural firing in response to stimulation. As single or even multi-unit recordings were impossible in this study, the nature of the subset of neurons that are stimulated is unclear. As described in previous studies[54], we surmise that it holds true that the axons of surrounding neurons are stimulated, leading to individual neuron depolarization and thereby accounting for the increased high gamma power as these neurons fire. Future studies may reveal whether the prevailing neural populations excited by stimulation are themselves excitatory, inhibitory, or mixed.
The high degree of regional connectivity of the cortex is evident from the broad spatial spread of high gamma responses, which could not be explained by volume conduction. Other studies have noted cortico-cortical evoked potentials centimeters away from the stimulation site[55,56], so it is not surprising that in driving cortical activity with DES we observe responses both near and far from the origin of stimulation. Resulting neural activation may cover a broad expanse (centimeters from the stimulation site) due to the interconnectivity of the stimulated site with other sites in the surrounding area.
While the high gamma power increase presented in this study strongly correlates with conscious perception, it cannot be definitively represented as a cause and effect relationship. It may be that the induced percept is causing high gamma response, for instance. In either case, it does not change the main claim of this work: that high gamma is a reliable biomarker of perceived stimuli.
In clinical practice, frequency and amplitude are two major parameters that are used to control stimulation effects. Charge delivered per unit time is the most relevant factor in whether stimulation exceeds threshold for a sensory percept. One example of this comes from microstimulation studies in rats, where it was shown that higher frequency and lower current may be used to achieve a similar sensation as lower frequency and higher current.[57,58] However, we have shown that predicting the outcome of stimulation using frequency and amplitude values is not as simple as summing the charge delivered. Equal increases in charge per time obtained using stimulation frequency as compared to stimulation amplitude will not have equal effects on either behavior or on neural activity, and frequency and amplitude may be tuned to deliver the same charge per time but affect perception differently. Importantly, a high frequency, low current stimulus may safely achieve the desired outcome without causing the damage to neural tissue that is associated with high currents. This knowledge is useful for achieving adequate stimulation levels while preserving the integrity of neural tissue in long-term devices.[59–61]
Finally, in this study, perception was assayed as a binary feature, but the behavioral response magnitude was not examined. Future work may include metrics of intensity or perceptual aspects associated with stimulation patterns or modulated stimulation profiles.[62,63]
5. Conclusion
We found that high gamma activation is a reliable biomarker of stimulation in sensory cortex that produces a subjective percept. Because in practice not every electrical stimulus causes a behavioral response, there are significant practical implications for future uses of this biomarker for clinical purposes. Furthermore, it is possible that DES induces high gamma activation at other brain regions where stimulation is expected to cause different behavioral effects. Therefore, high gamma power could be a biomarker for effective stimulation levels in many different applications. This would be especially useful for disorders in which there may not be obvious or immediate behavioral outcomes, such as in neuropsychiatric conditions.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Joseph O’Doherty for helpful discussion. This work was supported by grants from the NIH (U01NS098971 and R01-DC012379 to EFC, F32-DC013953 to JDR, F32-DC015966 to NPF). E.F.C is a New York Stem Cell Foundation-Robertson Investigator. This research was also supported by The New York Stem Cell Foundation, The McKnight Foundation, The Shurl and Kay Curci Foundation, and The William K. Bowes Foundation.
References
- 1.Penfield W, Boldrey E. Somatic Motor and Sensory Representation in the Cerebral Cortex of Man as studied by Electrical Stimulation. Brain. 1937 [Google Scholar]
- 2.Penfield W, Rasmussen T. The Cerebral Cortex of Man. A Clinical Study of Localization of Function.pdf. Academic Medicine. 1950;25:375. [Google Scholar]
- 3.Foerster O, Altenburger H. Elektrobiologische vorg{ä}nge an der menschlichen hirnrinde. 1934 [Google Scholar]
- 4.Purpura DP, Pool JL, Ransohoff J, Frumin MJ, Housepian EM. Observations on evoked dendritic potentials of human cortex. Electroencephalogr Clin Neurophysiol. 1957;9(3):453–9. doi: 10.1016/0013-4694(57)90034-2. [DOI] [PubMed] [Google Scholar]
- 5.Libet B. Electrical Stimulation of Cortex in Human Subjects, and Conscious Sensory Aspects BT - Somatosensory System. Somatosensory System. :743–90. [Google Scholar]
- 6.Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: A new door to brain plasticity. Brain. 2007;130:898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- 7.Duffau H. Acute functional reorganisation of the human motor cortex during resection of central lesions: a study using intraoperative brain mapping. J Neurol Neurosurg Psychiatry. 2001;70(4):506–13. doi: 10.1136/jnnp.70.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Taillandier L, et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg. 2003;98(4):764–78. doi: 10.3171/jns.2003.98.4.0764. [DOI] [PubMed] [Google Scholar]
- 9.Duffau H, Sichez JP, Lehéricy S. Intraoperative unmasking of brain redundant motor sites during resection of a precentral angioma: Evidence using direct cortical stimulation. Ann Neurol. 2000;47(1):132–5. [PubMed] [Google Scholar]
- 10.Berger MS, Rostomily AC. Low grade gliomas: Functional mapping resection strategies, extent of resection, and outcome. J Neurooncol. 1997;34(1):85–101. doi: 10.1023/a:1005715405413. [DOI] [PubMed] [Google Scholar]
- 11.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–64. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 12.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–45. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 13.Desmurget M, Song Z, Mottolese C, Sirigu A. Re-establishing the merits of electrical brain stimulation. Trends in Cognitive Sciences. 2013;17:442–9. doi: 10.1016/j.tics.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Leonard MK, Cai R, Babiak MC, Ren A, Chang EF. The peri-Sylvian cortical network underlying single word repetition revealed by electrocortical stimulation and direct neural recordings. Brain and Language. 2015 doi: 10.1016/j.bandl.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Front Hum Neurosci. 2010 Jan 4;(May):46. doi: 10.3389/fnhum.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winawer J, Parvizi J. Linking Electrical Stimulation of Human Primary Visual Cortex, Size of Affected Cortical Area, Neuronal Responses, and Subjective Experience. Neuron. 2016:1–7. doi: 10.1016/j.neuron.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borchers S, Himmelbach M, Logothetis N, Karnath H-O. Direct electrical stimulation of human cortex - the gold standard for mapping brain functions? Nat Rev Neurosci. 2012;13(1):63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- 18.Vincent M, Rossel O, Hayashibe M, Herbet G, Duffau H, Guiraud D, et al. The difference between electrical microstimulation and direct electrical stimulation - Towards new opportunities for innovative functional brain mapping? Rev Neurosci. 2016;27(3):231–58. doi: 10.1515/revneuro-2015-0029. [DOI] [PubMed] [Google Scholar]
- 19.Parvizi J, Jacques C, Foster BL, Withoft N, Rangarajan V, Weiner KS, et al. Electrical Stimulation of Human Fusiform Face-Selective Regions Distorts Face Perception. J Neurosci. 2012 Oct 24;32(43):14915–20. doi: 10.1523/JNEUROSCI.2609-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron. 2013 Dec 18;80(6):1359–67. doi: 10.1016/j.neuron.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangarajan V, Hermes D, Foster BL, Weiner KS, Jacques C, Grill-Spector K, et al. Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. J Neurosci. 2014;34(38):12828–36. doi: 10.1523/JNEUROSCI.0527-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–36. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121(Pt 1):2271–99. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- 24.Wahnoun R, Benson M, Helms-Tillery S, Adelson PD. Delineation of somatosensory finger areas using vibrotactile stimulation, an ECoG study. Brain Behav. 2015;5(10) doi: 10.1002/brb3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, et al. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131(7):1793–805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolston JD. A low-cost multielectrode system for data acquisition enabling real-time closed-loop processing with rapid recovery from stimulation artifacts. Front Neuroeng. 2009;2 doi: 10.3389/neuro.16.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards E, Soltani M, Kim W, Dalal SS, Nagarajan SS, Berger MS, et al. Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. J Neurophysiol. 2009;102(1):377–86. doi: 10.1152/jn.90954.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canolty RT, Soltani M, Dalal SS, Edwards E, Dronkers NF, Nagarajan SS, et al. Spatiotemporal dynamics of word processing in the human brain. Front Neurosci. 2007;1(1):185–96. doi: 10.3389/neuro.01.1.1.014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hambley AR. Pulse response. In: McDonald M, George DA, Disanno S, editors. Electrical Engineering: Principles and Applications. 4. Upper Saddle River, New Jersey: Pearson Education, Inc; 2008. pp. 546–8. [Google Scholar]
- 30.Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: Searching high and low. International Journal of Psychophysiology. 2011;79:9–15. doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crone NE, Miglioretti DL, Gordon B, Lesser RP, Crone N. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis II. Event-related synchronization in the gamma band. Brain. 1998;121(August 2016):2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 32.Lomb NR. Least-Squares Frequency-Analysis of Unequally Spaced Data. Astrophys Space Sci. 1976;39(2):447–62. [Google Scholar]
- 33.Scargle JD. Studies in Astronomical Time-Series Analysis .2. Statistical Aspects of Spectral-Analysis of Unevenly Spaced Data. Astrophys J. 1982;263(2):835–53. [Google Scholar]
- 34.Butovas S, Schwarz C. Spatiotemporal effects of microstimulation in rat neocortex: a parametric study using multielectrode recordings. J Neurophysiol. 2003;90(5):3024–39. doi: 10.1152/jn.00245.2003. [DOI] [PubMed] [Google Scholar]
- 35.Ectors L. Étude De L’Activité Électrique Du Cortex Cérébral. Arch Int Physiol. 1936 Jan 1;43(3):267–98. [Google Scholar]
- 36.Munk MH, Neuenschwander S. High-frequency oscillations (20 to 120 Hz) and their role in visual processing. J Clin Neurophysiol. 2000;17(4):341–60. doi: 10.1097/00004691-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Brosch M, Budinger E, Scheich H. Stimulus-related gamma oscillations in primate auditory cortex. J Neurophysiol. 2002;87(6):2715–25. doi: 10.1152/jn.2002.87.6.2715. [DOI] [PubMed] [Google Scholar]
- 38.Castelo-Branco M, Neuenschwander S, Singer W. Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retina in the anesthetized cat. J Neurosci. 1998;18(16):6395–410. doi: 10.1523/JNEUROSCI.18-16-06395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herculano-Houzel S, Munk MH, Neuenschwander S, Singer W. Precisely synchronized oscillatory firing patterns require electroencephalographic activation. J Neurosci. 1999;19(10):3992–4010. doi: 10.1523/JNEUROSCI.19-10-03992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuenschwander S, Singer W. Long-range synchronization of oscillatory light responses in the cat retina and lateral geniculate nucleus. Nature. 1996;379(6567):728–32. doi: 10.1038/379728a0. [DOI] [PubMed] [Google Scholar]
- 41.Cracco RQ, Cracco JB. Visual evoked potential in man: Early oscillatory potentials. Electroencephalogr Clin Neurophysiol. 1978;45(6):731–9. doi: 10.1016/0013-4694(78)90141-4. [DOI] [PubMed] [Google Scholar]
- 42.Goff GD, Matsumiya Y, Allison T, Goff WR. The scalp topography of human somatosensory and auditory evoked potentials. Electroencephalogr Clin Neurophysiol. 1977;42(1):57–76. doi: 10.1016/0013-4694(77)90151-1. [DOI] [PubMed] [Google Scholar]
- 43.Cobb WA, Dawson GD. The latency and form in man of the occipital potentials evoked by bright flashes. J Physiol. 1960;152:108–21. doi: 10.1113/jphysiol.1960.sp006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lachaux JP, George N, Tallon-Baudry C, Martinerie J, Hugueville L, Minotti L, et al. The many faces of the gamma band response to complex visual stimuli. Neuroimage. 2005;25(2):491–501. doi: 10.1016/j.neuroimage.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 45.Crone N, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clinical Neurophysiology. 2001 doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 46.Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol. 2005;94(6):4269–80. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- 47.Mesgarani N, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343(6174):1006–10. doi: 10.1126/science.1245994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung C, Chang EF. Real-time, time–frequency mapping of event-related cortical activation. J Neural Eng. 2012;9(4):46018. doi: 10.1088/1741-2560/9/4/046018. [DOI] [PubMed] [Google Scholar]
- 49.Crone NE, Hao L, Hart J, Boatman D, Lesser RP, Irizarry R, et al. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57(11):2045–53. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- 50.Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, et al. Attentional Stimulus Selection through Selective Synchronization between Monkey Visual Areas. Neuron. 2012;75(5):875–88. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309(5736):951–4. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 52.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 53.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RaW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309(5736):948–51. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 54.Ranck JB. Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Research. 1975;98:417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 55.Matsuzaki N, Juhász C, Asano E. Cortico-cortical evoked potentials and stimulation-elicited gamma activity preferentially propagate from lower- to higher-order visual areas. Clin Neurophysiol. 2013;124(7):1290–6. doi: 10.1016/j.clinph.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keller CJ, Bickel S, Entz L, Ulbert I, Milham MP, Kelly C, et al. Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc Natl Acad Sci U S A. 2011;108(25):10308–13. doi: 10.1073/pnas.1019750108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Butovas S, Schwarz C. Detection psychophysics of intracortical microstimulation in rat primary somatosensory cortex. Eur J Neurosci. 2007;25(7):2161–9. doi: 10.1111/j.1460-9568.2007.05449.x. [DOI] [PubMed] [Google Scholar]
- 58.Davidovics NS, Fridman GY, Chiang B, Santina CC Della. Effects of biphasic current pulse frequency, amplitude, duration, and interphase gap on eye movement responses to prosthetic electrical stimulation of the vestibular nerve. IEEE Trans Neural Syst Rehabil Eng. 2011;19(1):84–94. doi: 10.1109/TNSRE.2010.2065241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J Neurosci Methods. 2005 Feb 15;141(2):171–98. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 60.McCreery DB, Agnew WF, Yuen TGH, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng. 1990;37(10):996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- 61.Shannon RV. A Model of Safe Levels for Electrical Stimulation. IEEE Trans Biomed Eng. 1992;39(4):424–6. doi: 10.1109/10.126616. [DOI] [PubMed] [Google Scholar]
- 62.O’Doherty JE, Lebedev MA, Li Z, Nicolelis MAL. Virtual active touch using randomly patterned intracortical microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2012;20(1):85–93. doi: 10.1109/TNSRE.2011.2166807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Sci Transl Med. 2014 Oct 8;6(257):257ra138–257ra138. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.